Abstract
Hemophagocytic lymphohistiocytosis (HLH) is an often fatal hyperinflammatory syndrome. HLH may be inherited, but it more commonly arises secondary to Epstein-Barr virus (EBV) or other infections, hematologic malignancies, or rheumatologic diseases. We identified 17 patients diagnosed with HLH who had flow cytometric analysis of peripheral blood or bone marrow performed at the time of diagnosis. Two patients had primary HLH, and the others had HLH secondary to EBV infection, hematologic malignancies, rheumatologic conditions, or tuberculosis. The marrow typically showed a reactive lymphocytosis and a marked left shift in myelopoiesis regardless of the etiology. Qualitative abnormalities were also found in several cases, including T-cell abnormalities in the majority of the EBV-associated HLH cases. While not specific, flow cytometric findings in HLH are different from the findings in uninvolved marrow samples, and care should be taken not to overinterpret immunophenotypic findings in these cases as indicative of a primary marrow disorder or lymphoma.
Keywords: Hematopathology, Hematology, Flow cytometry, Hemophagocytic lymphohistiocytosis, Epstein-Barr virus
Hemophagocytic lymphohistiocytosis (HLH) is a relatively rare hyperinflammatory syndrome that is often fatal. This disorder is thought to result from impaired function of cytotoxic T cells and natural killer (NK) cells leading to inappropriately sustained immune activation and high cytokine levels. Patients with HLH have a characteristic constellation of signs, symptoms, and laboratory abnormalities, including fever, hepatosplenomegaly, cytopenias, hypofibrinogenemia, hypertriglyceridemia, and an elevated serum ferritin level.1 While this constellation of findings is diagnostic of HLH, many of these symptoms are nonspecific, and the diagnosis of HLH can be challenging, particularly in the early stages of disease.
HLH is divided into 2 broad categories: familial or primary HLH and secondary HLH that is related to an underlying disorder such as an infection, hematologic malignancy, or rheumatologic disease. The majority of familial HLH cases result from genetic defects that affect the function of NK and T cells.1,2 Numerous mutations have been implicated, all of which participate in the formation and intracellular transport of cytoplasmic granules.1 In addition to these familial diseases, several other inherited immunodeficiency syndromes can also result in the development of HLH. These include Chédiak–Higashi syndrome, Griscelli syndrome, and type II Hermansky-Pudlak syndrome. The mutations underlying these syndromes also disrupt granule formation or vesicle trafficking, similar to familial HLH.1–5 In addition, children and young adults who have X-linked lymphoproliferative disorder (XLP), which is associated with mutations in the SH2D1A (SH2 domain containing 1A) or XIAP (X-lined inhibitor of apoptosis) genes, are especially prone to the development of HLH as a result of Epstein-Barr virus (EBV) infection.6,7
Secondary HLH is much more common than primary HLH. Unlike primary HLH, secondary HLH tends to occur in older children and adults. EBV is the most common infection that triggers secondary HLH, but numerous other viral and bacterial infections can also precipitate HLH, including cytomegalovirus, herpes simplex virus, and Mycobacterium tuberculosis.8 HLH is also associated with malignancies (ie, malignancy-associated hemophagocytic syndrome), particularly lymphomas.9 Patients with rheumatologic diseases, including juvenile rheumatoid arthritis and Still disease, can also develop a form of HLH called macrophage activation syndrome.9 Regardless of the etiology, HLH can be rapidly fatal and requires prompt treatment with immunosuppressive drugs.
The striking cytopenias that are seen in HLH often prompt bone marrow examination. While the morphologic findings of HLH in bone marrow biopsies have been well characterized, the flow cytometric findings in the blood and bone marrow of patients with HLH have not been described in detail. In the present study, we reviewed the biopsy findings and flow cytometric analyses of blood or bone marrow samples from patients with a diagnosis of HLH at our institution during the past 10 years. We correlated these findings with the clinical history and laboratory data to ascertain abnormalities or patterns of antigen expression particular to primary and secondary HLH.
Materials and Methods
The surgical pathology database of the Johns Hopkins Hospital, Baltimore, MD, was searched for diagnoses containing the words “hemophagocytic lymphohistiocytosis,” “hemophagocytic syndrome,” “hemophagocytosis,” and “HLH” from January 2001 to July 2010. We identified 17 new diagnoses of HLH in which a bone marrow biopsy was done and flow cytometric data were obtained from bone marrow or peripheral blood samples at the time of diagnosis. Clinical data were reviewed by one of us (A.S.D.) in accordance with the institutional review board–approved protocol NA_00043502.
Flow cytometric immunophenotyping was performed on peripheral blood or fresh bone marrow aspirates. The material was collected in EDTA or heparin anticoagulant and processed routinely using a red cell lysis method. Cell suspensions were incubated with combinations of 4 monoclonal antibodies (Becton Dickinson, San Jose, CA) that were used at concentrations titrated for optimal staining. In most cases, the panel included antibodies specific for CD45, CD71, HLA-DR, CD33, CD2, CD3, CD4, CD5, CD7, CD8, T-cell receptor (TCR) αβ, TCR γδ, CD19, CD20, CD10, CD13, CD16, CD34, CD56, CD11b, CD15, and CD117, although some specimens were subjected to an abbreviated panel. Selected antibody combinations were conjugated to fluorescein isothiocyanate, phycoerythrin, peridinin-chlorophyll protein, and allophycocyanin fluorochromes. Specimens were analyzed on a BDIS FACSCalibur flow cytometry system (Becton Dickinson). List-mode data files were acquired and analyzed for each specimen using CellQuest and Paint-a-Gate software programs (Becton Dickinson). All flow cytometric data for patients with HLH were reviewed by 3 of us (C.M.M., M.J.B., and A.S.D.).
Results
Patient Characteristics
We identified 17 patients who had been diagnosed with HLH and had a bone marrow biopsy and corresponding flow cytometric analysis of bone marrow or peripheral blood performed at the time of diagnosis. Of the patients, 10 were male and 7 were female Table 1. The median age was 16 years, and the patients ranged in age from 6 months to 75 years. Of the patients, 7 had EBV-associated HLH, 1 had EBV-associated diffuse large B-cell lymphoma, and 1 had EBV infection and T-cell lymphoma. The remaining patients had a variety of disorders, including rheumatologic conditions (4 patients), precursor B-cell acute lymphoblastic leukemia (1 patient), tuberculosis (1 patient), and familial HLH (2 patients). Of the patients with familial HLH, one was known to have a truncation of the UNC13D gene (also known as unc-13 homolog D or Munc13-4), and the etiology was unknown for the other patient.
Table 1.
Clinicopathologic Data for 17 Patients With Hemophagocytic Lymphohistiocytosis
| Case No./ Sex/Age (y) |
Etiology | Pancytopenia | Fever | Elevated Ferritin Level |
Decreased Fibrinogen Level |
Elevated Triglycerides Level |
Outcome |
|---|---|---|---|---|---|---|---|
| 1/M/8 | EBV | Y | Y | Y | Y | N | Died |
| 2/F/10 | EBV | A/T | Y | Y | Y | Y | Alive |
| 3/M/15 | EBV | Y | Y | Y | Y | Y | Alive |
| 4/M/17 | EBV | L/T | Y | Y | Y | Y | Alive |
| 5/M/20 | EBV | Y | Y | Y | Y | Y | Died |
| 6/M/20 | EBV | Y | Y | Y | Y | Y | Died |
| 7/F/21 | EBV | Y | Y | Y | Y | Y | Died |
| 8/F/36 | EBV-associated DLBCL | Y | N | Y | Y | Y | Died |
| 9/M/71 | EBV + T-cell lymphoma | Y | Y | Y | Y | Y | Died |
| 10/F/0.5 | MUNC13 truncated | Y | Y | Y | N | Y | Alive |
| 11/M/1.5 | Familial HLH | Y | Y | Y | N | ND | Alive |
| 12/F/75 | Tuberculosis | Y | Y | Y | Y | ND | Died |
| 13/M/4 | B-ALL | Y | Y | ND | N | ND | Died |
| 14/M/31 | Still disease | Y | UNK | Y | N | Y | Alive |
| 15/M/4 | JRA | Y | Y | Y | N | Y | Died |
| 16/F/8 | JRA | Y | Y | Y | N | N | Alive |
| 17/F/16 | JRA | Y | Y | Y | Y | ND | Alive |
A/T, anemia + thrombocytopenia; B-ALL, B lymphoblastic leukemia; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein-Barr virus; JRA, juvenile rheumatoid arthritis; L/T, lymphopenia + thrombocytopenia; N, no; ND, not done; UNK, unknown; Y, yes.
All patients had a similar clinical picture regardless of etiology (Table 1). Of the 17 patients, 15 had pancytopenia; the remaining 2 patients had thrombocytopenia and anemia or lymphopenia. Of 16 patients, 15 were febrile at the time of diagnosis. Other laboratory values consistent with HLH were also found; 16 (100%) of 16 patients had an elevated ferritin level, 11 (65%) of 17 had a decreased fibrinogen level, and 11 (87%) of 13 had elevated levels of triglycerides.
Bone Marrow Biopsy Findings
Of the 17 patients, 16 had evidence of hemophagocytosis on bone marrow biopsy. The most common histologic findings were hypercellular marrow with markedly increased histiocytes and cytotoxic T cells Image 1. The patient who consistently had normocellular marrow with no evidence of hemophagocytosis in a series of 4 biopsies during a 6-month period was a 1.5-year-old boy with familial HLH of unknown etiology.
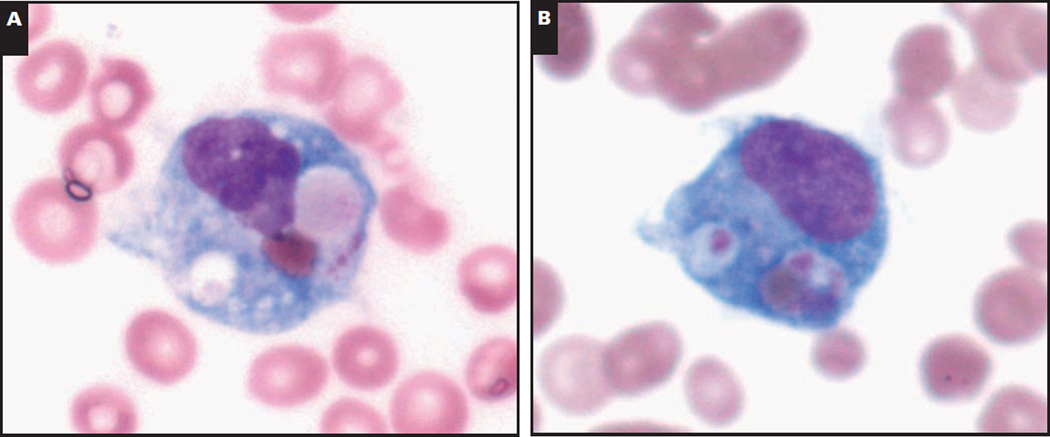
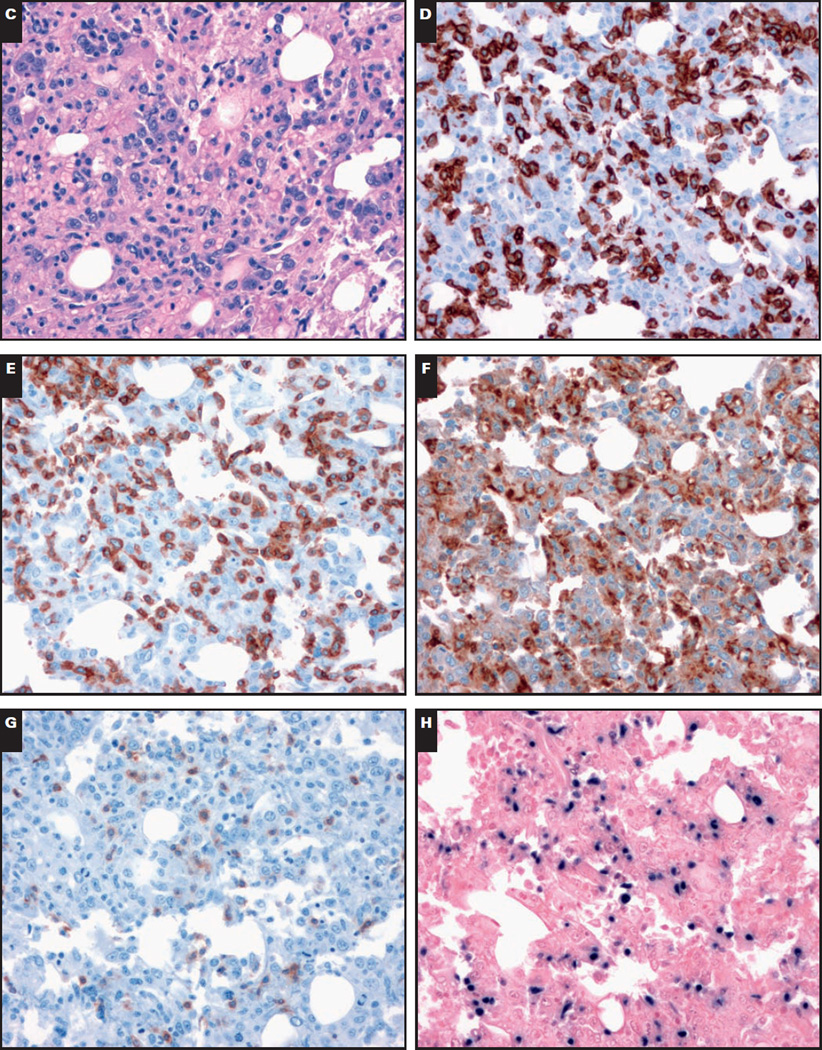
Image 1.
Representative morphologic and immunohistochemical findings in the bone marrow of a 20-year-old with Epstein-Barr virus (EBV)-associated hemophagocytic lymphohistiocytosis. A and B, Bone marrow aspirate. Prominent hemophagocytosis (A, modified Wright-Giemsa, ×100; B, modified Wright-Giemsa, ×100).
C–H, Bone marrow biopsy specimen. Normocellular bone marrow (C, H&E, ×40) with an abundance of cytotoxic T lymphocytes (D, CD8, ×40; E, CD3, ×40) and numerous histiocytes (F, CD68, ×40). The T lymphocytes show loss of CD5 (G, ×40). H, In situ hybridization for EBV (EBV-encoded RNA) is positive (×40).
Although most patients had only 1 bone marrow biopsy, we found that the bone marrow findings changed as the disease progressed in 1 patient with juvenile rheumatoid arthritis who had multiple marrow biopsies throughout the course of her illness. Initially, she had a fever of unknown origin and marked leukocytosis (WBC count, 54,450/µL [54.5 × 109/L]), but during the next 16 days, her WBC count dropped to 210/µL (210.0 × 109/L), and she developed anemia and thrombocytopenia. A bone marrow biopsy done the next day showed hypercellular marrow with marked megakaryocytic and histiocytic hyperplasia, although no significant hemophagocytosis was seen. A second biopsy 3 days later showed the first histologic evidence of hemophagocytosis, and treatment with etoposide, cyclosporine, and dexamethasone was initiated. A third bone marrow biopsy performed 2 weeks later showed that the marrow was predominantly composed of lymphocytes, and there was a marked decrease in histiocytosis and hemophagocytosis. One month later, the marrow was hypocellular but all normal hematopoietic elements were present and showed normal maturation, and within 3 months the marrow demonstrated complete recovery.
Flow Cytometric Findings
Flow cytometric analysis was performed on bone marrow or blood samples of all patients. Quantitative abnormalities were noted in the marrow regardless of etiology Table 2. There was a relative decrease in the fraction of myeloid cells (normal, 50%–60% in children; 50% –70% in adults) in the marrow (median, 42.5%; range, 4% –72%).10 Lymphoid cells in the marrow (normal, 20% –30% in children; 10% –15% in adults) were relatively increased (median, 39%; range, 11%–75%), with abundant T cells in most cases and increased NK cells in 1 case (Table 2).10 The increase in lymphocytes often reflected the high number of cytotoxic T cells, and the CD4/CD8 ratio was less than 1 in most cases of EBV-related HLH and two cases of HLH associated with rheumatologic conditions. Of 15 cases, 13 (87%) expressed the activation marker HLA-DR on the cytotoxic T cells.
Table 2.
Flow Cytometric Findings for 17 Patients With HLH
| Case No. |
Diagnosis | Lympho- cytes (%) |
Lymphocyte Subsets (%) |
Immunophenotype, CD8+ T Cells |
Characteristics of Myeloid Cells | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | NK | T | CD4/ CD8 |
CD3 Loss |
CD5 Loss |
CD7 Loss |
HLA- DR Gain |
Granulo- cytes (%) |
Abnormal Side Scatter |
CD13 Loss |
CD33 Loss |
Decreased CD10+ Cells |
HLA- DR Gain |
|||
| 1 | EBV | 22 | 2 | 1 | 19 | 0.85 | N | N | Y | ND | 36 | N | ND | N | Y | Y |
| 2 | EBV | 49 | 2 | 3 | 44† | 0.17 | N | N | N | Y | 33 | N | N | N | Y | Y |
| 3 | EBV | 37 | 1 | 1 | 35 | ND | N | N | N | Y‡ | 49 | N | N | N | Y | Y |
| 4 | EBV | 12 | 1 | <1 | 11 | 0.13 | Y | Y | N | Y | 72 | N | ND | N | Y | Y |
| 5 | EBV | 75 | 2 | 16 | 57 | 0.33 | N | Y | Y | Y‡ | 8 | N | ND | N | Y | Y |
| 6 | EBV | 66 | <1 | 7 | 59 | 0.21 | N | Y | Y | Y | 9 | N | N | N | Y | Y |
| 7 | EBV | 16 | 1 | <1 | 15 | 0.08 | N | Y | N | Y | 65 | N | Y | N | Y | Y |
| 8* | EBV/ DLBCL | 3 | <1§ | <1 | 2 | 3.09 | N | N | N | Y | 87 | N | ND | N | Y | Y |
| 9 | EBV/PTCL | 13 | <1 | <1 | 12‖ | 1.35 | N | Y | Y | ND | 54 | N | ND | N | Y | Y |
| 10* | MUNC13 mutation | 91 | 13 | 9 | 69 | 2.53 | N | N | Y¶ | Y | 6 | N | ND | N | N | N |
| 11 | Familial HLH | 24 | 4 | ND | 18 | 7.48 | N | N | N | Y‡ | 51 | N | ND | N | ND | N |
| 12* | Tuberculosis | 5 | <1 | <1 | 5 | 2.7 | N | N | ND | Y | 86 | N | ND | N | Y | Y |
| 13 | B-ALL | 93 | <1 | 82 | 11 | 2.7 | N | N | N | N | 4 | Y | ND | N | ND | Y |
| 14 | Still disease | 61 | 4 | 2 | 55 | 0.52 | N | N | N | Y | 8 | N | ND | N | N | N |
| 15 | JRAf | 41 | 8 | 1 | 41 | 0.31 | N | N | N | Y | 49 | N | ND | N | N | Y |
| 16 | JRA | 11 | 9 | 1 | 1 | 7.5 | N | N | N | Y | 68 | N | N | N | N | N |
| 17 | JRA | 41 | 34 | 0 | 7 | ND | N | N | N | N | 20 | Y | Y | Y | Y | Y |
B-ALL, precursor B acute lymphoblastic leukemia; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein-Barr virus; HLH, hemophagocytic lymphohistiocytosis; JRA, juvenile rheumatoid arthritis; N, no; ND, not done; NK, natural killer; PTCL, peripheral T-cell lymphoma; Y, yes.
Peripheral blood was used in cases 8, 10, and 12; in the other cases, bone marrow was used.
Rare large granular lymphocytes.
Immunophenotype of CD8+ T cells was not specifically determined, but this immunophenotype was seen on the majority of T cells.
Includes neoplastic B cells.
Includes neoplastic T cells.
Also seen on CD4+ T cells.
Immunophenotypic abnormalities were identified in many cases. Of 9 cases of EBV-associated HLH, 6 (67%) had expansions of CD8 T-cell populations with variable loss of expression of CD5, CD7, and/or CD3 Image 2. Of 8 cases of HLH that were not EBV-associated, 5 (63%) had increased T cells. However, only 1 case of HLH that was not associated with EBV (1/8 [13%]) had immunophenotypically abnormal cytotoxic T cells. In this case, a patient with familial HLH (a known Munc13-4 truncation) had a small population of CD8+ T cells that demonstrated loss of CD7. T-cell abnormalities were much more likely to be seen in EBV-associated than in non–EBV-associated cases of HLH (6/9 vs 1/8; P = .04).
Image 2.
Representative flow cytometric findings in lymphocytes of a 20-year-old with Epstein-Barr virus (bone marrow). A, There is a marked lymphocytosis with relatively few granulocytes. A significant subset of the lymphocytes are activated (gain of HLA-DR). B, The lymphocytes are predominantly T cells. A significant population of T cells lacks CD5. C, The T lymphocytes are predominantly cytotoxic T cells, many of which show loss of CD7. D, The T lymphocytes have appropriate expression of CD2 and express T-cell receptor (TCR) αβ. APC, allophycocyanin; FITC, fluorescein isothiocyanate; FSC, forward scatter; PE, phycoerythrin; PerCP, peridinin-chlorophyll protein; SSC, side scatter.
The myeloid series also showed quantitative and qualitative abnormalities. The percentage of myeloid cells was low (<40%) in 44% (4/9) and 50% (4/8) of EBV-associated and non–EBV-associated HLH cases, respectively. There were also immunophenotypic abnormalities in the myeloid cells, which were more common and pronounced in the cases of EBV-associated HLH. The EBV-associated cases showed a marked left shift in myelopoiesis, often with near complete absence of normal mature CD10+ granulocytes Image 3. All of these cases also showed expression of HLA-DR on the granulocytes, possibly in response to the massive cytokine release seen in HLH.11,12 Other abnormalities noted in the myeloid lineage included loss of CD13 on granulocytes in an EBV-associated case and loss of CD13 and CD33 on granulocytes in a case associated with juvenile rheumatoid arthritis. Abnormally low side scatter in the granulocytes was seen in 2 cases.
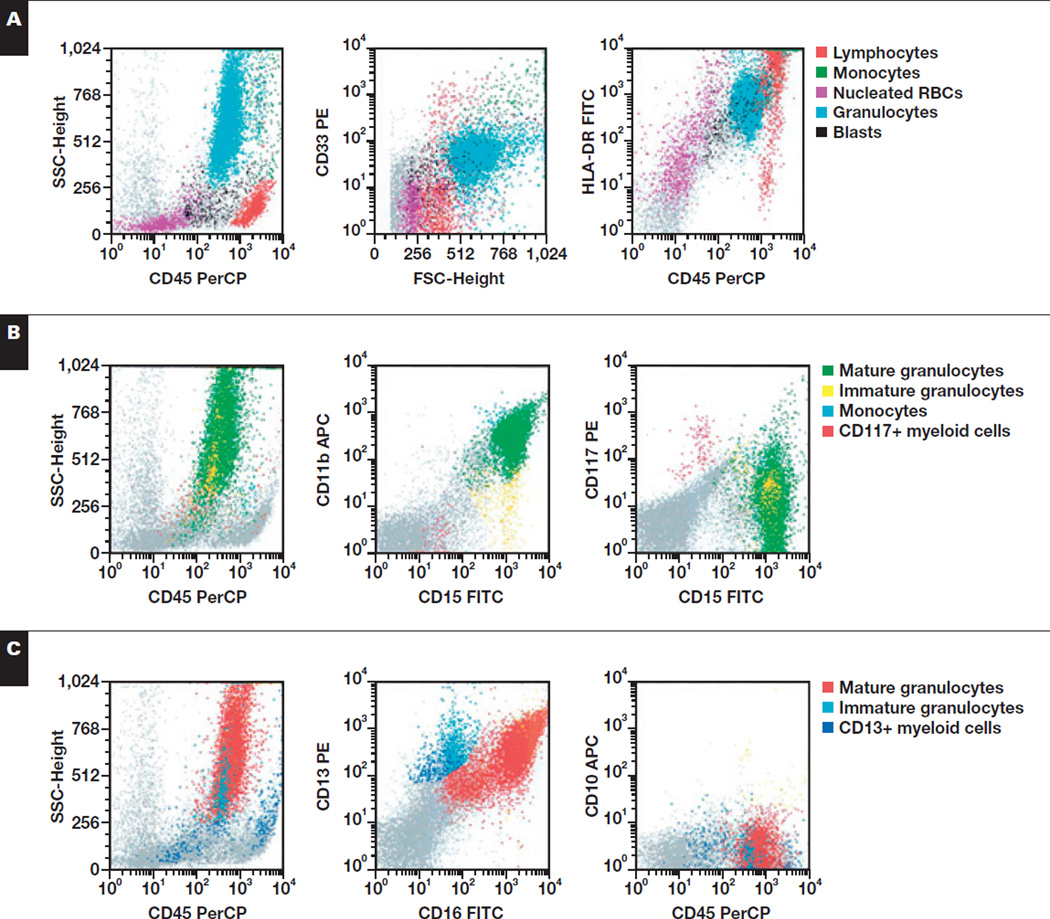
Image 3.
Representative flow cytometric findings in myeloid cells of a 21-year-old with Epstein-Barr virus–associated hemophagocytic lymphohistiocytosis (bone marrow). A, Granulocytes show normal SSC and appropriate expression of CD33 but are HLA-DR+. B, The granulocytes show appropriate expression of CD15 and CD11b and are CD117−. C, Myeloid cells show appropriate acquisition of CD13 and CD16 but express dim CD10. APC, allophycocyanin; FITC, fluorescein isothiocyanate; FSC, forward scatter; PE, phycoerythrin; PerCP, peridinin-chlorophyll protein; SSC, side scatter.
Discussion
A review of flow cytometric analyses of bone marrow and blood samples from patients with HLH demonstrated a range of quantitative and qualitative flow cytometric abnormalities in hematopoietic cells. The marrow typically showed a relative decrease in myeloid elements and an increase in lymphocytes. Prominent and unusual but phenotypically variable CD8+ T cells were often present, particularly in EBV-associated HLH. Many cases showed left-shifted myelopoiesis with occasional altered expression of myeloid antigens. These atypical findings on flow cytometric analysis are nonspecific but could potentially result in diagnostic confusion.
Flow cytometric analysis showed a population of atypical cytotoxic T cells in the majority of EBV-related cases of HLH. These atypical cells were largely absent in non–EBV-related HLH. These findings are in keeping with several previous reports of CD5 down-regulation on cytotoxic lymphocytes in patients with EBV-associated HLH but not patients with infectious mononucleosis.13–15 To our knowledge, this is the first report to describe the loss of other T-cell antigens in EBV-HLH, including CD7 and CD3. Previous studies have also found that clonal TCR gene rearrangements may be seen in the atypical cytotoxic T cells of patients with EBV-associated HLH.13,15,16 TCR gene rearrangement studies were performed on only 1 of the EBV-associated samples in the current study, which demonstrated a polyclonal population of T cells.
We also identified immunophenotypic changes in the myeloid lineage, including dim expression of CD10 and strong expression of HLA-DR on granulocytes. The altered expression of CD10 on granulocytes is likely the result of a left shift in myeloid maturation as the marrow replaces granulocytes that were engulfed by the activated histiocytes. Dim expression of CD10 was seen in all cases of EBV-associated HLH but in only 2 cases of non–EBV-associated HLH. Despite the pronounced left shift seen in many of the marrow samples, increased blasts were not seen in any case, so the presence of blasts in a patient with HLH should prompt evaluation for an underlying primary marrow disorder.
Strong expression of HLA-DR on granulocytes was seen in all cases of EBV-associated HLH and half of the non–EBV-associated HLH cases. It is interesting that the granulocytes expressed HLA-DR in 3 of the 4 patients who died of non–EBV-related HLH. The expression of HLA-DR on granulocytes has been seen in patients with high levels of cytokines, including interleukin-3, interferon-γ, and granulocyte/ macrophage colony-stimulating factor; thus, it may serve as a marker of cytokine release, and these high levels of cytokines could portend a poor outcome in non–EBV-associated HLH.11
Three cases also showed decreased side scatter or loss of the myeloid markers CD13 and CD33 in the granulocytes. There was, however, no morphologic evidence of dysplastic changes in the myeloid lineage in any of these cases. Two of the patients had a karyotype performed from unstimulated cultures while they had HLH. The karyotype of 1 patient with EBV-associated HLH was 46,XX,inv(9)(p12q13)[20], a variant that has been detected in healthy people and is not known to be clinically significant.17 A second patient who had HLH associated with juvenile rheumatoid arthritis had a normal female karyotype.
The immunophenotypic abnormalities seen in these samples can present diagnostic challenges if the clinical features of HLH are not well developed or bone marrow biopsy and/or aspirate samples are not available for morphologic examination. Decreased side scatter in the granulocytes and the unusual pattern of expression of myeloid antigens and HLA-DR can mimic flow cytometric findings in a myelodysplastic syndrome; in difficult cases, cytogenetic evaluation of the marrow may be helpful.
The loss of 1 or more T-cell antigens shown by flow cytometric analysis of the marrow, particularly in the setting of a T-cell clone, can suggest a T-cell lymphoma or a T-cell lymphoproliferative process such as large granular lymphocytosis. Recognition of concomitant abnormalities in the myeloid lineage may be helpful in suggesting a diagnosis of HLH based on flow cytometric analysis; however, to rule out a T-cell lymphoma, morphologic assessment of the marrow in conjunction with EBV studies of the serum or tissue may be necessary. The distinction between reactive and neoplastic populations of atypical T cells in HLH is particularly important because HLH can be associated with underlying T-cell lymphomas. The presence of 2 distinct atypical populations of T cells in a patient with HLH is worrisome for an underlying T-cell malignancy. A population of atypical CD4+ T cells, as noted in the 1 case in the present study with a concurrent T-cell lymphoma, is highly suggestive of marrow involvement by an underlying T-cell neoplasm since we did not see abnormal CD4+ T-cell populations arising as a consequence of HLH.
Distinguishing the atypical reactive CD8+ T cells seen in HLH from a population of neoplastic CD8+ T cells is more problematic. CD8+ T-cell lymphomas are uncommon, so care must be taken not to overinterpret such a population as evidence of malignancy, especially if the clinical picture is suggestive of HLH. Many T-cell lymphomas that express CD8, such as some nasal type NK-T cell lymphomas or intestinal T-cell lymphomas, have distinctive manifestations and can, therefore, be suspected on clinical grounds. One T-cell lymphoma, CD8+ subcutaneous panniculitis-like T-cell lymphoma, is often associated with the development of HLH, but fortunately essentially never becomes leukemic so that finding abnormal circulating T cells in this disease is most likely to support a diagnosis of HLH rather than disseminated lymphoma.18 The phenotypically abnormal cytotoxic T cells seen in HLH can also be confused with large granular lymphocytes (LGLs). In our experience, LGLs tend to show a comparatively subtle loss of CD5 or 2 CD3+ populations with dim and absent CD5. The LGLs also usually demonstrate at least partial coexpression of CD56 or CD57, and concomitant myeloid abnormalities are rarely seen. The clinical and laboratory findings are also typically quite different between HLH and large granular lymphocytosis.
Nearly all of the described immunophenotypic abnormalities were more prevalent in EBV-associated HLH, suggesting that EBV, rather than HLH per se, drives the phenotypic abnormalities in these cases. Two of the EBV-associated cases were seen in conjunction with lymphoid malignancies, but 7 of the EBV-associated HLH cases occurred in previously healthy people ranging in age from 8 to 21 years. While the male patients in this series were not tested for mutations associated with XLP, phenotypic abnormalities were similar between male and female patients, suggesting that XLP, if present, was not in itself a cause for the observed phenotypic abnormalities.
On receipt of a sample with clinical information that suggests a diagnosis of HLH, it is critical to characterize the myeloid and lymphoid lineages with comprehensive analysis of T-cell subsets. In addition, because HLH is associated with underlying hematopoietic neoplasms, it is important to rule out the possibility of an underlying B-cell lymphoma or acute leukemia. While flow cytometric findings may suggest HLH, the diagnosis of HLH requires correlation with clinical and laboratory findings, and these cases are typically signed out descriptively. The need to correlate with clinical and laboratory findings to make a definitive diagnosis of HLH means that it is also not possible to make this diagnosis based on morphologic findings in a marrow biopsy sample. Of note, hemophagocytosis can be seen in patients who do not have the constellation of signs and symptoms associated with HLH, and, conversely, patients with HLH may show no evidence of hemophagocytosis.
This case series presents a novel overview of flow cytometric abnormalities seen in HLH. The most striking abnormalities were seen most often, but not exclusively, in EBV-associated HLH. While no flow cytometric finding was specific for HLH, the findings were different from those seen in normal marrows, so that care should be taken not to overinterpret phenotypic findings in these cases as indicative of lymphoma or primary marrow disorders.
Upon completion of this activity you will be able to:
define primary and secondary hemophagocytic lymphohistiocytosis (HLH).
discuss the immunophenotypic abnormalities seen in flow cytometry of bone marrow in patients with HLH.
compare the T-cell abnormalities seen in patients with Epstein-Barr virus–associated HLH with those seen in HLH secondary to other disease states.
Footnotes
The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
References
- 1.Arceci RJ. When T cells and macrophages do not talk: the hemophagocytic syndromes. Curr Opin Hematol. 2008;15:359–367. doi: 10.1097/MOH.0b013e3282f97f88. [DOI] [PubMed] [Google Scholar]
- 2.Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Eur J Pediatr. 2007;166:95–109. doi: 10.1007/s00431-006-0258-1. [DOI] [PubMed] [Google Scholar]
- 3.Enders A, Zieger B, Schwarz K, et al. Lethal hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type II. Blood. 2006;108:81–87. doi: 10.1182/blood-2005-11-4413. [DOI] [PubMed] [Google Scholar]
- 4.Shiflett SL, Kaplan J, Ward DM. Chédiak-Higashi syndrome: a rare disorder of lysosomes and lysosome related organelles. Pigment Cell Res. 2002;15:251–257. doi: 10.1034/j.1600-0749.2002.02038.x. [DOI] [PubMed] [Google Scholar]
- 5.Klein C, Philippe N, Le DF, et al. Partial albinism with immunodeficiency (Griscelli syndrome) J Pediatr. 1994;125:886–895. doi: 10.1016/s0022-3476(05)82003-7. [DOI] [PubMed] [Google Scholar]
- 6.Rigaud S, Fondaneche MC, Lambert N, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 7.Coffey AJ, Brooksbank RA, Brandau O, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 8.Ishii E, Ohga S, Imashuku S, et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007;86:58–65. doi: 10.1532/IJH97.07012. [DOI] [PubMed] [Google Scholar]
- 9.Janka GE. Hemophagocytic syndromes. Blood Rev. 2007;21:245–253. doi: 10.1016/j.blre.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Foucar M. Bone Marrow Pathology. 2nd. Chicago, IL: ASCP Press; 2001. [Google Scholar]
- 11.Gosselin EJ, Wardwell K, Rigby WF, et al. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J Immunol. 1993;151:1482–1490. [PubMed] [Google Scholar]
- 12.Janka G. Hemophagocytic lymphohistiocytosis: when the immune system runs amok. Klin Padiatr. 2009;221:278–285. doi: 10.1055/s-0029-1237386. [DOI] [PubMed] [Google Scholar]
- 13.Toga A, Wada T, Sakakibara Y, et al. Clinical significance of cloned expansion and CD5 down-regulation in Epstein-Barr virus (EBV)-infected CD8+ T lymphocytes in EBV-associated hemophagocytic lymphohistiocytosis. J Infect Dis. 2010;201:1923–1932. doi: 10.1086/652752. [DOI] [PubMed] [Google Scholar]
- 14.Wada T, Kurokawa T, Toma T, et al. Immunophenotypic analysis of Epstein-Barr virus (EBV)-infected CD8+ T cells in a patient with EBV-associated hemophagocytic lymphohistiocytosis. Eur J Haematol. 2007;79:72–75. doi: 10.1111/j.1600-0609.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 15.Lin MT, Chang HM, Huang CJ, et al. Massive expansion of EBV+ monoclonal T cells with CD5 down regulation in EBV-associated haemophagocytic lymphohistiocytosis. J Clin Pathol. 2007;60:101–103. doi: 10.1136/jcp.2005.034371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn JS, Rew SY, Shin MG, et al. Clinical significance of clonality and Epstein-Barr virus infection in adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. 2010;85:719–722. doi: 10.1002/ajh.21795. [DOI] [PubMed] [Google Scholar]
- 17.Tawn EJ, Earl R. The frequencies of constitutional chromosome abnormalities in an apparently normal adult population. Mutat Res. 1992;283:69–73. doi: 10.1016/0165-7992(92)90124-z. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez CL, Medeiros LJ, Braziel RM, et al. T-cell lymphoma involving subcutaneous tissue: a clinicopathologic entity commonly associated with hemophagocytic syndrome. Am J Surg Pathol. 1991;15:17–27. doi: 10.1097/00000478-199101000-00002. [DOI] [PubMed] [Google Scholar]