Abstract
IMPORTANCE
Gastrointestinal stromal tumors (GISTs) are the most commonly diagnosed mesenchymal tumors of the gastrointestinal tract. The risk of recurrence following surgical resection of GISTs is typically reported from the date of surgery. However, disease-free survival (DFS) over time is dynamic and changes based on disease-free time already accumulated following surgery.
OBJECTIVES
To assess the comparative performance of established GIST recurrence risk prognostic scoring systems and to characterize conditional DFS following surgical resection of GISTs.
DESIGN, SETTING, AND PARTICIPANTS
A retrospective cohort study of 502 patients who underwent surgery for a primary, nonmetastatic GIST between January 1, 1998, and December 31, 2012, at 7 major academic cancer centers in the United States and Canada.
MAIN OUTCOMES AND MEASURES
Disease-free survival of the patients was classified according to 5 prognostic scoring systems, including the National Institutes of Health criteria, modified National Institutes of Health criteria, Memorial Sloan Kettering Cancer Center GIST nomogram, and American Joint Committee on Cancer gastric and nongastric categories. The concordance index (also known as the C statistic or the area under the receiver operating curve) of established GIST recurrence risk prognostic scoring systems. Conditional DFS estimates were calculated.
RESULTS
Overall 1-year, 3-year, and 5-year DFS following resection of GISTs was 95%, 83%, and 74%, respectively. All the prognostic scoring systems had fair prognostic ability. For all tumor sites, the American Joint Committee on Cancer gastric category demonstrated the best discrimination (C = 0.79). Using conditional DFS, the probability of remaining disease free for an additional 3 years given that a patient was disease free at 1 year, 3 years, and 5 years was 82%, 89%, and 92%, respectively. Patients with the highest initial recurrence risk demonstrated the greatest increase in conditional survival as time elapsed.
CONCLUSIONS AND RELEVANCE
Conditional DFS improves over time following resection of GISTs. This is valuable information about long-term prognosis to communicate to patients who are disease free after a period following surgery.
Gastrointestinal stromal tumors (GISTs) are the most commonly diagnosed mesenchymal tumors of the gastrointestinal tract, with an annual incidence of 10 to 15 cases per 1 million.1–3 For patients with primary GISTs, complete surgical resection remains the treatment modality that confers the best chance of cure.4 Factors associated with recurrence following resection of GISTs include tumor size, mitotic rate, tumor site, and tumor rupture.5–8 Several investigators have assembled recurrence risk stratification systems using these factors, with the goal of stratifying patients according to recurrence risk.6,9 In addition, a prognostic nomogram for GISTs has been created to numerically predict the recurrence-free survival for a given patient.10 To further standardize classification, GISTs were recently incorporated into the seventh edition of the American Joint Committee on Cancer (AJCC) TNM staging system11; however, the accuracy of this staging method has not been compared with established recurrence risk stratification systems.
Compared with traditional measures of estimating survival, conditional survival estimates have been identified as a more meaningful measure of the survival probability of patients who have an initial survival period.12 Currently available tools to predict recurrence risk of GISTs calculate the risk of recurrence from the time of surgery. However, the probability of future disease-free survival (DFS) changes based on DFS time accumulated. Conditional DFS (CDFS) estimates the probability that a patient will continue to remain disease free after a given length of DFS. Conditional survival estimates have been proposed as a more accurate way to predict prognosis in patients who have survived for a period following surgery.12–14 The objectives of the present study were to assess the comparative performance of established GIST risk stratification and prognostic scoring systems in patients undergoing surgery for a primary, nonmetastatic GIST and to characterize CDFS following surgical resection of GISTs.
Methods
Patient Population and Data Collection
The institutional review board of each participating institution approved the study. The institutional review board deemed that a separate informed consent was not necessary for this study. There were 609 patients who underwent surgery between January 1, 1998, and December 31, 2012, for a primary, nonmetastatic GIST retrospectively identified from 7 major academic cancer centers in the United States (The Johns Hopkins University, Baltimore, Maryland; Duke University, Durham, North Carolina; Emory University, Atlanta, Georgia; Medical College of Wisconsin, Milwaukee; and University of Virginia, Charlottesville) and Canada (University Health Network, Toronto, Ontario; and Sunnybrook Health Sciences Centre, Toronto, Ontario). From this database, 502 patients who underwent curative-intent surgical resection of a primary GIST were included in the present study. Patients with recurrent or metastatic disease were excluded. In addition, patients who underwent an operation for an indication other than GISTs who had an incidental finding of GISTs were excluded because their survival is more likely to be dictated by the indication for operation.
Standard demographic and clinicopathological data were collected and included the following: age, sex, tumor site, tumor size, tumor rupture, year of surgery, multivisceral resection (yes or no), operation type (open or minimally invasive), mitotic rate (number of mitoses per 50 high-power fields [HPFs]), and margin status (negative [R0], microscopically positive [R1], or macroscopically positive [R2]). Details of therapy with a tyrosine kinase inhibitor (TKI), the date of the last follow-up visit, and recurrence and survival information were collected. Recurrence was defined as a biopsy-proven recurrent GIST or a lesion deemed suspicious on cross-sectional imaging.
Statistical Analysis
Summary statistics for the study population are presented as proportions or as median values with interquartile ranges. Disease-free survival for the entire study population was generated using the Kaplan-Meier method, calculated with the date of surgery as the time origin.15 The association of relevant clinicopathological variables with DFS was assessed using Cox proportional hazards models; the prognostic power of covariates was expressed by calculating hazard ratios (HRs) with 95% CIs.16 The variables considered in our analysis were age, sex, tumor site, tumor size, tumor rupture, mitotic rate, neoadjuvant TKI, adjuvant TKI, and margin status.
Kaplan-Meier estimates of DFS and Cox proportional hazards models were used to explore differences among strata established by the National Institutes of Health (NIH) criteria,9 the modified NIH criteria,6 the Memorial Sloan Kettering Cancer Center (MSKCC) GIST nomogram,10 and the GIST staging in the seventh edition of the AJCC TNM staging system.11 For determining DFS based on the MSKCC GIST nomogram, the predicted probability of recurrence at 5 years was calculated using the nomogram, and patients were divided into roughly equal quartiles based on their predicted 5-year recurrence risk (patients with identical predicted 5-year recurrence risk were grouped in the same quartile). The discriminative abilities of each staging system were assessed using the concordance index (also known as the C statistic or the area under the receiver operating curve), a measure that quantifies the proportion of all patient pairs for whom the predicted and observed survival outcomes are concordant.17 A C statistic of 0.50 indicates no discriminatory ability, while a C statistic of 1.00 indicates perfect discrimination. Discrimination for the MSKCC GIST nomogram was calculated using the raw recurrence risk rather than the quartile grouping so as not to diminish the discriminative ability of the nomogram. C statistics were calculated for DFS for tumors at all sites according to the NIH criteria, modified NIH criteria, and MSKCC GIST nomogram. The AJCC TNM staging system has separate stratification for gastric and nongastric tumors. The AJCC gastric category includes GISTs arising in the stomach and omentum, and the AJCC nongastric category includes GISTs arising in the small bowel, esophagus, mesentery, colon, rectum, and peritoneum. To compare discrimination based on the AJCC TNM staging system with that of the other systems, C statistics were calculated separately for gastric and nongastric tumors according to the NIH criteria, modified NIH criteria, and MSKCC GIST nomogram. In addition, the ability of the NIH criteria, modified NIH criteria, MSKCC GIST nomogram, and AJCC gastric and nongastric categories to discriminate overall survival was assessed.
Conditional survival estimates characterize the probability that an individual will survive for an additional number of years given that he or she has already survived a specified amount of time.12–14,18 We calculated CDFS as the probability of remaining disease free for an additional 3 years (CDFS3) given that a patient had survived for x years. The 3-year CDFS for patients who had been disease free for x years was computed as CDFS3 = DFS(x + 3)/DFS(x). Conditional DFS estimates were stratified by prognostic groupings according to the NIH criteria, modified NIH criteria, MSKCC GIST nomogram, and AJCC gastric and nongastric categories. Changes in CDFS3 over time were assessed using linear regression. In addition, a sensitivity analysis was completed to determine if DFS and thus CDFS3 estimates were altered when patients who received neoadjuvant imatinib mesylate were excluded.
All analyses were performed with statistical software (STATA, version 12.0; StataCorp LP). All tests were 2-sided, and P < .05 was considered statistically significant.
Results
Demographic and Clinicopathological Characteristics
The characteristics of 502 patients included in our study are summarized in the eTable in the Supplement. The median age of our study population was 63 years, and 50% of the patients were female. Most tumors originated in the stomach (73%), while others originated in the small bowel (17%), rectum (2%), or elsewhere (8%). Eight percent of patients underwent neoadjuvant TKI therapy. Tumor size was less than 5.0 cm in 54% of patients, between 5.0 and 10.0 cm in 29% of patients, and greater than 10.0 cm in 18% of patients. Most patients (76%) had tumors of low mitotic rate (≤5 mitoses per 50 HPFs), while 14% of patients had tumors of high mitotic rate (>10 mitoses per 50 HPFs). Most patients (94%) underwent an R0 resection, and the incidence of tumor rupture was 1%. Approximately one-quarter (23%) of patients underwent adjuvant therapy with a TKI following resection.
Trends in DFS
Overall DFS after resection of primary GISTs was 95% at 1 year, 83% at 3 years, and 74% at 5 years; the median DFS was not reached (eFigure 1 in the Supplement). On univariate analysis, factors significantly associated with decreased DFS were the following: male sex (HR, 1.59), tumor size of 5.0 to 10.0 cm (HR, 2.49), tumor size larger than 10.0 cm (HR, 4.51), tumor originating in the small bowel (HR, 1.90) or rectum (HR, 4.37), mitotic rate exceeding 10 mitoses per 50 HPFs (HR, 6.32), treatment with neoadjuvant TKI (HR, 2.77), and treatment with adjuvant TKI (HR, 1.66) (P < .05 for all) (Table 1). On multivariable analysis, factors significantly associated with decreased DFS were older age (HR, 1.03), male sex (HR, 2.45), tumor size larger than 10.0 cm (HR, 2.55), tumor originating in the small bowel (HR, 2.34) or rectum (HR, 15.60), mitotic rate exceeding 10 mitoses per 50 HPFs (HR, 6.31), and no treatment with adjuvant TKI (HR, 2.17) (P < .05 for all).
Table 1.
Univariate and Multivariable Cox Proportional Hazards Models for Disease-Free Survival
| Variable | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | 1.02 (1.00–1.04) | .07 | 1.03 (1.01–1.06) | .005 |
| Sex | ||||
| Female | 1 [Reference] | NA | 1 [Reference] | NA |
| Male | 1.59 (1.00–2.52) | .049 | 2.45 (1.37–4.36) | .002 |
| Tumor site | ||||
| Stomach | 1 [Reference] | NA | 1 [Reference] | NA |
| Small intestine | 1.90 (1.12–3.22) | .02 | 2.34 (1.29–4.24) | .005 |
| Rectum | 4.37 (1.72–11.10) | .002 | 15.60 (3.18–76.47) | .001 |
| Other | 1.23 (0.56–2.74) | .61 | 1.21 (0.42–3.47) | .72 |
| Tumor rupture | 3.03 (0.95–9.67) | .06 | 1.81 (0.35–9.20) | .48 |
| Tumor size, cm | ||||
| <5.0 | 1 [Reference] | NA | 1 [Reference] | NA |
| 5.0–10.0 | 2.49 (1.36–4.58) | .003 | 1.33 (0.63–2.81) | .46 |
| >10.0 | 4.51 (2.51–8.10) | <.001 | 2.55 (1.17–5.57) | .02 |
| Mitotic rate, per 50 HPFs | ||||
| ≤5 | 1 [Reference] | NA | 1 [Reference] | NA |
| 6–10 | 1.35 (0.56–3.29) | .50 | 1.37 (0.52–3.59) | .53 |
| >10 | 6.32 (3.84–10.41) | <.001 | 6.31 (3.18–12.50) | <.001 |
| Margin status | ||||
| R0 | 1 [Reference] | NA | 1 [Reference] | NA |
| R1 | 1.99 (0.99–4.01) | .05 | 1.36 (0.63–2.95) | .44 |
| Neoadjuvant TKI | 2.77 (1.40–5.49) | .003 | 1.46 (0.45–4.77) | .53 |
| Adjuvant TKI | 1.66 (1.04–2.65) | .03 | 0.46 (0.24–0.87) | .02 |
Abbreviations: HPFs, high-power fields; HR, hazard ratio; NA, not applicable; TKI, tyrosine kinase inhibitor.
Comparative Performance of Prognostic Scoring Systems
Disease-free survival of the patients was classified according to 5 prognostic scoring systems, including the NIH criteria, modified NIH criteria, MSKCC GIST nomogram, and AJCC gastric and nongastric categories. These results are shown in Figure 1A and B and in eFigure 2A and eFigure 3A and B in the Supplement. The NIH criteria, modified NIH criteria, and AJCC gastric and nongastric categories stratify patients into groups based on recurrence risk, while the MSKCC GIST nomogram assigns a specific value for 5-year DFS. For quartile 1 of the MSKCC GIST nomogram, the actuarial DFS was better than that predicted by the nomogram; for each of quartiles 2, 3, and 4, the actuarial DFS for each quartile was similar to DFS predicted using the nomogram. Specifically, for quartile 1, the median predicted 5-year DFS was 7% (range, 1%–52%), while the actuarial 5-year DFS was 50%. For quartile 2, the median predicted 5-year DFS was 82% (range, 53%–87%), while the actuarial 5-year DFS was 90%. For quartile 3, the median predicted 5-year DFS was 92% (range, 88%–93%), while the actuarial 5-year DFS was 82%. For quartile 4, the median predicted 5-year DFS was 95% (range, 94%–96%), while the actuarial 5-year DFS was 92%.
Figure 1.

Disease-Free Survival (A and B) and 3-Year Conditional Disease-Free Survival (C and D)
A, Modified National Institutes of Health (NIH) criteria. B, Memorial Sloan Kettering Cancer Center (MSKCC) Gastrointestinal Stromal Tumor (GIST) nomogram. C, Modified NIH criteria. D, MSKCC GIST nomogram.
Disease-free survival was calculated for each of the 5 prognostic scoring systems, and discrimination of each system was assessed using C statistic (Table 2). Of the 3 risk stratification systems that examined patients with GISTs at all sites (NIH criteria, modified NIH criteria, and MSKCC GIST nomogram), the MSKCC GIST nomogram was superior for discrimination of DFS, with a C statistic of 0.72. The AJCC TNM staging system has separate stratification for gastric and nongastric tumors, so C statistics were calculated separately for gastric and nongastric tumors using the NIH criteria, modified NIH criteria, and MSKCC GIST nomogram to compare the AJCC system with the other systems. For gastric tumors, the AJCC system had the best discrimination, with a C statistic of 0.79; C statistics of the NIH criteria, modified NIH criteria, and MSKCC GIST nomogram when restricted to gastric tumors were 0.73, 0.73, and 0.72, respectively. For nongastric tumors, the modified NIH criteria, MSKCC GIST nomogram, and AJCC system all had equivalent discriminatory ability, with a C statistic of 0.68; the NIH criteria had a C statistic of 0.62. The prognostic scoring systems were superior for prediction of DFS than for overall survival. For overall survival, C statistics of the NIH criteria, modified NIH criteria, MSKCC GIST nomogram, and AJCC gastric and nongastric categories were 0.60, 0.58, 0.62, 0.72, and 0.60, respectively.
Table 2.
Disease-Free Survival by Prognostic Scoring Systema
| Variable | Disease-Free Survival, % | C Statistic | ||
|---|---|---|---|---|
| 1 y | 3 y | 5 y | ||
| Overall | 95 | 83 | 74 | NA |
| NIH criteria | 0.70 | |||
| Very low risk (n = 54) | 91 | 91 | 84 | |
| Low risk (n = 186) | 97 | 95 | 86 | |
| Intermediate risk (n = 120) | 99 | 94 | 89 | |
| High risk (n = 139) | 91 | 62 | 52 | |
| Modified NIH criteria | 0.69 | |||
| Very low risk (n = 54) | 91 | 91 | 84 | |
| Low risk (n = 184) | 97 | 95 | 86 | |
| Intermediate risk (n = 101) | 99 | 95 | 89 | |
| High risk (n = 160) | 92 | 65 | 56 | |
| MSKCC GIST nomogram | 0.72 | |||
| Quartile 4 (n = 103) | 95 | 92 | 92 | |
| Quartile 3 (n = 135) | 98 | 93 | 82 | |
| Quartile 2 (n = 122) | 98 | 97 | 90 | |
| Quartile 1 (n = 121) | 90 | 60 | 50 | |
| AJCC gastric category | 0.79 | |||
| IA (n = 170) | 97 | 94 | 89 | |
| IB (n = 71) | 98 | 96 | 96 | |
| II (n = 41) | 100 | 100 | 94 | |
| IIIA (n = 23) | 93 | 52 | 52 | |
| IIIB (n = 31) | 83 | 48 | 36 | |
| AJCC nongastric category | 0.68 | |||
| IA (n = 74) | 95 | 95 | 69 | |
| II (n = 12) | 100 | 83 | 83 | |
| IIIA (n = 11) | 100 | 80 | 80 | |
| IIIB (n = 27) | 85 | 57 | 39 | |
Abbreviations: AJCC, American Joint Committee on Cancer; GIST, gastrointestinal stromal tumor; MSKCC, Memorial Sloan Kettering Cancer Center; NA, not applicable; NIH, National Institutes of Health.
The total number of patients varies because of missing data. All percentages and numbers are relative to available data.
Conditional DFS
Overall DFS at 3 years was 83%, and this decreased to 74% at 6 years. In contrast, 3-year CDFS at 3 years (CDFS3) was 89%: this is the probability of remaining disease free for 3 additional years given that an individual is disease free for 3 years following surgery (Figure 2). Similarly, CDFS3 at 5 years (the probability of remaining disease free for an additional 3 years given that the patient is disease free at 5 years) was 92% compared with an actuarial DFS of 68%.
Figure 2.
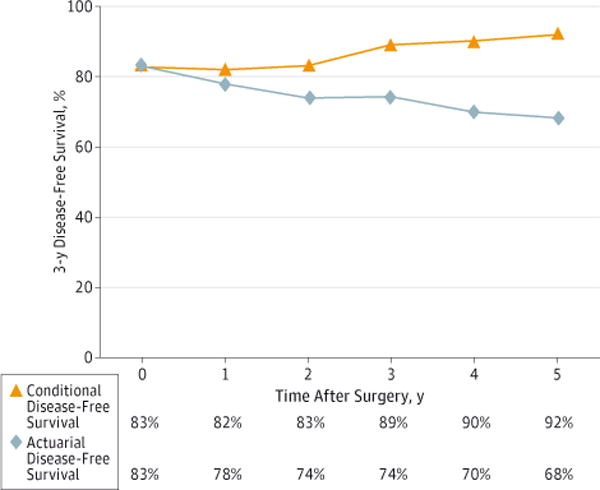
Conditional Disease-Free Survival Relative to Actuarial Disease-Free Survival
Conditional disease-free survival at 3 years was 89%. This is the probability of remaining disease free for 3 additional years given that an individual is disease free for 3 years following surgery.
The calculated CDFS3 for strata of the prognostic scoring systems exceeded the actuarial DFS, particularly for patients who were initially predicted to have poor outcomes (Figure 1C and D and eFigure 2B and eFigure 3C and D in the Supplement). For example, patients classified as having high risk of recurrence using the modified NIH criteria have a CDFS3 of 85% at 3 years compared with an actuarial DFS of 55% at 6 years (Figure 1C). Similarly, patients in quartile 1 of the MSKCC GIST nomogram have a CDFS3 of 83% at 3 years compared with an actuarial DFS of 51% at 6 years (Figure 1D). The differences between actuarial DFS and CDFS were smaller in patients predicted to have better outcomes: patients in quartile 4 of the MSKCC GIST nomogram have a CDFS3 of 92% at 3 years compared with an actuarial DFS of 84% at 6 years.
The differences in CDFS3 over time were more pronounced for patients with higher risk of recurrence. Using the NIH criteria, patients with very low recurrence risk had a smaller change over time in CDFS3 (91%–100%, Δ9%) relative to patients with high recurrence risk (62%–94%, Δ32%). Similar differences in CDFS3 were seen over time between very low vs high risk strata of the modified NIH criteria (91%–100%, Δ9% vs 65%–94%, Δ29%) and between quartile 4 vs quartile 1 of the MSKCC GIST nomogram (91%–100%, Δ9% vs 62%–94%, Δ32%).
The calculated CDFS3 estimates were stratified by modified NIH category, MSKCC GIST nomogram quartile, tumor site, tumor size, and mitotic rate for the first 5 years following surgery for primary GISTs. These results are summarized in Table 3.
Table 3.
Three-Year Conditional Disease-Free Survival From Years 0 to 5 After Surgery
| Variable | 3-y Conditional Disease-Free Survival, % | |||||
|---|---|---|---|---|---|---|
| 0 y | 1 y | 2 y | 3 y | 4 y | 5 y | |
| Modified NIH criteria | ||||||
| Very low risk | 91 | 92 | 92 | 92 | 100 | 100 |
| Low risk | 95 | 91 | 90 | 90 | 97 | 100 |
| Intermediate risk | 95 | 90 | 92 | 94 | 92 | 79 |
| High risk | 65 | 68 | 71 | 85 | 83 | 94 |
| MSKCC GIST nomogram | ||||||
| Quartile 4 | 91 | 92 | 92 | 92 | 100 | 100 |
| Quartile 3 | 95 | 91 | 90 | 90 | 97 | 100 |
| Quartile 2 | 94 | 90 | 91 | 95 | 92 | 79 |
| Quartile 1 | 62 | 65 | 68 | 83 | 82 | 94 |
| Tumor size, cm | ||||||
| <5.0 | 95 | 92 | 92 | 92 | 98 | 100 |
| 5.0–10.0 | 80 | 78 | 80 | 88 | 89 | 85 |
| >10.0 | 62 | 64 | 69 | 83 | 81 | 90 |
| Tumor site | ||||||
| Gastric | 85 | 85 | 88 | 93 | 91 | 90 |
| Nongastric | 78 | 74 | 69 | 77 | 87 | 100 |
| Mitotic rate, per 50 HPFs | ||||||
| ≤5 | 93 | 91 | 91 | 92 | 94 | 91 |
| 6–10 | 85 | 88 | 88 | 92 | 92 | 100 |
| >10 | 48 | 50 | 55 | 74 | 69 | 83 |
Abbreviations: GIST, gastrointestinal stromal tumor; HPFs, high-power fields; MSKCC, Memorial Sloan Kettering Cancer Center; NIH, National Institutes of Health; TKI, tyrosine kinase inhibitor.
Sensitivity Analysis
A sensitivity analysis was completed to determine the effect of the exclusion of patients who received neoadjuvant imatinib on DFS and thus on CDFS3 estimates. When patients who received neoadjuvant imatinib were excluded, DFS and CDFS3 estimates were the same or very similar if patients were classified by the NIH criteria, modified NIH criteria, or MSKCC GIST nomogram quartile. Larger changes in both DFS and CDFS3 were seen in the groups of patients with AJCC stage 3 GISTs after removal of patients who received neoadjuvant imatinib; however, the remaining numbers of patients in these groups were small.
Discussion
Although surgical resection remains the treatment of choice for patients with primary GISTs, the risk of recurrence following resection is significant. Nevertheless, prognosis for patients undergoing resection of GISTs is heterogeneous and depends on several prognostic factors. Accurately defining prognosis following resection for patients with GISTs is important for patients, family members, and care providers. This study is important because it defines DFS for a large, multi-institutional cohort of patients who have undergone resection of GISTs in the imatinib era. We demonstrate that commonly used GIST prognostic scoring systems, including the NIH criteria, modified NIH criteria, MSKCC GIST nomogram, and AJCC gastric and nongastric categories, demonstrate fair prognostic discriminatory ability. In addition, we demonstrate that CDFS can more accurately predict DFS for patients with GISTs who have survived for a period following surgery compared with traditional DFS derived using Kaplan-Meier curves.
In a pooled analysis of 2459 patients with GISTs, the estimated 5-year DFS was 70.5%.19 The overall 1-year, 3-year, and 5-year DFS in the present study was 95%, 83%, and 74%, respectively. Herein, we identified the following 6 independent risk factors for recurrence: older age, male sex, tumor size larger than 10 cm, tumor originating in the small bowel or rectum, mitotic rate exceeding 10 mitoses per 50 HPFs, and no treatment with adjuvant TKI. These risk factors are consistent with previously documented risk factors for recurrence following resection of GISTs, including tumor size, tumor site, mitotic rate, and tumor rupture.5–8
Conditional DFS may be superior to traditional survival estimates and prognostic scoring systems to estimate DFS over time. By considering the time already survived since surgery, CDFS provides a more dynamic estimate of the probability of DFS.14,20 Conditional DFS is particularly useful in counseling patients with an initial high risk of recurrence who have remained disease free for a certain period.21,22 Specifically, 3-year conditional survival estimates increased the most over time for patients in the poorest prognostic groups. These data allow one to tailor estimates of DFS to the individual patient by accurately predicting DFS, taking into consideration both clinicopathological characteristics of the tumor and the time an individual has remained disease free following resection.
To our knowledge, this is the first study that estimates CDFS for GISTs. Conditional DFS has been used for a range of other malignancies. Mayo et al20 examined conditional survival for pancreatic adenocarcinoma after curative-intent resection and found that patients with high lymph node ratios or positive margins had the greatest increase in 2-year conditional survival as time elapsed. Similarly, Nathan et al14 studied conditional survival in a multi-institutional cohort of patients undergoing surgical resection of colorectal liver metastases. In that study, conditional survival improved substantially over time for patients who were initially predicted to have poor survival at the time of surgery. Similarly, in the present study, we noted that CDFS3 for GISTs increased as a function of time survived for patients initially predicted to have the poorest prognosis. For example, using the modified NIH criteria, patients with high recurrence risk had a 32% increase in CDFS3 from the time of surgery to year 5, while patients with very low recurrence risk had only a 9% increase in CDFS3 during that period. Similar trends were seen for the NIH criteria, MSKCC GIST nomogram, and AJCC gastric and nongastric categories. This suggests that traditional DFS estimates are not optimally informative when trying to predict future DFS for patients with high-risk tumors who remain disease free and are seen in follow-up.
The present study had several limitations. Despite its being a large series of primary, nonmetastatic GISTs, the study still had a small sample size (<1000). In addition, collaboration with multiple institutions limited the ability to easily standardize diagnostic and treatment criteria. However, benefits of the multi-institutional study design herein were higher statistical power and greater generalizability of the results.
Conclusions
In conclusion, we demonstrated in this study that DFS estimates following surgical resection of GISTs changed as a function of the time a patient has remained disease free since surgery. For all patients, the probability of remaining disease free for an additional 3 years given that a patient was disease free at 1 year, 3 years, and 5 years was 82%, 89%, and 92%, respectively. In addition, relative CDFS improved the most among patients predicted to have increased risk of recurrence at the time of surgery. Therefore, CDFS can provide accurate information about how DFS changes over time for patients following GIST resection. Conditional DFS estimates should be used to better inform patients of their predicted DFS as they undergo surveillance for recurrence following resection of GISTs.
Supplementary Material
Footnotes
Previous Presentation: This study was presented as a poster at the Society of Surgical Oncology 2014 Annual Cancer Symposium; March 11–12, 2014; Phoenix, Arizona.
Author Contributions: Drs Bischof and Pawlik had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bischof, Kim, Dodson, Cocieru, Kooby, Pawlik.
Acquisition, analysis, or interpretation of data: Bischof, Kim, Jimenez, Behman, Cocieru, Fisher, Groeschl, Squires, Maithel, Blazer, Kooby, Gamblin, Bauer, Quereshy, Karanicolas, Law.
Drafting of the manuscript: Bischof, Jimenez, Gamblin.
Critical revision of the manuscript for important intellectual content: Bischof, Kim, Dodson, Behman, Cocieru, Fisher, Groeschl, Squires, Maithel, Blazer, Kooby, Bauer, Quereshy, Karanicolas, Law, Pawlik.
Statistical analysis: Bischof, Kim, Behman, Cocieru, Groeschl, Squires, Kooby.
Administrative, technical, or material support: Jimenez, Fisher, Squires, Gamblin, Pawlik.
Study supervision: Groeschl, Maithel, Gamblin, Bauer, Pawlik.
Conflict of Interest Disclosures: Dr Bischof reported completing this work when supported by the Detweiler Travelling Fellowship from the Royal College of Physicians and Surgeons of Canada. No other disclosures were reported.
References
- 1.Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438(1):1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era: a population-based study in western Sweden. Cancer. 2005;103(4):821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 3.Tryggvason G, Gíslason HG, Magnússon MK, Jónasson JG. Gastrointestinal stromal tumors in Iceland, 1990–2003: the icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005;117(2):289–293. doi: 10.1002/ijc.21167. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. von Mehren MG, Meyer C, Riedel R, Van Tine B. Soft tissue sarcoma. NCCN Clinical Practice Guidelines in Oncology. 2013 http://www.nccn.org/professionals/default.aspx. Accessed 2013.
- 5.Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST) Cancer. 2008;112(3):608–615. doi: 10.1002/cncr.23199. [DOI] [PubMed] [Google Scholar]
- 6.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39(10):1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30(4):477–489. doi: 10.1097/00000478-200604000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29(1):52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33(5):459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 10.Gold JS, Gönen M, Gutiérrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10(11):1045–1052. doi: 10.1016/S1470-2045(09)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Joint Committee on Cancer. Cancer Staging Atlas: A Companion to the Seventh Editions of the AJCC Cancer Staging Manual and Handbook. 2nd. New York, NY: Springer; 2012. [Google Scholar]
- 12.Merrill RM, Henson DE, Ries LA. Conditional survival estimates in 34,963 patients with invasive carcinoma of the colon. Dis Colon Rectum. 1998;41(9):1097–1106. doi: 10.1007/BF02239430. [DOI] [PubMed] [Google Scholar]
- 13.Henson DE, Ries LA, Carriaga MT. Conditional survival of 56,268 patients with breast cancer. Cancer. 1995;76(2):237–242. doi: 10.1002/1097-0142(19950715)76:2<237::aid-cncr2820760213>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Nathan H, de Jong MC, Pulitano C, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210(5):755–764. 764–766. doi: 10.1016/j.jamcollsurg.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 16.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187–220. [Google Scholar]
- 17.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Bouvier AM, Remontet L, Hédelin G, et al. Association of the French Cancer Registries (FRANCIM) Conditional relative survival of cancer patients and conditional probability of death: a French national database analysis. Cancer. 2009;115(19):4616–4624. doi: 10.1002/cncr.24489. [DOI] [PubMed] [Google Scholar]
- 19.Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265–274. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 20.Mayo SC, Nathan H, Cameron JL, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer. 2012;118(10):2674–2681. doi: 10.1002/cncr.26553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi M, Fuller CD, Thomas CR, Jr, Wang SJ. Conditional survival in ovarian cancer: results from the SEER dataset 1988–2001. Gynecol Oncol. 2008;109(2):203–209. doi: 10.1016/j.ygyno.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 22.Kato I, Severson RK, Schwartz AG. Conditional median survival of patients with advanced carcinoma: surveillance, epidemiology, and end results data. Cancer. 2001;92(8):2211–2219. doi: 10.1002/1097-0142(20011015)92:8<2211::aid-cncr1565>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


