Abstract
A lack of effective treatment for mitochondrial diseases prompts scientists to investigate the molecular processes that underlie their development. The major cause of mitochondrial diseases is dysfunction of the sole mitochondrial DNA polymerase, DNA polymerase γ (Pol γ). The development of treatment strategies will require a detailed characterization of the molecular properties of Pol γ. A novel technique, biolayer interferometry, allows one to monitor molecular interactions in real time, thus providing an insight into the kinetics of the process. Here, we present an application of the biolayer interferometry technique to characterize the fundamental reactions that Pol γ undergoes during the initiation phase of mitochondrial DNA replication: holoenzyme formation and binding to the primer-template.
Keywords: Biolayer interferometry, Protein–protein interactions, Mitochondria, Mitochondrial replisome
1 Introduction
Mitochondria are essential organelles that play a key role in fundamental cellular processes, such as oxidative phosphorylation (OXPHOS), calcium signaling, and apoptosis. Human mitochondria cotntain a semi-independent genome that encodes only 13 polypeptides, all of which are crucial components of the OXPHOS machinery [1]. Thus, defects in the mitochondrial genome often result in a shortage of ATP, which affects tissues with high energy requirements (such as the nervous system or muscle tissue) and can lead to the development of so-called mitochondrial diseases [2]. There is no known cure for any of the diseases resulting from mtDNA dysfunction and only symptom treatment strategies are currently available [3–7]. The majority of human mitochondrial disorders originate from dysfunction of the sole mitochondrial DNA polymerase, DNA polymerase γ (Pol γ). To date, nearly 250 disease-causing mutations have been identified within the POLG gene, encoding the catalytic subunit of Pol γ, Pol γα (http://tools.niehs.nih.gov/polg/). On the molecular level, Pol γ dysfunction causes mtDNA depletion and multiple mtDNA deletions. The mechanisms of pathogenesis of the POLG syndromes are largely unknown and can result from various Pol γ-related issues, such as defects in assembly of the Pol γ holoenzyme, problems in recognition of the primer-template, decreased processivity, etc. Each of these functions is based on specific molecular interactions between all the factors contributing to Pol γ function. Understanding these interactions is thus fundamental to elucidate the molecular basis of disease, and to develop treatments [1].
Pol γα is an ~140 kDa subunit that catalyzes the nucleotide polymerization reaction. It has a weak intrinsic single-stranded DNA (ssDNA) binding ability, but it is able to recognize and bind primer-template and synthesize ~200 nt of nascent strand per turnover [8]. It has also been suggested that Pol γα plays an independent role in mtDNA repair processes [9]. In the replication process, Pol γα binds to its homodimeric accessory subunit, Pol γβ2 (55 kDa/promoter), to form the Pol γ holoenzyme [10]. Pol γβ subunit enhances the ssDNA-binding affinity of Pol γα by approximately 100-fold, and increases its processivity by 50–100-fold [11, 12]. Pol γβ also has the ability to bind dsDNA on its own [13], though this appears to be dispensable for its function as a component of Pol γ; as a part of the holoenzyme, the Pol γβ dimer does not contact the DNA template directly [14]. The low abundance of both of these proteins in vivo limits studies of the early steps of mtDNA replication. Thus, little is known about the mechanisms leading to initiation of the replication process.
In this chapter, we describe the use of biolayer interferometry to study two crucial stages of the initiation process: the formation of Pol γ holoenzyme and replication site recognition. Biolayer interferometry is a technique based on the optical phenomenon of wave interference. It utilizes a novel type of biosensor in the form of a tip with two specific layers at its end. The first external layer, called the biolayer, is coated with molecules of interest and the second layer is an internal reference optical layer. During the measurement, white light is streamed constantly along the biosensor fiber and is reflected from the reference optical layer, as well as from the biolayer. Superposition of waves reflected from both layers forms the interference pattern. Binding of a ligand to molecules on the biolayer changes its optical properties and thus alternates the phase of the reflected light. This effect causes a wavelength shift in the interference pattern, which correlates directly with the thickness of the biosensor tip. The interference pattern is measured constantly, allowing one to monitor changes of the biosensor thickness (nm) in real time [15, 16]. In practice, executing an experiment is limited to the preparation of solutions with and without ligands of interest, in a 96-or 384-well black plate. The Octet device places biosensors into solutions as programmed and displays the results of the analysis in real time, enabling the determination of binding kinetics and affinity. The biosensors are available with diverse modifications of the biolayer, e.g., streptavidin, Ni-NTA, anti-GST antibody, protein A, etc. (http://www.fortebio.com/). This relatively novel technique has already been applied successfully in studies of protein–protein interactions, such as interactions between protein components of the iron-sulfur cluster assembly pathway, or protein–DNA interactions, such as binding of the XPD helicase to ssDNA [17, 18].
Interaction of the Pol γα and Pol γβ2 subunits is fundamental to the formation of Pol γ holoenzyme, and has been studied in the past [11, 19]. To evaluate biolayer interferometry for studies of the mtDNA replisome, we load His-tagged Pol γα onto Ni-NTA-coated biosensors, move the sensors to a reaction buffer containing bovine serum albumin (BSA), which limits non-specific interactions with any unbound sites. BSA is also thought to inhibit weak protein–protein interactions, and is thus considered useful to block non-specific binding between proteins. The response reached with the blocking step serves as a baseline for subsequent steps. Next, sensors are placed into solutions of various Pol γβ concentrations to evaluate the association step, and subsequently placed back into the blocking/baseline solution for the determination of dissociation properties (Fig. 1). The data collected are processed and analyzed with the Octet Data Analysis Software.
Fig. 1.
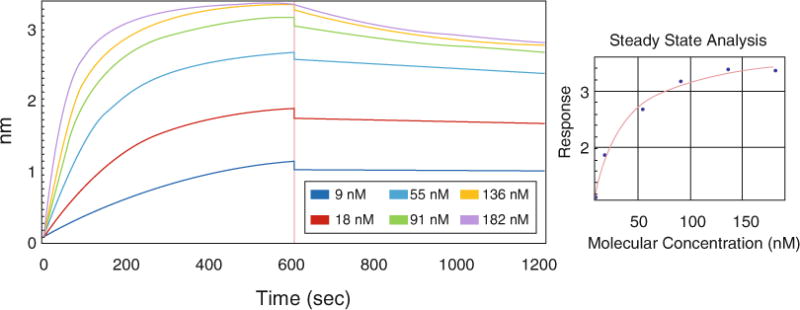
Biolayer interferometry analysis of the Pol γ α–β interaction. Ni-NTA sensors were loaded with Pol γα-his6exo− (10 μg/mL) and blocked with BSA. During the association step (from 0 to 600 s), the sensors were placed into Pol γβ solutions of various concentrations: 9, 18, 55, 91, 136, and 182 nM. Subsequently, the sensors were moved to the buffer without Pol γα or β for dissociation analysis (from 600 to 1200 s). The data were processed and analyzed with the Octet Data Analysis Software. A control without Pol γβ was subtracted from remainder of the data collected. The right panel represents steady-state analysis of the response (nm) to Pol γβ concentrations (nM)
Earlier studies of Pol γ holoenzyme formation reported that the subunits interact with apparent dissociation constant (KD) of 35 nM (±16 nM) [19]. This value was estimated as a function of Pol γβ concentration and its measured biochemical activity of stimulating maximal DNA binding and nucleotide polymerization by Pol γα. For comparison, we utilized a similar approach and used a steady-state analysis for KD estimation, measuring the association response that yielded a value of 20 nM (±1.5 nM). The interaction of the subunits was also analyzed with surface plasmon resonance (SPR) in an earlier study [11]. On a basis of kinetic analysis of association and dissociation rate constants, the authors estimated the KD value to be 27 nM, which is similar to the value we obtain with the steady-state analysis by biolayer interferometry. Because the real-time measurements by biolayer interferometry enable kinetic analysis as well, we fit our data to 1:1 model and obtained association (kon = 1.03 × 105 1/M × s) and dissociation rates (koff = 3.02 × 10−4 1/s), with a resulting KD value of 2.95 nM (±0.03 nM). Although the binding affinity determined by the two different fitting methods vary substantially, they both indicate a tight interaction between the subunits in good agreement with earlier studies. Notably, the data presented here suggest that once assembled, the Pol γ subunits dissociate at a very limited rate. The stability of the Pol γ holoenzyme is further supported by previous studies utilizing crystallography and other biophysical techniques [10, 20]. In comparison to the previous SPR studies [11], in the experiments presented here Pol γβ associates faster with Pol γα to reach the equilibrium state, and dissociates significantly slower. The main difference between our approach and those in the previous studies lies in the preparation and regeneration of the Pol γα-coated sensors (see Note 1). In the SPR experiments the authors used an amino-coupling approach and regeneration with 3 mM NaOH, which might impact the native conformation of the enzyme. The use of Ni-NTA sensors allow us to prepare Pol γα-coated sensors by a gentle immobilization strategy, and the low cost of sensors makes it possible to eliminate a regeneration step.
The Octet biosensor also makes it possible to study protein–DNA interactions. To study the interaction of Pol γ with a primer-template DNA, we used a 5′-biotin-labeled 40-mer oligonucleotide annealed to an 80-mer oligonucleotide, forming a template of 40 nt dsDNA with a free 3′-OH end followed by 40 nts stretch of ssDNA. Streptavidin biosensors were loaded with the primer-template DNA and blocked with BSA. The affinity of the Pol γ holoenzyme to the primer-template DNA was measured by placing the sensors into solution of various Pol γ holoenzyme concentrations. After the association step the sensors are placed back to the baseline solution for the determination of dissociation (Fig. 2), and the data collected are processed and analyzed with the Octet Data Analysis Software.
Fig. 2.
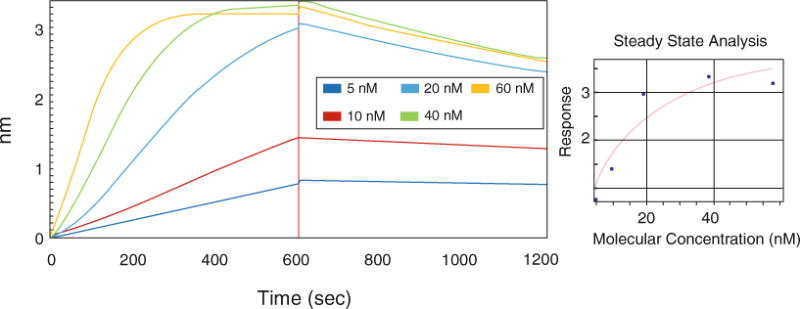
Biolayer interferometry analysis of the Pol γ–primer-template DNA interaction. Streptavidin biosensors were presoaked and loaded with 25 nM 5′-biotinylated 40/80-mer primer-template DNA. After blocking, sensors were placed into Pol γ solutions of 5, 10, 20, 40, and 60 nM, prepared as a mixture of α to β2 subunits in a 1–1.5 molar ratio (association—0–600 s). The dissociation rate was determined after the association step by placing the sensors back into the buffer without Pol γα or β (600–1200 s). The data obtained were processed and analyzed with the Octet Data Analysis Software. A control without Pol γ subunits was subtracted from the remaining set of raw data. The right panel represents steady-state analysis of the response (nm) to Pol γβ concentrations (nM)
Previously published KD values for the Pol γ–primer-template DNA exo− interaction vary significantly depending on the method and conditions used (see Note 2). Kinetic analysis using SPR yielded a value of 0.06 nM, whereas results of gel mobility shift assay and analysis of the stimulation of DNA binding of Pol γα by Pol γβ yielded a value of ~10 nM [11, 12, 19]. The results we obtained with biolayer interferometry analyzed as a function of concentration and association response yielded a KD of 16 nM (±6.5 nM). In the kinetic analysis we determined association and dissociation rates (kon = 1.06 × 105 1/M × s, koff = 3.99 × 10−4 1/s), fitting the results to 1:1 binding model, and obtained a KD value of 3.76 nM (±0.06 nM). Dissociation constant values obtained with both approaches are thus similar to those published previously, except for those determined by SPR. The difference obtained could be linked to the immobilization strategy. In our approach, streptavidin sensor places the DNA strands sparsely and the layer thickness is low (according to manufacturer the biosensor surface may be considered almost flat). We also noticed that the Pol γ–DNA binding data show some deviation from a 1:1 binding model, which could indicate more a complex binding mechanism. Therefore, further studies are needed to understand all the details of the interaction.
In summary, these examples demonstrate that biolayer interferometry provides an effective tool for studies of the mitochondrial replisome. Future studies could be developed to examine how mutations in either of the Pol γ subunits affect holoenzyme formation or the primer-template binding properties of the enzyme, or to test small molecules that may stimulate or inhibit mtDNA replication.
2 Materials
The biolayer interferometry analyses were performed with the ForteBio Octet RED 384 system. The data collected were processed and analyzed using the Octet Data Analysis Software.
2.1 Pol γ Holoenzyme Formation
Pol γα-his6 exo−, 0.04 μg/μL (see Note 1).
Pol γβ, 0.05 μg/μL.
Bovine serum albumin (BSA).
1 M Tris–HCl, pH 7.5.
2 M Potassium chloride (KCl).
1 M Magnesium chloride (MgCl2).
Tween 20.
Glycerol.
Ni-NTA (NTA) biosensors.
384-Well, tilted-bottom black plate (see Note 3).
Pol γα buffer: 30 mM Tris–HCl, pH 7.5, 100 mM KCl, 2 mM EDTA, 20 % glycerol, 5 mM β-Mercaptoethanol.
Reaction buffer 1: 50 mM Tris–HCl, pH 7.5, 1 μg/μL BSA, 4 mM MgCl2, 0.02 % Tween 20 (see Note 1).
2.2 Binding of Pol γ to Primer-Template DNA
Pol γα-his6, 0.08 μg/μL (see Note 2).
Pol γβ, 0.05 μg/μL.
1 μM solution of 5′-biotin-labeled 40 bases oligonucleotide: 5′-ATT ATG AAT TAA TTT AAT AAT TTT TTT TTT TTT TTT TTT T-3′.
1.2 μM solution of 80-mer oligonucleotide: 5′-GGG GGG GGG GGG GGG GGG GGC TGA CTG GGC GGC TCA CTG GAA AAA AAA AAA AAA AAA AAA TTA TTA AAT TAA TTC ATA AT-3′.
Streptavidin (SA) biosensors.
384-Well, tilted-bottom black plate (see Note 3).
Phosphate-buffered saline (PBS): 135 mM NaCl, 10 mM Na2HPO4, 2 mM KCl, 2 mM KH2PO4.
Pol γα buffer: 30 mM Tris–HCl, pH 7.5, 100 mM KCl, 2 mM EDTA, 20 % glycerol, 5 mM β-Mercaptoethanol.
Reaction Buffer 2: 50 mM Tris–HCl, pH 8.0, 0.4 μg/μL BSA, 10 mM DTT, 50 mM KCl 4 mM MgCl2 (see Note 2).
3 Methods
3.1 Pol γ Holoenzyme Formation
Place NTA sensors in a tray and soak them in Pol γα buffer for 30 min.
Prepare a master solution of 10 μg/mL Pol γα-his6 exo in Pol γα buffer (see Note 3).
Prepare Pol γβ solutions of 9, 18, 55, 91, 136, and 182 nM in reaction buffer 1. Also include a control without Pol γβ.
- Pipette solutions for each step in individual wells of a 384-well, tilted-bottom black plate. Measure the following steps:
- Baseline—40 μL Pol γα buffer.
- Loading—40 μL Pol γα solution.
- Baseline 2 and dissociation—40 μL reaction buffer 1.
- Association—40 μL Pol γβ solutions of various concentrations.
- Set the kinetic experiment parameters to 1000 rpm for each step and set duration of steps:
- Baseline—60 s.
- Loading—300 s.
- Baseline 2–300 s.
- Association—600 s.
- Dissociation—600 s.
Start the measurement.
- Process the data as follows:
- Set data from no-Pol γβ control well as reference, and subtract it from the remaining wells.
- Align ϒ axis to the last 5 s of baseline 2.
- Set the inter-step correlation to dissociation.
- Apply the Savitzky–Golay transformation.
- Analyze the data by applying the following settings:
- Set both association and dissociation to be analyzed.
- 1:1 Binding model.
- Apply global fitting with Rmax Unlinked.
3.2 Binding of Pol γ to Primer-Template DNA
Prepare template master solution by annealing 40-mer and 80-mer oligos. Mix equal volumes of the oligomer solutions and incubate for 30 min at 90 °C. After incubation allow the sample to cool down to room temperature.
Prepare 25 nM primer-template solution in PBS (1 pmol/40 μL).
Place SA sensors in a tray and soak them in PBS for 30 min.
Prepare 60 nM Pol γ solution mixing Pol γα and Pol γβ2 solutions in a 1–1.5 molar ratio, respectively, and then prepare dilutions in reaction buffer 2 of 5, 10, 20, and 40 nM. Include a control without Pol γ.
- Pipette solutions for each step into individual wells of a 384-well, tilted-bottom black plate:
- Baseline—40 μL PBS.
- Loading—40 μL of primer-template solution.
- Baseline 2 and dissociation—40 μL reaction buffer 2.
- Association—40 μL of individual Pol γ solutions.
- Set the kinetic experiment parameters by setting the shaking rate at 1000 rpm and the duration of each step as follows:
- Baseline—60 s.
- Loading—300 s.
- Baseline 2–300 s.
- Association—600 s.
- Dissociation—600 s.
Start the measurement.
Process and analyze the data as in the previous section using the no-Pol γ control as a reference.
Acknowledgments
This work was supported by grant GM45295 from the National Institutes of Health, and funds from the University of Tampere to L.S.K. G.C. was supported in part by Biocenter Finland. V.H. was supported by the Academy of Finland (grants 136288 and 273192). We acknowledge infrastructure support from Biocenter Finland.
Footnotes
The Pol γ holoenzyme formation experiment was performed under conditions similar to those used previously for SPR studies [11].
The Pol γ–primer-template DNA interaction experiment was performed under conditions similar those used for the Pol γ activity assay [12].
The 96-well black plate could be used as well, but in that case every solution should be scaled up to 200 μL. The 384-tilted well plate may improve the quality of the baseline and reduce the deviation in the baseline level between the wells.
References
- 1.Copeland WC. Defects of mitochondrial DNA replication. J Child Neurol. 2014;29(9):1216–1224. doi: 10.1177/0883073814537380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raimundo N. Mitochondrial pathology: stress signals from the energy factory. Trends Mol Med. 2014;20:282–292. doi: 10.1016/j.molmed.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Tuppen HA, Blakely EL, Turnbull DM, Taylor RW. Mitochondrial DNA mutations and human disease. Biochim Biophys Acta. 2010;1797:113–128. doi: 10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Fujii T, Nozaki F, Saito K, Hayashi A, Nishigaki Y, Murayama K, Tanaka M, Koga Y, Hiejima I, Kumada T. Efficacy of pyruvate therapy in patients with mitochondrial disease: a semiquantitative clinical evaluation study. Mol Genet Metab. 2014;112:133–138. doi: 10.1016/j.ymgme.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Pirinen E, Canto C, Jo YS, Morato L, Zhang H, Menzies KJ, Williams EG, Mouchiroud L, Moullan N, Hagberg C, Li W, Timmers S, Imhof R, Verbeek J, Pujol A, van Loon B, Viscomi C, Zeviani M, Schrauwen P, Sauve AA, Schoonjans K, Auwerx J. Pharmacological inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014;19:1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagano G, Talamanca AA, Castello G, Cordero MD, d’Ischia M, Gadaleta MN, Pallardo FV, Petrovic S, Tiano L, Zatterale A. Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies: toward mitochondria-targeted clinical strategies. Oxid Med Cell Longev. 2014;2014:541230. doi: 10.1155/2014/541230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell O, Turnbull D. Mitochondrial DNA disease-molecular insights and potential routes to a cure. Exp Cell Res. 2014;325:38–43. doi: 10.1016/j.yexcr.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graves SW, Johnson AA, Johnson KA. Expression, purification, and initial kinetic characterization of the large subunit of the human mitochondrial DNA polymerase. Biochemistry. 1998;37:6050–6058. doi: 10.1021/bi972685u. [DOI] [PubMed] [Google Scholar]
- 9.Kazak L, Reyes A, Holt IJ. Minimizing the damage: repair pathways keep mitochondrial DNA intact. Nat Rev Mol Cell Biol. 2012;13:659–671. doi: 10.1038/nrm3439. [DOI] [PubMed] [Google Scholar]
- 10.Lee YS, Kennedy WD, Yin YW. Structural insight into processive human mitochondrial DNA synthesis and disease-related polymerase mutations. Cell. 2009;139:312–324. doi: 10.1016/j.cell.2009.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yakubovskaya E, Chen Z, Carrodeguas JA, Kisker C, Bogenhagen DF. Functional human mitochondrial DNA polymerase gamma forms a heterotrimer. J Biol Chem. 2006;281:374–382. doi: 10.1074/jbc.M509730200. [DOI] [PubMed] [Google Scholar]
- 12.Fan L, Kim S, Farr CL, Schaefer KT, Randolph KM, Tainer JA, Kaguni LS. A novel processive mechanism for DNA synthesis revealed by structure, modeling and mutagenesis of the accessory subunit of human mitochondrial DNA polymerase. J Mol Biol. 2006;358:1229–1243. doi: 10.1016/j.jmb.2006.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrodeguas JA, Pinz KG, Bogenhagen DF. DNA binding properties of human pol gammaB. J Biol Chem. 2002;277:50008–50014. doi: 10.1074/jbc.M207030200. [DOI] [PubMed] [Google Scholar]
- 14.Lee YS, Lee S, Demeler B, Molineux IJ, Johnson KA, Yin YW. Each monomer of the dimeric accessory protein for human mitochondrial DNA polymerase has a distinct role in conferring processivity. J Biol Chem. 2010;285:1490–1499. doi: 10.1074/jbc.M109.062752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Concepcion J, Witte K, Wartchow C, Choo S, Yao D, Persson H, Wei J, Li P, Heidecker B, Ma W, Varma R, Zhao LS, Perillat D, Carricato G, Recknor M, Du K, Ho H, Ellis T, Gamez J, Howes M, Phi-Wilson J, Lockard S, Zuk R, Tan H. Label-free detection of biomolecular interactions using BioLayer interferometry for kinetic characterization. Comb Chem High Throughput Screen. 2009;12:791–800. doi: 10.2174/138620709789104915. [DOI] [PubMed] [Google Scholar]
- 16.Wallner J, Lhota G, Jeschek D, Mader A, Vorauer-Uhl K. Application of bio-layer interferometry for the analysis of protein/liposome interactions. J Pharm Biomed Anal. 2013;72:150–154. doi: 10.1016/j.jpba.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Prischi F, Konarev PV, Iannuzzi C, Pastore C, Adinolfi S, Martin SR, Svergun DI, Pastore A. Structural bases for the interaction of frataxin with the central components of iron-sulphur cluster assembly. Nat Commun. 2010;1:95. doi: 10.1038/ncomms1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuper J, Wolski SC, Michels G, Kisker C. Functional and structural studies of the nucleotide excision repair helicase XPD suggest a polarity for DNA translocation. EMBO J. 2012;31:494–502. doi: 10.1038/emboj.2011.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson AA, Tsai Y, Graves SW, Johnson KA. Human mitochondrial DNA polymerase holoenzyme: reconstitution and characterization. Biochemistry. 2000;39:1702–1708. doi: 10.1021/bi992104w. [DOI] [PubMed] [Google Scholar]
- 20.Lim SE, Longley MJ, Copeland WC. The mitochondrial p55 accessory subunit of human DNA polymerase gamma enhances DNA binding, promotes processive DNA synthesis, and confers N-ethylmaleimide resistance. J Biol Chem. 1999;274:38197–38203. doi: 10.1074/jbc.274.53.38197. [DOI] [PubMed] [Google Scholar]


