Abstract
The large increase in the incidence of esophageal adeno-carcinoma in the West during the past 30 years has stimulated interest in screening for Barrett’s esophagus (BE), a precursor to esophageal cancer. Effective endoscopic treatments for dysplasia and intramucosal cancer, coupled with screening programs to detect BE, could help reverse the increase in the incidence of esophageal cancer. However, there are no accurate, cost-effective, minimally invasive techniques available to screen for BE, reducing the enthusiasm of gastroenterologists. Over the past 5 years, there has been significant progress in the development of screening technologies. We review existing and developing technologies, new minimally invasive imaging techniques, nonendoscopic devices for cell collection, and biomarkers that can be measured in blood or stool samples. We discuss the status of these approaches, data from clinical studies of their effects, and their anticipated strengths and weaknesses in screening. The area is rapidly evolving, and new tools will soon be ready for prime time.
Keywords: Biomarkers, Biophotonics, Cytology, Endoscopy, FISH
Screening is defined as performing a test on a group of people to look for evidence of a medical condition.1 Screening programs are effective for diseases that can be identified at early stages of development and that affect a significant proportion of the population. A screening program only works if it significantly reduces the burden of disease in the population and is cost effective.
Esophageal adenocarcinoma (EAC) is the most common esophageal malignancy in Western countries and has a poor prognosis; less than 15% of patients are alive 5 years after diagnosis.2-4 The standardized incidence rates of EAC have increased greatly over the past 30 years (approximately 7% per year), although more recent evidence indicates a smaller increase in incidence rate (2% per year).3,5 It seems possible to screen for patients at risk for esophageal cancer. The premalignant stage, Barrett’s esophagus (BE), has a relatively long time of progression to cancer.6,7 Transformation of BE to cancer occurs via intermediate disease stages, including low-grade and high-grade dysplasias; endoscopic interventions have been proven in randomized controlled trials to prevent disease progression.8,9 Furthermore, the risk factors for BE and EAC are well defined, so it should be possible to screen a high-risk population.
Gastroesophageal reflux disease (GERD) is the most common risk factor for EAC, with central obesity, smoking, male sex, white race, hiatal hernia, and aging contributing to risk.10-12 There are rare families with inherited forms of EAC,13 and genome-wide association studies have associated some low-penetrance susceptibility alleles with sporadic cases.14,15 BE usually progresses to cancer without changes in symptoms; the cancer is not usually detected until the tumor has advanced to a stage that cannot be treated.2 A recent population study of patients with EAC associated a prior diagnosis of BE with detection of lower-stage tumors and longer survival.16
The population prevalence of BE is believed to be approximately 1.5%.17,18 This value increases to 15% for individuals with a long history of reflux symptoms.19-21 Most cases of BE are identified during endoscopy for symptomatic gastroesophageal reflux. A diagnosis of BE is generally followed by endoscopic surveillance to identify cancer at an early stage, when it can be treated.22 However, more that 60% of patients who present with dysphagia and are found to have EAC have long histories of uninvestigated symptoms.23 This could be because heartburn and indigestion can be reduced with over-the-counter medications, causing patients to wait long periods before finally seeing a physician. As a consequence, most cases of BE are undiagnosed; <5% of patients undergoing resection for EAC have a previous diagnosis of BE.24 Screening for BE, especially in patients with multiple risk factors, might therefore be justified because it can lead to early diagnosis and management of BE.
Nonetheless, many factors have dampened the enthusiasm of gastroenterologists for screening for BE.25 Population-based studies and a large meta-analysis found the incidence of cancer among patients with BE to be lower than previously believed (currently estimated at approximately 0.3% per year).26-28 Also, more than one-third of patients with EAC deny a previous history of symptomatic reflux disease, posing a problem for screening strategies based exclusively on reflux symptoms.10,29 Increased understanding of the risk factors related to BE and EAC should help plan more effective programs that select high-risk patients.30 One recent study found that a combination of a long duration of reflux exposure, older age, the presence of central obesity, and a history of smoking better predicted which patients would develop BE than did reflux symptoms alone.31
Some cases of EAC develop in people without BE,32,33 so screening only for BE could miss some patients who will still develop this cancer. However, a pathologic study of patients with EAC before and after chemotherapy found that the tumor can grow over areas of premalignant disease, so BE is not always detected. In this study, BE was revealed after neoadjuvant treatment of up to 90% of cases with no previous signs of BE.34
The only validated screening approach for BE involves standard white light endoscopy with collection of biopsy specimens, which is invasive and expensive. Gastroenterology societies therefore recommend against screening unselected populations, because modeling studies have shown that this approach is not cost effective (Table 1).35-39 On the other hand, in light of the improved outcomes of patients with early-stage cancer40 and the increasing evidence for the efficacy of endoscopic therapies before invasion of the submucosa,9,41-43 increased screening and early diagnosis could reduce EAC mortality. For screening to be justified, it would need to include mechanisms to identify patents at highest risk for cancer progression without increasing the burden on endoscopic surveillance programs.
Table 1.
Recommendations for BE Screening
| Society | Year | Screening for general population |
Targeted population for screening |
|---|---|---|---|
| American College of Gastroenterology | 2008 | Not recommended | To be established |
| American Gastroenterological Association | 2011 | Not recommended | Patients with multiple risk factors (age older than 50 years, white race, male sex, chronic GERD, hiatal hernia, obesity) |
| American Society for Gastrointestinal Endoscopy | 2012 | Not recommended | Patients with multiple risk factors |
| British Society of Gastroenterology | 2013 | Not recommended | Patients with GERD and at least 3 of the following risk factors: age older than 50 years, white race, male sex, and obesity; threshold to be lowered in case of family history |
NOTE. Other European society guidelines are currently under preparation.
Although multiple societies recommend forms of selective screening, this approach has never been validated in a prospective population-based study. To advance the case for screening, we will consider the availability and efficacy of diagnostic tests for BE that might be acceptable to patients, affordable, and practical for the health care systems to implement.44,45 We will also discuss the technologies that have the potential to satisfy these criteria, including standard endoscopy coupled with biopsies or cytology, new imaging techniques, nonendoscopic cell collection devices, and biomarkers that can be measured in blood or stool.
Endoscopy and Biopsy Collection
Screening for EAC has traditionally involved endoscopy and collection of biopsy specimens from any suspicious lesions in the esophagus. Typically, patients have been selected for screening based on their higher risk of BE than that of the general population. The patients are sedated, and oral endoscopy is performed. Additional, indirect costs include time taken off work by both patients and those who accompany them.46 In screening procedures, it is recommended that biopsy specimens be collected only from patients with endoscopically visible columnar tissue in the tubular esophagus; otherwise, the presence of intestinal metaplasia can be difficult to distinguish from gastric cardia intestinal metaplasia.47 Collection of normal-appearing squamocolumnar junction tissues for histopathologic analysis generates additional costs. The examination should be conducted with high-definition endoscopes, with careful observation of the mucosa in the tubular esophagus. In expert hands, assessment of the mucosal pattern, in conjunction with dye chromoendoscopy or virtual chromoendoscopic imaging, can help to identify areas of potential intestinal metaplasia. Mucosal patterns have been identified that correlate with the presence of intestinal metaplasia.48 However, advanced imaging techniques with endoscopic magnification have not been found to consistently increase the yield of intestinal metaplasia detection compared with careful standard white light endoscopy.35 Biopsy specimens of any visible lesion should also be performed to exclude potential malignancy.
Screening endoscopy and histological findings could be used to stratify patients for risk and management. Overall, even though standard endoscopy with biopsy collection has the highest degree of diagnostic accuracy and is used as the standard in research studies, it is limited by its high costs. Previous modeling studies showed that endoscopy could be a cost-effective screening tool, with an incremental cost-effectiveness ratio that ranges from approximately $10,000 to $24,000 per quality-adjusted life year.49,50 These ratios, however, were determined based on the assumption that BE has a much higher rate of progression to cancer than is currently estimated, and the model did not take into account subsequent surveillance. In conclusion, there is noevidence that standard endoscopy is cost effective for screening unselected populations for BE or EAC.51
More recently, narrow field imaging techniques such as confocal laser endomicroscopy and probe-based Raman spectroscopy have had increased interest because they can identify intestinal metaplasia in near real time.52-54 Despite the theoretical advantage of obtaining optical biopsy specimens during endoscopy, these technologies are expensive and require dedicated equipment. These techniques are most likely to be used in determining the prognosis of high-risk patients with BE rather than for screening the general population.
Standard Endoscopy With Cytological Analysis
Brush cytology sampling of the mucosa during endoscopy has been advocated as an alternative to endoscopic collection of biopsy specimens. This technique allows larger areas of esophageal epithelium to be sampled. However, there is a tendency for dysplastic cells to break free more than normal cells, so this technique might enrich the sample for dysplasia.
Brush cytology sampling could reduce the initial cost of sample processing compared with that of multiple biopsy specimens.55 Findings from cytological analysis have 70% to 80% concordance with those from histological analysis and have been shown to identify high-grade dysplasia and cancer with more than 80% sensitivity and 95% specificity.56,57 However, it only detects nondysplastic BE with 33% to 60% sensitivity, according to different studies, and it is not likely to accurately detect low-grade dysplasia.58,59 Additionally, acute inflammation can produce false-positive results; in cytological preparations, some cells look abnormal, with features of cancer cells.60
In addition to routine cytological analysis, cells collected can be analyzed by molecular markers that can be used in diagnosis and risk stratification. Immunocytochemical assays can detect increases in markers such as cyclin A, and fluorescence in situ hybridization can detect gains or losses in chromosomes.58,61-63 The combination of cytological and molecular analyses of cells could increase the diagnostic power of a simpler sampling method and be used to identify the best therapeutic strategies for patients. However, it could significantly increase the costs of screening, particularly if complex laboratory methodologies such as those used in immunohistochemistry or fluorescence in situ hybridization are involved; these require expensive sets of fluorescent probes, manual assessment by experienced technicians, or automated analysis with elaborate equipment.62,63
A computer-assisted brush biopsy technique (EndoCDx; CDx Diagnostics, New York, NY) has been devised to aid the diagnosis of BE. In this procedure, a stiff endoscopic brush device can sample deeper layers of the esophageal epithelium and provide information about cells and some structures. The brush is proprietary and has the ability to remove small strips of mucosa. These samples are sent to a central processing location in New Jersey for analysis. The samples are analyzed by a high-speed automated process, and artificial intelligence software assists a trained pathologist in identifying abnormal cells and glandular structures with features of BE or dysplasia. In 2 prospective studies, computer-assisted brush biopsy analysis increased the diagnostic yield for BE by approximately 70% and for dysplasia by 87% compared with standard biopsy protocols.64,65 However, the technology has to be combined with conventional endoscopy, and pathologists have to be specially trained in the computer analysis. The method could be used to screen patients, as well as predict their outcomes and determine their management, based on the degree of dysplasia.
Although endoscopic cytological analysis has some advantages over biopsy analysis, it is still limited by sampling errors and does not overcome the need for conventional endoscopy. Cytology also has a high-false positive rate for detection of dysplasia where inflammation coexists.
Transnasal Endoscopy
Transnasal intubation can increase patient tolerance for the endoscopy procedure. The procedure can be performed with only topical anesthetic because it avoids contact with the root of the tongue, preventing gagging during oral intubation. Two crossover studies have shown that transnasal videoendoscopy can accurately diagnose BE. Furthermore, biopsy specimens can be collected that are of sufficient quality for histological analysis.66,67 In addition, objective acceptability tools have indicated that transnasal endoscopy (TNE) is generally preferred by patients and produces lower levels of anxiety compared with conventional endoscopy.67 All of the major endoscope manufacturers have produced ultrathin endoscopic instruments for transnasal use; these can be used in conjunction with standard endoscopy towers (Table 2). Electronic enhancements such as narrow band imaging are available with these instruments, although magnification is not.
Table 2.
Ultrathin Transnasal Endoscopes
| Office based | Channel diameter |
Distal tip | Working length | Angulation up/down |
Angle of view | |
|---|---|---|---|---|---|---|
| Pentax, EE1580K | No | 2 | 5.5 | 600 | 210/120 | 140° |
| Fuji, EG-530NP | No | 2 | 4.9 | 1100 | 210/120 | 120° |
| Olympus, GIFXP 190N/290N | No | 2.2 | 5.4 | 1100 | 210/90 | 140° |
| Vision Sciences, EndoSheath | Yes | 2.1 | 4.7 | 650 | 145/220 | 120° |
| IntroMedic, EG scan II | Yes | Not applicable | 6 | 1088 | 160/160 | 125° |
NOTE. These are the best available models from Pentax, Fuji, and Olympus.
A randomized crossover study that compared a small-caliber 1-knob 5.1-mm flexible endoscope (Olympus, Melville, NY) with conventional endoscopy in patients with GERD or BE reported “moderate” agreement in identification of BE by these 2 methods (k = 0.59). However, the biopsy specimens collected with the small-caliber endo-scope were significantly smaller in size due to its small operating channel. On the other hand, more than 70% of patients preferred TNE to conventional endoscopy.68
The EndoSheath (Vision Sciences, Orangeburg, NY) is an ultrathin endoscope with a 2-way angulation system (Table 2). It is used in combination with a disposable sheath, which has a working channel for biopsy forceps. In a study of 426 patients with GERD who underwent the EndoSheath procedure, 38% were found to have lesions (34% with erosive esophagitis and 4% with BE).69
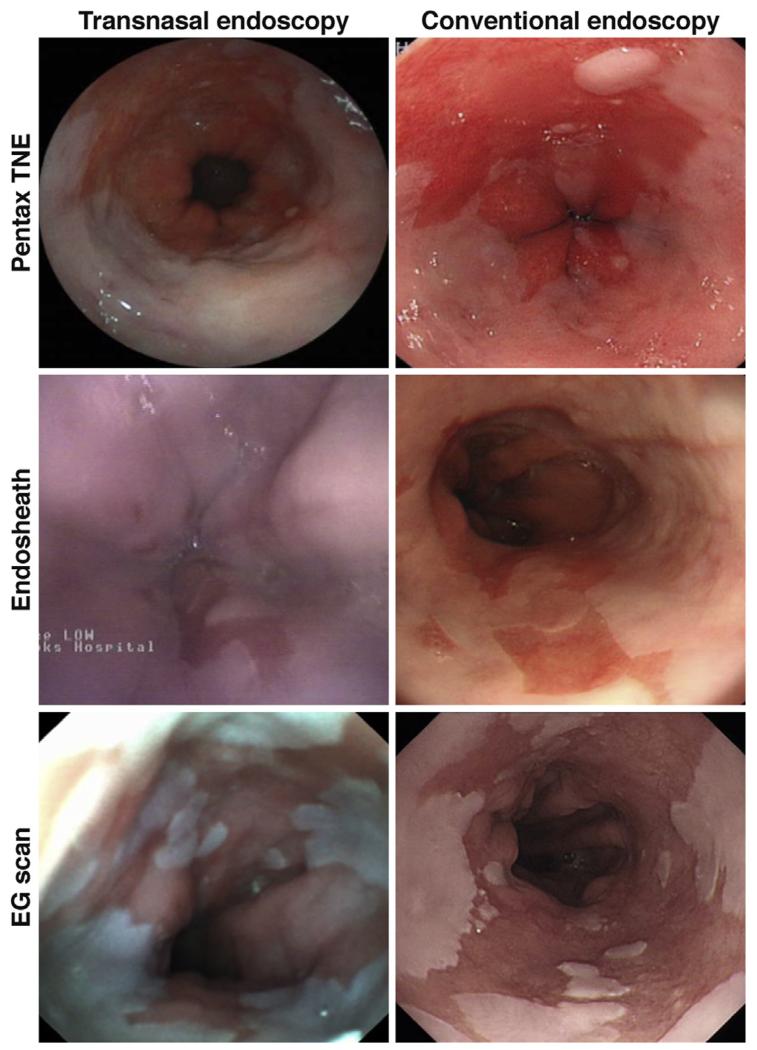
A disposable esophagoscope (EG scan; IntroMedic, Seoul, South Korea) equipped with a 2-way angulation system and an air channel for insufflation is commercially available. In a prospective study of 96 patients, it detected BE with a good level of agreement with standard endoscopy(k = 0.619 ± 0.123). In general, disposable systems generate images of lower quality (Figure 1) than conventional high-definition videoendoscopes, and the effect on diagnostic accuracy needs to be carefully addressed.
Figure 1.
Three cases of BE imaged with 3 different types of TNE systems (left panel), with a corresponding picture taken with a conventional white light Olympus system (right panel images). (Top left panel, Pentax TNE; middle left panel, office-based EndoSheath; bottom left panel, office-based EG scan).
Even though TNE was preferred to standard endoscopy in the studies performed, it is difficult to predict what proportion of the population would accept it within a primary care screening program, given that it is performed without sedation. Another important limitation of this technology is that it must be performed by a physician, which limits its potential for population-based screening. Advantages of this technology include its ability to collect biopsy specimens that, even though smaller than those collected by conventional endoscopes, can nonetheless help determine disease stage. Ultrathin TNE appears to be a promising tool for screening for BE, although more data on diagnostic accuracy and the cost-effectiveness of disposable systems are needed.
Single-Fiber Endoscope
A more sophisticated, small-diameter instrument called a single-fiber endoscope can be passed orally and allows excellent visualization of the esophageal mucosa. It is similar in diameter to that of a capsule, although its sheath has a smaller diameter. This instrument differs from standard endoscopes in that it does not require image sensors because there is only a single fiber, which is rapidly rotated to create a rasterized image of the mucosa. Its single-fiber design greatly reduces the size of the instrument to an outside diameter of only 1.6 mm. It uses laser sources for illumination, allowing for fluorescence and narrow band imaging. Multiple sophisticated optical tools could theoretically be added to the platform, such as 2-photon or confocal microscopes. This technology has been tested in pilot studies in humans, but a commercialized version is not available.
Limitations are that the device does not permit operator control of the endoscope and there is no capability for collection of biopsy specimens.70 A tethered version is also available, which could produces images in real time. If a commercialized version includes optical biopsy capabilities, this technology could be used in diagnosis as well as prognosis, especially if used in conjunction with in vivo molecular markers. However, it is likely to have a high cost.
Volume Laser Endomicroscopy
Optical coherence tomography has been used to identify subsquamous BE and areas of dysplasia; it involves a probe operated much like the B mode.71 However, it has no multi-dimensionality, which is required to scan large mucosal surfaces. A newer generation of this technology, called volume laser endomicroscopy (VLE), has incorporated a rotating optical frequency domain imaging probe into a catheter centered inside a balloon to provide large-volume microscopic reconstructions of tubular organs (such as the esophagus or coronary arteries) at a depth of 2 mm.72 A VLE-tethered capsule has also been created, which uses a rotating optical frequency domain imaging probe to circumferentially scan the esophageal lumen at a 30-μm lateral and 7-μm axial resolution.73
After the capsule is swallowed and delivered by esophageal peristalsis to the stomach, the operator can withdraw it, along the esophageal body, to generate cross-sectional images of the mucosa. This technology generated high-quality endomicroscopic images of the esophageal mucosa in a proof-of-principle study of 7 controls and 6 patients with BE.73
Even though this technology was conceived for screening, it can also detect dysplasia in the mucosa. Its resolution is lower than that of laser confocal endomicroscopy, but it has greater depth of penetration into the submucosa. Dysplasia is assessed based on analysis of surface maturation patterns caused by increased scattering of light by dysplastic cells with enlarged nuclei and the presence of abnormal submucosal glandular structures (Figure 2). These structures appear to be the result of an altered submucosal microenvironment with activated stromal fibroblasts. One advantage of this technique is that it could be used to scan the entire BE segment at high resolution.
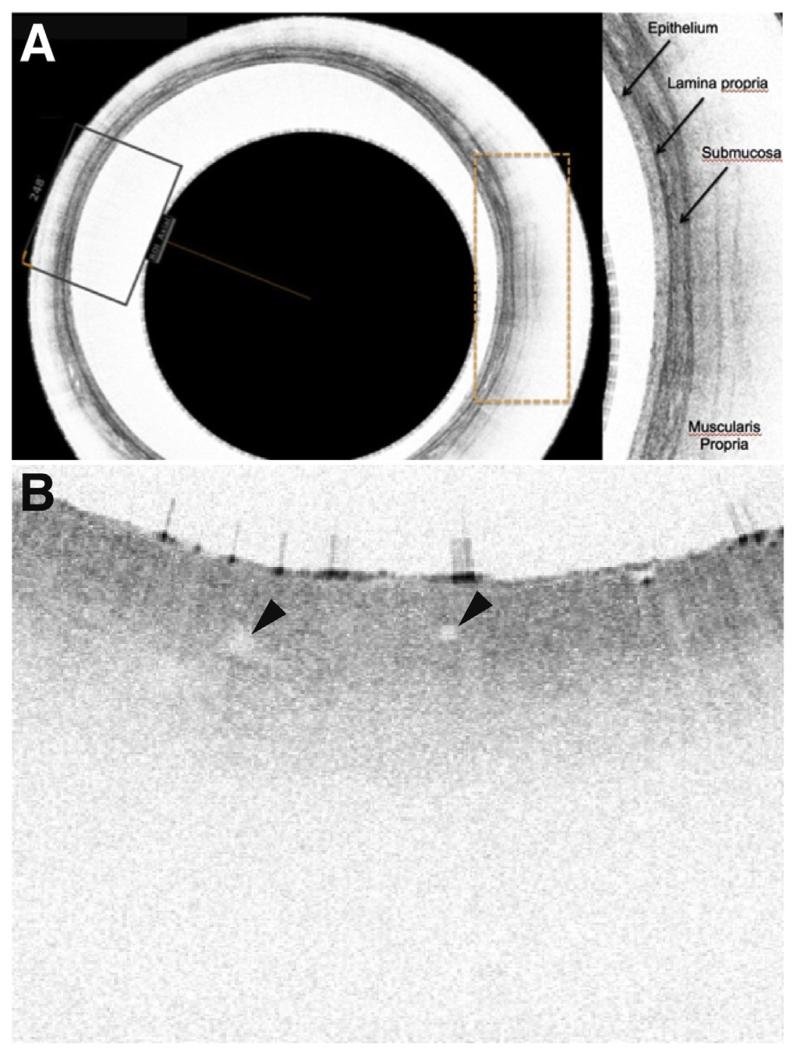
Figure 2.
Examples of VLE. (A) A VLE circular scan with imaging of the multilayered squamous esophagus and identification of the layered structure. Sections of the scan can be expanded in the device. It is apparent that this resolution is superior than can be seen with even the highest-resolution endoscopic ultrasound probes. (B) Sector magnification of VLE shows a mucosa without a great degree of surface scattering, indicating a likelihood of nondysplastic BE. The glands indicated by the arrowheads suggest the presence of an abnormal submucosal microenvironment.
Cost will depend on the ability to reuse the capsule, a fixed-diameter device. Although image analysis is complex, it could be performed by a medical technician, which would be advantageous over endoscopic techniques. Overall, this technology represents a new interesting avenue for research, but it is not currently available for the clinic. Further studies are required to show a correlation between imaging patterns and histological findings and its accuracy in identification of BE in a screening setting.
Nonendoscopic Technologies
Nonendoscopic devices are being developed to collect cells for cytological analysis. For example, a catheter that can be passed transorally has an inflatable balloon at the end equipped with soft cones to increase collection of cells by mechanical scraping. Two comparative studies investigated the accuracy of identification of BE using cells collected using this technology. Even though analyses of cells collected using balloon technology detected high-grade dysplasia and cancer with 80% sensitivity, the accuracy of identification of benign BE was low,74,75 so this device is not suitable for BE screening.
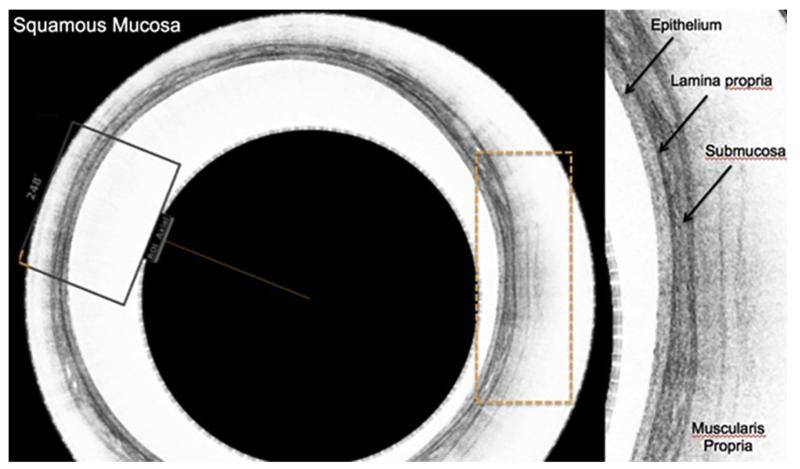
More promising results have been obtained with the Cytosponge, a cell collection device developed with Medical Research Council technology. Cells collected with this technology can be used in immunohistochemical assays. The Cytosponge device is composed of reticulated foam (approximately 30 mm in diameter) compressed within a gelatin capsule and attached to a simple string. The capsule is swallowed by the patient; after a maximum of 5 minutes to allow dissolution of the gelatin and expansion of the polyester foam, the device is retrieved by the operator (usually a nurse). During the passage of the sponge, cells are absorbed from the entire length of the esophagus. The cells are retrieved from the sponge, and immunohistochemistry is performed to detect expression of markers such as trefoil factor 3 (TFF3). TFF3 was found in a gene expression microarray study to be a marker that can distinguish intestinal cells of BE from other columnar cells derived from the normal gastric cardia and upper airways (Figure 3).
Figure 3.
Layered epithelium in the cross sectional view on the left with a much enhanced sector view on the right showing the individual layers of the squamous mucosa.
In a feasibility study of more than 500 patients with GERD, analysis of cells collected with the Cytosponge detected BE of more than 1 cm with 73% sensitivity and BE of more than 2 cm with 90% sensitivity; specificity values were more than 90%. The procedure could be performed in the primary care setting and was well tolerated by patients; 82% had only low levels of anxiety before and after the procedure.20 However, due to the low prevalence of BE in this cohort (3%), this feasibility study was not sufficient to determine diagnostic accuracy. A large multicenter case-control cohort study (BEST2 study) involving more than 1000 patients was recently completed in the United Kingdom and will provide information on the diagnostic accuracy of BE. A recent cost analysis using a micro-simulation model confirmed that, compared with conventional endoscopy, collection of cells by Cytosponge and analysis led to an equal number of quality of life years gained but was more cost effective. Furthermore, when coupled with endoscopic therapy, Cytosponge collection and analysis is cost effective in reducing EAC mortality.76
Cell collection by Cytosponge and subsequent TFF immunohistochemical analysis seem to have the characteristics required for a clinically applicable screening tool, including potentially low cost, suitability for primary care, excellent tolerability, and potentially good diagnostic accuracy. Similar to endoscopic brush collection of cytology specimens, cells collected by Cytosponge can be assessed by histological and molecular analyses to simultaneously provide information such as grade of dysplasia and potential for transformation into cancer cells. However, the Cytosponge is not commercially available, and the lower sensitivity of detection of shorter segments of BE is a potential issue.
Untethered endoscopic microgrippers, designed by a research group at Johns Hopkins University (Baltimore, MD), have been developed for random collection of cytology samples from large gastrointestinal organs.77 The micro-grippers are star-shaped nickel appendages smaller than 1 mm with flexible joints that close and grip at 37 °C (Figure 4). Hundreds can be dispersed in water and then swallowed. They distribute randomly along the mucosal surface and can then be retrieved with a magnet. Tissue microspecimens collected from porcine esophagi contained tissues suitable for cytological analysis, and squamous cells were detected by microscopy.77 This technology might someday be used in screening for gastrointestinal disorders but has not yet been tested in humans.
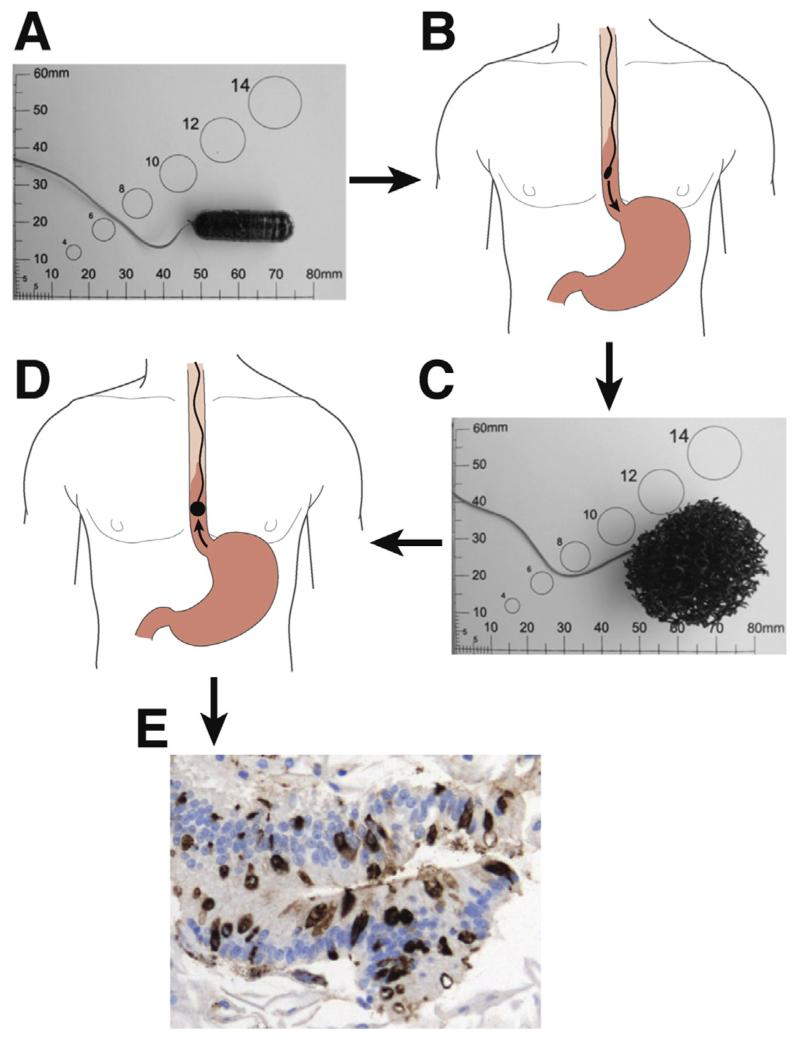
Figure 4.
Use of the Cytosponge. (A) Cytosponge embedded in a gelatin capsule. (B) The Cytosponge is swallowed and descends to the stomach through the esophagus. (C) The expanded Cytosponge. (D) The Cytosponge is retrieved by an assistant who pulls the string. (E) Immunohistochemical analysis of TTF3 in cells collected by the Cytosponge as well as BE cells (arrows).
Capsule Endoscopy
Capsule-based procedures have been used increasingly as a minimally invasive method of visualizing the lumen of the gastrointestinal tract. Although first applied for imaging of the small bowel, newer devices have been configured specifically for visualization of the esophagus. Esophageal capsule endoscopy (ECE) was approved by the US Food and Drug Administration and became commercially available as the PillCam ESO in 2004. The second generation, ESO2, became available in 2007. The devices are marketed by Covidien (Dublin, Ireland). They comprise a wireless ingestible capsule that images the esophagus at approximately 7 (ESO) or 9 (ESO2) images per second. Dual imagers (rather than single imagers in the small bowel capsule) at each end capture 14 (ESO) or 18 (ESO2) images per second. The devices also include a data recorder, comprising a recorder belt and sensor array, and a workstation for processing and real-time interpretation of transmitted images.78-80
ECE has an advantage over standard endoscopy in that it can be administered without sedation and is generally better tolerated.81 In initial studies, the PillCam ESO detected BE with 97% sensitivity and 99% specificity.82 However, further studies showed less than optimal sensitivity (60%–67%) and specificity (84%–100%) in detection of BE.79,83,84 The second-generation ESO2 device, which has a wider viewing angle and can generate 18 images per second, detected BE with 100% sensitivity and 74% specificity. This study, however, included only 28 patients.85
There have been variations in the design of the esophageal capsule, such as adding a tethering feature that allows the capsule to be held in place to improve visualization of the gastroesophageal junction. String capsule endoscopy was used to screen 100 veterans with chronic reflux symptoms for BE. BE was detected with 78% sensitivity and 83% specificity.86 A meta-analysis of these studies found that ECE detects BE with pooled values of 77% sensitivity and 86% specificity.87
A cost-benefit analysis that compared routine endoscopy with capsule endoscopy found that routine endoscopy was more cost effective in screening of patients with reflux symptoms because of the high prevalence of BE in this group.88 The costs of screening with ECE were found to be nearly equivalent to those of EGD, with sensitivity and specificity values of 78% and 90%, respectively.46,87,88 Thus, ECE is not routinely used to screen for BE, and its routine use was not recommended by the American College of Gastroenterology in their 2008 practice guidelines for BE.37 ECE has been modified such that the capsule can be steered by magnetic fields to allow greater control of image acquisition, but this approach has only been tested in research settings. It is important to consider that tissues cannot be collected by this method, so it must be followed by endoscopy to obtain biopsy specimens.
Biomarkers
Genetic Factors
Seven percent of patients with BE or EAC have other family members with these diseases, and a family history of BE or EAC has been reported in up to 28% of cases.13,89 Researchers have searched for genetic factors that increase the risk of BE and EAC. A genome-wide association study of a large cohort of patients in the United Kingdom identified 2 susceptibility loci for BE at positions 6p21 and 16q24.15 A subsequent study of patients with BE and EAC in western Europe, North America, and Australia identified 3 additional risk loci at positions 3p13, 9q22, and 19p13.14 Interestingly, this study showed that BE and EAC have common susceptibility loci.
It is tempting to speculate how this information could be used in screening for those at risk for BE and EAC. Development of BE appears to depend on a combination of multiple low-penetrance susceptibility loci and exposure to other environmental factors, so screening would not be straightforward. Large cohort studies can find important gene associations, but their clinical significance is minor; odds ratios for disease are fairly low. For example, the 6p21 locus increases the risk of BE by only 21%.
Blood biomarkers
MicroRNAs (miRNAs) are approximately 21 to 25 nucleotides long and regulate many cellular processes; their alteration has been associated with the pathogenesis of many diseases, including cancer.90 miRNAs are stable and can be detected in circulating plasma, so they are good biomarkers.91 BE and EAC tissues were shown to have different patterns of miRNAs compared with control squamous tissues.92 Levels of specific circulating miRNAs can be used to detect esophageal squamous carcinoma.93,94 Studies are required to determine whether patients with BE have a distinct miRNA profile.
Large-scale proteome and metabolome screening studies can uncover distinct proteins, polypeptides, or metabolites linked to specific cancers. Studies of esophageal cancer have mainly been conducted in patients with squamous cell carcinoma; peptide and metabolite panels can accurately identify these patients.95-97 Fan et al identified 5 peptides in blood that could distinguish patients with squamous cell carcinoma from healthy volunteers; the peptide panel was validated in an independent cohort.96 Using a mass spectroscopy approach, Kelly et al associated levels of 3 proteins (apolipoprotein A-I, serum amyloid A, and transthyretin) with increased survival times of patients with EAC.98 It is therefore feasible to detect BE and EAC using blood-based protein markers, but further studies are needed.
Stool Biomarkers
Cells are constantly shed from esophageal neoplasias and have been detected in the stool using fecal DNA extraction techniques. Tests have been developed to screen for potential upper gastrointestinal malignancies as well as for colonic neoplasms that detect DNA, methylation, and protein markers unique to cancerous cells. Assays that detect changes in DNA methylation patterns are particularly well studied for detection of esophageal cancers99-103 and hold promise for minimally invasive screening. However, it is likely that these assays detect established tumors rather than preinvasive lesions. They might also detect esophageal cancer with low specificity; positive results would need to be followed up with evaluation of the entire gastrointestinal tract, including the colon.
Conclusions
There is a strong rationale for screening for BE to reduce mortality from EAC and reverse the increasing incidence of esophageal cancer in Western countries. However, screening tests must be simple, minimally invasive procedures.4,45 Although minimally invasive imaging technologies and molecular diagnostic tools are continually being developed, nonendoscopic tools that collect cells and tissues for analysis, such as the Cytosponge and office-based transnasal esophagoscopes, appear to be the most promising. However, the Cytosponge has only been tested in the United Kingdom (Table 3). We await results from a large case-control study of the Cytosponge that will reveal the suitability of this technology for diagnosis of BE. Additional studies are needed to assess the ability of disposable office-based ultrathin endoscopes to accurately identify patients with BE. A new version of the EG scan (EG scan II) is under investigation in an ongoing multicenter study (ISRCTN 70595405) (Table 2).
Table 3.
Screening Tests for BE
| Type of study | Diagnostic accuracy |
Patient acceptability |
Costs | Primary care |
|
|---|---|---|---|---|---|
| Standard endoscopy | Population-based cross-sectional | Excellent | Poor | High | No |
| Endoscopic brush cytology | Retrospective cohort | Good | Poor | High | No |
| EndoCDx | Prospective comparative | Gooda | Poor | High | No |
| TNE | Randomized | Excellent | Moderate | High | No |
| Office-based TNE | Randomized | Good | Moderate | Moderate | Yes |
| Capsule endoscopy | Prospective comparative | Moderate | Moderate | High | Partly |
| Single-fiber endoscopy | Animal model | Unknown | Moderate | Unknown | Unknown |
| VLE | Small cross-sectional | Unknown | Moderate | Unknown | No |
| Cytosponge | Prospective comparative | Gooda | Good | Low | Yes |
| Balloon cytology | Prospective comparative | Poor | Moderate | Low | Yes |
| Microgrippers | Animal model | Unknown | Good | Unknown | No |
| Genetic susceptibility markers (blood) | Genome-wide association studies | Unknown | Excellent | Unknown | Yes |
| Stool markers (eg, methylation) | Discovery preclinical | Unknown | Excellent | Unknown | Yes |
| Blood markers (eg, proteins, miRNAs) | Discovery preclinical | Unknown | Excellent | Unknown | Yes |
More data are required.
Ultimately, these technologies must tested in the primary care setting with robust study designs. Large randomized trials should include those at risk for BE and determine the effects of screening on cancer incidence, stage of cancers detected, and cost per treatable cancer detected. When the best screening approaches are identified, screening campaigns can be launched in countries with a high incidence of EAC. Tests for genetic factors and blood-based biomarkers could be included, alone or in combination with other tests.
However, screening for BE will be of benefit at the population level only if it is coupled with effective surveillance programs. Corley et al showed that current surveillance practices for BE do not significantly affect EAC mortality.104 These findings are at odds with those from retrospective studies, which showed increased survival times among patients diagnosed with EAC in surveillance programs, and from a more recent population-based study, which reported increased survival times of patients with cancer who had with a prior diagnosis of BE, even after correction for lead and length time bias.16,38,40 Furthermore, many of the subjects in the study by Corley et al had advanced EAC, indicating that community-based practices (outside of clinical studies) perform suboptimal endoscopic examinations of patients with BE. Previous studies have also shown that community endoscopists have poor adherence to guidelines for endoscopic surveillance.105,106
Increased centralization of surveillance programs and new advanced imaging techniques to detect dysplasia should help improve the management of patients found to have BE through screening programs. Minimally invasive endoscopic techniques for ablation of high-risk BE could, with accurate screening strategies, reverse the increase in the incidence of EAC in Western countries. It is important to further develop biomarkers that can separate the relatively small group of patients at high risk for cancer who require endoscopic therapy from the larger proportion who are at low risk and can be evaluated at longer time intervals.
Acknowledgments
Funding
Supported by core funding from the Medical Research Council (to R.C.F.) and National Cancer Institute grants U01 CA182940, U54 CA163004, and U54 CA163059 (to K.K.W.).
Abbreviations used in this paper
- BE
Barrett’s esophagus
- EAC
esophageal adenocarcinoma
- ECE
esophageal capsule endoscopy
- GERD
gastroesophageal reflux disease
- miRNA
microRNA
- TFF
trefoil factor 3
- TNE
transnasal endoscopy
- VLE
volume laser endomicroscopy
Footnotes
Conflicts of interest
The authors disclose the following: R.C.F. developed the Cytosponge technology, which has been licensed by MRC Technology to Covidien. R.C.F. has no direct financial arrangement with Covidien. K.K.W. has research and consulting agreements with Ninepoints Medical and a patent with Abbott Molecular Diagnostics for FISH in Barrett’s esophagus. The remaining authors disclose no conflicts.
References
- 1.Wilson JMG, Jungner G. Principles and practice of screening for disease. World Health Organization; Geneva, Switzerland: 1968. [Google Scholar]
- 2.Eloubeidi MA, Mason AC, Desmond RA, et al. Temporal trends (1973-1997) in survival of patients with esophageal adenocarcinoma in the United States: a glimmer of hope? Am J Gastroenterol. 2003;98:1627–1633. doi: 10.1111/j.1572-0241.2003.07454.x. [DOI] [PubMed] [Google Scholar]
- 3.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 4.CancerResearchUK [Accessed September 2012];Oesophageal cancer statistics. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/oesophagus/?script=true.
- 5.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155–3162. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 6.Reid BJ, Li X, Galipeau PC, et al. Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer. 2010;10:87–101. doi: 10.1038/nrc2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenglinger J, Riegler M, Cosentini E, et al. Review on the annual cancer risk of Barrett’s esophagus in persons with symptoms of gastroesophageal reflux disease. Anticancer Res. 2012;32:5465–5473. [PubMed] [Google Scholar]
- 8.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radio-frequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209–1217. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 9.Shaheen NJ, Sharma P, Overholt BF, et al. Radio-frequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 10.Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 11.Tramacere I, La Vecchia C, Negri E. Tobacco smoking and esophageal and gastric cardia adenocarcinoma: a meta-analysis. Epidemiology. 2011;22:344–349. doi: 10.1097/EDE.0b013e31821092cd. [DOI] [PubMed] [Google Scholar]
- 12.Turati F, Tramacere I, La Vecchia C, et al. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann Oncol. 2013;24:609–617. doi: 10.1093/annonc/mds244. [DOI] [PubMed] [Google Scholar]
- 13.Chak A, Ochs-Balcom H, Falk G, et al. Familiality in Barrett’s esophagus, adenocarcinoma of the esophagus, and adenocarcinoma of the gastroesophageal junction. Cancer Epidemiol Biomarkers Prev. 2006;15:1668–1673. doi: 10.1158/1055-9965.EPI-06-0293. [DOI] [PubMed] [Google Scholar]
- 14.Levine DM, Ek WE, Zhang R, et al. A genome-wide association study identifies new susceptibility loci for esophageal adenocarcinoma and Barrett’s esophagus. Nat Genet. 2013;45:1487–1493. doi: 10.1038/ng.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su Z, Gay LJ, Strange A, et al. Common variants at the MHC locus and at chromosome 16q24.1 predispose to Barrett’s esophagus. Nat Genet. 2012;44:1131–1136. doi: 10.1038/ng.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhat SK, McManus DT, Coleman HG, et al. Oesophageal adenocarcinoma and prior diagnosis of Barrett’s oesophagus: a population-based study. Gut. 2015;64:20–25. doi: 10.1136/gutjnl-2013-305506. [DOI] [PubMed] [Google Scholar]
- 17.Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oeso-phageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57:1354–1359. doi: 10.1136/gut.2007.145177. [DOI] [PubMed] [Google Scholar]
- 18.Ronkainen J, Aro P, Storskrubb T, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–1831. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 19.Connor MJ, Weston AP, Mayo MS, et al. The prevalence of Barrett’s esophagus and erosive esophagitis in patients undergoing upper endoscopy for dyspepsia in a VA population. Dig Dis Sci. 2004;49:920–924. doi: 10.1023/b:ddas.0000034549.55326.67. [DOI] [PubMed] [Google Scholar]
- 20.Kadri SR, Lao-Sirieix P, O’Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: cohort study. BMJ. 2010;341:c4372. doi: 10.1136/bmj.c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winters C, Jr, Spurling TJ, Chobanian SJ, et al. Barrett’s esophagus. A prevalent, occult complication of gastro-esophageal reflux disease. Gastroenterology. 1987;92:118–124. [PubMed] [Google Scholar]
- 22.Lagergren J. Adenocarcinoma of oesophagus: what exactly is the size of the problem and who is at risk? Gut. 2005;54(suppl 1):i1–i5. doi: 10.1136/gut.2004.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chak A, Faulx A, Eng C, et al. Gastroesophageal reflux symptoms in patients with adenocarcinoma of the esophagus or cardia. Cancer. 2006;107:2160–2166. doi: 10.1002/cncr.22245. [DOI] [PubMed] [Google Scholar]
- 24.Dulai GS, Guha S, Kahn KL, et al. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122:26–33. doi: 10.1053/gast.2002.30297. [DOI] [PubMed] [Google Scholar]
- 25.Dellon ES, Shaheen NJ. Does screening for Barrett’s esophagus and adenocarcinoma of the esophagus prolong survival? J Clin Oncol. 2005;23:4478–4482. doi: 10.1200/JCO.2005.19.059. [DOI] [PubMed] [Google Scholar]
- 26.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desai TK, Singh J, Samala N, et al. The incidence of esophageal adenocarcinoma in Barrett’s esophagus has been overestimated. Am J Gastroenterol. 2011;106:1364–1365. doi: 10.1038/ajg.2011.145. author reply 1365-1366. [DOI] [PubMed] [Google Scholar]
- 28.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 29.Farrow DC, Vaughan TL, Sweeney C, et al. Gastro-esophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control. 2000;11:231–238. doi: 10.1023/a:1008913828105. [DOI] [PubMed] [Google Scholar]
- 30.Rubenstein JH, Scheiman JM, Sadeghi S, et al. Esophageal adenocarcinoma incidence in individuals with gastro-esophageal reflux: synthesis and estimates from population studies. Am J Gastroenterol. 2011;106:254–260. doi: 10.1038/ajg.2010.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubenstein JH, Morgenstern H, Appelman H, et al. Prediction of Barrett’s esophagus among men. Am J Gastroenterol. 2013;108:353–362. doi: 10.1038/ajg.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson J, Johnsson F, Walther B, et al. Adenocarcinoma in the distal esophagus with and without Barrett esophagus. Differences in symptoms and survival rates. Arch Surg. 1996;131:708–713. doi: 10.1001/archsurg.1996.01430190030008. [DOI] [PubMed] [Google Scholar]
- 33.Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett’s adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–640. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 34.Theisen J, Stein HJ, Dittler HJ, et al. Preoperative chemotherapy unmasks underlying Barrett’s mucosa in patients with adenocarcinoma of the distal esophagus. Surg Endosc. 2002;16:671–673. doi: 10.1007/s00464-001-8307-3. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 36.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 37.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 38.Rubenstein JH, Sonnenberg A, Davis J, et al. Effect of a prior endoscopy on outcomes of esophageal adenocarcinoma among United States veterans. Gastrointest Endosc. 2008;68:849–855. doi: 10.1016/j.gie.2008.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans JA, Early DS, Fukami N, et al. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76:1087–1094. doi: 10.1016/j.gie.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Cooper GS, Kou TD, Chak A. Receipt of previous diagnoses and endoscopy and outcome from esophageal adenocarcinoma: a population-based study with temporal trends. Am J Gastroenterol. 2009;104:1356–1362. doi: 10.1038/ajg.2009.159. [DOI] [PubMed] [Google Scholar]
- 41.Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology. 2011;141:460–468. doi: 10.1053/j.gastro.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett’s esophagus with early neoplasia. Clin Gastroenterol Hepatol. 2010;8:23–29. doi: 10.1016/j.cgh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endo-scopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–1206. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 44.UK National Screening Committee [Accessed February 2015];Criteria for appraising the viability, effectiveness and appropriateness of a screening programme. 2009. Available at: http://www.screening.nhs.uk/criteria.
- 45.Wilson JM, Jungner YG. Principles and practice of mass screening for disease. Bol Oficina Sanit Panam. 1968;65:281–393. [PubMed] [Google Scholar]
- 46.Rubenstein JH, Inadomi JM, Brill JV, et al. Cost utility of screening for Barrett’s esophagus with esophageal capsule endoscopy versus conventional upper endoscopy. Clin Gastroenterol Hepatol. 2007;5:312–318. doi: 10.1016/j.cgh.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus—the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol. 1998;93:1033–1036. doi: 10.1111/j.1572-0241.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 48.Kara MA, Ennahachi M, Fockens P, et al. Detection and classification of the mucosal and vascular patterns (mucosal morphology) in Barrett’s esophagus by using narrow band imaging. Gastrointest Endosc. 2006;64:155–166. doi: 10.1016/j.gie.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 49.Soni A, Sampliner RE, Sonnenberg A. Screening for high-grade dysplasia in gastroesophageal reflux disease: is it cost-effective? Am J Gastroenterol. 2000;95:2086–2093. doi: 10.1111/j.1572-0241.2000.02173.x. [DOI] [PubMed] [Google Scholar]
- 50.Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 51.Barbiere JM, Lyratzopoulos G. Cost-effectiveness of endoscopic screening followed by surveillance for Barrett’s esophagus: a review. Gastroenterology. 2009;137:1869–1876. doi: 10.1053/j.gastro.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Canto MI, Anandasabapathy S, Brugge W, et al. In vivo endomicroscopy improves detection of Barrett’s esophagus-related neoplasia: a multicenter international randomized controlled trial (with video) Gastrointest Endosc. 2014;79:211–221. doi: 10.1016/j.gie.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma P, Meining AR, Coron E, et al. Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2011;74:465–472. doi: 10.1016/j.gie.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergholt MS, Zheng W, Ho KY, et al. Fiberoptic confocal raman spectroscopy for real-time in vivo diagnosis of dysplasia in Barrett’s esophagus. Gastroenterology. 2014;146:27–32. doi: 10.1053/j.gastro.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Falk GW. Cytology in Barrett’s esophagus. Gastrointest Endosc Clin North Am. 2003;13:335–348. doi: 10.1016/s1052-5157(03)00016-3. [DOI] [PubMed] [Google Scholar]
- 56.Geisinger KR, Teot LA, Richter JE. A comparative cyto-pathologic and histologic study of atypia, dysplasia, and adenocarcinoma in Barrett’s esophagus. Cancer. 1992;69:8–16. doi: 10.1002/1097-0142(19920101)69:1<8::aid-cncr2820690105>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 57.Kumaravel A, Lopez R, Brainard J, et al. Brush cytology vs. endoscopic biopsy for the surveillance of Barrett’s esophagus. Endoscopy. 2010;42:800–805. doi: 10.1055/s-0030-1255710. [DOI] [PubMed] [Google Scholar]
- 58.Rygiel AM, Milano F, Ten Kate FJ, et al. Assessment of chromosomal gains as compared to DNA content changes is more useful to detect dysplasia in Barrett’s esophagus brush cytology specimens. Genes Chromosomes Cancer. 2008;47:396–404. doi: 10.1002/gcc.20543. [DOI] [PubMed] [Google Scholar]
- 59.Robey SS, Hamilton SR, Gupta PK, et al. Diagnostic value of cytopathology in Barrett esophagus and associated carcinoma. Am J Clin Pathol. 1988;89:493–498. doi: 10.1093/ajcp/89.4.493. [DOI] [PubMed] [Google Scholar]
- 60.Hardwick RH, Morgan RJ, Warren BF, et al. Brush cytology in the diagnosis of neoplasia in Barrett’s esophagus. Dis Esophagus. 1997;10:233–237. doi: 10.1093/dote/10.4.233. [DOI] [PubMed] [Google Scholar]
- 61.Lao-Sirieix P, Lovat L, Fitzgerald RC. Cyclin A immuno-cytology as a risk stratification tool for Barrett’s esophagus surveillance. Clin Cancer Res. 2007;13:659–665. doi: 10.1158/1078-0432.CCR-06-1385. [DOI] [PubMed] [Google Scholar]
- 62.Fahmy M, Skacel M, Gramlich TL, et al. Chromosomal gains and genomic loss of p53 and p16 genes in Barrett’s esophagus detected by fluorescence in situ hybridization of cytology specimens. Mod Pathol. 2004;17:588–596. doi: 10.1038/modpathol.3800088. [DOI] [PubMed] [Google Scholar]
- 63.Pacha A, Rygiel AM, Westra W, et al. Novel FISH biomarker assay detects barrett progressors: a phase IV five year prospective follow up study. Gastroenterology. 2012;142:S–72. [Google Scholar]
- 64.Anandasabapathy S, Sontag S, Graham DY, et al. Computer-assisted brush-biopsy analysis for the detection of dysplasia in a high-risk Barrett’s esophagus surveillance population. Dig Dis Sci. 2011;56:761–766. doi: 10.1007/s10620-010-1459-z. [DOI] [PubMed] [Google Scholar]
- 65.Johanson JF, Frakes J, Eisen D. Computer-assisted analysis of abrasive transepithelial brush biopsies increases the effectiveness of esophageal screening: a multicenter prospective clinical trial by the EndoCDx Collaborative Group. Dig Dis Sci. 2011;56:767–772. doi: 10.1007/s10620-010-1497-6. [DOI] [PubMed] [Google Scholar]
- 66.Saeian K, Staff DM, Vasilopoulos S, et al. Unsedated transnasal endoscopy accurately detects Barrett’s metaplasia and dysplasia. Gastrointest Endosc. 2002;56:472–478. doi: 10.1067/mge.2002.128131. [DOI] [PubMed] [Google Scholar]
- 67.Shariff MK, Bird-Lieberman EL, O’Donovan M, et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett’s esophagus. Gastrointest Endosc. 2012;75:954–961. doi: 10.1016/j.gie.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 68.Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol. 2006;101:2693–2703. doi: 10.1111/j.1572-0241.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 69.Peery AF, Hoppo T, Garman KS, et al. Feasibility, safety, acceptability, and yield of office-based, screening transnasal esophagoscopy (with video) Gastrointest Endosc. 2012;75:945–953. e2. doi: 10.1016/j.gie.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seibel EJ, Carroll RE, Dominitz JA, et al. Tethered capsule endoscopy, a low-cost and high-performance alternative technology for the screening of esophageal cancer and Barrett’s esophagus. IEEE Trans Biomed Eng. 2008;55:1032–1042. doi: 10.1109/TBME.2008.915680. [DOI] [PubMed] [Google Scholar]
- 71.Poneros JM. Diagnosis of Barrett’s esophagus using optical coherence tomography. Gastrointest Endosc Clin North Am. 2004;14:573–588. doi: 10.1016/j.giec.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Yun SH, Tearney GJ, Vakoc BJ, et al. Comprehensive volumetric optical microscopy in vivo. Nat Med. 2006;12:1429–1433. doi: 10.1038/nm1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gora MJ, Sauk JS, Carruth RW, et al. Tethered capsule endomicroscopy enables less invasive imaging ofgastrointestinal tract microstructure. Nat Med. 2013;19:238–240. doi: 10.1038/nm.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Falk GW, Chittajallu R, Goldblum JR, et al. Surveillance of patients with Barrett’s esophagus for dysplasia and cancer with balloon cytology. Gastroenterology. 1997;112:1787–1797. doi: 10.1053/gast.1997.v112.pm9178668. [DOI] [PubMed] [Google Scholar]
- 75.Fennerty MB, DiTomasso J, Morales TG, et al. Screening for Barrett’s esophagus by balloon cytology. Am J Gastroenterol. 1995;90:1230–1232. [PubMed] [Google Scholar]
- 76.Benaglia T, Sharples LD, Fitzgerald RC, et al. Health benefits and cost-effectiveness of endoscopic and non-endoscopic cytosponge screening for Barrett’s esophagus. Gastroenterology. 2013;144:62–73. doi: 10.1053/j.gastro.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 77.Gultepe E, Yamanaka S, Laflin KE, et al. Biologic tissue sampling with untethered microgrippers. Gastroenterology. 2013;144:691–693. doi: 10.1053/j.gastro.2013.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Franchis R, Rondonotti E, Villa F. Capsule endoscopy—state of the art. Dig Dis. 2007;25:249–251. doi: 10.1159/000103895. [DOI] [PubMed] [Google Scholar]
- 79.Galmiche JP, Sacher-Huvelin S, Coron E, et al. Screening for esophagitis and Barrett’s esophagus with wireless esophageal capsule endoscopy: a multicenter prospective trial in patients with reflux symptoms. Am J Gastroenterol. 2008;103:538–545. doi: 10.1111/j.1572-0241.2007.01731.x. [DOI] [PubMed] [Google Scholar]
- 80.Waterman M, Gralnek IM. Capsule endoscopy of the esophagus. J Clin Gastroenterol. 2009;43:605–612. doi: 10.1097/MCG.0b013e3181aabd93. [DOI] [PubMed] [Google Scholar]
- 81.di Pietro M, Fitzgerald RC. Screening and risk stratification for Barrett’s esophagus: how to limit the clinical impact of the increasing incidence of esophageal adenocarcinoma. Gastroenterol Clin North Am. 2013;42:155–173. doi: 10.1016/j.gtc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 82.Eliakim R, Sharma VK, Yassin K, et al. A prospective study of the diagnostic accuracy of PillCam ESO esophageal capsule endoscopy versus conventional upper endoscopy in patients with chronic gastroesophageal reflux diseases. J Clin Gastroenterol. 2005;39:572–578. doi: 10.1097/01.mcg.0000170764.29202.24. [DOI] [PubMed] [Google Scholar]
- 83.Lin OS, Schembre DB, Mergener K, et al. Blinded comparison of esophageal capsule endoscopy versus conventional endoscopy for a diagnosis of Barrett’s esophagus in patients with chronic gastroesophageal reflux. Gastrointest Endosc. 2007;65:577–583. doi: 10.1016/j.gie.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 84.Sharma P, Wani S, Rastogi A, et al. The diagnostic accuracy of esophageal capsule endoscopy in patients with gastroesophageal reflux disease and Barrett’s esophagus: a blinded, prospective study. Am J Gastroenterol. 2008;103:525–532. doi: 10.1111/j.1572-0241.2007.01233.x. [DOI] [PubMed] [Google Scholar]
- 85.Gralnek IM, Adler SN, Yassin K, et al. Detecting esophageal disease with second-generation capsule endoscopy: initial evaluation of the PillCam ESO 2. Endoscopy. 2008;40:275–279. doi: 10.1055/s-2007-995645. [DOI] [PubMed] [Google Scholar]
- 86.Ramirez FC, Akins R, Shaukat M. Screening of Barrett’s esophagus with string-capsule endoscopy: a prospective blinded study of 100 consecutive patients using histology as the criterion standard. Gastrointest Endosc. 2008;68:25–31. doi: 10.1016/j.gie.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 87.Bhardwaj A, Hollenbeak CS, Pooran N, et al. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barrett’s esophagus in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2009;104:1533–1539. doi: 10.1038/ajg.2009.86. [DOI] [PubMed] [Google Scholar]
- 88.Gerson L, Lin OS. Cost-benefit analysis of capsule endoscopy compared with standard upper endoscopy for the detection of Barrett’s esophagus. Clin Gastroenterol Hepatol. 2007;5:319–325. doi: 10.1016/j.cgh.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 89.Juhasz A, Mittal SK, Lee TH, et al. Prevalence of Barrett esophagus in first-degree relatives of patients with esophageal adenocarcinoma. J Clin Gastroenterol. 2011;45:867–871. doi: 10.1097/MCG.0b013e31821f44a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 91.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sakai NS, Samia-Aly E, Barbera M, et al. A review of the current understanding and clinical utility of miRNAs in esophageal cancer. Semin Cancer Biol. 2013;23:512–521. doi: 10.1016/j.semcancer.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 93.Kurashige J, Kamohara H, Watanabe M, et al. Serum microRNA-21 is a novel biomarker in patients with esophageal squamous cell carcinoma. J Surg Oncol. 2012;106:188–192. doi: 10.1002/jso.23064. [DOI] [PubMed] [Google Scholar]
- 94.Komatsu S, Ichikawa D, Takeshita H, et al. Circulating microRNAs in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer. 2011;105:104–111. doi: 10.1038/bjc.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fan NJ, Gao CF, Wang XL. Tubulin beta chain, filamin A alpha isoform 1, and cytochrome b-c1 complex subunit 1 as serological diagnostic biomarkers of esophageal squamous cell carcinoma: a proteomics study. OMICS. 2013;17:215–223. doi: 10.1089/omi.2012.0133. [DOI] [PubMed] [Google Scholar]
- 96.Fan NJ, Gao CF, Zhao G, et al. Serum peptidome patterns for early screening of esophageal squamous cell carcinoma. Biotechnol Appl Biochem. 2012;59:276–282. doi: 10.1002/bab.1024. [DOI] [PubMed] [Google Scholar]
- 97.Xu J, Chen Y, Zhang R, et al. Global and targeted metabolomics of esophageal squamous cell carcinoma discovers potential diagnostic and therapeutic bio-markers. Mol Cell Proteomics. 2013;12:1306–1318. doi: 10.1074/mcp.M112.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kelly P, Paulin F, Lamont D, et al. Pre-treatment plasma proteomic markers associated with survival in oesophageal cancer. Br J Cancer. 2012;106:955–961. doi: 10.1038/bjc.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zou H, Osborn NK, Harrington JJ, et al. Frequent methylation of eyes absent 4 gene in Barrett’s esophagus and esophageal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2005;14:830–834. doi: 10.1158/1055-9965.EPI-04-0506. [DOI] [PubMed] [Google Scholar]
- 100.Zou H, Molina JR, Harrington JJ, et al. Aberrant methylation of secreted frizzled-related protein genes in esophageal adenocarcinoma and Barrett’s esophagus. Int J Cancer. 2005;116:584–591. doi: 10.1002/ijc.21045. [DOI] [PubMed] [Google Scholar]
- 101.Jin Z, Cheng Y, Gu W, et al. A multicenter, double-blinded validation study of methylation biomarkers for progression prediction in Barrett’s esophagus. Cancer Res. 2009;69:4112–4115. doi: 10.1158/0008-5472.CAN-09-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schulmann K, Sterian A, Berki A, et al. Inactivation of p16, RUNX3, and HPP1 occurs early in Barrett’s-associated neoplastic progression and predicts progression risk. Oncogene. 2005;24:4138–4148. doi: 10.1038/sj.onc.1208598. [DOI] [PubMed] [Google Scholar]
- 103.Alvi MA, Liu X, O’oDnovan M, et al. DNA methylation as an adjunct to histopathology to detect prevalent,inconspicuous dysplasia and early-stage neoplasia in Barrett’s esophagus. Clin Cancer Res. 2013;19:878–888. doi: 10.1158/1078-0432.CCR-12-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Corley DA, Mehtani K, Quesenberry C, et al. Impact of endoscopic surveillance on mortality from Barrett’s esophagus-associated esophageal adenocarcinomas. Gastroenterology. 2013;145:312–319. e1. doi: 10.1053/j.gastro.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol. 2009;7:736–742. doi: 10.1016/j.cgh.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Curvers WL, Peters FP, Elzer B, et al. Quality of Barrett’s surveillance in The Netherlands: a standardized review of endoscopy and pathology reports. Eur J Gastroenterol Hepatol. 2008;20:601–607. doi: 10.1097/MEG.0b013e3282f8295d. [DOI] [PubMed] [Google Scholar]