Abstract
Objective
The incidence of central line associated blood stream infections (CLABSI) attributed to central venous catheters (CVC) inserted in the Emergency Department (ED) is not widely reported. Our goal was to report the incidence of ED CLABSI. Secondary goals included determining the impact of a CVC bundle introduced by infection prevention to decrease CLABSI during our surveillance period.
Methods
This was a prospective observational study over a 28-month period at an academic tertiary care center. A standardized electronic CVC procedure note identified CVC insertions in the ED. Abstractors reviewed inpatient records to determine ED CVC catheter-days. An infection prevention specialist identified CLABSIs originating in the ED using National Hospital Safety Network (NHSN) definitions from blood culture results collected up to 2 days after ED CVC removal. During the period of surveillance a hospital-wide CVC insertion bundle was introduced to standardize insertion practices and prevent CLABSIs. Institutional CLABSI rates were determined by infection prevention from routine surveillance data.
Results
Over the 28-month study period, 98 ED physicians inserted 994 CVCs in 940 patients. The ED CVC remained in place for more than 2 days in 679 patients and the median number of days an ED CVC remained in use during the hospital stay was 3 (IQR, 2-7). There were 4,504 ED catheter-days and 9 CLABSIs attributed to an ED CVC. The ED CLABSI rate was 2.0/1,000 catheter-days (95%CI, 1.0 to 3.8). The concurrent institutional intensive care unit (ICU) CLABSI rate was 2.3/1,000 catheter days (95%CI 1.9-2.7). The ED CLABSI rate pre-bundle was 3.0/1,000 catheter days and post-bundle was 0.5/1,000 catheter days (p = .038).
Conclusions
ED CLABSI rates in this academic medical center were in the range of those reported by the ICUs. The impact of ED CLABSI prevention practices requires further research dedicated to surveying ED CLABSI rates.
Introduction
Recent estimates suggest nearly 2 of every 1,000 general ED visits and 270 of every 1,000 visits for sepsis or respiratory arrest will result in the insertion of a central venous catheter (CVC) by an emergency physician. 1,2 Additionally, emergency physicians are placing greater number of CVCs than a decade ago.1,2 The reported incidence of acute mechanical complications from CVC insertion in the ED ranges from 1-5 per 100 CVC insertions.3,4 However, less is known regarding the incidence of central line associated blood stream infections (CLABSIs) attributed to CVCs placed in the ED. Because of the delayed nature of diagnosing CLABSIs, emergency physicians may not be aware that a CLABSI occurred after the patient is admitted.
Since CLABSIs are considered largely preventable the $2 billion spent annually to treat CLABSIs has attracted the attention of policymakers.5 The Centers for Medicare and Medicaid Services (CMS) has introduced payment incentives to encourage institutions to lower CLABSI rates as close to zero as possible.6 The Joint Commission has proposed reporting data on compliance with evidence-based prevention recommendations for CVC insertion as criteria for accreditation.7 The leading evidence-based CLABSI prevention recommendations focus on optimizing CVC insertion practices by creating CLABSI prevention bundles that include all essential equipment, optimizing hand hygiene, ensuring strict sterile technique, avoiding the femoral vein insertion site and using a checklist to ensure compliance with all recommended processes. 8-10
Publicly reported CLABSI rates and successful prevention strategies have focused largely on ICU patients. There are few data on CLABSI rates in EDs or successful ED CLABSI prevention strategies. The National Healthcare Safety Network (NHSN) does not track or attribute CLABSIs to EDs because EDs are not considered inpatient units.11 Only one large study from an academic medical center has compared ED and ICU CLABSI rates and found them to be similar. 12 However the data on CVC insertions originated from billing records and predated the widespread attention now placed on CLABSI prevention efforts. Also, the CLABSI definition used in this study has been updated since publication to exclude blood cultures with common skin flora when they are collected more than 1 calendar day apart and clarify the definition of primary blood stream infections to avoid erroneously associating secondary bloodstream infections with CVCs.11 Surveillance for ED CLABSI and demonstration of the effectiveness of CLABSI prevention techniques in the ED is necessary to help inform clinical operations and guideline recommendations in the very different environment of the ED.13
The primary purpose of this study was to determine ED CLABSIs rates using prospectively identified ED CVC insertions in an ED at an academic tertiary care medical center. Since a “bundle” intervention was introduced during our period of surveillance we also sought to measure the impact of introducing the CVC insertion bundle in the ED.
Methods
This prospective observational study took place over 28 months from March 2008 to June 2010 in a large academic, urban, tertiary care hospital with over 90,000 annual ED visits. The Washington University Human Research Protection Office approved the study.
During our surveillance period all units of the hospital implemented “bundled” CVC insertion kits. The commercial kits (Cardinal Health, Waukegan, IL) were created specifically for the institution with input from the institution's infection prevention department. Beginning in March 2009, each kit contained infection prevention supplies including a 2% chlorehexidine gluconate in 70% isopropyl alcohol skin antiseptic (ChloraPrep; Caridan Health, Dublin, OH), sterile gown, cap, mask, sterile ultrasound probe cover with sterile gel, a checklist to ensure compliance with aseptic techniques and all equipment necessary to perform the CVC insertion using the Seldinger technique. The catheter itself was in a separate package so a variety of CVC types could be used with the insertion kit. To perform the procedure the operator had to collect sterile gloves, the preferred catheter, and ultrasound machine, if deemed necessary. There were no additional resources (staffing or otherwise) devoted to ensuring adherence with the specific elements of the bundle.
In conjunction with the Division of Emergency Medicine's information technology (IT) section, we created a standardized electronic CVC procedure note template for the electronic health record that allowed us to query ED visits in which physicians working in the ED documented the insertion of any CVC.14 Every ED chart indicating an insertion of a CVC (including “crash lines”) was selected for abstraction of demographic data, the procedure date and time, indication, site, and method of insertion.
Research assistants were trained to retrospectively review ED and inpatient medical charts and assign the number of days an ED CVC remained in place. Nurses detailed vascular access status daily in the medical chart including the date of removal of central lines and present method of venous access. If this information was missing we searched radiology records and physician records for details regarding CVC removal. If no record indicated CVC presence or removal we defined the last day of CVC use to be the last day nursing records indicated use of a CVC. During training, each research assistant abstracted 15 charts chosen at random; inter-rater agreement between the principal investigator and research assistant abstraction of catheter-days was measured and feedback was provided. All standardized abstraction forms were flagged for inconsistencies and adjudicated during periodic meetings between the research assistant and principal investigator. During chart abstractor training, the kappa statistics for catheter-days between the principal investigator and the two research assistants were 0.9 (95%CI 0.69-1.0) and 0.9 (95%CI 0.69-1.0). Research assistants were not blinded to the purpose of the study, however, they were blinded to the study outcomes.
Blood culture and hospital discharge dates for cases of ED CVC were obtained from the hospital Medical Informatics database. Candidate CLABSIs included all positive blood cultures from patients with CVCs inserted in the ED, from two calendar days after CVC insertion until two calendar days after that line was removed. Single positive blood cultures with suspected skin contaminants (e.g., coagulase-negative Staphylococci) were excluded. CLABSIs were identified by an experienced infection prevention specialist using the National Healthcare Safety Network (NHSN) April 2013 definitions from the list of candidates.11The primary outcome is expressed as the number of ED CLABSI per 1,000 catheter-line days. Institutional ICU catheter-days and CLABSI rates were obtained from infection prevention's routine surveillance practices of ICUs according to updated NHSN guidelines. Duration of ED stay with a CVC in place was calculated as the time between documentation of CVC insertion and time of patient transport from the ED to a hospital bed. Discharge diagnoses were categorized using ICD-9 CM codes and the Clinical Classification Software (CCS) developed by the Agency for Healthcare Research and Quality.15
Data Analysis
Since, at the study start date, the ED had no formal CLABSI surveillance or prevention program, we estimated an ED CLABSI rate of 3.0/1,000 line days (similar to reported ICU rates prior to large scale CLABSI prevention programs) for the sample size calculation.16 We estimated we needed to capture 4,400 ED catheter-days to obtain a significant difference compared to the institutional target rate of 0.5 CLABSI/1,000 catheter-days with 80% power and α = .05. We present descriptive statistics and 95% confidence intervals for CLABSI rates. Kappa statistics were used to characterize chart reviewer performance. The Chi Square test or Fisher's Exact test were used for categorical variable comparisons, as appropriate, and the Mann-Whitney U test was used to compare non-normally distributed variables. A p<.05 was considered significant. Since the primary objective of the paper was descriptive we did not adjust the alpha for multiple comparisons. SAS version 9.2 (Cary, NC) was used for all analyses.
Results
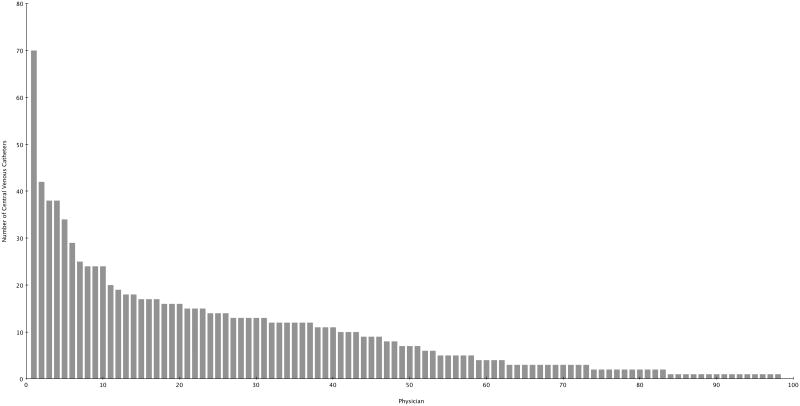
Over the 28-month study period, 98 ED physicians inserted 994 CVCs in 940 patients (35 patients had more than 1 CVC inserted on separate ED visits). The median number of insertions by physician was 7 (interquartile range (IQR), 2-14) (Figure 1). Among these, 491 (49%) were inserted in females, 756 (76%) were inserted due to shock, and 238 (24%) were inserted due to lack of peripheral access. Central lines were placed in the internal jugular in 539 (54%) of cases, subclavian in 172 (17%) cases, and in the femoral vein in 283 (28%) cases. Ultrasound guidance was used in 687 (69%) cases. The median number of hours between ED CVC insertion and patient transfer was 2.2 (IQR, 1-4) and ranged from 8 minutes to 26 hours. A total of 798 (80%) patients were admitted from the ED to an ICU while 196 (20%) were admitted to a general floor. The median number of days a CVC placed in the ED remained in use during the hospital stay was 3 (IQR, 2-7) and the median length of hospital stay for patients with a CVC inserted in the ED was 8 days (IQR, 4-13). ED CLABSI rates were determined from 679 patients in whom the ED CVC remained in place for more than 2 days.
Figure 1. Number of Central Venous Catheters Inserted by Emergency Physicians During the 28-Month Study Period.
Table 1 shows the number of CLABSIs, catheter-days attributed to individual ICUs and to the ED, and CLABSI rates. There were a total of 4,504 ED catheter-days and 68,033 ICU catheter-days during the 28 month time period. Total catheter-days for each of the ICUs varied and ranged from a low of 6,975 catheter-days (Medical ICU) to 15,321 catheter-days (Surgical ICU). The ED contributed 7.7% of total institutional ICU catheter-days.
Table 1. Institutional and Individual Unit Central Line Associated Blood Stream Infection (CLABSI) Rates From March, 2008 to May, 2010.
| Hospital Unit | No. of CLABSI | Catheter-Days | CLABSI/1,000 Catheter-Days | 95% Confidence Interval |
|---|---|---|---|---|
| Neurological ICU | 34 | 7,479 | 4.6 | 3.2-6.3 |
| Medical ICU #1 | 23 | 5,760 | 4.0 | 2.5-6.0 |
| Cardiac Care Unit | 21 | 6,252 | 3.4 | 2.1-5.1 |
| Medical ICU #2 | 24 | 11,118 | 2.2 | 1.4-3.2 |
| Emergency Department | 9 | 4,504 | 2.0 | 0.9-3.8 |
| Surgical ICU | 18 | 12,002 | 1.5 | 0.9-2.4 |
| Cardiothoracic ICU | 4 | 11,727 | 0.3 | 0.1-0.8 |
| Composite ICU | 124 | 54,338 | 2.3 | 1.9-2.7 |
Table 2 shows characteristics of the 9 ED CLABSIs. The median age of patients with ED CLABSI was 52 years and ranged from 26 to 90. CLABSIs occurred at all three catheter insertion sites. There were no cases of ED CLABSIs associated with mucosal barrier injury as defined by updated NHSN definitions. Among the CLABSI cases, the median number of days from insertion to diagnosis was 7.5 and ranged from 2 to 21 days. In 6 of 9 cases a CLABSI was diagnosed in 8 days or less. The median number of ED catheter-days for patients who developed a CLABSI was 10 (IQR, 9-12) while the number of ED catheter-days among patients who did not develop a CLABSI was 5 (IQR, 3-8, p =.005). Median length of hospital stay of patients with ED CLABSI was 13 (IQR, 10-28) days while the median length of stay among ED CVC patients without CLABSI was 8 days (IQR, 5-14, p = .006). Twenty-two percent of the ED CLABSI group (n = 2) died while 14% of the ED CVC group without CLABSI died (n=91, p =.356).
Table 2. Emergency Department Central Catheter Associated Blood Stream Infections: Clinical Characteristics of 9 cases.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Age | 61 | 55 | 51 | 26 | 90 | 46 | 34 | 73 | 52 |
| Sex | Female | Male | Female | Male | Male | Female | Male | Female | Female |
| CCS Admission Diagnosis | Respiratory Failure | Sepsis | Diabetes w/Complications | Spinal Cord Injury | Chronic Kidney Disease | Sepsis | Acute Renal Failure | Brain Injury | Endocrine Disorder |
| Emergency Physician Indication for placement | No Peripheral Access | Shock | No Peripheral Access | Shock | Shock | Shock | Shock | Shock | No Peripheral Access |
| Location of ED CVC | Femoral | Internal Jugular | Internal Jugular | Femoral | Subclavian | Subclavian | Subclavian | Femoral | Subclavian |
| Ultrasound Assisted | No | Yes | Yes | No | No | No | Yes | No | Yes |
| Needle Sticks | 3 | 1 | 1 | 1 | 3 | 2 | 1 | 2 | 1 |
| Level of Operator | Resident | Attending | Resident | Resident | Resident | Resident | Resident | Resident | Attending |
| Duration of catheter in ED, hours | 9 | 2 | 4 | <1 | 3 | 9 | 7 | 4 | 7 |
| Accepting Admitting Unit | Medical Intensive Care Unit | Cardiac Care Unit | Neurologic Intensive Care Unit | Surgical Intensive Care Unit | Medical Intensive Care Unit | General Floor | Medical Intensive Care Unit | Cardiac Care Unit | General Floor |
| Duration of ED catheter, days | 4 | 15 | 9 | 12 | 5 | 28 | 9 | 10 | 10 |
| LOS | 13 | 68 | 9 | 32 | 11 | 28 | 10 | 10 | 20 |
| CLABSI Organism | Streptococcus | Enteroccoccus | MRSA | S. Epidermidis | S. Capitis | S. Epidermidis | C. Albicans | C. Albicans | MRSA |
| Outcome | Discharge | Discharge | Discharge | Discharge | Discharge | Death | Discharge | Death | Discharge |
CCS = Clinical Classification Software; CVC = central venous catheter; LOS = length of stay; MRSA = methicillin-resistant Staphylococcus aureus
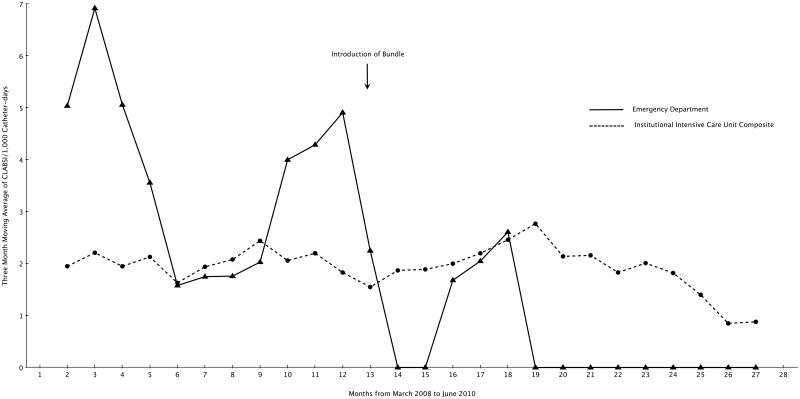
Table 3 shows the characteristics of CVCs inserted in the ED and ED CLABSI rates before and after the introduction of the bundle in March, 2009. ED physicians inserted 497 (50%) before the bundle intervention and 497 CVCs (50%) after the intervention. Before the bundle 8 CLABSIs attributable to CVCs inserted in the ED occurred compared to 1 after the introduction of the bundle (p = .038). Figure 2 shows the plot of the 3-month moving average ED CLABSI rate and the 3-month pooled moving average CLABSI rate of all institutional ICUs during the study period.
Table 3. Characteristics of central venous catheters inserted in the emergency department before and after the introduction of a central venous catheter bundle.
| Pre-Bundle | Post-Bundle | Total | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Mean Age, years (± SD) | 60 | (±17) | 59 | (±18) | |
| Male | 257 | (51) | 246 | (49) | 503 |
| Race | |||||
| African American | 293 | (51) | 273 | (48) | 566 |
| Caucasian | 189 | (47) | 212 | (53) | 401 |
| Other | 15 | (56) | 12 | (44) | 27 |
| Indication | |||||
| Shock | 370 | (49) | 386 | (51) | 756 |
| No Access | 127 | (54) | 111 | (46) | 238 |
| Site of Insertion | |||||
| Internal Jugular | 258 | (48) | 281 | (52) | 539 |
| Subclavian | 103 | (60) | 69 | (40) | 172 |
| Femoral | 136 | (48) | 147 | (52) | 283 |
| Ultrasound Assisted | 324 | (47) | 363 | (53) | 687 |
| Level of Operator | |||||
| Resident | 463 | (50) | 454 | (50) | 917 |
| Attending | 34 | (44) | 43 | (56) | 77 |
| Inserted for >2 days | 351 | (52) | 328 | (48) | 679 |
| Median Emergency Department catheter-days, (IQR) | 178 | (159-212) | 162 | (127-181) | |
| Central Line Associated Blood Stream Infections (CLABSIs) /1,000 catheter days | 3.0 | 0.5 | |||
IQR = interquartile range
Figure 2.
Three Month Moving Average of Central Line Associated Blood Stream Infection (CLABSI) Rates in the Emergency Department and Three Month Moving Average of Pooled Institutional Intensive Care Unit Rates from March, 2008 to June, 2010.
Discussion
In this study the rate of CLABSI from CVCs inserted in the ED was within the range of individual institutional ICU CLABSI rates providing evidence that CVCs inserted in the ED are not at greater risk of infection than CVCs inserted in the ICU. Using updated CLABSI definitions, the ED CLABSI rate of 2.00/1,000 catheter-days was similar to that reported in Lemaster's study of 1.93/1,000 catheter-days.12 ED CLABSIs occurred more frequently in CVCs left in place for longer periods of time, as described previously.12 The rate of CLABSIs was lowest in the cardiothoracic ICU. Though not systematically studied, we suspect many CVCs assigned to this unit are placed during elective and semi-elective procedures in the operating room prior to the patient's ICU stay. The capacity to maximize aseptic technique in the sterile operating room environment potentially explains the low CLABSI rate in the cardiothoracic ICU.17,18
Only 1 ED CLABSI was identified in the last 14 months of the study after infection prevention efforts to package necessary CVC equipment into a bundle were introduced hospital-wide. This intervention may partly explain the lower ED CLABSI rates observed in the latter part of the surveillance period. Lower CLABSI rates have been found when operators strictly adhere to hand hygiene, maximize aseptic technique, use maximal sterile barrier precautions, perform skin anti-sepsis with >0.5% chlorhexidine with alcohol, avoid the femoral vein as a cannulation site, and cover the site with a sterile semi-permeable dressing. 8-10,19 It is important to note there were no efforts to track compliance with specific elements of the CLABSI prevention bundled elements or the rate of adoption by the operators. Furthermore, the study was not powered to explore the effect of this intervention, and other temporal factors may have influenced the decline in the ED CLABSI rate. Determining the adherence to CVC bundles in the ED and the impact on CLABSIs should be a subject of further research.
A significant gap exists in how best to implement CLABSI prevention efforts in settings outside of the ICU.20 Staffing and staff culture, patient volume, the undifferentiated nature of critical illness and timing issues may pose a significant challenge to the implementation of conventional CLABSI prevention techniques in the ED. Since NHSN excludes EDs from formal CLABSI reporting there are no widely reportable data. Determining ED CLABSI rates requires special collaboration with hospital infection prevention personnel to track CVCs placed in the ED and provide timely follow-up.21 Improving feedback and knowledge of both positive and negative CVC outcomes is an important implementation strategy which emphasizes the importance of active surveillance systems to track outcomes.22-25
Active surveillance will require collaborative efforts between infection prevention specialists and ED personnel if ED CVC insertion rates continue to increase1,2,20,26-28 The findings from the PROCESS and ARISE trials in ED patients with septic shock may influence ED CVC insertion rates. In both trials aggressive early quantitative goal-directed therapy, guided by the insertion of a CVC in the ED, achieved similar results to patients treated conservatively with fewer CVC insertions. However, in those studies, half of patients in the less aggressive arms of the protocols still underwent CVC placement.29,30 This far outpaced insertion rates in population-based studies, suggesting approximately 20-25% of septic patients undergo early CVC potentially in the ED.2,26 Without surveillance and feedback mechanisms, ED staff may not realize the impact of CLABSI prevention initiatives or become motivated to adopt practices that disrupt traditional workflow. 20 Determining how to integrate CLABSI prevention methods into ED workflow and which interventions work best will require further research and collaboration.
Limitations
There were limitations to our methodology. This study was constrained by its observational design. While effort was made to capture consecutive CVCs originating in the ED, lack of documentation in the medical record may have occurred resulting in loss of follow up. Catheters lost to follow up may have increased or decreased the ED CLABSI rate depending on whether they resulted in an ED CLABSI or contributed CLABSI free catheter-days. Furthermore, the study was not powered to specifically address the effect of the “bundled” intervention, but we sought to assess its impact to inform future studies. Our statistical analyses assumed independent observations and we did not adjust for the small number of patients included more than once. Lastly, we did not adjust for multiple comparisons so a p<.05 must be interpreted conservatively.
Our definition of ED CLABSI did not account for CVC care that occurred once the patient left the ED. CVCs remained assigned to the ED regardless of how long the ED CVC remained in place. The new NHSN definitions are designed to minimize the reporting of infections unrelated to insertion practices, however, it is possible some CLABSIs might occur from CVC management unrelated to CVC placement. At this institution, infection prevention specialists attribute a CLABSI occurring within 7-10 days after the insertion to placement practices.31 Furthermore, the NHSN rule attributes CLABSIs to units only if the qualifying criteria are present on the same day of transfer or next calendar day. For the purposes of this study, we did not apply the transfer rule to the ED. This could artificially increase the rate of CLABSI attributed to the ED, however, there is no guidance as to how to classify CLABSIs possibly caused by ED CVC insertion or maintenance practices.
Conclusions
The ED CLABSI rate was in the range of rates reported by individual ICUs within our institution. Further resources dedicated to surveying ED CLABSI rates are necessary to determine the impact of CLABSI prevention practices in the ED.
Acknowledgments
Grant: This work was sponsored by a grant from the Agency for Research Healthcare and Quality (AHRQ) K08 HS18092 (DLT), by 5K12RR023249 (VF), and by the Center for Administrative Data Research at Washington University School of Medicine. The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ), and Grant Number KM1CA156708 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH). This research was also supported by the Washington University Emergency Care Research Core (ECRC) which receives funding from the Foundation for Barnes-Jewish Hospital.
Footnotes
Meetings: This work was presented at the Great Plains Society of Academic Emergency Medicine Meeting in October, 2014.
Mesh: Emergency Care, Central Venous Catheters, Catheters, Indwelling/adverse effects, Bacteremia/prevention & control, Bacteremia/epidemiology, Adult
References
- 1.Glickman SW, Krubert C, Koppenhaver J, Glickman LT, Schulman KA, Cairns CB. Increased rate of central venous catheterization procedures in community EDs. The American journal of emergency medicine. 2010;28(2):208–212. doi: 10.1016/j.ajem.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Theodoro D, Owens PL, Olsen MA, Fraser V. Rates and timing of central venous cannulation among patients with sepsis and respiratory arrest admitted by the emergency department*. Critical care medicine. 2014;42(3):554–564. doi: 10.1097/CCM.0b013e3182a66a2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milling TJ, Jr, Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Critical care medicine. 2005;33(8):1764–1769. doi: 10.1097/01.ccm.0000171533.92856.e5. [DOI] [PubMed] [Google Scholar]
- 4.Leung J, Duffy M, Finckh A. Real-Time Ultrasonographically-Guided Internal Jugular Vein Catheterization in the Emergency Department Increases Success Rates and Reduces Complications: A Randomized, Prospective Study. Annals of emergency medicine. 2006;48(5):540–547. doi: 10.1016/j.annemergmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 5.O'Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51(RR-10):1–29. [PubMed] [Google Scholar]
- 6.Inpatient Prospective Payment System (IPPS) Fiscal Year (FY) 2009 Final Rule. Services CfMM. 2008 [Google Scholar]
- 7.National Patient Safety Goals. Washington, D.C.: Joint Commission; 2014. http://www.jointcommission.org/assets/1/6/HAP_NPSG_Chapter_2014.pdf. [Google Scholar]
- 8.Pronovost P, Needham D, Berenholtz S, et al. An Intervention to Decrease Catheter-Related Bloodstream Infections in the ICU. N Engl J Med. 2006;355(26):2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 9.Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Critical care medicine. 2004;32(10):2014–2020. doi: 10.1097/01.ccm.0000142399.70913.2f. [DOI] [PubMed] [Google Scholar]
- 10.Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864–1868. doi: 10.1016/S0140-6736(00)02291-1. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention: National Healthcare Safety Network Device Associated Module: Central Line Associated Bloodstream Infection Event, July 2013. [accessed March 17, 2014];2013 Updated protocol available online at http://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf.
- 12.LeMaster CH, Schuur JD, Pandya D, et al. Infection and natural history of emergency department-placed central venous catheters. Annals of emergency medicine. 2010;56(5):492–497. doi: 10.1016/j.annemergmed.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Cairns CB, Maier RV, Adeoye O, et al. NIH Roundtable on Emergency Trauma Research. Annals of emergency medicine. 2010;56(5):538–550. doi: 10.1016/j.annemergmed.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Theodoro D, Bausano B, Lewis L, Evanoff B, Kollef M. A Descriptive Comparison of Ultrasound-guided Central Venous Cannulation of the Internal Jugular Vein to Landmark-based Subclavian Vein Cannulation. Academic Emergency Medicine. 2010;17(4):416–422. doi: 10.1111/j.1553-2712.2010.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agency for Healthcare Research and Quality; Rockville, MD: 2006-2009. [Accessed July 11, 2011]. HCUP Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP) http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. [Google Scholar]
- 16.Vital signs: central line-associated blood stream infections--United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243–248. [PubMed] [Google Scholar]
- 17.Maki DG, Ringer M, Alvarado CJ. Prospective randomised trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet. 1991;338(8763):339–343. doi: 10.1016/0140-6736(91)90479-9. [DOI] [PubMed] [Google Scholar]
- 18.Raad II, Hohn DC, Gilbreath BJ, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 1994;15(4 Pt 1):231–238. [PubMed] [Google Scholar]
- 19.Warren DK, Yokoe DS, Climo MW, et al. Preventing Catheter Associated Bloodstream Infections: A Survey of Policies for Insertion and Care of Central Venous Catheters From Hospitals in the Prevention Epicenter Program. Infection Control and Hospital Epidemiology. 2006;27(1):8–13. doi: 10.1086/499151. [DOI] [PubMed] [Google Scholar]
- 20.LeMaster CH, Hoffart N, Chafe T, Benzer T, Schuur JD. Implementing the Central Venous Catheter Infection Prevention Bundle in the Emergency Department: Experiences Among Early Adopters. Annals of emergency medicine. 2013(0) doi: 10.1016/j.annemergmed.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 21.The National Healthcare Safety Network (NHSN) Manual. Atlanta: Centers for Disease Control and Prevention; pp. 2010pp. 41–47. [Google Scholar]
- 22.Anderson J, Gosbee LL, Bessesen M, Williams L. Using human factors engineering to improve the effectiveness of infection prevention and control. Critical care medicine. 2010;38(8 Suppl):S269–281. doi: 10.1097/CCM.0b013e3181e6a058. [DOI] [PubMed] [Google Scholar]
- 23.Kretzer EK, Larson EL. Behavioral interventions to improve infection control practices. Am J Infect Control. 1998;26(3):245–253. doi: 10.1016/s0196-6553(98)80008-4. [DOI] [PubMed] [Google Scholar]
- 24.Render ML, Hasselbeck R, Freyberg RW, Hofer TP, Sales AE, Almenoff PL. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725–732. doi: 10.1136/bmjqs.2010.048462. [DOI] [PubMed] [Google Scholar]
- 25.Powell BJ, McMillen JC, Proctor EK, et al. A compilation of strategies for implementing clinical innovations in health and mental health. Medical care research and review : MCRR. 2012;69(2):123–157. doi: 10.1177/1077558711430690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walkey AJ, Wiener RS, Lindenauer PK. Utilization Patterns and Outcomes Associated With Central Venous Catheter in Septic Shock: A Population-Based Study*. Critical care medicine. 2013;41(6):1450–1457. doi: 10.1097/CCM.0b013e31827caa89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quan H, Parsons G, Ghali WA. Validity of Procedure Codes in International Classification of Diseases, 9th revision, Clinical Modification Administrative Data. Medical Care. 2004;42(8):801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 28.Moles E, Andrews RM. Emergency Department Data Evaluation. HCUP Methods Series Report #2005-2. ONLINE June 3 2005 US Agency for Healthcare Research and Quality. 2005 Available: http://www.hcup-us.ahrq.gov/reports/EmergencyDepartmentDataEvaluation.pdf.
- 29.ARISE Investigators; ANZICS Clinical Trials Group. Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 30.ProCess Investigators. Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raad I, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, Bodey GP. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J Infect Dis. 1993;168(2):400–407. doi: 10.1093/infdis/168.2.400. [DOI] [PubMed] [Google Scholar]