Abstract
Purpose
Identify facilitators and barriers to physical activity (PA), and explore the utility of Social Cognitive Theory (SCT) and Transactional Model of Stress and Coping (TMSC) in understanding PA behaviour among persons with multiple sclerosis (MS).
Methods
Thirteen participants from a clinical trial were interviewed and classified as physically active, sometimes active, or inactive based on the Health-Promoting Lifestyle Profile-II. Interviews were analysed using analytical induction, which consisted of coding data into pre-established categories and then exploring similarities and differences between groups. Pre-established coding categories were constructs from SCT (i.e. environment, expectations, self-efficacy, and self-regulation) and TMSC (i.e. stress appraisal and coping style).
Results
Inactive and active participants differed in their self-regulation skills, self-efficacy, and coping styles. Common barriers to PA included symptoms and the physical and social environment. Facilitators of PA included strong self-regulation skills, confidence to overcome symptoms to engage in PA (i.e. barrier self-efficacy), and positive coping styles.
Conclusion
Results from this pilot study suggest that PA interventions will need to implement multiple strategies that target self-efficacy, social environment, and coping styles. We found SCT and TMSC useful in understanding PA behaviour among persons with MS; however, a limitation to these theories is that they are not explicit in the relationship between health and cognitions. Future research will need to explore how to incorporate models of health and function into existing behaviour change theories.
Introduction
Multiple sclerosis (MS) is an immune-mediated inflammatory disease of the central nervous system [1]. The prevalence rate for MS is approximately 58 per 100,000 people in the United States [2]. Common symptoms of MS include fatigue, cognitive impairment, and problems with balance and mobility [3]. Symptoms are caused by T-cells attacking the myelin sheath of nerves, which can ultimately destroy axonal neurons [4, 5]. Managing symptoms, slowing progression, and preventing comorbidities are common medical treatment goals [3]. These goals may be facilitated by promoting routine physical activity (PA), which has been shown to reduce fatigue and improve quality of life [6–9]. There are also some indications that PA may slow progression of MS [10]. Nevertheless, a meta-analysis found that people with MS are below population averages for engaging in PA [11].
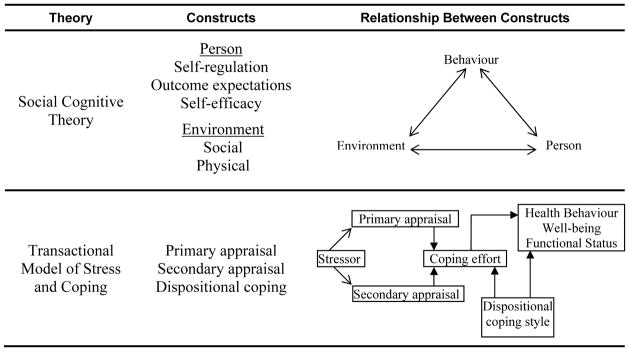
Understanding how to promote PA for persons with MS may be achieved by utilising behaviour change theories [12]. These theories describe factors that regulate behaviour and can be used to guide the development and implementation of interventions [13]. Two theories that show promise in understanding health behaviour for persons with disabling conditions are Social Cognitive Theory (SCT) [12, 14, 15] and Transactional Model of Stress and Coping (TMSC) [16–19]. The central concept of SCT is reciprocal determinism, which is the interaction of person, environment, and behaviour [14]. TMSC shows how stress and coping strategies influence behaviour [16].
Self-regulation from SCT is argued to be the basis of purposeful action [20]. Self-regulation has three main subfunctions: self-observation, judgmental process, and self-reaction. Self-observation is the monitoring of one’s own behaviour and the effects that it produces (e.g. the perceived influence of PA on symptoms), and how one sets goals and becomes motivated to accomplish these goals. The judgmental process is the way that people evaluate their behaviour through personal standards and social comparisons. Self-reaction is the way that people use incentives to accomplish goals. Important influences on these subfunctions include self-efficacy (i.e. one’s confidence to engage in PA) and outcome expectations (i.e. expected benefits).
According to TMSC, stress and coping efforts influence health behaviours [16]. Stress leads to primary and secondary appraisal. Primary appraisal is the evaluation of the significance of the stressor; secondary appraisal is the evaluation of whether the stressor can be controlled. Appraisals result in coping efforts (i.e. engagement in problem management and emotional regulation). Coping efforts are influenced by dispositional coping styles, which are stable characteristics [21]. Examples of positive coping styles are optimism, information seeking, humour, and the use of problem-solving techniques. Examples of negative coping styles are avoidance, denial, and pessimism.
Selecting and combining the components from different behaviour change theories that are pertinent for persons with disabling conditions may increase our understanding of how to promote PA [22]. SCT and TMSC have related concepts (e.g. efficacy and control), but each theory emphasises different factors that can influence PA. Reciprocal determinism from SCT can guide our understanding of how the interaction between functional limitations (e.g. mobility impairments) and environmental factors (e.g. access to a recreational facility) influence PA [12]. TMSC can aid our understanding of how coping with MS influences PA [21, 23].
Our understanding of the relevance and utility of theories for people with disabling conditions can be informed by qualitative research [24, 25]. For example, Beverly et al.[26] conducted a qualitative analysis to show how efficacy from SCT influenced PA behaviour in persons with diabetes. Qualitative studies in persons with MS have explored how fatigue, mobility impairments, and other MS-related symptoms influence social roles [27–30]. Two qualitative studies have indirectly explored PA behaviour among persons with MS. Wihite et al. [31] described the problem-solving strategies used by those with MS to engage in wellness activities. Dodd et al. [32] explored how persons with MS tolerated a progressive resistance exercise programme. However, no qualitative study to date has utilised SCT or TMSC to understand PA behaviour among those with MS.
Thus the objective of our study was to identify facilitators of and barriers to PA behaviour in persons with MS. Specifically, we aimed to understand how the person-environment interaction and coping with MS influence PA behaviour from the perspective of people with MS. The secondary aim of the study was to explore the relevance and utility of SCT and TMSC in understanding PA behaviour among persons with MS. We defined PA as bodily movement for the purpose of improving fitness, health, and/or well-being [33].
Methods
Overview
Thirteen participants were purposefully selected for interviews after the intervention phase of a clinical trial to explore their experience of engaging in PA [9]. The aim of the clinical trial was to compare the efficacy of two interventions designed to promote health and PA. After completion of the interviews, data from the clinical trial were used to categorise the 13 participants as active, sometimes active, and inactive. We then conducted a qualitative analysis to explore the similarities and differences between these groups. The trial is briefly described below and is described in detail elsewhere [9]. The University of Minnesota IRB approved this study.
Clinical trial
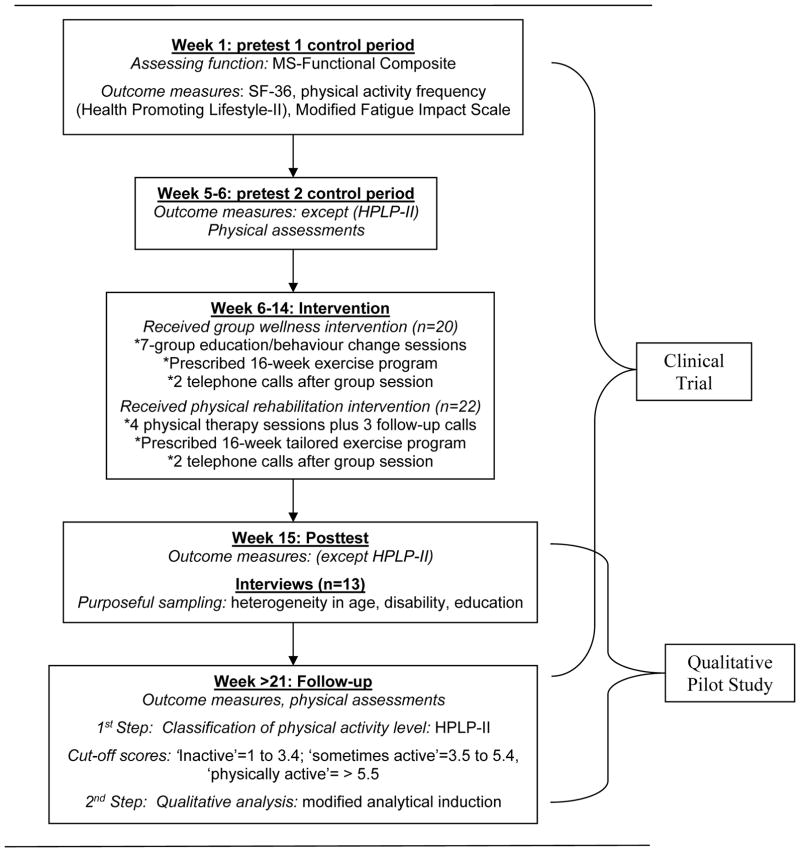
Fifty participants with MS were recruited through the MS Society and neurologists’ offices. Inclusion criteria were a physician-confirmed diagnosis of MS and the ability to walk with or without an assisting device. Exclusion criteria were pregnancy, cardiovascular disease, more than two falls in the past month, and/or inability to understand the study. Participants were randomly assigned into an individualized rehabilitation regimen or a group wellness programme. Participants were prescribed a 16-week home exercise programme, which consisted of stretching, indoor bicycling, and strength training; however, they were allowed to engage in other types of PA and exercise that were deemed equivalent to the home exercise programme (e.g. biking outside or going to a gym). Participants were given a structured activity log to keep track of their programme and to note strategies they used to engage in PA. Outcome measures were administered twice pre-intervention and twice post-intervention. Figure 1 shows an overview of the study design and when data were collected. Measures used to describe participants in this study were: SF-36 [34], PA frequency subscale of the Health-Promoting Lifestyle Profile (HPLP-II) [35], Modified Fatigue Impact Scale [36], MS-Functional Composite [37], physical assessments [38], and activity logs.
Figure 1.
Timeline of study
Participant selection
Thirteen participants were purposely selected to participate in an interview that took place between the post-test and follow-up assessments of the clinical trial. Selection of these participants was based on a demonstrated willingness to tell their story and achieving heterogeneity in age, education, and disability. The first author believed that there was sufficient heterogeneity in PA levels in this group of participants (i.e. engaging in PA daily to engaging in PA rarely) based upon conversations with the 13 participants and the interventionists.
The first author conducted semi-structured interviews, which lasted approximately 1.5 hours. Participants were asked to describe how they initially coped with their diagnosis of MS, how they were coping at present, their experience with the exercise programme, and their beliefs in general about exercise and PA (i.e. theoretical constructs). Interviews were audiotaped and transcribed verbatim. A question in the activity log asked participants once a week to note any strategies they used to engage in PA. The interviews and notes from the activity log were analysed using a modified analytical induction approach that is described below [39, 40].
Participant classification by physical activity level
After the interviews were completed and before the qualitative analysis was conducted, the interview participants were classified by activity level: active, sometimes active, or inactive. Since three of the participants who were interviewed did not complete their activity log, we were unable to use the log to classify participants. Instead, we used a modified version of the PA frequency subscale of HPLP-II that was administered at follow-up.
We modified the HPLP-II by changing the response options from 1–4 to 1–7 and omitting three questions. The scale was labeled as 1 (never), 4 (sometimes), and 7 (routinely). Two questions on reaching a target heart rate zone and checking pulse rate were omitted because the topic was not emphasised when prescribing the exercise programme (i.e. prescription was based on Rate of Perceived Exertion). One question on incorporating PA into daily activities was omitted because it contradicted energy conservation topics taught in the wellness course and participants could have included non-purposeful PA not associated with the prescribed programmes when answering this question. Therefore the following five remaining questions were averaged together: (1) followed a planned exercise programme, (2) exercised vigorously for 20 minutes or more, (3) took part in light to moderate PA, (4) engaged in leisure-time PA, and (5) stretched.
The internal consistency of the modified HPLP-II using data from a sample of 42 participants was adequate (Cronbach’s alpha=0.77) [41], indicating that the revised scale was internally consistent and robust in spite of the changes. We also examined concurrent validity by determining the correlation of the modified HPLP-II with the activity log (% sessions completed). The correlation was 0.52, which included 33 participants who had complete data on the activity log.
Classification of participants PA levels was based on rounding average scores to the nearest whole digit and corresponding scores to the labels on the scale. Thus subjects were classified into the ‘physically active’ group if their score was 5.5 or higher, into the ‘sometimes active’ group if their score was 3.5 to 5.4, and into the ‘inactive’ group if their score was 1 to 3.4. Based on average scores, participants #1 thru #3 were active, #4 thru #10 were sometimes active, and #11 thru #13 were inactive.
Participant characteristics
The SF-36, Modified Fatigue Impact Scale, and MS-Functional Composite obtained from the clinical trial were used to describe the health status of participants. Participant follow-up scores on the SF-36 were compared to general population norms [42], while pretest scores on the MS-Functional Composite were compared to MS-specific population norms [37]. Body mass index was calculated from follow-up physical assessment data.
Data analysis
The interviews were analysed using modified analytical induction [39, 40]. The aim of this approach is to develop a descriptive hypothesis or model that identifies patterns of behaviour, perceptions, or interactions [39, 40]. As recommended by Patton, we first conducted a deductive analysis by coding data into pre-established categories specified by SCT and TMSC [39]. We then conducted an inductive comparative analysis to identify similarities and differences between active, sometimes active, and inactive participants. A descriptive model was then generated from our findings.
Deductive analysis
Theoretical constructs, shown in Figure 2 and described below, guided the deductive analysis. The selection of constructs was based on capturing the central components of SCT and TMSC. The theoretical constructs selected for the pre-established coding categories from SCT were social and physical environment, self-regulation subfunctions, self-efficacy, and outcome expectation. Coding categories from TMSC were primary appraisal, secondary appraisal, and dispositional coping style.
Figure 2.
Selected Theories and Constructs Implemented for Qualitative Analysis
The first author used the pre-determined coding categories to analyse the interviews and statements written in the activity log by selecting quotations from each participant that illustrated the category. An overall description was then generated to summarize the participant’s quotes in the category. To help ensure analytic rigour, an audit trail was generated by citing each coding exemplar to a transcript page number. Thus the two co-authors, from the diverse disciplines of sociology and rehabilitation science, could review the coding scheme and verify the accuracy of the analysis.
Inductive analysis
The inductive analysis consisted of finding similarities and differences between active, sometimes active, and inactive participants by using descriptions and quotes from the deductive analysis. The first author reviewed descriptions and quotes from the deductive analysis repeatedly until patterns emerged within groups. Once such patterns were detected, comparisons were made between PA classification groups (i.e. inactive, sometimes active and active participants). We also explored whether there were differences in constructs between intervention groups (i.e. group wellness and individual rehabilitation). In accordance with Patton’s recommendation [39], the simplicity of a part (i.e. theoretical construct) was compared to the complexity of the whole (i.e. relationship between constructs). To ensure the rigour of the inductive analysis, the three authors discussed and reached agreement on within-group and between-group comparisons.
Results
Participant characteristics
We found no consistent patterns among or between classification groups in terms of demographic characteristics, type of MS, and years-since diagnosis (shown in Table 1). Study participants were predominantly white middle-class females, with an average age of 48 years and an education equivalent to two-years of college. Six participants worked full or part-time and five participants were unable to work due to disability. Most participants had relapsing-remitting MS, which is characterised by acute attacks with full or partial recovery and little to no progress between exacerbations. Two participants had secondary progressive MS, which is characterized by decreased function after an exacerbation and a slow decline between exacerbations. Participant #5, #11, and #13 reported an exacerbation during the study.
Table 1.
Demographic Characteristics, Year-Since Diagnosed with MS and Type of MS
| PA classification | Participant # (group assignment) | Gender | Age | Years of Education | Employment | Year-since Diagnosed | Type of MS |
|---|---|---|---|---|---|---|---|
| Active | #1(W) | F | 55 | 14 | Full-time | 2 | RR |
| #2(IR) | M | 34 | 14 | Unable to work | 3 | RR | |
| #3(IR) | F | 55 | 13 | Unable to work | 28 | RR | |
|
| |||||||
| Sometimes | #4(W) | F | 41 | 18 | Full-time | 16 | RR |
| #5(IR) | F | 44 | 10 | Unable to work | 1 | SP | |
| #6(IR) | F | 34 | 18 | Full-time | 1 | RR | |
| #7(IR) | F | 18 | 12 | Student/part-time | 2 | RR | |
| #8(W) | F | 68 | 14 | Retired/volunteer | 43 | Unknown | |
| #9(W) | M | 47 | 14 | Unable to work | 1 | RR | |
| #10(W) | F | 60 | 16 | Part-time | 18 | SP | |
|
| |||||||
| Inactive | #11(W) | F | 44 | 18 | Full-time | 3 | RR |
| #12(IR) | F | 61 | 16 | Retired | 32 | RR | |
| #13(W) | F | 46 | 13 | Unable to work | 9 | Unknown | |
Key: PA=physical activity; W=wellness & IR=Individual Rehab. F=female; M=male; RR=relapsing-remitting; SP=secondary-progressive
We found no consistent patterns between PA classification groups in terms of indicators of health status, as shown in Table 2. Active participants had a higher mean score on the MSFC and a lower BMI than inactive participants. However, inactive participants had a higher SF-36 mental composite score and a lower fatigue impact score. The SF-36 indicated that as a group (n=13) they were comparable to the general population in mental health, but showed one standard deviation below the general population in physical health [40]. Body mass index showed that five participants were obese. Two active, two sometimes active, and one inactive participant regularly used a mobility device (e.g. cane, scooter, or walker). All but four reported having other conditions of which depression was the most common.
Table 2.
Indicators of Health Status
| PA classification | Participant | SF-36 Mental | SF-36 Physical | MFIS | MSFC | BMI | Mobility device | Other Conditions |
|---|---|---|---|---|---|---|---|---|
| Active | #1 | 55.1 | 36.3 | 24 | −0.28 | 33.2 | No | Anxiety |
| #2 | 37.12 | 30.6 | 66.5 | 0.12 | 25.0 | Yes | Back problems | |
| #3 | 61.04 | 40.2 | 31 | 0.78 | 25.0 | Yes | None | |
|
| ||||||||
| Sometimes | #4 | 39.00 | 48.8 | 36 | 1.01 | 38.3 | No | Depression |
| #5 | 33.6 | 21.3 | 45 | −0.52 | 33.1 | Yes | Anemic | |
| #6 | 61.00 | 43.4 | 24.5 | 0.52 | 27.8 | No | Depression | |
| #7 | 39.7 | 48.00 | 24.5 | −0.08 | 29.5 | No | Depression | |
| #8 | 61.0 | 41.9 | 32.5 | −1.20 | 21.6 | Yes | None | |
| #9 | 49.6 | 43.8 | 31 | −0.34 | 21.5 | No | None | |
| #10 | 60.4 | 35.5 | 35.5 | −0.12 | 25.7 | No | Depression | |
|
| ||||||||
| Inactive | #11 | 55.8 | 48.9 | 34.5 | 0.72 | 20.9 | No | None |
| #12 | 57.2 | 43.3 | 24.5 | −0.81 | 33.6 | No | Arthritis | |
| #13 | 55.8 | 15.00 | 59.5 | 0.13 | 45.1 | Yes | Diabetes | |
Note: MFIS=Modified Fatigue Impact Scale (higher score worse fatigue); MS-Functional Composite (MSFC); BMI=body mass index
Social Cognitive Theory (SCT)
Physical environment (SCT)
The study took place over the summer. Thus hot weather, which exacerbates fatigue [43], was frequently cited as a PA barrier. Participants in all three groups reported difficulty exercising, even indoors, when it was hot outside. Participant #1 said, ‘It was very hard to persevere with the extreme heat. I lacked energy and therefore motivation’. Participant #7 reported that exercising, even in an air-conditioned room, such as a gym, was difficult when the weather was hot.
Active participants and sometimes active participants reported going to recreational facilities to engage in PA. However, participants cited several barriers to exercising at recreational facilities. Some reported that the swimming pool was too warm or that the facility was not easily accessible, and that getting there increased their fatigue. For example, participant #4 reported, ‘I have tried to workout at a gym, but by the time you park your car, change your clothes, and fight your way to the equipment, I am too tired to exercise’.
Social environment (SCT)
While most participants had a story of how the social environment interfered with PA, sometimes active and inactive participants more often cited family and friends as barriers to PA. Participant #6 described how other social activities interfered with exercise: ‘[My friends] want to do social stuff sometimes. “Oh let’s get a coffee”. You know, and I’m really likely to just give up and drink coffee’. Inactive participants described how the social environment was a tipping point that either facilitated or hindered PA. Participant #11 described how a family crisis led to her non-adherence after a period of exercising: ‘I finally got myself up to that [exercising five days a week] before my brother called. I get this phone call [saying my brother had cancer] and everything had changed’.
In participant’s #13 case, the social environment was a tipping point that helped facilitate PA. Participant #13 was motivated by one particular in-home nursing aid who provided support, such as moving a portable mini-cycle by her chair and helping her perform stretches. Several nursing aids came to the house, but not all provided this type of tangible social support.
Self-regulation (SCT)
Self-monitoring
The self-monitoring of PA behaviour and symptoms as well as the observed affects of PA on symptoms were important differences between groups. Active participants consistently wrote in their activity log and often noted problem-solving techniques that helped them engage in PA Participant #1 wrote: ‘I have kept a wet towel on my neck to stay cool. On days when I was tired, I reduced the number of reps. I sometimes space exercise over the day’. Active participants also focused on the positive aspects of engaging in PA and the consequences of not engaging in PA. For example, participant #2 wrote that although his fatigue levels increased after PA, he liked the feeling associated with engaging in PA. Participant #3 observed that if she did not stretch everyday she would become stiff and would have difficulty walking. Because of these self-monitoring cognitions, active participants had a high level of commitment to accomplishing PA goals. Participant #2 provided an example of a determination statement: ‘I have to drive through it [symptoms], because if I don’t do it, it won’t get done. And it has to get done. And I would do everything I have to do to get it done. If it doesn’t kill me…it will get done’.
Sometimes active and inactive participants described how symptoms, such as fatigue, were worsened after engaging in PA and perceived that symptoms were often too severe to engage in PA. For example, participant #13 thought that using the mini-cycle may have caused her exacerbation. Participant #5 had a goal of exercising three times a week because she felt it would help reduce fatigue. However, she felt that some days her fatigue was so severe that she could not exercise. On the other hand, participant #6 recognised that she used her anxiety about possibly exacerbating her fatigue in hot weather, as an excuse not to exercise: ‘Yeah, it’s totally motivation. I mean, I come up with a lot of reasons not to exercise like, “Oh the weather’s bad. It’s too hot or you know, I’m too busy”, but I just kind of come up with those excuses’.
Judgmental Process
Active participants typically described moderate to vigorous activity as PA. They believed that they were more physically active then their peers, and that they needed to engage in PA 3 to 7 days a week to stay healthy. For example, participant #2 said, ‘Once you break a sweat, then you’re being physically active…I think you need to exercise everyday to stay healthy’. Participant #1 referring to how physically active she thought she was compared with other people, said: ‘I think I’m on the level of my peers…I think I’m more active than my husband and co-workers, cause they are kind of couch potatoes’.
In contrast to active participants, sometimes active and inactive participants often described mild to moderate activities as PA. For example, participant #13 described self-care activities, such as showering and dressing, as PA. Sometime active participants gave a wide range of responses on how physically active they viewed themselves compared with their peers, whereas all three inactive participants thought they were as active or even more active compared with their peers. For example, even though participant #11 recognised that her PA levels had declined, she thought she was still more active than her co-workers and family. Similar to active participants, sometimes active and inactive participants felt that they needed to engage in PA 3 to 7 times per week to stay healthy.
Self-reaction
No clear differences emerged between groups on the use of incentives to engage in PA. The most common incentive that participants stated was a sense of accomplishment because of recognition by interventionists in the study. For several participants, the only response in their activity logs for the question about PA strategies was the word ‘study’. The other most common incentive stated by participants was the desire to meet their personal health goals, such as losing weight, maintaining flexibility, and preventing cardiovascular disease. None of the participants mentioned short-term incentives for engaging in PA.
Influences on self-regulation (SCT)
Self-efficacy
Many participants referred to how they managed their symptoms in order to engage in PA. Active participants often indicated that they were confident in managing their symptoms so that they could exercise, whereas inactive participants often indicated low self-efficacy. For example, participant #1 said: ‘I know that my fatigue will always be a problem, but I know that I can fight through it to accomplish my goals’. Participant #13, talking about her cognitive impairment, said: ‘the ability is limited, the ability to remember or not remember [to exercise] is problematic’.
Outcome Expectations
Most participants thought exercise was beneficial, but many had mixed feelings on how well exercise helped them to manage symptoms and/or prevent MS from progressing. Participant #2 said: ‘As far as the disease itself is concerned, I’m completely indifferent [to the effects of exercise], but exercise always benefits me, its exercise.’ Participant #13 felt that exercise was beneficial in improving mental health, but not beneficial in improving physical health. Participant #6, who was newly diagnosed, was waiting to see if the disease progressed before concluding that exercise was beneficial: ‘I just don’t know yet. The jury’s kind of out’.
Reciprocal determinism (SCT)
Sometimes active and inactive participants described how fatigue (i.e. person), hot weather (i.e. physical environment), and social obligations (i.e. social environment) interrelated to form a barrier to PA. The comments of Participant #4, a single mother taking care of three children, illustrate the interaction between fatigue, hot weather, and family obligations:
This is one of the worst summers I have ever had. I think it’s because I’m in the position of having to do it all myself. If my husband was there, I would probably complain enough to have him change the air-conditioning. But because the stress level was high, the responsibility was more. Not just because of the heat but because of everything, the stress, just the whole, all of it built into one summer. I was like ehhh; you know the summer from Hades made it difficult to exercise.
Transactional Model of Stress and Coping (TMSC)
Primary appraisal (TMSC)
No patterns emerged between groups on how participants initially evaluated their diagnosis. Most were devastated. For example, participant #11 feared, ‘losing everything that I’d worked so hard for. I put myself through college and advanced education and now I get hit with MS. It just freaked me out….And I thought my brain was going to turn to mush and I was going to be a vegetable; that was scary’. However, some were relieved to discover why their symptoms were occurring. For example, participant #8 said: ‘I was thinking it’s just in my head. So when I found out that it was something -- that it was a real disease, I was happy’.
Secondary appraisal (TMSC)
A pattern did emerge in the perception of control over the disease by active and inactive participants. Active participants perceived that they had control over the disease. For instance, Participant #1 believed that she could fight the disease by ‘staying positive and engaging in healthy behaviours’. In contrast, sometimes active and inactive participants expressed both feelings of control over symptoms as well as feelings of helplessness. Participant #11 and #12, for example, had very strong feelings about controlling the disease process through exercise, healthy eating and taking medications, whereas participant #13 and #10 felt that they had little control over symptoms and disease progression.
Dispositional coping styles (TMSC)
Unlike inactive participants, active participants were optimistic, information seekers, and often used humour to help them cope with the disease. For example, participant #1 said: ‘I think its [MS] empowered me to be a better person spiritually, physically, and mentally’. Participant #2 was frustrated with many of the limitations caused by MS, but often used humour to help him cope. He joked about ‘walking like a drunken sailor’ and would often decorate his cane with amusing ornaments. All three active participants were self-motivated to manage symptoms of the disease by seeking information from books and health professionals (i.e. information seekers).
Inactive participants more frequently reported a range of negative coping styles. For example, participant #12 was aware of her denial: ‘I’m not so accepting of it I guess. Basically in my mind I just put it on the back burner. I don’t think about it. I’d just rather almost forget about it’. Participant #13 expressed feelings of helplessness, which a therapist had helped her to recognise: ‘I had anxiety, paranoia and all that stuff too and as far as I was concerned I wasn’t being properly sympathised with. I did feel that I had this devastating illness and you got to feel sorry for me’.
Interaction between stress, coping, and health behaviour (TMSC)
All three inactive participants reported considerable stress in their life. For example, participant #11 felt guilty about not exercising, but also felt that coping with her brother having cancer, changing jobs, and recovering from an exacerbation made exercise too difficult. Participant #12 frequently had to take care of her grandchild, which she believed increased her stress and fatigue. In addition to these stressful events, participant #11, #12, and #13 displayed negative dispositional coping styles, such as being in denial about MS, avoiding MS-related functions, and expressing helplessness over the disease. On the other hand, active participants displayed positive and engaging coping styles, which enabled them to participate in social roles that they found meaningful. For example, participant #2 stated: ‘I have coped with not being able to work by taking care of the kids in the neighborhood. After school, my house becomes the local hangout spot’. While he had very little experience in caring for children, he sought out information from books.
Difference between intervention groups
We found no consistent differences in constructs or PA behaviour between participants who had received individual rehabilitation regimens and group wellness programmes. However, active participants typically had favorable attitudes towards the interventions, whereas inactive participants had mixed attitudes towards the interventions. For example, participant #13 did not like going to the wellness group sessions because of her fatigue, whereas participant #11 liked going to the group sessions because she was able to share stories with other participants.
Summary
A persistent theme throughout the data was the relation between the desire to engage in PA and barriers that would overwhelm desire. We found that participants had a threshold or tipping point where barriers would often exceed desire and commitment to engage in PA. Sometimes active and inactive participants described similar barriers to PA. However, the magnitude of the barriers, and the strength of commitment to overcome these, differed among participants. Table 3 summarises similarities and differences between the three PA classification groups.
Table 3.
Similarities and Differences in Key Constructs Between Groups
| Coding Categories | Active | Sometimes Active | Inactive |
|---|---|---|---|
| Self-efficacy | High | Variable | Low |
| Outcome expectations | Variable | Variable | Variable |
| Self-regulation | High | Variable | Low |
| Physical environment | Moderate barriers | Moderate barriers | Moderate barriers |
| Social environment | Low barriers | Moderate barriers | High barriers |
| Primary appraisal | Variable | Variable | Variable |
| Secondary appraisal | Positive | Variable | Variable |
| Coping style | Information seeking Problem-solving Optimism | Variable | Denial Avoidance |
Discussion
This pilot study contributes to the growing literature on understanding how to promote PA behavior among those with MS [44–46]. We found several differences between physically active and inactive participants, whereas sometimes active participants often shared characteristics with the other two groups. Barriers to PA included symptoms, accessibility of recreational facilities, and social obligations. Facilitators of PA included self-regulation, barrier self-efficacy, and positive coping styles. These findings have important implications for developing PA interventions for those with mild to moderate MS. This pilot research also demonstrates the utility of combining different components of SCT and TMSC, in order to understand PA behaviour among persons with MS.
Barriers to PA
Physical environment
Hot weather increased fatigue, which made engaging in PA difficult for members of all three groups. Participants found that exercising indoors helped, but they also cited many barriers to exercising at recreational facilities. Accessibility difficulties at gyms, such as having to park too far away or having to move through a cluttered gym, fatigued participants before they had an opportunity to exercise. These findings are consistent with those of Rimmer et al. [47], who examined the accessibility of 35 recreational facilities and reported low to moderate levels of accessibility for persons with disability.
Social environment
Caring for family members, fulfilling social role obligations, and persuasive arguments by family members and friends to convince participants not to exercise, were common barriers to PA. Numerous studies have found that perceived levels of social support are associated with PA behaviour [48]. In the clinical trial from which the interviewed participants were selected, we found low scores on the Social Support for Exercise Scale. The average score, post-intervention, for social support of friends and family was 2.2 on a 7-point scale. The regression model that was conducted from the clinical trial indicated that social support was a significant independent correlate of PA (standard β=0.31) [44].
Health & Symptoms
Participants in all three groups experienced mild to moderate symptoms, with fatigue being the most common symptom and depression being the most common secondary condition. We found fatigue to be a common barrier to PA, while the influence of depression on PA was less apparent because only sometimes active participants reported depression; future research will need to explore this issue. Although fatigue was a common barrier, we still found examples of persons overcoming fatigue to engage in PA. Self-efficacy may be an important factor in determining whether people overcome their fatigue. Motl et al. [45, 46] has demonstrated that symptoms and self-efficacy influence PA behaviour among persons with MS.
Facilitators of PA
Self-regulation & self-efficacy
Active participants were much more committed to engaging in PA even when symptoms were present. Active participants describe self-regulation subfunctions that were conducive to engaging in PA and were also confident that they could take steps to overcome symptoms that were barriers to PA (i.e. barrier self-efficacy). Sometimes active and inactive participants had little self-motivation to engage in PA, or indicated a strong desire to engage in PA, but felt that their symptoms were too severe. These finding are consistent with Albert Bandura’s argument that [16]: ‘The more capable people judge themselves to be, the higher the goals they set for themselves and the more firmly committed they remain to them’.
Coping strategies
Active participants were optimistic, information seekers, and used problem-solving techniques to engage in PA. These coping strategies may have helped to prevent social environmental stress from becoming a barrier to PA. Inactive participants, on the other hand, used avoidance strategies, which may have increased social environmental stress and resulted in a barrier to PA. Others have reported that positive coping styles, such as optimism, are associated with better health outcomes [49]. Possible mechanisms for better health outcomes may be an increased ability to cope with stress and increased adherence to healthy/self-care behaviours [49, 50].
Utility of SCT & TMSC
In general, we found the SCT and TMSC useful in understanding PA behaviour among persons with MS. Combing constructs from these two theories allowed us to explore how strategies of coping with MS and the person-environment interaction influence PA behaviour. A limitation to these theories is that they are not explicit in the relationship between health, function, and cognitions. This relationship may be particularly important among persons with MS because of the progressive nature of the disease. An increase in symptoms and a decrease in function may influence both actual physical abilities to engage in PA as well as PA cognitions. Furthermore, PA cognitions may also negatively influence health and function. For example, participant #2 stated that he would engage in PA even if it killed him. Although this indicates high self-regulation to engage in PA, this could also indicate poor self-monitoring of symptoms, which is probably not conducive to managing one of his most problematic symptoms, fatigue.
Thus future research will need to explore the relation between symptoms, cognitions, and PA behaviour. For example, which constructs of control and efficacy are most important in influencing PA? Do cognitions, such as barrier self-efficacy, mediate PA behaviour and symptoms severity and are there any factors that moderate this relationship? Systematically examining how to integrate models of health and function (e.g. International Classification of Health and Functioning) with existing theories of behavior change may be a crucial next step in understanding how to promote PA for persons with MS.
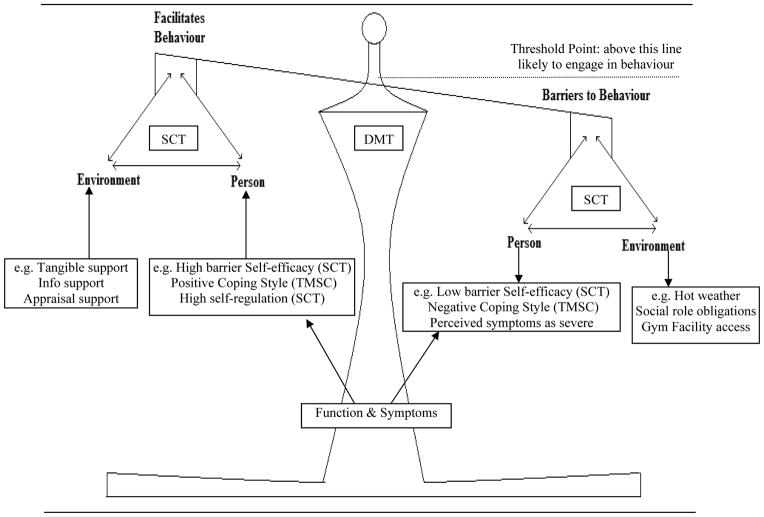
Descriptive model
The relation between barriers overcoming facilitators, and conceptualisation of a threshold or tipping point (described in the summary section of the results), is consistent with Decision-Making Theory (DMT), which states that people are more likely to engage in health behaviour when pros/motivators outweigh cons/barriers [51, 52]. DMT may be particularly useful in accounting for the daily variability in symptoms that persons with MS experience. For example, a person may consistently engage in PA, but then experience an exacerbation (i.e. tipping point) and now has several new barriers to contend with in order to engage in PA. DMT may provide a general framework for integrating concepts from SCT and TMSC. DMT provides insight into how a person decides to engage in PA (i.e. the ratio of barriers to facilitators), while SCT and TMSC provide insights into specific barriers (e.g. unsupportive social environment) and facilitators (e.g. self-regulation) that may influence PA behaviour. Figure 3 depicts a model integrating concepts from the SCT, TMSC, and DMT. The figure also highlights how symptoms and function may influence both facilitators and barriers to engage in PA.
Figure 3.
Integrating Concepts from Decision-Making Theory (DMT), Social Cognitive Theory (SCT), & Transactional Model of Stress and Coping (TMSC). Boxes summarize factors that were found to influence physical activity behaviour.
Implications for physical activity interventions
Findings from this study indicate the need to implement multiple intervention strategies that address both the environment and cognitions, in order to promote PA among those with mild to moderate MS. Unlike interventions designed for the general population, PA interventions for persons with MS will need to address issues of symptom management and improving efficacy to manage symptoms. Bandura [53] suggests that self-efficacy can be improved through verbal persuasion (e.g. talking about the benefits of physical activity), vicarious experience (e.g. encouraging PA with peers who have MS), performance accomplishment (e.g. identifying reinforcement behaviors), and emotional arousal (e.g. differentiating fatigue felt after exercise from fatigue resulting from the disease).
Verbal persuasion
We found that most participants expected positive benefits from engaging in PA, but had mixed feelings on whether PA slowed progression and/or helped in managing the symptoms of MS. Participants may have been reflecting on perceived outcomes from their prior experience with PA [54]. Unfortunately, MS may still progress, regardless of how much a person engages in PA, possibly leading to the conclusion that PA is not beneficial in managing symptoms even though PA may actually be slowing progression [10]. Thus an important aspect of a PA intervention for those with MS will be emphasising that although the disease may progress, PA can help prevent and reduce limitations (i.e. emphasising MS-specific benefit of PA).
Vicarious experience
Encouraging those with MS to engage in PA with friends and encouraging interaction with those who are physically active and have MS may help address social environment barriers and judgmental processes that are not conducive to PA. We found that inactive participants viewed themselves as active and that they had much lower standards on what constitutes PA. Encouraging vicarious experiences, conveying the importance of social support for exercise, and empowering people to seek out supportive social networks may help address social environmental barriers, and help alter judgmental processes [55].
Performance accomplishment
Participants could identify the incentives that encouraged them to engage in PA, but most were extrinsic and long-term. Incentives created by not wanting to disappoint interventionists in the study will cease to be incentives once the study is complete. Furthermore, because of the delayed gratification of PA, and the immediate discomforts possibly associated with it, participants may need to develop short-term incentives (e.g. buying something they desire when their PA goals for the week are met). Thus interventions will need to foster intrinsic motivation (e.g. prescribing a PA program to meet personal health goals) as well as encourage participants to reward themselves when they meet their short-term PA goals [56].
Emotional arousal
Another unique aspect of a PA intervention for those with MS is differentiating the ability to engage in PA from poor self-regulation skills. This will require participants to reflect on their emotional state and why they have low motivation to engage in PA [57]. Is low motivation due to feelings associated with actual physical ability to engage in PA as well as anxiety about making symptoms worse or is low motivation resulting from not making PA a priority? Participants will need to understand when they can push through their symptoms in order to engage in PA, and when they should not because symptoms are too severe. Having persons with MS use an activity log to rate severity of symptoms and mood as well as list pros/cons for engaging PA may help persons differentiate feelings of low motivation from actual physical ability.
Descriptive model (Figure 3)
Determining a person’s threshold point may help tailor a multi-strategic intervention to individual needs. Some participants may have a low threshold because of poor self-regulation skills. In this case, the intervention would focus on improving self-regulation by implementing strategies that increase self-efficacy and change self-regulation subfunctions. On the other hand, some participants may have a high threshold due to high self-regulation skills, but barriers such as fatigue or stress are at too great an intensity to allow adherence. Thus the intervention would focus on removing these barriers by teaching self-management strategies. Future research will need to explore how to incorporate self-management strategies into a PA intervention. For example, energy conservation techniques, such as using the elevator rather than the stairs and taking frequent rest breaks, may be contradictory to promoting PA. How energy conservation techniques are framed in relation to PA needs to be explored.
Limitations
This study was limited by a small sample size in the active and inactive groups. The inclusion of additional participants might have accentuated contrasts between intervention groups (i.e. wellness and individual rehabilitation) and between PA classifications (i.e. inactive, sometimes active and active). Initially, we relied upon conversations with interventionist/participants to confirm heterogeneity of PA levels among participants selected for interviews. Subsequently, we used follow-up PA subscale scores (HPLP-II) to classify participants by PA groups. This methodology resulted in unequal group sizes. Furthermore, analysing data as the interviews were being conducted would have allowed for the purposeful selection of participants and the addition of new interview questions to explore specific contrasts as they appeared in the analysis. These two limitations may have diminished the contrasts between intervention groups and between PA classifications. Another limitation was our method of classification of participants by using self-report levels of PA. The use of a pedometer or an accelerometer may have resulted in a more accurate classification of participants. Despite these limitations, we found similarities within the active and inactive groups, and found differences between these two groups.
Conclusions
The results of this pilot study indicate that PA interventions for those with mild to moderate MS will need to implement multiple strategies to create a supportive social environment, increase barrier self-efficacy to self-manage symptoms, foster positive coping styles, and change cognitions associated with poor self-regulation functions. We found that sometimes active and inactive participants had a threshold or a tipping point at which the magnitude of barriers seemed too large to engage in PA. Determining this point in each case could help tailor a multi-strategic intervention. This study also demonstrates the utility of combining different components of SCT and TMSC to help understand PA behaviour in those with MS. Future research will need to explore how to incorporate models of health and function into existing behaviour change theories, in order to further our understanding of how to promote PA among persons with disabling conditions.
Acknowledgments
This research was supported by Grant #1 R36 HS015554-01 from the Agency for Healthcare Research and Quality (AHRQ), and Grant #HS00011-18 from AHRQ in supporting time for writing the manuscript.
Contributor Information
Matthew A. Plow, Email: mplow@uic.edu.
Linda Resnik, Email: Linda_Resnik@brown.edu.
Susan Allen, Email: Susan_Allen@Brown.edu.
References
- 1.Chitnis T, Khoury SJ. 20. Immunologic neuromuscular disorders. Journal of Allergy Clinical Immunology. 2003;111(2 Suppl):S659–68. doi: 10.1067/mai.2003.92. [DOI] [PubMed] [Google Scholar]
- 2.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmune Review. 2003;2(3):119–25. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 3.Joy J, Johnston R. Multiple sclerosis: current status and strategies for the future. Washington (DC): National Academy Press; 2001. [PubMed] [Google Scholar]
- 4.Cui JY. Multiple sclerosis: an immunologic perspective. Physical Medicine and Rehabilitation Clinics of North America. 2005;16(2):351–8. doi: 10.1016/j.pmr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Cifelli A, Matthews PM. Cerebral plasticity in multiple sclerosis: insights from fMRI. Multiple Sclerosis. 2002;8(3):193–9. doi: 10.1191/1352458502ms820oa. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland G, Andersen MB. Exercise and multiple sclerosis: physiological, psychological, and quality of life issues. Journal of Sports Medicine and Physical Fitness. 2001;41(4):421–32. [PubMed] [Google Scholar]
- 7.Petajan JH, Gappmaier E, White AT, Spencer MK, Mino L, Hicks RW. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Annals of Neurology. 1996;39(4):432–41. doi: 10.1002/ana.410390405. [DOI] [PubMed] [Google Scholar]
- 8.Surakka J, Romberg A, Ruutiainen J, Aunola S, Virtanen A, Karppi SL, Maentaka K. Effects of aerobic and strength exercise on motor fatigue in men and women with multiple sclerosis: a randomized controlled trial. Clinical Rehabilitation. 2004;18(7):737–46. doi: 10.1191/0269215504cr780oa. [DOI] [PubMed] [Google Scholar]
- 9.Plow M, Mathiowetz V, Lowe D. Comparing individualized rehabilitation to a group wellness intervention for persons with multiple sclerosis. American Journal of Health Promotion. doi: 10.4278/ajhp.071211128. In-Press. [DOI] [PubMed] [Google Scholar]
- 10.Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: A longitudinal study of persons with multiple sclerosis. Archives of Physical Medicine and Rehabilitation. 2006;87(7):935–43. doi: 10.1016/j.apmr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Multiple Sclerosis. 2005;11(4):459–63. doi: 10.1191/1352458505ms1188oa. [DOI] [PubMed] [Google Scholar]
- 12.Nieuwenhuijsen ER, Zemper E, Miner KR, Epstein M. Health behavior change models and theories: contributions to rehabilitation. Disability and Rehabilitation. 2006;28(5):245–56. doi: 10.1080/09638280500197743. [DOI] [PubMed] [Google Scholar]
- 13.Rothman AJ. “Is there nothing more practical than a good theory?”: Why innovations and advances in health behavior change will arise if interventions are used to test and refine theory. International Journal of Behavioral Nutrition and Physical Activity. 2004;1(1):11. doi: 10.1186/1479-5868-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs (NJ): Prentice-Hall; 1986. [Google Scholar]
- 15.Stuifbergen AK, Becker H, Blozis S, Timmerman G, Kullberg V. A randomized clinical trial of a wellness intervention for women with multiple sclerosis. Archives of Physical Medicine and Rehabilitation. 2003;84(4):467–76. doi: 10.1053/apmr.2003.50028. [DOI] [PubMed] [Google Scholar]
- 16.Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer Publication Company; 1984. [Google Scholar]
- 17.Gillen G. A comparison of situational and dispositional coping after a stroke. Occupational Therapy in Mental Health. 2006;22(2):31–59. [Google Scholar]
- 18.Harzke AJ, Williams ML, Nilsson-Schonnesson L, Ross MW, Timpson S, Keel KB. Psychosocial factors associated with adherence to antiretroviral medications in a sample of HIV-positive African American drug users. AIDS Care. 2004;16(4):458–470. doi: 10.1080/09540120410001683394. [DOI] [PubMed] [Google Scholar]
- 19.Laubmeier KK, Zakowski SG, Bair JP. The role of spirituality in the psychological adjustment to cancer: a test of the transactional model of stress and coping. International Journal of Behavioral Medicine. 2004;11(1):48–55. doi: 10.1207/s15327558ijbm1101_6. [DOI] [PubMed] [Google Scholar]
- 20.Bandura A. Social cognitive theory of self-regulation. Organizational Behavior and Human Decision Processes. 1991;50(2) [Google Scholar]
- 21.Wenzel L, Glanz K, Lerman C. Stress, Coping, and Health Behavior. In: Glanz K, Rimer BK, Lewis BA, editors. Health behavior and health education: theory, research and practice. San Francisco: Jossey-Bass; 2002. pp. 210–239. [Google Scholar]
- 22.van der Ploeg HP, van der Beek AJ, van der Woude LH, van Mechelen W. Physical activity for people with a disability: a conceptual model. Sports Medicine. 2004;34(10):639–49. doi: 10.2165/00007256-200434100-00002. [DOI] [PubMed] [Google Scholar]
- 23.Sharpe L, Curran L. Understanding the process of adjustment to illness. Social Science and Medicine. 2006;62(5):1153–66. doi: 10.1016/j.socscimed.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Stuifbergen AK. Building health promotion interventions for persons with chronic disabling conditions. Family and Community Health. 2006;29(1 Suppl):28S–34S. doi: 10.1097/00003727-200601001-00006. [DOI] [PubMed] [Google Scholar]
- 25.Corrrigan M, Cupples ME, Smith SM, Byrne M, Leathem CS, Clerkin P, Murphy AW. The contribution of qualitative research in designing a complex intervention for secondary prevention of coronary heart disease in two different healthcare systems. BMC Health Services Research. 2006;6:90. doi: 10.1186/1472-6963-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beverly EA, Wray LA. The role of collective efficacy in exercise adherence: a qualitative study of spousal support and Type 2 diabetes management. Health Education Research. 2008 doi: 10.1093/her/cyn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finlayson M, Van Denend T, Hudson E. Aging with multiple sclerosis. Journal of Neuroscience Nursing. 2004;36(5):245–51. 259. doi: 10.1097/01376517-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Finlayson M, van Denend T. Experiencing the loss of mobility: perspectives of older adults with MS. Disability and Rehabilitation. 2003;25(20):1168–80. doi: 10.1080/09638280310001596180. [DOI] [PubMed] [Google Scholar]
- 29.Olsson M, Lexell J, Soderberg S. The meaning of fatigue for women with multiple sclerosis. Journal of Advance Nursing. 2005;49(1):7–15. doi: 10.1111/j.1365-2648.2004.03258.x. [DOI] [PubMed] [Google Scholar]
- 30.Somerset M, Sharp D, Campbell R. Multiple sclerosis and quality of life: a qualitative investigation. Journal of Health Services and Research Policy. 2002;7(3):151–9. doi: 10.1258/135581902760082454. [DOI] [PubMed] [Google Scholar]
- 31.Wihite B, Keller J, Hodges J, Caldwell L. Enhancing human development and optimizing health and well-being in persons with MS. Therapeutic Recreation Journal. 2004;38(2):167–187. [Google Scholar]
- 32.Dodd KJ, Taylor NF, Denisenko S, Prasad D. A qualitative analysis of a progressive resistance exercise programme for people with multiple sclerosis. Disability and Rehabilitation. 2006;28(18):1127–34. doi: 10.1080/09638280500531842. [DOI] [PubMed] [Google Scholar]
- 33.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Reports. 1985;100(2):126–31. [PMC free article] [PubMed] [Google Scholar]
- 34.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 35.Walker SN, Sechrist KR, Pender NJ. The Health-Promoting Lifestyle Profile: development and psychometric characteristics. Nursing Research. 1987;36(2):76–81. [PubMed] [Google Scholar]
- 36.Ritvo P, Fischer JS, Miller DM, Andrews H, Paty D, LaRocca N. Multiple Sclerosis Quality of Life Inventory: a user’s manual. New York: National Multiple Sclerosis Society; 1997. [Google Scholar]
- 37.Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force Multiple Sclerosis. 1999;5(4):244–50. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 38.Baumgartner T, Jackson A, Mahar M, Rowe D. Measurement for evaluation in physical education and exercise science. 7. Athens: McGraw-Hill; 2003. [Google Scholar]
- 39.Patton MQ. Qualitative research & evaluation methods. 3. Thousand Oaks, Calif: Sage Publications; 2002. [Google Scholar]
- 40.Bogdan R, Biklen SK. Qualitative research for education: an introduction to theory and methods. 5. Boston, MA: Pearson Allyn and Bacon; 2007. [Google Scholar]
- 41.Portney L, Watkins M. Foundations of clinical research: application to practice. 2. Upper Saddle River (NJ): Prentice-Hall; 2000. [Google Scholar]
- 42.Gandek B, Ware JE., Jr Methods for validating and norming translations of health status questionnaires: the IQOLA project approach. International Quality of Life Assessment Journal of Clinical Epidemiology. 1998;51(11):953–9. doi: 10.1016/s0895-4356(98)00086-9. [DOI] [PubMed] [Google Scholar]
- 43.Davis FA, Michael JA, Tomaszewski JS. Fluctuation of motor function in multiple sclerosis related to circadian temperature variations. Diseases of the Nervous System. 1973;34(1):33–6. [PubMed] [Google Scholar]
- 44.Plow M, Mathiowetz V, Resnik L. Multiple sclerosis: impact of physical activity on psychosocial constructs. American Journal of Health Behavior. 2008;32(6):614–626. doi: 10.5555/ajhb.2008.32.6.614. [DOI] [PubMed] [Google Scholar]
- 45.Motl RW, Snook EM, McAuley E, Scott JA, Douglass ML. Correlates of physical activity among individuals with multiple sclerosis. Annual of Behavioral Medicine. 2006;32(2):154–61. doi: 10.1207/s15324796abm3202_13. [DOI] [PubMed] [Google Scholar]
- 46.Motl RW, Snook EM, McAuley E, Gliottoni RC. Symptoms, self-efficacy, and physical activity among individuals with multiple sclerosis. Research in Nursing and Health. 2006;29(6):597–606. doi: 10.1002/nur.20161. [DOI] [PubMed] [Google Scholar]
- 47.Rimmer JH, Riley B, Wang E, Rauworth A. Accessibility of health clubs for people with mobility disabilities and visual impairments. American Journal of Public Health. 2005;95(11):2022–8. doi: 10.2105/AJPH.2004.051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Medicine and Science in Sports and Exercise. 2002;34(12):1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 49.de Ridder D, Fournier M, Bensing J. Does optimism affect symptom report in chronic disease? What are its consequences for self-care behaviour and physical functioning? Journal of Psychosomatic Research. 2004;56(3):341–50. doi: 10.1016/S0022-3999(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 50.Rasmussen HN, Wrosch C, Scheier MF, Carver CS. Self-regulation processes and health: the importance of optimism and goal adjustment. Journal of Personality. 2006;74(6):1721–47. doi: 10.1111/j.1467-6494.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 51.Janis IL, Mann L. Decision making: a psychological analysis of conflict, choice, and commitment. New York: Free Press; 1977. [Google Scholar]
- 52.Marcus BH, Rakowski W, Rossi JS. Assessing motivational readiness and decision making for exercise. Health Psychology. 1992;11(4):257–61. doi: 10.1037//0278-6133.11.4.257. [DOI] [PubMed] [Google Scholar]
- 53.Bandura A. Self-Efficacy: The exercise of control. New York: W.H. Freeman; 1997. [Google Scholar]
- 54.Rothman AJ. Toward a theory-based analysis of behavioral maintenance. Health Psychology. 2000;19(1 Suppl):64–9. doi: 10.1037/0278-6133.19.suppl1.64. [DOI] [PubMed] [Google Scholar]
- 55.McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Social Science and Medicine. 2006;63(4):1011–22. doi: 10.1016/j.socscimed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Roberts G. Advances in motivation in sport and exercise. Champaign, IL: Human Kinetics; 2001. [Google Scholar]
- 57.Bandura A. Human agency in social cognitive theory. American Psychologist. 1989;44(9):1175–84. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]