Abstract
Nanoscience has matured significantly during the last decade as it has transitioned from bench top science to applied technology. Presently, nanomaterials are used in a wide variety of commercial products such as electronic components, sports equipment, sun creams and biomedical applications. There are few studies of the long-term consequences of nanoparticles on human health, but governmental agencies, including the United States National Institute for Occupational Safety and Health and Japan’s Ministry of Health, have recently raised the question of whether seemingly innocuous materials such as carbon-based nanotubes should be treated with the same caution afforded known carcinogens such as asbestos. Since nanomaterials are increasing a part of everyday consumer products, manufacturing processes, and medical products, it is imperative that both workers and end-users be protected from inhalation of potentially toxic NPs. It also suggests that NPs may need to be sequestered into products so that the NPs are not released into the atmosphere during the product’s life or during recycling. Further, non-inhalation routes of NP absorption, including dermal and medical injectables, must be studied in order to understand possible toxic effects. Fewer studies to date have addressed whether the body can eventually eliminate nanomaterials to prevent particle build-up in tissues or organs. This critical review discusses the biophysicochemical properties of various nanomaterials with emphasis on currently available toxicology data and methodologies for evaluating nanoparticle toxicity.
1. Introduction
The substantial differences in physicochemical properties of nanomaterials compared to the bulk phase has been recognized in numerous scientific and technological areas.1 Nanomedicine is a new field of science based on the significantly enhanced properties of nanoparticles (NPs) (e.g. semiconducting-, metallic-, magnetic-, and polymeric-nanosystems) that make possible the early diagnosis and new treatments for catastrophic diseases, such as multiple sclerosis, atherosclerosis, and cancer.2–5 For instance, one of the most promising NP systems is superparamagnetic iron oxide NPs (SPIONs), which are in clinical development as imaging agents6 and preclinical studies for theranosis applications (i.e. simultaneous diagnosis and treatment).7–10 In addition, SPIONs have been used for magnetic labeling, cell isolation, hyperthermia and controlled drug release.11–21 Several commercial nano-agents are already available for biomedical applications and many nanomedicine-products are near obtaining final approval for clinical use.22
Besides biomedical applications, NPs are used commercially in products such as electronic components, scratch-free paint, sports equipment, cosmetics, food color additives, and surface coatings.23 Hence, our exposure to nanomaterials is significant and increasing, yet there is little understanding of the unique toxicological properties of NPs and their long-term impact on human health.24,25 Because of their very small size, NPs are capable of entering the human body by inhalation, ingestion, skin penetration or injections, and NPs have the potential to interact with intracellular structures and macromolecules for long periods of time.
The number of nanomaterials-based publications has increased significantly over the years; however, the majority of publications are focused on the synthesis and development of novel nanomaterials and less than one percent have focused on NPs’ biological impact. While the toxicity of many bulk materials is well understood, it is not known at what concentration or size they can begin to exhibit new toxicological properties due to nanoscopic dimensions. There is a considerable gap between the available data on the nanomaterials production and toxicity evaluations. The lack of toxicity data can prohibit the safe design of NPs.
This review presents a broad overview of the available in vivo toxicity assessments of NPs. In addition, the biophysicochemical properties of NPs in vivo are discussed in detail.
2. Mechanism of toxicity
Several different mechanisms can cause NP toxicity in body, but most intracellular and in vivo toxicities from NPs arise from the production of excess reactive oxygen species (ROS).26–28 One mechanism of NP-induced oxidative stress occurs during the dissolution of iron-based NPs, which catalyzes ROS generation and formation of OOH• and OH• radicals from H2O2 via the Fenton reaction. Furthermore, some inert nanomaterials do not give rise to spontaneous ROS production, yet are capable of inducing ROS production under biological conditions, based on the ability of the NPs to target mitochondria.29 ROS are both physiologically necessary and potentially destructive. Moderate levels of ROS play specific roles in the modulation of several cellular events, including signal transduction, proliferative response, gene expression and protein redox regulation.30,31 High ROS levels are indicative of oxidative stress and can damage cells by peroxidizing lipids, altering proteins, disrupting DNA, interfering with signaling functions, and modulating gene transcription32 and finally ending up in cancer, renal disease, neurodegeneration, cardiovascular or pulmonary disease. ROS can steal electrons from lipids in cell membrane resulting in decline in physiological function and cell death.33 Oxidative stress associated with TiO2 NPs, for example, results in early inflammatory responses, such as an increase in polymorph nuclear cells, impaired macrophage phagocytosis, and/or fibro proliferative changes in rodents.34 TiO2 NPs also can cause proinflammatory effects in human endothelial cells. Carbon NPs have been shown to induce oxidative stress in fish brain cells and pulmonary inflammation in rats.35,36 Exposure of human keratinocytes to insoluble carbon NPs was associated with oxidative stress and apoptosis.
Toxicity from ROS can be more pronounced in the central nervous system (CNS) due to the high content of unsaturated fatty acids, which are susceptible to peroxidation.37 ROS also play a role in the development of vasculopathies, including those that define atherosclerosis, hypertension, and restenosis after angioplasty.38 Accumulation of NPs in the organs of the reticuloendothelial system (RES) along with the prevalence of numerous phagocytic cells, imbalances ROS homeostasis and antioxidant defenses, which makes the liver and spleen main targets of oxidative stress.
Nanoparticle-induced oxidative stress affects cell signaling in three stages as described by Nel et al.39 A low level of oxidative stress enhances transcription of defense genes through transcription factor nrf2. A higher level of oxidative stress activates inflammation signaling through NFκB, and very high levels are connected with activation of apoptotic pathways and necrosis. Changing these signaling pathways in cells is associated with the carcinogenic effects of NPs.40 Peterson and Nelson reviewed the ROS toxicity of NPs towards the cell nucleus and DNA material. The accumulation of single strand breaks and oxidative induced base lesions can lead to double strand breaks, which are considered the most lethal type of oxidative damage to DNA.41 Excess amounts of ROS can damage the mitochondrial DNA (mtDNA) as well.42 Damage to mtDNA is reported to associate with several clinical syndromes such as neurogenic muscle weakness, ataxia and retinitis pigmentosa, mitochondrial encephalomyopathy lactic acidosis, stroke like episodes, retinitis pigmentosa, cardiac conduction defect and elevated cerebrospinal fluid protein.43 To mitigate ROS effects, some new steps have been taken in NP design. Recently, cerium oxide nanoparticles have been developed that incorporate oxygen defects which scavenge free radicals. It was found that the cerium oxide NPs prevented oxidative stress similarly as well as N-acetyl cystine in mice with tetrachloride-induced liver toxicity. Apart from ROS effects, certain physicochemical properties of NP can also induce toxicity. For instance, recently Minchin et al. showed that some NPs cause unfolding of fibrinogen, hence promoting its interaction with the integrin receptor, Mac-1. Activation of this receptor upregulates the NFκB signaling pathway, resulting in the release of inflammatory cytokines.44
3. Analysis of nanomaterials toxicity
3.1. In vitro assay vs. in vivo assay
The in vitro methods are ideal in nanotoxicology research because they can produce reproducible results rapidly and inexpensively without the use of animals.45 Simple in vitro methods that produce specific and quantitative measurements of toxicity are extremely valuable for initially evaluating the expected biocompatibility of new NPs. Widely cited examples include the LDH assay of cell membrane integrity, the MTT assay of mitochondrial function, and immunochemistry markers for apoptosis and necrosis. However, these methods provide little information on the mechanism or cause of cellular toxicity and death. For example, cell viability is measured as a function of metabolic activity in many tetrazolium-based toxicity assays, but the mechanism underlying mitochondrial inactivity and cell death cannot be elucidated from this assay. In fact, any lethal consequences from NP exposure including membrane lysis, cell cycle arrest and apoptosis may stop mitochondrial activity. Other colorimetric assays such as Live/Dead, Trypan Blue and Neutral also provide little information regarding the mechanisms of cell death, as they just discriminate live cells from dead cells.
The accuracy and precision of colorimetric assays for in vitro toxicity of NPs are also affected by the interactions of the nanoparticles with the colour-generating dyes. For example, it has been found that CNTs interact with MTT formazan crystals or other dyes such as Neutral Red or Alamar Blue by physisorption and produce conflicting results.46–49 Similarly absorption of key proteins such as albumin or LDH can lead to confounding endpoint measurements.50–52 In addition to these problems, inherent issues including dose effect, time course effect, cell–cell and cell–matrix interaction and physico-chemical characteristics of NPs in cell culture conditions also contribute in false results. Since higher doses are usually used in in vitro experiments, toxicity is usually higher in vitro compared with in vivo where lower doses are used. Short term in vitro test results cannot be used as a good prognostic of long-term physiological effects. Recently Lee et al. demonstrated that common 2D cell cultures may not accurately reflect the actual toxicity of NPs as they do not adequately represent the functions of 3D tissues that have extensive cell–cell and cell–matrix interactions with obvious different diffusion/transport conditions.53 Using CdTe nanoparticles, they observed significantly reduced toxicity compared to 2D culture system.
Recent studies have shown little correlation between the in vitro and in vivo toxicity of some nanomaterials. Sayes et al.54 assessed the reliability of in vitro screening studies to predict in vivo pulmonary toxicity of several NPs in rats, including carbonyl iron, crystalline and amorphous silica, and zinc oxide. The comparisons of in vivo and in vitro measurements demonstrated little correlation between groups. Thus the in vitro systems are mainly useful to identify specific characteristics of nanomaterials that can be used as indicators of toxicity and in order to establish a ranking of NP toxicity for mechanistic studies (Fig. 1). Animal models would be particularly useful to study aspects that cannot be obtained with in vitro systems, such as toxico-kinetics in the body, i.e. absorption, distribution, metabolism, and elimination. In vivo tests are time-consuming, expensive, and invoke ethical issues. Nevertheless, these studies can provide information on the carcinogenicity, pulmonary, dermal and gastrointestinal toxicities related to the initial deposition of nanomaterials by various exposure routes. In addition, these studies can evaluate the immunological, neurological, reproductive, cardiovascular and developmental toxicity to determine the chronic systemic toxicity of nanomaterials.55
Fig. 1.
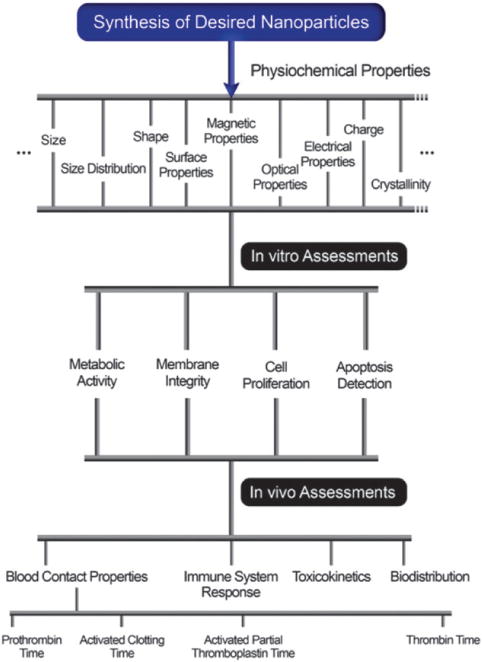
In vivo and in vitro studies for nanotoxicity research.
3.2. In silico assays for nanotoxicity
In silico methods to predict the toxicity of nanomaterials can supplement or replace some expensive and time-consuming assays, especially early in the design process of new materials. Quantitative structure–activity relationships, collectively referred to as (Q)SARs, are theoretical models that can be used to predict the physicochemical and biological properties of molecules.56 According to the QSAR paradigm, if the molecular parameters have been calculated for a group of compounds, but experimental data on the activity of those compounds are available for only part of the group, it is possible to interpolate the unknown activity of the other compounds from the molecular descriptors using a suitable mathematical model.57 In silico predictive toxicology techniques are a fast and cost efficient alternative (or supplement) to bioassays for the identification of toxic effects of nanomaterials.58 Sayes et al. used the QSAR method to develop mathematical models to predict cellular membrane damage resulting from several nanoparticle physicochemical features. They found that the size, concentration and zeta potential of particles in ultra-pure aqueous medium are among the most influential factors on cytoplasm leaking.59 Puzyn et al. applied nano-QSAR to predict the toxicity of 17 different metal oxides nanoparticles. Their theoretical model along with experimental data was able to describe the relationship between NP structure and cytotoxicity to E. coli cells.57 In silico methods can be applied to both in vivo and in vitro data, hence the quality of the in vivo or in vitro data is of fundamental importance. However, the uncertainty of the in vivo data limits the accuracy of the model. In fact, the results from in silico methods cannot be expected to exceed the accuracy of the data used to construct the model.60
4. In vivo toxicity
The increasing production and use of NPs, has given rise to many concerns and debates among public, scientific and regulatory authorities regarding their safety and final fate in biological systems. In vitro and in silico methods for acute chemical toxicity are able to provide adequate data for many bulk materials; however, the in vivo interaction of NPs and biological system is quite complicated and dynamic. In addition, in the absence of sufficient in vivo data to correlate with the in vitro and in silico assays, these methods are of limited use.
Nanoparticles can be administrated by six principal routes: intravenous, transdermal, subcutaneous, inhalation, intraperitoneal and oral.61 Although inhalation, ingestion, skin contact and intravenous injection are the predominant routes of exposure for human, existing in vivo data were largely collected from inhalation and intra-tracheal instillation in rodents, including the bulk of toxicity data for Au, C, CdO, Fe, Mn2O3, Ni, TiO2 and carbon nanotubes.62 A limited number of studies concern the intravenous and oral routes of administration, which are more relevant for most NPs of interest in nanomedicine.63
When the nanostructures enter into the body, absorption can occur through interactions with biological components such as proteins and cells; afterwards, they can distribute into various organs where they may remain in the same structure or become modified or metabolized.64 NPs may enter cells of the organ and reside in the cells for an unknown amount of time before leaving to other organs or to be excreted. Most of the recent studies in this area have focused on the absorption of nanostructures via inhalation or dermal exposure. Some studies of NP toxicity have focused on NPs with known toxic characteristics, such as asbestos and carbon black.65–67 However, these studies are inadequate to predict the biological interactions of substantially more complex nanoparticles. For example, the absorption of quantum dots (QDs) through porcine skin is highly variable with surface coating chemistry, with periodic variation in cellular uptake.68 Orally administered nanostructures do not seem to be significantly absorbed and are recovered in feces.69,70 These studies suggest the importance of exposure route and physical properties of the nanostructures on absorption behavior. The importance of in vivo studies in nanomaterial toxicology and the challenges encountered in such studies have been discussed in detail by Fischer et al.71
A complete analysis of the pharmacokinetics (PK) of NPs is necessary to understand their activity and their potential toxicity. PK includes absorption, distribution, metabolism, and excretion of nanostructures and gives quantitative information about their behavior in biological systems. The data can lead (i) to improvements in design of NPs for diagnostic and therapeutic applications, (ii) a better understanding of nanostructures non-specificity toward tissues and cell types, and (iii) assessments of basic distribution and clearance that serve as the basis in determining their toxicity and their future investigative directions.
4.1. Blood contact properties
Blood compatibility is an essential property for the in vivo functions of most NPs. Lack of blood compatibility may trigger coagulation and clot formation through adsorption of plasma proteins, platelet adhesion and activation of complement cascades. The coagulation of NPs is thermodynamically driven to minimize the contact surfaces areas of hydrophobic domains with the aqueous milieu. Therefore, the blood contact properties of NPs should always be evaluated prior to clinical use to gauge their safety.72 NPs in contact with blood can induce hemolysis, palate aggregation or blood coagulation, so the hemolysis assay using mammalian erythrocytes is a primary screening of NP toxicity.73,74 Erythrocytes are the predominant cell type in the blood and play a crucial role in transporting oxygen. These cells are vulnerable to toxicity with deformation, agglutination and membrane damage.75 NPs exert hemolytic effects through multiple mechanisms, including enzymatic modifications, changes to the rheological properties, oxidative damage of cell membranes, changes in osmotic stability and endotoxin and/or microbial contamination.
There is some evidence that some carbon nanoparticles and microparticles have the ability to activate platelets and enhance vascular thrombosis and initiate thrombosis.76,77 Radomski et al. showed that both urban dusts and engineered carbon particles, such as CNT and carbon black, except C60CS, stimulated platelet aggregation and accelerated the rate of vascular thrombosis in rat carotid arteries with a similar rank order of efficacy. All particles resulted in up-regulation of GPIIb/IIIa in human platelets. In contrast, particles differentially affected the release of platelet granules, as well as the activity of thromboxane-, ADP-, matrix metalloproteinase- and protein kinase C-dependent pathways of aggregation.77 Consistent with the in vitro results, exposure to nano Ag (0.05–0.1 mg kg−1 intravenously or 5–10 mg kg−1 intratracheal instillation) in vivo enhanced platelet aggregation and promoted venous thrombus formation in rats.78 Similarly, Seaton et al. have shown that exposure to ambient airborne particulate matter with aerodynamic diameter of 10 nm or less results in increased plasma fibrinogen levels.79 In addition, tracheal inhalation of diesel exhaust particles caused peripheral thrombosis in experimental animals.80
The coagulation properties of NPs can be evaluated with several routine and widely available clinical assays,63 including prothrombin time (PT), activated clotting time (ACT), activated partial thromboplastin time (APTT) and thrombin time (TT). In addition, Neun et al. developed detailed procedures for measuring the thrombogenicity of NPs.81 Since NPs may affect the intrinsic coagulation pathway, NP treatment should be subject to the APTT testing prior to use in nanomedical application.82,83 Table 1 provides a summary of procedures for testing blood coagulation. Such clotting assays are useful as screening tests to evaluate the intrinsic and extrinsic effects of NPs on the blood coagulation cascades.
Table 1.
Common blood compatibility test (coagulation test) of NPs
| Blood test | Test description | Measured parameter | Test procedure |
|---|---|---|---|
| Prothrombin time (PT) | Evaluates extrinsic coagulation pathway | Coagulation time | Thromboplastin and Ca2+ are added to a blood sample containing NPs. The time for clot formation is measured. |
| Activated clotting time (ACT) | Evaluates coagulation in heparin-treated blood | Coagulation time | Similar to PT using heparinized blood |
| Activated partial thromboplastin time (APTT) | Evaluates intrinsic coagulation pathway | Coagulation time | NPs are added to a plasma sample, in which the intrinsic pathway is activated by the addition of phospholipid, an activator (kaolin, or micronized silica), and Ca2+.84,85 |
| Thrombin time (TT) | Evaluates blood clot formation rates after thrombin treatment compared with that of a normal plasma control | Coagulation time | Addition of a standard amount of thrombin to plasma that has been depleted of platelets but contains NPs. Clotting time is measured. |
4.2. Pharmacokinetics study of NPs
4.2.1. Absorption
The surfaces of NPs are rapidly covered by selective sets of blood plasma proteins after injection, forming the so-called “protein corona”. For other routes of exposure, NPs must pass through additional physiological barriers before entering the blood (e.g., the skin, gastrointestinal tract, or the lungs), picking up additional biomolecules as they are transported.86 The absorption of biomolecules to such surfaces confers a new “biological identity” in the biological milieu, which determines the subsequent cellular and tissue responses.87 The protein corona is a complex mixture of absorbed proteins in equilibrium on the surface of NPs88,89 which play an important role in determining what surface is actually presented to cells that take the nanostructure up and activate signaling pathways.90,91 The protein corona is composed of an inner layer of selected proteins with a lifetime of several hours in slow exchange with the environment (hard corona) and an outer layer of weakly bound proteins which are characterized by a faster exchange rate with the free proteins (the soft corona).86 The biological impact of protein-coated NPs is mainly related to the hard corona and their specificity and suitable orientation for a particular receptor. Although low-affinity high-abundance proteins may initially adsorb to the surface of NPs, lower abundance but higher affinity proteins quickly replace them.
Nanoparticle size, shape, surface charge, and solubility are among the contributing factors, which determine the interaction of the NPs with proteins.92 Protein absorption strongly influences NPs fate and biodistribution in body. For instance, adsorption of fibrinogen, IgG, or complement factor, is believed to promote phagocytosis with removal of the NPs from the bloodstream.93 On the other hand, binding of human serum albumin or apolipoproteins promotes prolonged circulation time in blood.94
A combination of analytical techniques is needed to understand the binding kinetics between nanostructures and the proteins on cells.90,95 Conformational changes of proteins adsorbed onto nanostructure surfaces could alter the function of the protein88,96 and could affect the fate of the nanostructures.94,102 Many of the subset of serum proteins that interact with nanosystems are immunoactive, such as complement factors97 and immunoglobulins. A correlation of nanostructure–protein interaction with in vivo PK data permits the assembly of a structure–activity relationship; this represents an important next step for evaluating nanotoxicity.71
4.2.2. Distribution
After absorption, NPs can be distributed to various organs, tissues, and cells. Only a few recent studies have focused on the biodistribution of engineered nanostructures. In these studies, the key is to quantitatively map the location of the nanostructures at different time points and at different doses, as size, shape, aggregation state and surface chemistry may affect nanostructure biodistribution. Thus it is difficult to predict the in vivo behavior of NPs. When injected intravenously, NPs are cleared rapidly from the circulation, predominantly by the Kupffer cells of the liver and the spleen macrophages.98
In the distribution phase, the density and permeability of blood vessels are key factors that determine the speed at which equilibrium and organ-specific concentrations are reached, where highly vascularized areas reach equilibrium more rapidly than poorly vascularized areas. In studies with (QDs) and single-walled carbon nanotubes (SWCNT), it was discovered that a high dose percentage can be sequestered in the liver, dependent upon the surface modification.70,99,100 Other organs such as the spleen, lymph node, or bone marrow can take up nanostructures. All of these organs contain large concentrations of macrophages, which are part of the reticuloendothelial system (RES). The RES system, also called the mononuclear phagocyte system (MPS), is a part of the immune system that consists of monocytes and macrophages involved in the uptake and metabolism of foreign molecules and particulates.101 Nanostructures coated with polyethylene glycol—can avoid RES uptake.102 In another example, MWCNT were shown to evade the RES when their surface is coated with ammonium and chelator functional groups103 but were taken up when coated with taurine.69 Aside from the surface chemistry, the core nanostructure can also impact the bio-distribution behavior. Polymer-based nanostructures and superparamagnetic iron oxide nanosystems for MRI contrast agents are known to degrade in vivo, but there is no clear indication whether fullerenes or silica NPs degrade in vivo.70,103–105 Fischer et al.99 and Ballou et al.105 showed that core ZnS-capped CdSe QDs remain intact and fluorescent in vivo after one month; however, neither study analyzed the metabolism of the organic coating on the nanostructure surface. The breakdown of the nanostructures could elicit molecular responses that are not predictable, and thus, an understanding and cataloging of what, when, and how much nanostructures degrade is extremely important.
4.2.3. Metabolism
There are very few reports regarding the metabolism of NPs. Polymer-based nanostructures and superparamagnetic iron oxide nanostructures have been shown to degrade in tissues; while QDs, fullerenes and silica NPs did not degrade in vivo.70,103–105 Although it is usually considered implausible that enzymes could effectively metabolize inert nanomaterials such as gold and silver NPs, recent study showed that generally considered bio-persistent CNT is degraded by neutrophil myeloperoxidase.106 Likewise, coatings, capping materials, and surface functional groups could be metabolized. For example, the protein cap of a functionalized QD could be cleaved by proteases.107
Nanoparticles could be metabolized in liver through phase I and II metabolic pathways. Phase I reactions involve formation of a new or altered functional group by oxidation, reduction, or hydrolysis reactions to increase reactivity or polarity. Phase II reactions involve conjugation of an endogenous compound, such as glucuronic acid orglycine, to ensure higher water solubility and lowered chemical reactivity. Frequently, phase II reactions occur after the NPs have been rendered more reactive by phase I metabolism. The metabolites of these processes have a higher polarity and are excreted at a higher rate than the original molecule through the kidneys via the urine or the liver via the bile. For example the metallic core of QDs and other metal oxides could be sequestered by metallothionen and excreted. These enzymes, present in liver and kidney, can bind metals and restore the cellular metal homeostasis.108
The most important enzymatic system of phase I metabolism is the microsomal family of isoenzymes, cytochrome P450, which can transfer electrons supplied by flavoproteins to catalyze oxidation. However, there is evidence that NPs can inhibit activity of this enzyme.109 Since NPs breakdown may elicit unique unpredictable molecular responses, understanding the exact mechanisms of degradation or alternation of NPs is extremely important.
4.2.4. Elimination
Elimination can occur via multiple routes, including perspiration, seminal fluids, mammary glands, saliva, and exhaled breath, although the urine via the kidneys103 and the feces via the biliary duct110,111 are the expected primary routes of NP elimination. Hydroxyl functionalized SWCNT dosed intra-peritoneally accumulate in the liver and kidneys and are excreted in the urine within 18 days;112 whereas, ammonium functionalized SWCNT dosed intravenously showed no liver uptake and much faster renal excretion.103
For nanostructures such as QD, two initial studies showed they are not excreted and remain intact in vivo.70,99 Reddy et al.113 showed that QDs smaller than 5.5 nm and coated with cysteine are excreted in the urine of mice. If not excreted in this manner, how long they reside and their long-term behavior in vivo remains unclear. For example, since the liver is involved in nanostructure uptake, biochemical indicators of liver stress were examined in response to multi-walled CNTs. No negative effect was observed after 28 days despite accumulation in that organ.69 Inflammation in response to nanostructures has been observed, though, with gene expression analysis of rat lungs showing upregulation of transcription factors associated with cellular responses to oxidative stress.114 QD have activated astrocytes in the brain upon direct injection, depending on surface functionalization,115 and nanostructure size has been shown to influence the ability to produce CD8 and CD4 type 1 T cell responses, with those between 40 and 50 nm causing a maximum effect.116,117 These specific studies can identify the organs that could be stressed by exposure to nanostructures and can provide a molecular basis of the stress. If these responses can be associated with specific organ cells and NP characteristics (e.g. surface chemistry, size, shape, aggregation and composition), then it would be possible to correlate the toxic effects of NP to specific nanostructure properties. Demonstrated PK studies of various NP systems are shown in Table 2.
Table 2.
Pharmacokinetics studies63
| Study | NPs | Main findings | Ref. |
|---|---|---|---|
| PK in BALB/c mice | DTPA-CNT with radiotracer [111In] | Functionalized SWNT are not retained in any of the reticuloendothelial system organs (liver or spleen) and are rapidly cleared from systemic blood circulation through the renal excretion route | 103 |
| PK in A/J mice | SiRNA DOTAP/DOPE complexes (250 nm) SiRNA RGD-PEG-PEI complexes (130 nm) |
Complexation of siRNA with DOTAP/DOPE or RGD-PEG-PEI did not affect siRNA blood levels Complexes distributed mainly in liver and kidney with a rapid renal clearance by glomerular filtration DOTAP/DOPE: highest tissue levels were found in the liver, lung and kidney RGD-PEG-PEI: accumulated in the liver, lung, kidney, spleen |
118 |
| PK in BALB/c Mice | SPIO 20 nm, nanoferrite 30 nm and 100 nm, radioimmuno NPs | Clearance of the NPs and mean concentrations in lung, kidney and lymph nodes were similar to 111In-ChL6-NP Similar mean uptake levels in tumors |
119 |
| PK and biodistribution in Wistar rats | USPIO and USPIO-PHO | Long elimination half-life (255 min for USPIO and 776 min for USPIO-PHO) Accumulation in lungs and liver |
120 |
| Distribution in Wistar rats | Gold NPs | 10 nm particles were present in various organ systems (liver, spleen, kidney, thymus, heart, testis) Larger particles were only detected in blood, liver and spleen |
121 |
| Distribution in ICR mice | 50 nm MNP-SiO2 core–shell structure (RITC) | The particles were distributed in all organs, and the distribution pattern was time dependent | 122 |
| Distribution at ICR mice | Water soluble, hydroxylated SWCNT with radioactive 125I | Accumulated in the liver and kidneys, excreted in the urine after 18 days | 112 |
5. Effect of physicochemical properties of NPs on toxicity
Characteristic parameters of NPs, including dissolution, chemical composition, size, shape, agglomeration state, crystal structure, specific surface area, surface energy, surface charge, surface morphology and surface coating, influence the biological interaction of NPs, and hence it is important to evaluate these properties in determining toxic potential of nanomaterials. In the following section we review the effect of abovementioned parameters on in vivo toxicity of nanomaterials.
5.1. Effect of size
Particle size and surface area are crucial material characteristics from a toxicological point of view, as interactions between nanomaterials and biological organisms typically take place at the surface of the NP. As the particles’ size decreases, the surface area exponentially increases and a greater proportion of the particles’ atoms or molecules will be displayed on the surface rather than within the bulk of the material. Thus, the nanomaterial surface becomes more reactive toward itself or surrounding biological components with decreasing size, and the potential catalytic surface for chemical reactions increases. Since it is known that endocytic mode, cellular uptake and efficiency of particle processing in the endocytic pathway are dependent on size of material,26,123–125 size plays a key role in physiological response, distribution, and elimination of materials.126,127 In vitro cytotoxicity studies of NPs of different size using various cell types, culture conditions and time course of exposure are being reported increasingly.2,127–136 Although some aspects of size dependent NP toxicity can be reasonably predicted by in vitro techniques, the wide range of NP concentrations and exposure times makes it difficult to determine when the observed cytotoxicity is clinically relevant. In addition, the uniqueness of each nanomaterial type being investigated for medical applications makes generalization of nanomaterial toxicity rather complicated. While in vitro NP applications requires less strict toxicological characterization, in vivo use of NPs requires a comprehensive understanding of the kinetics and toxicology of the particles.136 To our knowledge, few data are available in the literature regarding the in vivo size dependent evaluation of nanomaterials. Thus a better understanding of the relationship between the physicochemical properties of the nano systems and their in vivo behavior would provide a basis for assessing toxic response and more importantly could lead to predictive models for assessing toxicity.71 In the following section, existing research regarding the effect of nanomaterial size on the in vivo toxicity is discussed.
Since inhalation is the most important route of human exposure to NPs, the early characterizations of in vivo toxicity of NPs have been conducted in respiratory systems. In general it is observed that as the particle size decreases, there is a tendency for pulmonary toxicity to increase, even if the same material is relatively inert in a bulkier form. For example, Oberdorster et al.137 showed that TiO2 particles with a size of 25 nm when instilled or inhaled into the human lungs produced a much greater inflammatory response compared to larger particles of 250 nm.
The nature of the interface between nanomaterials and biological systems affects the in vivo biocompatibility and toxicity of NPs. A series of studies in rodents using a variety of different NPs showed that surface area is a critical factor in provoking lung and other epithelial-induced inflammatory responses.138 When equal-mass doses of fine or ultrafine particles of the same composition were inhaled by rats, the latter caused greater pulmonary inflammation. However, there was not any difference between them when the particle dose was normalized to the equivalent total particle surface area.
The lung is an effective barrier against the uptake and distribution of NPs. Within the human respiratory tract, inhaled particles of different sizes exhibit different fractional depositions, as ultrafine particles with diameters smaller than 100 nm deposit in all regions, whereas particles smaller than 10 nm deposit in the tracheobronchial region, and particles between 10 and 20 nm deposit in the alveolar region. Particles smaller than 20 nm also deposit efficiently in the nasopharyngeal-laryngeal region.139,140
In order to show toxic effects, NPs first need to traverse the epithelial barrier. NPs usually enter cells through energy-dependent endocytosis, non-phagocytic mechanisms or through receptor mediated endocytosis. There is evidence that translocation or distribution of NPs is size dependent in rats. Kreyling et al.141 showed that instillation of Ir192-particles with a diameter of 80 nm resulted in 0.1% being translocated to the liver, but with particles of 15 nm, this increased to 0.3–0.5%. Translocation of NPs across the alveolar-capillary barrier is still a matter of debate in other animals and in humans.142 Although it seems that size can be useful for assessing the toxic potential of some NPs, there is a consensus among experts that NP surface area or size is not the only physicochemical property that determines toxicity. Usually there is no precision in size determination as particle aggregation and agglomeration and the physicochemical properties of dispersion medium can also influence the ultimate particle size and related toxicity. For example, the hydrodynamic diameters of TiO2 and ZnO particles are significantly greater in phosphate buffer than in water, thus their sizes are often significantly larger than the primary particle size.143 Aggregation is more common in CNT, which have a tendency to form bundle-like agglomerates because of their geometry and hydrophobic surface. In vivo aggregation has been observed for both SWCNT and MWCNT with the difference that SWCNT agglomerates remained at same size with translocation, but MWCNT agglomerates grew larger without any translocation from their administration site.144
Studies have implicated size, length, and impurities of aggregated CNTs as primary determinants for toxicity, as the CNT cellular uptake mechanism may differ depending on the functionalization and size of the CNTs, including endocytosis and passive diffusion.145–147
Several studies conducted on the in vivo distribution of intravenously administered CNT showed that CNT are mainly accumulated in liver, spleen and lungs without acute toxicity; however, cytotoxic effects induced by aggregates and accumulation have been observed in long-term studies.148 Wick et al.149 reported that agglomerated CNTs have more adverse effects than well-dispersed CNTs, and they changed the morphology and performance of a mesothelioma cell line similar to asbestos. Mercer et al.150 also showed that a well-dispersed preparation of CNT with a mean diameter of 0.69 μm had a better interstitial distribution with rare macrophage phagocytosis after pharyngeal aspiration to mice. However, as improved dispersion of the CNTs caused both increased aspiration of smaller structures as well as their easy entrance into the alveolar walls, the dispersed CNT enhanced pulmonary interstitial fibrosis. Taking it all together, it is likely that the most appropriate means of expressing size related toxicity for NPs must be determined on an individual basis.151
Generally speaking, the harmfulness of NPs may arise from their size-related ability to readily enter biological systems152 and modify the structure of proteins through formation of new NP–protein complexes or enhanced protein degradation.153,154 Clinical and experimental studies indicated that small size, and consequently a large surface area, enhance the generation of ROS. The electron donor or acceptor sites on the NPs react with molecular oxygen, resulting in formation of superoxide anions or hydrogen peroxide, which subsequently oxidizes other molecules. This phenomenon plays a role in the ability of NPs to induce another tissue injury. Recently Jiang et al.155 showed that binding and activation of membrane receptors and subsequent protein expression strongly depend on NP size. Using gold NPs between 2 and 100 nm, they found that the NPs actively engage and mediate the molecular processes that are essential for regulating cell functions. Redistribution of NPs from their site of deposition156,157 or deposition into renal tissues158 and escape from normal phagocytic defenses159,160 also may lead to toxicity.
Apart from size dependent toxicity of NPs toward respiratory organs, oral toxicity of NPs has been shown to have significant correlation with size in spite of the fact that the gastrointestinal tract offers physical, chemical, and cell-based barriers against the uptake and spread of NPs. Chen et al.161 showed that the oral toxicity of copper particles of 17 μm to 23.5 nm increased with decreasing size; larger particles were non-toxic at high doses (>5000 mg kg−1) whereas the smallest particles were moderately toxic (LD50 of 413 mg kg−1). The toxicity of copper NPs was attributed to the accumulation of copper ions culminating in metabolic alkalosis and copper ion overload. The much larger copper microparticles were chemically inert, due to their lower specific surface area. Quantitative studies of oral administration of gold NPs of 4, 10, 28, and 58 nm diameter in mice also showed that uptake is dependent on particle size, as smaller particles cross the gastrointestinal tract more readily.162
Nanoparticle size has an important effect on the rate and route of clearance from the body, especially in parenteral dosage forms. For example, although the inert nature of bulk gold suggests it is a safe substrate for nanomaterials, NPs smaller than 50 nm administrated by intravenous injection are potentially toxic and disperse quickly to nearly all tissues, accumulating in blood, heart, lungs, liver, spleen, kidney, thymus, brain and reproductive organs. Larger particles (100–200 nm) were found in the RES tissues but not as widely dispersed into other tissues as were the smaller particles.163,164 Chen et al. reported that Au NPs of 3, 5, 50, and 100 nm are nontoxic when injected weekly into mice, whereas Au NPs between 8 and 37 nm caused severe toxicity and death within 3 weeks.165 However, these toxicities were reduced after incorporating immunogenic peptides on the NP surface that induced an enhanced antibody response. The in vivo toxicity of gold and silver NPs has been investigated with zebra fish,166 which are a useful in vivo model for toxicity evaluation because of the high degree of homology to the human genome and the rapid development of a transparent embryo. Using colloidal silver and gold NPs of different sizes (3, 10, 50, and 100 nm), it was found that Ag NPs produce size-dependent mortality after 120 h post fertilization, while the behavior of Au NPs was independent of size and caused less than 3% mortality at the same time point. This implies that although NP surface area is important in toxicity, other factors such as chemistry play a role.167
At present, QDs are considered intrinsically harmful because divalent cations and heavy metals in their structures can cause nephro-toxicity or acute and chronic toxicities in vertebrates.168 Surface coatings that limit the leakage of heavy metal ions can reduce the toxic potential of QDs, but size may also play a role in toxicity and distribution of these particles. For example, Shiohara et al.169 reported that the cytotoxicity of CdSe/ZnS QD with carboxyl groups on the surface is correlated with a decrease in QD size. The size of QDs is a determining factor in sub-cellular distribution as it was observed that 5.2 nm cationic CdTe QDs localized throughout the cytoplasm of N9 cells, whereas smaller 2.2 nm QDs accumulated in the nuclear compartment.152
Polyacrylate NPs were among the first NPs studied for controlled delivery of biological agents, with their introduction in the 1970s. Recently, Song et al.170 investigated the human toxicity of polyacrylate NPs prepared from polymerization of unsaturated monomers, such as methyl methacrylate, methacrylic amide or cyanoacrylates.171 These NPs were between 40 and 250 nm in size, which is relatively large compared to SPIONS, QDs, carbon blacks or metal oxides.172,173 The toxicity of the larger cyanoacrylate NPs was correlated with chemical properties and molecule chain length and was independent of particle size.174 However, smaller polyacrylate NPs produced toxic effects independently of chemistry; pathological examination indicated nonspecific pulmonary inflammation, pulmonary fibrosis and foreign-body granulomas of pleura after exposure.170 Thus, the safety of polyacrylate NP is still of debate and further study is warranted in biomedical applications.175
Generally NPs formed from biodegradable materials are expected to demonstrate fewer toxic events than non-biodegradable materials. Semete et al. investigated the in vivo toxicity and biodistribution of PLGA (poly(D,L-lactic-co-glycolic acid)) NPs with a size of 200–300 nm. Seven days after oral administration in mice, nearly 40% percent of PLGA particles were localized in the liver, and the rest were localized in brain and kidney without apparent toxicity.176 However, because of large size, it is unlikely that these NPs show any size-dependent toxicity. The chemical composition of biodegradable NPs and the subsequent degradation products will influence the biological effects. Polyesters such as PLGA or polycaprolactone (PCL) undergo hydrolysis and enzymatic degradation after implantation into the body, forming lactic acid, glycolic or capronic acid, which are biologically compatible moieties. Apart from size dependent toxicity due to ROS-generating capability, particle size can affect the degradation of the polymer matrix. As the particle size is reduced, the surface area to volume ratio increases, resulting in a large surface area available for penetration of physiological liquids into the particles and also faster release of the polymer degradation products.177 The size effects of various NPs are shown in Table 3.
Table 3.
Size-dependent in vivo study on nanomaterials
| Nanomaterial | Toxicity study | Species | Study parameters | Toxicity mechanism | Administration route | Ref. |
|---|---|---|---|---|---|---|
| Copper | Biochemistry analysis (ceruloplasmin, serum copper and urine copper) | Mouse | Changes in blood gas and plasma electrolyte content of copper elements in renal tissues, serum and urine | Accumulation of alkalescent substance | Oral | 158, 161 |
| Silica | Immunohistochemistry | Mouse | Tissue distribution, excretion in urine and feces | Inflammatory response of the liver for the 100 and 200 nm particles. All particles remained as aggregates in the spleen through macrophage trapping | Intravenous | 178, 179 |
| TiO2 | Morphometric, micro-array gene expression, and pathway analyses | Mouse | Up-regulation of placenta growth factor and other chemokines (CXCL1, CXCL5, and CCL3) | Pulmonary emphysema, macrophages accumulation, extensive disruption of alveolar septa, type II pneumocyte hyperplasia, and epithelial cell apoptosis | Intratracheal | 180, 181 |
| Gold | Percent mortality | Zebra-fish, mouse | Size dependent distribution in liver, lung, spleen, kidneys and brain | Au NP of sizes of 3, 10, 50, and 100 nm did not show toxicity | Intravenous (mouse), exposure of zebra fish embryo to particles | 163, 165, 167 |
| Carbon nanotube | Mouse | Translocation of NP from lungs to blood circulation and other organs | Significantly less translocation and accumulation with 80 nm than with 20 nm NP | Inhalation | 171 | |
| Silver | Blood biochemistry; histopathology measurement of cytokines and IGE and immuno phenotyping | Mouse | Distribution in the body | Organ toxicity and inflammatory responses, cytokine production, increased B cell distribution, and inflammatory cell infiltrates | Oral | 182 |
| CdTe | MTT assay, acute toxicity | Rat | Urinary or blood changes | Intravenous | 183 | |
| Polystyrene | LDH assay | Rat | Protein assay | Reactive oxygen species | Sub-conjunctival, instillation | 184, 185 |
| PLGA | Histopathology assays, tissue distribution | Mouse | Histopathology assays | No toxicity | Oral | 176 |
| PCL | Histological evaluation of heart, liver, spleen, lung, and kidneys | Rats | No toxicity | Intravenous | 186 |
5.2. Effect of particle shape
Particle shapes and aspect ratios are two additional key factors that determine the toxicity of NPs. Nanomaterials can have very different shapes including fibers, spheres, tubes, rings, and planes. Most of the knowledge about shape dependent toxicity is based on in vitro experiments. In vivo, shape dependent toxicity of nanomaterials is usually imparted through its adverse effect on endocytosis or clearance by macrophages, as shape can influence the membrane warping process during endocytosis or phagocytosis.187 For example, it had been suggested that endocytosis of spherical NPs is easier and faster compared to rod-shaped or fiber-like NPs.188 Rod-shaped or needle-like NPs can have a larger contact area with the cell membrane receptors than spherical NPs when the longitudinal axis of the rods interacts with the receptors. Hence, the ends with high curvature at the half-cup stage of endocytosis are very likely to cause a higher membrane surface energy, resulting in a large distorting force that exceeds the maximum force provided by the actin polymerization. This effect stalls the growing ends of the phagocytic cup and results in impaired phagocytosis and the macrophage spreading onto the material rather than internalizing it.189,190 Because of this, disc-like, cylindrical and hemispherical particles substantially outperform spherical particles in terms of evading uptake by phagocytic cells; consequently these non-spherical particles are more disposed to flow through capillaries and adhere to blood vessel walls, thus causing other biological consequences.191 For example, Radomski et al.192 showed that in contrast to fullerenes, SWCNT and MWCNT with tubular structure stimulate plate aggregation and vascular thrombosis in rat carotid arteries. Park et al.193 showed that SWCNT with rod structure can block potassium ion channels two to three times more efficiently than spherical carbon fullerenes. The length of CNTs has been shown to result in inefficient phagocytosis and damage to macrophages. Since full phagocytosis is hampered and a full phagosome is not formed, the macrophage’s harmful oxygen radicals and hydrolytic enzymes are released extra-cellularly. Poland et al. reported that after intra-abdominal instillation of long MWCNTs, the MWCNTs could cause inflammation of the abdominal wall, with formation of so-called foreign body giant cells. No inflammatory response was observed with short MWCNT, as they were effectively taken up by macrophages with efficient phagocytosis.67 If the particles are bio-persistent, the resulting chronic inflammation could lead to additional mutagenic events and ultimately the formation of mesothelioma. After intra-tracheal administration, SWCNTs induced lung granulomas, and the presence of multifocal granulomatous lesions without accompanying inflammation, cell proliferation or cytotoxicity, indicated a potential new mechanism of pulmonary toxicity and injury.194–196 Donaldson et al. have studied the relationship between fiber physicochemistry and pathogenicity and three fundamental attributes, namely, dimension, durability and dose, referred to as the 3D’s, have emerged as paramount to the pathogenicity of a fiber.197 Very recently, Donaldson et al. showed that instilled particles are rapidly drawn cranially in the lymph flow through the diaphragm via stomata, which are pore-like structures less than 10 mm in diameter, to the parathymic lymph nodes. Long fibers such as long carbon nanofibers block stomata pores and meanwhile damage mesothelial and endothelial cell. Accumulation of pleural macrophages attempting to phagocytose these retained fibers results in frustrated phagocytosis. The macrophages release cytokines and oxidants that cause further inflammation, fibrosis and genotoxicity to the bystander mesothelial cells in these areas of congestion around the stomatal entrances.197,198
A shape dependent toxicity has been observed with silica and TiO2 allotropes as well. For example, amorphous silica is an FDA-approved food additive, whereas crystalline silica is a suspected human carcinogen and is involved in the pathogenesis of silicosis.41
Different toxicity behavior has also been observed for TiO2 NPs with different crystal structures. For instance, Gurr et al. reported that Rutile TiO2 NPs can induce oxidative DNA damage, lipid peroxidation, and micronuclei formation in the absence of light, where anatase NPs of the same size and chemical composition are inert.199 Contrast to these results, Petkovic et al. found that TiO2-anatase was significantly stronger ROS inducer than TiO2-rutile.200 Despite the contradictory results, both studies show that the intrinsic ability of anatase and rutile TiO2 to induce ROS is related to their structure, which influence toxicity.
Shape dependent toxicity of nickel NPs has been reported. Ispas et al.201 observed that nickel dendritic clusters consisting of aggregated 60 nm particles resulted in higher toxicity in zebra fish compared to spherical ones, suggesting that differences in shape and aggregation is responsible for increased toxicity. They hypothesized that Ni NPs in the cluster form adhere more readily and are retained for longer periods in the intestinal lumen, which increases cellular stress.
Shape dependent toxicity also has been observed in gold and titanium nanomaterials.136 Chithrani et al.202 reported that uptake of Au nanorods of 74 × 14 nm is slower than spherical nanospheres of radius 14 or 74 nm. The uptake of Au nanorods reaches a maximum when the size nears 50 nm and the aspect ratio approaches unity.165 Studies with TiO2 also demonstrated that fibrous structures with higher aspect ratios are more cytotoxic than more spherical structures. Hamilton et al.203 showed that TiO2 fibers with a length of 15 mm are highly toxic compared to fibers with a length of 5 mm, and the longer ones initiate an inflammatory response by alveolar macrophages in mice. As conclusion of this part, the role of the shape in nanotoxicity is summarized in Table 4.
Table 4.
Effect of NPs’ shape on biological response
| Particle shape | Particle examples | Toxicity mechanism | Physiological response | Ref. |
|---|---|---|---|---|

|
Iron oxide, gold | Internalization and membrane disruption. Highest cellular uptake with least membrane disruption among all shapes, thus least shape dependent toxicity | Cell division dysfunction and disturbed cellular trafficking; Mechanical interference with the mitotic spindle and DNA | 204–206 |

|
SWCNT, MWCNT, TiO2, gold, mesoporous silica | Internalization and membrane disruption. Severe influence on initiation of phagocytosis. Blockage of transport channels. Highest distorting force on cell membrane among all shapes. Smaller aspect ratios lead to faster internalization and less cell membrane disruption | Chronic inflammation due to frustrated phagocytosis, mutagenic events, mesothelioma formation | 207–213 |

|
Gold | Dependent on the average radius of curvature. Disruption of membrane integrity and transport may occur | Toxicity due to chronic inflammation or impaired phagocytosis | 202 |

|
Nickel, carbon black, TiO2 | Aggregation or agglomeration changes size of particles thus increasing their visibility to macrophages | Aggregation changes retention time of particles; changes in size may increase or decrease toxicity | 143, 214, 215 |

|
ZnO, iron oxide | Aggregation and cell membrane disruption may be dependent on the prevalence of high aspect ratio particles | Combinational effect similar to aggregated particles and fibrous particles | 16, 216, 217 |

|
Quantum dots | Similar to spherical NPs | Similar to spherical NPs | 218 |
5.3. Effect of surface charge
Surface charge also plays a role in toxicity, as it influences the adsorption of ions and biomolecules that may change organism or cellular responses toward particles. In addition, surface charge is a major determinant of colloidal behavior, which influences the organism response by changing the shape and size of NPs through aggregate or agglomerate formation.219
In general, it is believed that cationic surfaces are more toxic than anionic surfaces, and cationic surfaces are more likely to induce hemolysis and platelet aggregation, whereas neutral surfaces are the most biocompatible.220 This may be due to the affinity of cationic particles to the negative phospholipid head groups or protein domains on cell membranes. In addition surface charge influences plasma protein binding, which in turn affects the in vivo organ distribution and clearance of NPs from the circulation. For example Saxena et al. showed that acid-functionalized SWCNTs caused markedly significant in vivo toxicity compared to pristine SWCNTs. This higher toxicity could result either from a possible greater bioavailability of well dispersed AF-SWCNT preparations, or from the high negative charge on AF-SWCNTs, or both.221 Pietroiusti et al. found that AF-SWCNTs had a marked embryotoxic effect compared to pristine SWCNTs in pregnant mice models. Similarly, increased toxicity was attributed to a higher percentage of monodispersed SWCNTs in acid functionalized SWCNTs and higher negative charge and hydrophilicity.222
Nanoparticle surface charge has been observed to alter blood–brain barrier integrity and permeability.223 It is suggested that high concentrations of anionic NPs and cationic NPs are able to disrupt the integrity of the blood brain barrier.
Particle surface charge can also impact transdermal permeation of charged NPs. It was found that after dermal administration, negatively charged NPs of about 50 and 500 nm permeated the skin, while positively charged and neutral particles of all sizes did not.223 NPs of 50 nm permeate the skin due to the small size and large specific surface area, whereas 500 nm particles permeate the skin because the high number and density of charged groups leads to a high charge concentration that overcomes the skin barrier.224 The lipophilicity of the outer skin layers also limits the permeation of smaller charged NPs due to the presence of ionized groups, similarly to small molecule drugs. Geys et al.203 investigated the in vivo toxicity of positively charged (amine-QDs) and negatively charged (carboxyl-QDs) quantum dots after intravenous injection in mice. They found that the carboxyl-QDs caused more pulmonary vascular thrombosis than amine-QDs at high doses. The presence of fibrin fibers in the thrombi suggests that negatively charged QDs activate the coagulation cascade via contact activation. Hoshino et al.219 studied a series of QDs with different surface coatings (carboxyl, hydroxyl, amine or their combinations). They found that the highly negatively charged QDs with carboxyl groups induced DNA damage after 2 h, while the other types did not induce significant cellular damage. In a similar study, positively charged Si NPs (Si–NP–NH2) proved to be more cytotoxic than neutral Si (NP–N3) in terms of reduced mitochondrial metabolic activity and phagocytosis, while negatively charged Si (Si–NP–COOH) had very little or no cytotoxicity.225 Heiden et al.226,227 reported that surface charge also impacts the toxicity of dendrimers. Positively charged PAMAM dendrimers (G4) showed time-dependent toxicity toward zebrafish embryos; however, anionic PAMAM dendrimers had no toxicity. Similar results have been reported when anionic PAMAM dendrimers were administrated to mice.226,227 As a summary, the role of the surface charge in NP toxicity is shown in Fig. 2.
Fig. 2.
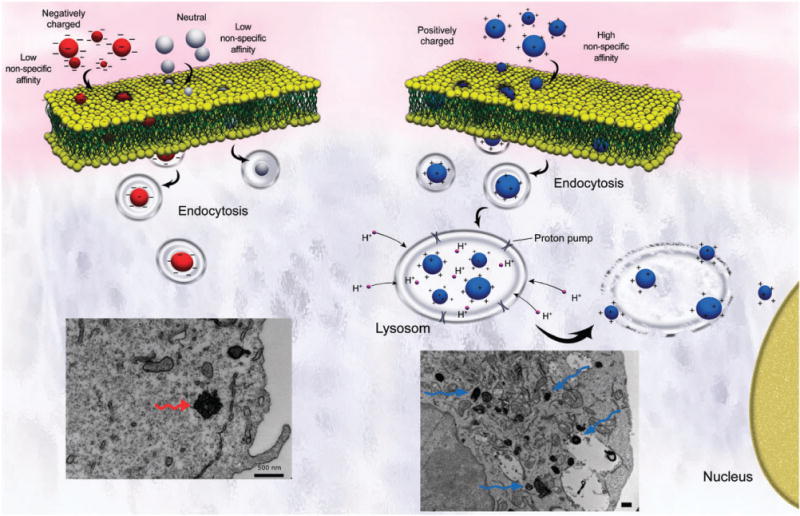
Scheme showing the importance of the surface charge on the yield of cell uptake. Positively charged NPs illustrate significant cellular uptake, in comparison with negative and neutral ones, due to the attractive electrostatic interactions with the cell membrane. In addition, positively charged NPs are capable of acting as “proton sponges” that disrupt the lysosomes to enhance cytoplasmic delivery and induce cell death signaling cascades. The bottom left and right panels show TEM images of HeLa cells which have been exposed to negatively and positively charged SPIONs, respectively (unpublished work by M. Mahmoudi).
5.4. The effect of composition
Although it has been suggested that size or surface area may be more important than chemical composition in conferring NPs toxicity, particle chemistry is more relevant in relation to cell molecular chemistry and oxidative stress. Harper et al.228 evaluated the effect of NP composition on toxicity using eleven commercially available dispersions of NPs with similar particle size in an embryonic zebrafish model, including positively charged-aluminium oxide, titanium oxide, zirconium oxide, gadolinium oxide, dysprosium oxide, holmium oxide, samarium oxide and erbium oxide, negatively charged yttrium oxide, silicon dioxide, and alumina doped and cerium oxide. Significant mortality was observed after a 5-day continuous waterborne exposure at 50 ppm for erbium oxide and samarium oxide, and at 250 ppm for holmium oxide and dysprosium oxide. Waterborne exposure to yttrium oxide, samarium oxide and dysprosium oxide at concentrations of 10, 50 and 250 ppm caused significant morphological malformations in embryonic zebrafish. In contrast, no significant morbidity or mortality was observed for the other metal oxide NPs when embryos were injected with approximately 0.5 ng of NPs. Griffitt et al.229 used zebrafish, daphnids, and algal species as models of various trophic levels and feeding strategies to evaluate the toxicity of similarly sized silver, copper, aluminium, nickel, cobalt and titanium dioxide NPs and their corresponding soluble salts. The authors found that nanosilver, nanocopper, and their soluble forms caused toxicity in all organisms tested; however, titanium dioxide did not show any toxicity.229 They also observed that filter-feeding invertebrates are more susceptible to NP exposure compared to zebrafish. Although the NPs were of similar size but different surface charges, the chemical composition of NPs appeared to be the most important factor in toxicity. Contrary to these results, Chen et al.230 reported acute toxicity of titanium dioxide NPs in mice after intraperitoneal injection. They found that the TiO2 NPs were mainly retained in spleen, lung, kidney and liver tissues, leading to serious lesions. According to these reports, it appears that the toxicity of NPs is not a generic response to nanoscopic dimensions; rather, it seems that multiple particular characteristics affect toxicity, including but not limited to chemical composition, surface charge, size, and shape.
5.5. Effects of coatings
The adverse effects of NPs maybe mitigated or eliminated by incorporation of surface coatings. Proper surface coatings can stabilize particles and avoid agglomeration. Coating is also an effective means of preventing the dissolution and release of toxic ions.231 Furthermore the steric hindrance of coatings can retard the cellular uptake and accumulation of NPs, or coatings can facilitate NP endocytosis.232–235 Surface coatings can modify the surface charge or surface composition, which can impact intracellular distribution and the production of ROSs that cause further toxicity. Many coatings are environmentally labile or degradable and may shed or degrade after exposure to biological media, thus rendering an initially nontoxic material a hazardous one.
Several studies in animals have shown that after a large dose of iron-based NPs (2.5 mmol), no life-threatening side effects appeared after a 7-day treatment, according to histology and serological blood tests. However, severe inflammatory and immunological responses can occur dependent on the density and type of surface coating.236–238 Normally, magnetic NPs are surrounded by coatings to prevent the presence of free iron oxide, but the coating may be metabolized after some time.239
In some NPs such as QDs, a coating is unavoidable as the metallic core is hydrophobic, and the core itself is toxic as it is composed of heavy metals such as cadmium. Thus, a secondary coating is needed to increase the QD core’s durability, prevent ion leaching, and increase water dispersibility.240–243 The type of secondary surface coating may affect the toxicity of the QD complex. For example, Chen et al. coated QDs with silica and the lack of genotoxicity was related to the silica coating, which successfully prevented the interaction of Cd, Se, Zn, and sulfur with proteins and DNA in the nucleus.244 Coatings may not be stable under oxidative or photolytic conditions thus exposing the metalloid core, which may be toxic or pave the way for unforeseen reactions of the QD inside the body.107,245 The charge of surface coatings may affect the toxicity of QD NPs. At high doses in mice, carboxyl-coated CdSe/ZnS QDs activated the coagulation cascade via contact activation and caused pulmonary vascular thrombosis.246 Fisher et al.247 investigated QDs coated with the negatively charged serum protein albumin. They observed a higher liver uptake (99%) and faster blood clearance relative to the QDs without albumin (40%).247–251
Polyethylene glycol is a FDA approved biocompatible polymer that generally does not induce any toxicity, so PEG has been used extensively for coating QDs. Ballou et al. applied PEG coatings of different molecular weights (methoxy-terminated 750 Da PEG, carboxy-terminated 3400 Da PEG, and ethoxy-terminated 5000 Da PEG), and the NPs were observed for differential tissue and organ deposition in mice in a time- and size-(MW) dependent manner. The particles coated with lower molecular weight PEG were eliminated from circulation 1 h after injection, but QDs coated with PEG 5000 remained in the blood circulation for 3 h.105,252–256
Biocompatible polymers are widely used as coating materials for SPIONs to accomplish multiple objectives, including colloidal stabilization, delivering biologically active agents with a controlled release profile, and targeting specific tissues via conjugation with specific ligands.1,3,4,8–10,12–18,22,257–261 In fact, uncoated iron oxide NPs have very low solubility, which can lead to precipitation during storage and a high rate of agglomeration under physiological conditions that can impede blood vessels. Similar to QD coatings, the stability and toxicity of the SPION coating is important. It has been shown that dextran-magnetite (Fe3O4) NPs cause cell death and reduced proliferation similar to uncoated iron oxide particles, which was attributed to the breakdown of the dextran shell exposing the cellular components to chains or aggregates of iron oxide NPs.262 Xie et al.263 also showed that coating PEG on monodisperse Fe3O4 NPs produced negligible aggregation in cell culture conditions and reduced nonspecific uptake by macrophage cells. Although PEGylation may reduce the potential of harmful biological interactions, Cho et al. found that 13-nm sized Au NPs coated with PEG 5000 induce acute inflammation and apoptosis in the mouse liver.264 These NPs were found to accumulate in the liver and spleen for up to 7 days after injection and to have a long blood circulation time of about 30 h. A relatively high concentration of PEG on the NPs surface alone does not lead to a lower NP uptake, but rather the spatial configurational freedom of PEG chains on the particle surface plays a determinant role.265
Coatings and functionalization can also reduce the in vivo toxicity of carbon nanotubes. Lacerda et al.266 intravenously injected MWCN functionalized with diethylene triamine penta-acetic di-anhydride, which resulted in stable dispersions with high excretion rates. Altogether, most studies have indicated that surface coatings can alter the pharmacokinetics, distribution, accumulation, and toxicity of NPs.
5.6. Effect of surface roughness
Physical surface properties of nanomaterials play a critical role in determining the outcome of their interactions with cells. Contrary to specific receptor–ligand interactions (e.g. endocytic uptake), surface roughness along with hydrophobicity and cationic charge are the main factors involved in nonspecific binding forces that promote cellular uptake.257,267 Small-radii surface coarseness dictates the strength of NP–cell interactions at the nanoscale, as it greatly minimizes electrostatic or hydrophobic–hydrophilic repulsive interactions therefore promoting cell adhesion. Particles may pass through cell membranes by disrupting the phospholipid bilayer of the plasma membrane and generating transient holes usually associated with cytotoxicity.268 Shen et al.269 investigated the hemolytic activity of nonporous and porous-silica NPs of varied sizes. They observed that the size-dependent hemolysis effect of mesoporous silica NPs is only present when the NPs have long-range ordered porous structure, revealing that pore structure is critical in cell–NP interactions. The extent of hemolysis by mesoporous silica NPs increases with particle age as phosphate-buffered solutions compromise the pore structure. Although the reduced cytotoxicity can be correlated to less penetrating force of the particles through the membrane, the authors suggest the effect is mainly due to fewer silanol groups on the cell-contactable surface of the porous silica NPs.269 Angelis et al.270 showed that nanoporous silicon NPs with a pore size of about 2 nm did not have any toxicity in mouse-models, as serum levels of both inflammatory cytokine IL1-b and hepato-toxicity markers LDH and GSH were normal, and there was no histological evidence of tissue pathology in the liver, kidney, spleen, lungs and heart. Similarly, Park et al.271 reported no in vivo toxicity using biodegradable luminescent porous silicon NPs.
The significant factors that impact new biological applications of NPs are summarized in Table 5. The enhancements of certain physicochemical properties of NPs can create new applications for these materials, but these new properties may also cause significant toxicities.
Table 5.
Significant enhancements on the new biological applications of various NPs
| NPs | Physicochemical properties | Animal model | Possible toxicity mechanism | Remarks | Ref. |
|---|---|---|---|---|---|
| Silica Nanorattle | Size: 125 nm with very narrow size distribution; detectable by doped fluorescent agents | Mice (liver cancer) | ROS production, however much less than non-porous silica due to less surface area | High drug loading capability; good conjugation potential with biomolecules; suitable contrast for in vivo detection using fluorescent agents; coating with polymeric materials (e.g. PEG) improves therapeutic efficacy, and reduces the toxicity of antitumor agents. | 272, 273 |
| Silicon nanocrystals | Overall size: 50 to 120 nm; formed by spherical aggregates of crystalline particles; good functionalization capability with organic molecules | Mice (different organs) | ROS production due to crystals and surface defects; membrane disruption | Better in vivo compatibility in comparison with QDs; suitable luminescent properties and long tumor accumulation times (> 40 h); targeted cancer imaging and sentinel lymph node mapping; oxidant injury and toxicity can be reduced by increasing size (e.g. aggregation) or changing charge | 271, 274 |
| Fullerenes | Size: 160 ±50 nm; zeta potential: −30 mV | Rat (lung tissue) | ROS production; oxidant injury to cellular membranes | Addition of ionic groups decreases systematic toxicity due to radical generation by the Fullerene surface (e.g. C60(OH)24) | 275 |
| Gold | Size 20 nm with very narrow size distribution; capability to be conjugated with variety of biochemical compounds | Athymic nude mice (tumor phantom model) | AU NPs can cause nephrotoxicity and eryptosis, or they can interact with DNA. Nevertheless, they are amongst the safest NPs reported to date. | Used as near-infrared fluorescence (NIRF) imaging probes by adding variable surface compositions of dye-labeled peptide substrates (for example Quasar 670); the probe enhances particle circulation time and image contrast in vivo. Cytotoxicity can be mitigated by modifying size or surface charge | 276 |
| Gold nanorods | Aspect ratio: ~ 3 (diameter of 15 nm and length of 50 nm) | Athymic nude mice (breast cancer tumors) | Nanorods are more internalized in the intracellular environment compared to spherical particles, as their high aspect ratio may retard initiation of phagocytosis. The slight toxicity of gold nanorods is due to toxic capping agents (e.g. ethyltrimethylammonium). | In comparison to spherical NPs, nanoroads have better capabilities as a probe for molecular imaging purposes; in order to reduce the toxicity of capping agents and to increase the blood circulation time of nanorods, biocompatible polymers (e.g. PEG) should be used as coating materials. | 277 |
| Semiconductor quantum dots (QDs) | Size: 80 nm | Mice (liver tissue), and lymphatic vessel | Release of toxic heavy metal ions (Cd and Se ions) | They usually belong to group II–VI elements of the periodic table, and have core-shell morphology. They are traceable by in vivo targeted fluorescence imaging. Their major short comings are the short circulation time and removal by reticuloendothelial system (RES) which causes reduction of tumor-specific signal, and the heavy metals used in their construction can cause long-term toxicity. Their fluorescence intensity in tumors can be enhanced significantly (more than 50%) by conjugation of targeting agents, e.g. epidermal growth factors. To reduce toxicity, the surface should be coated with biocompatible polymers or inorganic materials. | 278, 279 |
| Single-walled carbon nanohorns | Diameter 2–5 nm, and length 40–50 nm. Single-walled carbon nanohorns (diameter 100 nm) | Mice (liver and spleen tissues) | Production of ROS (metal impurities) followed by inflammatory effects | Determination of their biodistribution is inconvenient; however, in order to overcome this problem, Gd2O3NPs can be embedded within single-walled carbon nanohorns (i.e. Gd exhibits a high electron scattering ability). For drug delivery purposes, drugs can be loaded and stored inside open oxidant holes. | 280, 281 |
| Calcium phosphate | Size: <50 nm (10–50 nm) | Breast cancer | Possible ROS production due to crystal defects if crystalline, or surface defects if amorphous | Good biocompatibility; indocyanine green (ICG) molecules can be embedded as a near-infrared (NIR) emitting fluorophore to enhance in vivo contrast. The ICG-encapsulating NPs have noticeably higher fluorescence and stability in vivo compared to the free fluorophore. Suitable for deep tissue imaging. In order to enhance the circulation time, the surface of the NPs should be coated or functionalized (e.g. PEG or carboxylate). | 282 |
| Silver | Size: 5–46 nm | Zebrafish embryos | Cell suffocation due to inhibitory effect of silver ions on respiratory enzymes; generation of ROS and membrane disruption | Ag NPs have high quantum yields. Ag NPs can be used to probe inside embryos. Optical properties were fully dependent on the size and shape of Ag NPs. There was no trace of particle aggregation or photo decomposition, thus continuous imaging for long periods of time is possible. | 283 |
| SiO2 and Al2O3 | Sizes: 19 and 12 nm for SiO2 and Al2O3, respectively; shape: spherical | Zebrafish embryos | ROS production | No physiological and morphological defects. Usually coating can reduce toxicity | |
| Pt | Size: 13 nm; shape: spherical | Zebrafish embryos | ROS production | Inhibitory effects on cardiac rate. Pericardial edema, low heart beat and mortality were observed. | |
| ZnO | Size: 10 nm; shape: Spherical | Zebrafish embryos | ROS production, release of toxic cations with damage to cell membranes | The most prominent lethalities were morphological abnormalities, or interference with embryo hatching, unhatched embryos, and disintegrated embryos. |
5.7. Effect of the medium that contains NPs
Proper and stable dispersion of nanoparticles in the delivery medium is very important for their biological distribution and subsequent activity. Due to agglomeration, NPs may not form a stable suspension in the physiological solutions suitable for in vivo exposure. Medium condition such as ionic strength and pH can affect particles dispersion. For instance particles of TiO2, ZnO or carbon black have been shown to have significantly greater size in PBS than in water.143 Similarly TiO2 NPs also have been shown to have different diameters in biological systems.285 Colvin pointed out that the behavior of NPs systems depends on the medium that they are suspended in286 Fig. 3 shows the diversity of fullerene (C60) NP preparations: dried, dissolved in a nonpolar solvent and chemically modified C60 NPs dispersed as a colloid in water. The toxic effect of NPs in these three different cases may be different. For example, dried NPs may be dispersed into the air by forced or natural convection and can pose a hazard when inhaled into the lungs. Single NPs or clustered NPs may have different biological reactivities. Further, liquid media may affect the dermal uptake of NPs.
Fig. 3.
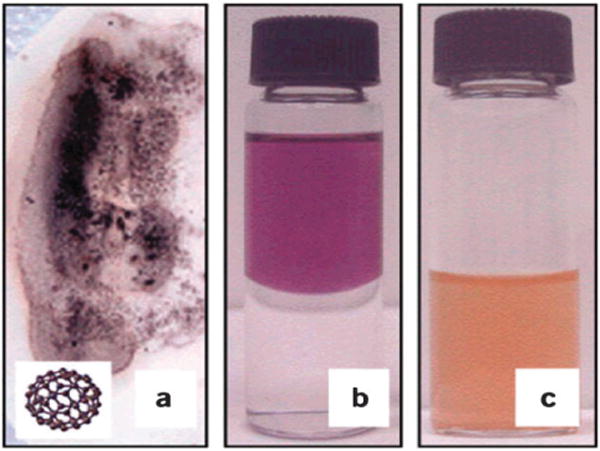
The diverse forms of engineered nanomaterials: (a) C60 dried onto filter paper is a black powder (inset: molecular structure of C60); (b) fullerenes dissolved in a nonpolar solvent form a purple solution (top layer); and (c) with relatively mild chemical treatments, such as evaporation of the nonpolar phase, C60 becomes water stable in the yellow aqueous phase. The material is present as colloidal aggregates that contain between 100–1000 fullerene molecules. (Reproduced with permission from ref. 286)
Dispersion or wetting agents in media also may adversely affect the toxicity of nanomaterials. For example, Sager et al. showed that the addition of dipalmitoylphosphatidylcholine in PBS improved dispersion of TiO2 and carbon nanoparticles; however, it significantly increases the inflammatory response of rats after intratracheal instillation.143 Therefore, dispersion agents may improve the physicochemical and solution properties of nanomaterials formulations, but they may have adverse effects on the safety of these materials.
6. Conclusions and future challenges
The toxicity of nanomaterials is affected by their composition, much like the parent bulk materials. However, additional physicochemical properties play a crucial role in determining the toxicity of nanomaterials, such as size, surface chemistry, shape, protein absorption gradient and surface smoothness or roughness. Thus, the toxicity of chemically identical materials can be altered significantly by the manipulation of several physicochemical properties. In order to reduce the considerable knowledge gap between the development and in vivo toxicity of NPs, a considerable effort is needed by the scientific community to study the physiological effects of acute and chronic exposure to NPs. A fundamental understanding of the biological interactions of NPs with cells, proteins, and tissues, is vital to the future design of safe nanotechnologies. Prior to their wider adoption in everyday products and their clinical use, NP-products must be shown to have a high degree of biocompatibility, with minimal negative effects on blood components, genetic material, and cell viability.
From limited research conducted in the last several years, inhaled nanoparticles from airborne sources are of concern, included carbon-based materials. During the manufacturing of nano-based materials, it is imperative that workers are adequately protected from inhaling NPs, as the long-term effects of exposure are still unknown. This also suggests that NPs should be properly incorporated or sequestered into products to prevent subsequent release during use or disposal. Further, dermal contact and other non-inhalation routes of exposure to nanoparticles must be studied to understand possible toxic effects.
Biographies

Shahriar Sharifi
Dr Shahriar Sharifi received his PhD in 2007 from the Amirkabir University of Technology (Poly Technique of Tehran) with specialization in the design and development of biodegradable biomaterials and nanocomposites for drug delivery and tissue engineering applications. He has received several awards, such as Dux bachelor and master student and national grant for the Elite, 2008. After his graduation, he worked as a researcher in Iran polymer and petrochemical institute (IPPI). In 2009, he moved to the University of Groningen, Netherlands. His current research involves development of smart materials including nanomaterials for tissue engineering applications

Shahed Behzadi
Shahed Behzadi is a senior research scientist in Professor Mahmoudi’s Laboratory (www.biospion.com). His current research is focused on the safe use of nanoparticles for biomedical applications. He obtained his M.Sc. degree in 2010 from Sharif University of Technology.

Sophie Laurent
Dr Sophie Laurent was born in 1967. Her studies were performed at the University of Mons-Hainaut (Belgium) where she received her PhD in Chemistry in 1993. She joined then Prof R. N. Muller’s team and was involved in the development (synthesis and physicochemical characterization) of paramagnetic Gd complexes and super paramagnetic iron oxide nanoparticles as contrast agents for MRI. She is currently working on the vectorization of contrast agents for molecular imaging. She is lecturer and co-author around 120 publications and more than 200 communications in international meetings.

M. Laird Forrest
M. Laird Forrest received his B.S. (1998) in Chemical Engineering from Auburn University. He then completed his MS (2001) and PhD (2003) in Chemical and Biomolecular Engineering at the University of Illinois-Urbana/Champaign. Dr Forrest completed a postdoctoral fellowship at the University of Wisconsin (2006) before beginning his position as assistant professor of Pharmaceutical Chemistry at the University of Kansas in 2007. Dr Forrest’s research focuses on the development of nanomaterials for locoregional drug delivery and imaging agents for detecting cellular response to treatments.

Pieter Stroeve
Pieter Stroeve is a Distinguished Professor of Chemical Engineering and Materials Science at the University of California Davis. Professor Stroeve conducts fundamental work on colloid and surface science, self-assembled monolayers, Langmuir–Blodgett films, supramolecular structures on surfaces, supported lipid bilayers, transport in colloids and tissues, nanotechnology, bio-nanotechnology, electrochemistry, solar energy and nanoporous membrane separations. He has developed theoretical models and compared predictions to his experimental results for reactive transport in cellular suspensions and in colloidal solutions, particularly oxygen and carbon dioxide transport in blood. In colloid science, he is working on the nanostructure of polyion-surfactant complexes, polycation–polyanion complexes, self-assembled monolayers, and nanoparticle–polymer composites. His group developed a method on the nucleation and growth of nanoparticles in ultrathin layer-by-layer polyion films. Further, his group has synthesized nanoparticles and modified their surface properties with polymers.

Morteza Mahmoudi
Professor Morteza Mahmoudi is a director and principal investigator of NanoBio Interactions Laboratory (www.biospion.com). The NanoBio Interactions Laboratory is in collaboration with several prestigious universities such as Harvard, MIT, Stanford, UBC, McGill, EPFL, and UCD. He obtained his PhD in 2009 from Sharif University of Technology with specialisation on the cytotoxicity of superparamagnetic iron oxide nanoparticles (SPIONs). He has received many awards such as the Shahid Chamran Award (National Endowment for the Elite; 2011), Distinguished Researcher at Pasteur Institute of Iran (2010), the Dr Mojtahedi Innovation Award for Distinguished Innovation in Research and Education at Sharif University of Technology (2010) and Kharazmi Young Festival Award (2009). His current research involves the magic SPION for simultaneous diagnosis and therapeutic applications.
References
- 1.Mahmoudi M, Stroeve P, Milani AS, Arbab A. Superparamagnetic iron oxide nanoparticles for biomedical applications. Nova Science Publishers, Inc; 2010. [Google Scholar]
- 2.Karlsson HL, Gustafsson J, Cronholm P, Moller L. Size-dependent toxicity of metal oxide particles—A comparison between nano- and micrometer size. Toxicol Lett. 2009;188:112–118. doi: 10.1016/j.toxlet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Mahmoudi M, Sahraian MA, Laurent S. Superparamagnetic Iron Oxide Nanoparticles: Promises for Diagnosis and Treatment of Multiple Sclerosis. ACS Chem Neurosci. 2011;2:118–140. doi: 10.1021/cn100100e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmoudi M, Hosseinkhani M, Laurent S, Simchi A, Hosseinkhani H, Journeay WS, Subramani KD, Broutry S. Magnetic Resonance Imaging Tracking of Stem Cells in vivo Using Iron Oxide Nanoparticles as a Tool for the Advancement of Clinical Regenerative Medicine. Chem Rev. 2011;112:253–280. doi: 10.1021/cr1001832. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv Drug Delivery Rev. 2010 doi: 10.1016/j.addr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Nchimi A, Defawe O, Brisbois D, Broussaud TK, Defraigne JO, Magotteaux P, Massart B, Serfaty JM, Houard X, Michel JB, Sakalihasan N. MR imaging of iron phagocytosis in intraluminal thrombi of abdominal aortic aneurysms in humans. Radiology. 2010;254:973–981. doi: 10.1148/radiol.09090657. [DOI] [PubMed] [Google Scholar]
- 7.Mahmoudi M, Sardari S, Shokrgozar MA, Laurent S, Stroeve P. Interaction of superparamagnetic iron oxide nanoparticles with human transferrin: Irreversible changes in human transferrin conformation. Nanoscale. 2010 doi: 10.1039/c0nr00733a. Revised. [DOI] [PubMed] [Google Scholar]
- 8.Mahmoudi M, Shokrgozar MA, Simchi A, Imani M, Milani AS, Stroeve P, Vali H, Hafeli UO, Bonakdar S. Multiphysics flow modeling and in vitro toxicity of iron oxide nanoparticles coated with poly(vinyl alcohol) J Phys Chem C. 2009;113:2322–2331. [Google Scholar]
- 9.Mahmoudi M, Simchi A, Imani M. Cytotoxicity of uncoated and polyvinyl alcohol coated superparamagnetic iron oxide nanoparticles. J Phys Chem C. 2009;113:9573–9580. [Google Scholar]
- 10.Mahmoudi M, Simchi A, Imani M. Recent advances in surface engineering of superparamagnetic iron oxide nanoparticles for biomedical applications. J Iran Chem Soc. 2010;7:S1–S27. [Google Scholar]
- 11.Xu C, Sun S. Monodisperse magnetic nanoparticles for biomedical applications. Polym Int. 2007;56:821–826. [Google Scholar]
- 12.Mahmoudi M, Simchi A, Imani M, Hafeli UO. Superparamagnetic iron oxide nanoparticles with rigid cross-linked polyethylene glycol fumarate coating for application in imaging and drug delivery. J Phys Chem C. 2009;113:8124–8131. [Google Scholar]
- 13.Mahmoudi M, Simchi A, Imani M, Milani AS, Stroeve P. Optimal design and characterization of superparamagnetic iron oxide nanoparticles coated with polyvinyl alcohol for targeted delivery and imaging. J Phys Chem B. 2008;112:14470–14481. doi: 10.1021/jp803016n. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoudi M, Simchi A, Imani M, Milani AS, Stroeve P. An in vitro study of bare and poly(ethylene glycol)-co-fumarate-coated superparamagnetic iron oxide nanoparticles: A new toxicity identification procedure. Nanotechnology. 2009;20:225104. doi: 10.1088/0957-4484/20/22/225104. [DOI] [PubMed] [Google Scholar]
- 15.Mahmoudi M, Simchi A, Imani M, Shokrgozar MA, Milani AS, Hafeli UO, Stroeve P. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surf, B. 2010;75:300–309. doi: 10.1016/j.colsurfb.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 16.Mahmoudi M, Simchi A, Imani M, Stroeve P, Sohrabi A. Templated growth of superparamagnetic iron oxide nanoparticles by temperature programming in the presence of poly(vinyl alcohol) Thin Solid Films. 2010;518:4281–4289. [Google Scholar]
- 17.Mahmoudi M, Simchi A, Milani AS, Stroeve P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J Colloid Interface Sci. 2009;336:510–518. doi: 10.1016/j.jcis.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoudi M, Simchi A, Vali H, Imani M, Shokrgozar MA, Azadmanesh K, Azari F. Cytotoxicity and cell cycle effects of bare and poly(vinyl alcohol)-coated iron oxide nanoparticles in mouse fibroblasts. Adv Eng Mater. 2009;11:B243–B250. [Google Scholar]
- 19.Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, Zywicke H, Miller B, van Gelderen P, Moskowitz BM, Duncan ID, Frank JA. Magnetoden-drimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141–1147. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 20.Bulte JW, Duncan ID, Frank JA. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab. 2002;22:899–907. doi: 10.1097/00004647-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R. Tat peptide derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 22.Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv Drug Delivery Rev. 2011;63:24–46. doi: 10.1016/j.addr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Mahmoudi M, Laurent S, Azadmanesh K, Journeay WS. Effect of nanoparticles on the cell life cycle. Chem Rev. 2010 doi: 10.1021/cr1003166. proposal accepted. [DOI] [PubMed] [Google Scholar]
- 24.Aillon KL, Xie Y, El-Gendy N, Berkland CJ, Forrest ML. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv Drug Delivery Rev. 2009;61:457–466. doi: 10.1016/j.addr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papp T, Schiffmann D, Weiss D, Castranova V, Vallyathan V, Rahman Q. Human health implications of nanomaterial exposure. Nanotoxicology. 2008;2:9–27. [Google Scholar]
- 26.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 27.Moller P, Jacobsen NR, Folkmann JK, Danielsen PH, Mikkelsen L, Hemmingsen JG, Vesterdal LK, Forchhammer L, Wallin H, Loft S. Role of oxidative damage in toxicity of particulates. Free Radical Res. 2010;44:1–46. doi: 10.3109/10715760903300691. [DOI] [PubMed] [Google Scholar]
- 28.Unfried K, Albrecht C, Klotz LO, Von Mikecz A, Grether-Beck S, Schins RPF. Cellular responses to nanoparticles: Target structures and mechanisms. Nanotoxicology. 2007;1:52–71. [Google Scholar]
- 29.Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 30.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4th. Oxford University Press, Inc; New York: 2007. [Google Scholar]
- 31.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-Glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayes CM, Gobin AM, Ausman KD, Mendez J, West JL, Colvin VL. Nano-C-60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26:7587–7595. doi: 10.1016/j.biomaterials.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 34.Bermudez E, Mangum JB, Wong BA, Asgharian B, Hext PM, Warheit DB, Everitt JI, Moss OR. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci. 2004;77:347–357. doi: 10.1093/toxsci/kfh019. [DOI] [PubMed] [Google Scholar]
- 35.Oberdörster E. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ Health Perspect. 2004;112:1058–1062. doi: 10.1289/ehp.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;1:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 37.Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signaling. 2010;12:125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- 38.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 39.Rallo R, France B, Liu R, Nair S, George S, Damoiseaux R, Giralt F, Nel A, Bradley K, Cohen Y. Self-organizing map analysis of toxicity-related cell signaling pathways for metal and metal oxide nanoparticles. Environ Sci Technol. 2011 doi: 10.1021/es103606x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radical Biol Med. 2008;44:1689–1699. doi: 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen EJ, Nelson BC. Mechanisms and measurements of nanomaterial-induced oxidative damage to DNA. Anal Bioanal Chem. 2010;398:613–650. doi: 10.1007/s00216-010-3881-7. [DOI] [PubMed] [Google Scholar]
- 42.Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci U S A. 1999;96:4820–4825. doi: 10.1073/pnas.96.9.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirkinezos IG, Moraes CT. Reactive oxygen species and mitochondrial diseases. Semin Cell Dev Biol ogy. 2001;12:449–457. doi: 10.1006/scdb.2001.0282. [DOI] [PubMed] [Google Scholar]
- 44.Deng ZJ, Liang MT, Monteiro M, Toth I, Minchin RF. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat Nanotechnol. 2011;6:39–44. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- 45.Sabbioni M. In vitro toxicology of nanoparticles. Proceedings of the Workshop: Research Needs on Nanoparticles; Brussels: 2005. [Google Scholar]
- 46.Monteiro-Riviere NA, Inman AO. Carbon. 2006;44:1070. [Google Scholar]
- 47.Casey A, Herzog E, Davoren M, Lyng FM, Byrne HJ, Chambers G. 2007;45:1425. [Google Scholar]
- 48.Worle-Knnirsch JM, Pulskamp K, Krug HF. Nano Lett. 2006;6:1261. doi: 10.1021/nl060177c. [DOI] [PubMed] [Google Scholar]
- 49.Herzog E, Casey A, Lyng FM, Chambers G, Byrne HJ, Davoren M. Toxicol Lett. 2007;174:49. doi: 10.1016/j.toxlet.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Brown DM, Stone V, Findlay P, MacNee W, Donaldson K. Occup Environ Med. 2000;57:685. doi: 10.1136/oem.57.10.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim H, Liu X, Kobayashi T, Kohyama T, Wen FQ, Romberger DJ, Conner H, Gilmour PS, Donaldson K, MacNee W, Rennard SI. Ultrafine carbon black particles inhibit human lung fibroblast-mediated collagen gel contraction. Am J Respir Cell Mol Biol. 2003;28:111. doi: 10.1165/rcmb.4796. [DOI] [PubMed] [Google Scholar]
- 52.Dutta D, Sunddaram SK, Teeguarden JG, Riley BJ, Fifiels LS, Jacobs JM, Addleman SR, Kaysen GA, Moudgil BM, Weber TJ. Toxicol Sci. 2007;100:303. doi: 10.1093/toxsci/kfm217. [DOI] [PubMed] [Google Scholar]
- 53.Lee J, Lilly GD, Doty RC, Podsiadlo P, Kotov NA. In vitro toxicity testing of nanoparticles in 3D cell culture. Small. 2009;5:1213–1221. doi: 10.1002/smll.200801788. [DOI] [PubMed] [Google Scholar]
- 54.Sayes CM, Reed KL, Warheit DB. Toxicol Sci. 2007;97:163. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- 55.Environmental Protection Agency. 2008 [Google Scholar]
- 56.Hansch C. A quantitative approach to biochemical structure– activity relationships. Acc Chem Res. 1969;2:232–&. [Google Scholar]
- 57.Puzyn T, Rasulev B, Gajewicz A, Hu XK, Dasari TP, Michalkova A, Hwang HM, Toropov A, Leszczynska D, Leszczynski J. Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles. Nat Nanotechnol. 2011;6:175–178. doi: 10.1038/nnano.2011.10. [DOI] [PubMed] [Google Scholar]
- 58.Puzyn T, Leszczynska D, Leszczynski J. Toward the development of “Nano-QSARs”: Advances and challenges. Small. 2009;5:2494–2509. doi: 10.1002/smll.200900179. [DOI] [PubMed] [Google Scholar]
- 59.Sayes C, Ivanov I. Comparative study of predictive computational models for nanoparticle-induced cytotoxicity. Risk Anal. 2010;30:1723–1734. doi: 10.1111/j.1539-6924.2010.01438.x. [DOI] [PubMed] [Google Scholar]
- 60.Benfenati E, Gini G, Hoffmann S, Luttik R. Comparing in vivo, in vitro and in silico methods and integrated strategies for chemical assessment: problems and prospects. Altern Lab Anim. 2010;38:153–166. doi: 10.1177/026119291003800201. [DOI] [PubMed] [Google Scholar]
- 61.Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Variables influencing interactions of untargeted quantum dot nanoparticles with skin cells and identification of biochemical modulators. Nano Lett. 2007;7:1344–1348. doi: 10.1021/nl070375j. [DOI] [PubMed] [Google Scholar]
- 62.Oberdörster G. Principles for characterizing the potential human health effects from exposure to nanomaterials:elements of a screening strategy. Part Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sahu SC, Casciano DA. Nanotoxicity: from in vivo and in vitro models to health risks. Wiley; United Kingdom: 2009. [Google Scholar]
- 64.Borm P, Klaessig FC, Landry TD, Moudgil B, Pauluhn J, Thomas K, Trottier R, Wood S. Research strategies for safety evaluation of nanomaterials, Part V: Role of dissolution in biological fate effects of nanoscale particles. Toxicol Sci. 2006;90:23–32. doi: 10.1093/toxsci/kfj084. [DOI] [PubMed] [Google Scholar]
- 65.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsuji JS, Maynard AD, Howard PC, James JT, Lam CW, Warheit DB, Santamaria AB. Research strategies for safety evaluation of nanomaterials, part IV: Risk assessment of nanoparticles. Toxicol Sci. 2006;89:42–50. doi: 10.1093/toxsci/kfi339. [DOI] [PubMed] [Google Scholar]
- 67.Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, Stone V, Brown S, MacNee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 68.Lee HA, Imran M, Monteiro-Riviere NA, Colvin VL, Yu WW, Riviere JE. Biodistribution of quantum dot nanoparticles in perfused skin: Evidence of coating dependency and periodicity in arterial extraction. Nano Lett. 2007;7:2865–2870. doi: 10.1021/nl071563c. [DOI] [PubMed] [Google Scholar]
- 69.Deng X, Jia G, Wang H, Sun H, Wang X, Yang S, Wang T, Liu Y. Translocation and fate of multi-walled carbon nanotubes in vivo. Carbon. 2007;45:1419–1424. [Google Scholar]
- 70.Yang RSH, Chang LW, Wu JP, Tsai MH, Wang HJ, Kuo YC, Yeh TK, Yang CS, Lin P. Persistent tissue kinetics and redistribution of nanoparticles, quantum Dot 705, in Mice: ICP-MS quantitative assessment. Environ Health Perspect. 2007;115:1339–1343. doi: 10.1289/ehp.10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fischer HC, Chan WCW. Nanotoxicity: the growing need for in vivo study. Curr Opin Biotechnol. 2007;18:565–571. doi: 10.1016/j.copbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 72.De Joug WH, Bonn PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomed. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dobrovolskaia MA, Clogston JD, Neun BW, Hall JB, Patri AK, McNeü SE. Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett. 2008;8:2180–2187. doi: 10.1021/nl0805615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin S, Du F, Wang Y, Ji S, Liang D, Yu L, Li Z. An acid-labile block copolymer of PDMAEMA and PEG as potential carrier for intelligent gene delivery systems. Biomacromolecules. 2008;9:109–115. doi: 10.1021/bm7008747. [DOI] [PubMed] [Google Scholar]
- 75.Kim D, El-Shall H, Dennis D, Morey T. Interaction of PLGA nanoparticles with human blood constituents. Colloids Surf, B. 2005;40:83–91. doi: 10.1016/j.colsurfb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 76.Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ Health Perspect. 2005;113:934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, Radomski MW. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146:882–893. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jun EA, Lim KM, Kim K, Bae ON, Noh JY, Chung KH, Chung JH. Silver nanoparticles enhance thrombus formation through increased platelet aggregation and procoagulant activity. Nanotoxicology. 2011;5:157–167. doi: 10.3109/17435390.2010.506250. [DOI] [PubMed] [Google Scholar]
- 79.Seaton A, Soutar A, Crawford V, Elton R, MeNerlan S, Cherrie J, Watt M, Agius R, Stout R. Particulate air pollution and the blood. Thorax. 1999;54:1027–1032. doi: 10.1136/thx.54.11.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nemmar A, Hoet PH, Dinsdale D, Vermylen J, Hovlaerts MF, Nemery B. Diesel exhaust particles in lung acutely enhance experimental peripheral Chrombosis. Circulation. 2003;107:1202–1208. doi: 10.1161/01.cir.0000053568.13058.67. [DOI] [PubMed] [Google Scholar]
- 81.Neun BW, Dobrovolskaia MA. Method for in vitro analysis of nanoparticle thrombogenic properties. Methods Mol Biol. 2011;697:225–235. doi: 10.1007/978-1-60327-198-1_24. [DOI] [PubMed] [Google Scholar]
- 82.Jiao Y, Ubrich N, Marchand-Arvier M, Vigneron CV, Hoffman M, Lecompte T, Maincent P. In vitro and in vivo evaluation of oral heparin-loaded polymeric nanoparticles in rabbits. Circulation. 2002;105:230–235. doi: 10.1161/hc0202.101988. [DOI] [PubMed] [Google Scholar]
- 83.Cenni E, Granchi D, Avnet S, Fotia C, Salerno M, Micieli D, Sarpietro MG, Pignatello R, Castelli F, Baldini N. Biocompatibility of poly(DL-lactide-co-glycolide) nanoparticles conjugated with alendronate. Biomaterials. 2008;29:1400–1411. doi: 10.1016/j.biomaterials.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 84.Ray M, Carroll P, Smith I, Hawson G. An attempt to standardize APTT reagents used to monitor heparin therapy. Blood Coagulation Fibrinolysis. 1992;3:743–748. doi: 10.1097/00001721-199212000-00007. [DOI] [PubMed] [Google Scholar]
- 85.Dragoni F, Minotti C, Palumbo G, Faillace F, Redi R, Bongarzoni V, Avvisati G. As compared to kaolin clotting time, silica clotting time is a specific and sensitive automated method for detecting lupus anticoagulant. Thromb Res. 2001;101:45–51. doi: 10.1016/s0049-3848(00)00374-1. [DOI] [PubMed] [Google Scholar]
- 86.Mahmoudi M, Lynch I, Ejtehadi MR, Monopoli MP, Bombelli FB, Laurent S. Protein-nanoparticle interactions: opportunities and challenges. Chem Rev. 2011;111:5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 87.Lynch I, Dawson KA. Protein–nanoparticle interactions. Nano Today. 2008;3:40–47. [Google Scholar]
- 88.Lundqvist M, Sethson I, Jonsson BH. Protein adsorption onto silica nanoparticles: Conformational changes depend on the particles’ curvature and the protein stability. Langmuir. 2004;20:10639–10647. doi: 10.1021/la0484725. [DOI] [PubMed] [Google Scholar]
- 89.Monopoli MP, Bombelli FB, Dawson KA. Nanobiotechnology: Nanoparticle coronas take shape. Nat Nanotechnol. 2011;6:11–12. doi: 10.1038/nnano.2011.267. [DOI] [PubMed] [Google Scholar]
- 90.Cedervall T, Lynch I, Lindman S, Berggrd T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle-protein corona using methods to quntify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci U S A. 2007;104:2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lynch I, Cedervall T, Lundqvist M, Cabaleiro-Lago C, Linse S, Dawson KA. The nanoparticle-protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv Colloid Interface Sci. 2007;134–135:167. doi: 10.1016/j.cis.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 92.Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Bombelli FB, Dawson KA. Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc. 2011;133:2525–2534. doi: 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- 93.Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 94.Ishida T, Harashima H, Kiwada H. Interactions of liposomes with cells in vitro and in vivo: Opsonins and receptors. Curr Drug Metab. 2001;2:397–409. doi: 10.2174/1389200013338306. [DOI] [PubMed] [Google Scholar]
- 95.Cedervall T, Lynch I, Foy M, Berggård T, Donnelly SC, Cagney G, Linse S, Dawson KA. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew Chem, Int Ed. 2007;46:5754–5756. doi: 10.1002/anie.200700465. [DOI] [PubMed] [Google Scholar]
- 96.De M, You CC, Srivastava S, Rotello VM. Biomimetic interactions of proteins with functionalized nanoparticles: A thermodynamic study. J Am Chem Soc. 2007;129:10747–10753. doi: 10.1021/ja071642q. [DOI] [PubMed] [Google Scholar]
- 97.Reddy ST, Van Der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 98.Moghimi SM, Hunter AC, Murray JC. Long circulating and target specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 99.Fischer HC, Liu L, Pang KS, Chan WCW. Pharmacokinetics of nanoscale quantum dots: In vivo distribution, sequestration, and clearance in the rat. Adv Funct Mater. 2006;16:1299–1305. [Google Scholar]
- 100.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 101.Saba TM. Physiology and physiopathology of the reticuloendothelial system. Arch Intern Med. 1970;126:1031–1052. [PubMed] [Google Scholar]
- 102.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Delivery: J Delivery Targeting Ther Agents. 2004;11:169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 103.Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci U S A. 2006;103:3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khan MK, Nigavekar SS, Minc LD, Kariapper MST, Nair BM, Lesniak WG, Balogh LP. In vivo biodistribution of dendrimers and dendrimer nanocomposites–Implications for cancer imaging and therapy. Technol Cancer Res Treat. 2005;4:603–613. doi: 10.1177/153303460500400604. [DOI] [PubMed] [Google Scholar]
- 105.Ballou B, Lagerholm BC, Ernst LA, Bruchez MP, Waggoner AS. Noninvasive imaging of quantum dots in mice. Bioconjugate Chem. 2004;15:79–86. doi: 10.1021/bc034153y. [DOI] [PubMed] [Google Scholar]
- 106.Kagan VE, Konduru NV, Feng WH, Allen BL, Conroy J, Volkov Y, Vlasova II, Belikova NA, Yanamala N, Kapralov A, Tyurina YY, Shi JW, Kisin ER, Murray AR, Franks J, Stolz D, Gou PP, Klein-Seetharaman J, Fadeel B, Star A, Shvedova AA. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotechnol. 2010;5:354–359. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hardman R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frohlich E, Kueznik T, Samberger C, Roblegg E, Wrighton C, Pieber TR. Size-dependent effects of nanoparticles on the activity of cytochrome P450 isoenzymes. Toxicol Appl Pharmacol. 2010;242:326–332. doi: 10.1016/j.taap.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 110.Hardonk MJ, Harms G, Koudstaal J. Zonal heterogeneity of rat hepatocytes in the in vivo uptake of 17 nm colloidal gold granules. Histochemistry. 1985;83:473–477. doi: 10.1007/BF00509211. [DOI] [PubMed] [Google Scholar]
- 111.Renaud G, Hamilton RL, Havel RJ. Hepatic metabolism of colloidal gold-low-density lipoprotein complexes in the rat: Evidence for bulk excretion of lysosomal contents into bile. Hepatology. 1989;9:380–392. doi: 10.1002/hep.1840090307. [DOI] [PubMed] [Google Scholar]
- 112.Wang H, Wang J, Deng X, Sun H, Shi Z, Gu Z, Liu Y, Zhao Y. Biodistribution of carbon single-wall carbon nanotubes in mice. J Nanosci Nanotechnol. 2004;4:1019–1024. doi: 10.1166/jnn.2004.146. [DOI] [PubMed] [Google Scholar]
- 113.Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kooter IM, Pennings JLA, Opperhuizen A, Cassee FR. Gene expression pattern in spontaneously hypertensive rats exposed to urban particulate matter (EHC-93) Inhalation Toxicol. 2005;17:53–65. doi: 10.1080/08958370590885717. [DOI] [PubMed] [Google Scholar]
- 115.Maysinger D, Behrendt M, Lalancette-Hébert M, Kriz J. Real-time imaging of astrocyte response to quantum dots: In vivo screening model system for biocompatibility of nanoparticles. Nano Lett. 2007;7:2513–2520. doi: 10.1021/nl071611t. [DOI] [PubMed] [Google Scholar]
- 116.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IFC, Plebanski M. Size-dependent immunogenicity: Therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 117.Gamvrellis A, Leong D, Hanley JC, Xiang SD, Mottram P, Plebanski M. Vaccines that facilitate antigen entry into dendritic cells. Immunol Cell Biol. 2004;82:506–516. doi: 10.1111/j.0818-9641.2004.01271.x. [DOI] [PubMed] [Google Scholar]
- 118.de Wolf HK, Snel CJ, Verbaan FJ, Schiffelers RM, Hennink WE, Storm G. Effect of cationic carriers on the pharmacokinetics and tumor localization of nucleic acids after intravenous administration. Int J Pharm. 2007;331:167–175. doi: 10.1016/j.ijpharm.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 119.Natarajan A, Gruettner C, Ivkov R, Denardo GL, Mirick G, Yuan A, Foreman A, DeNardo SJ. Nanoferrite particle based radioimmunonanoparticles: Binding affinity and in vivo pharmacokinetics. Bioconjugate Chem. 2008;19:1211–1218. doi: 10.1021/bc800015n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Larbanoix L, Burtea C, Laurent S, Van Leuven F, Toubeau G, Elst LV, Muller RN. Potential amyloid plaque-specific peptides for the diagnosis of Alzheimer’s disease. Neurobiol Aging. 2010;31:1679–1689. doi: 10.1016/j.neurobiolaging.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 121.De Jong WH, Borm PJA. Drug delivery and nanoparticles: Applications and hazards. Int J Nanomed. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim JS, Yoon TJ, Yu KN, Kim BG, Park SJ, Kim HW, Lee KH, Park SB, Lee JK, Cho MH. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol Sci. 2006;89:338–347. doi: 10.1093/toxsci/kfj027. [DOI] [PubMed] [Google Scholar]
- 123.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lanone S, Boczkowski J. Biomedical applications and potential health risks of nanomaterials: Molecular mechanisms. Curr Mol Med. 2006;6:651–663. doi: 10.2174/156652406778195026. [DOI] [PubMed] [Google Scholar]
- 125.Aillon KL, Xie YM, El-Gendy N, Berkland CJ, Forrest ML. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv Drug Delivery Rev. 2009;61:457–466. doi: 10.1016/j.addr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Powers KW, Brown SC, Krishna VB, Wasdo SC, Moudgil BM, Roberts SM. Research strategies for safety evaluation of nanomaterials. Part VI. Characterization of nanoscale particles for toxicological evaluation. Toxicol Sci. 2006;90:296–303. doi: 10.1093/toxsci/kfj099. [DOI] [PubMed] [Google Scholar]
- 127.Powers KW, Palazuelos M, Moudgil BM, Roberts SM. Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies. Nanotoxicology. 2007;1:42–51. [Google Scholar]
- 128.Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, Schmid G, Brandau W, Jahnen-Dechent W. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 129.Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112:13608–13619. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- 130.Yin H, Too HP, Chow GM. The effects of particle size and surface coating on the cytotoxicity of nickel ferrite. Biomaterials. 2005;26:5818–5826. doi: 10.1016/j.biomaterials.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 131.Hu Y, Xie JW, Tong YW, Wang CH. Effect of PEG conformation and particle size on the cellular uptake efficiency of nanoparticles with the HepG2 cells. J Controlled Release. 2007;118:7–17. doi: 10.1016/j.jconrel.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 132.Yang H, Liu C, Yang DF, Zhang HS, Xi ZG. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol. 2009;29:69–78. doi: 10.1002/jat.1385. [DOI] [PubMed] [Google Scholar]
- 133.Clift MJD, Rothen-Rutishauser B, Brown DM, Duffin R, Donaldson K, Proudfoot L, Guy K, Stone V. The impact of different nanoparticle surface chemistry and size on uptake and toxicity in a murine macrophage cell line. Toxicol Appl Pharmacol. 2008;232:418–427. doi: 10.1016/j.taap.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 134.Napierska D, Thomassen LCJ, Rabolli V, Lison D, Gonzalez L, Kirsch-Volders M, Martens JA, Hoet PH. Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small. 2009;5:846–853. doi: 10.1002/smll.200800461. [DOI] [PubMed] [Google Scholar]
- 135.Wang SG, Lu WT, Tovmachenko O, Rai US, Yu HT, Ray PC. Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem Phys Lett. 2008;463:145–149. doi: 10.1016/j.cplett.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 137.Oberdorster G, Ferin J, Lehnert BE. Correlation between particle-size, in-vivo particle persistence, and lung injury. Environ Health Perspect. 1994;102:173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Holgate ST. Exposure, uptake, distribution and toxicity of nanomaterials in humans. J Biomed Nanotechnol. 2010;6:1–19. doi: 10.1166/jbn.2010.1098. [DOI] [PubMed] [Google Scholar]
- 139.Oberdorster G, Stone V, Donaldson K. Toxicology of nanoparticles: A historical perspective. Nanotoxicology. 2007;1:2–25. [Google Scholar]
- 140.Asgharian B, Price OT. Deposition of ultrafine (NANO) particles in the human lung. Inhalation Toxicol. 2007;19:1045–1054. doi: 10.1080/08958370701626501. [DOI] [PubMed] [Google Scholar]
- 141.Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, Oberdorster G, Ziesenis A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health, Part A. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- 142.Wiebert P, Sanchez-Crespo A, Falk R, Philipson K, Lundin A, Larsson S, Moller W, Kreyling WG, Svartengren M. No significant translocation of inhaled 35-nm carbon particles to the circulation in humans. Inhalation Toxicol. 2006;18:741–747. doi: 10.1080/08958370600748455. [DOI] [PubMed] [Google Scholar]
- 143.Sager TM, Porter DW, Robinson VA, Lindsley WG, Schwegler-Berry DE, Castranova V. Improved method to disperse nanoparticles for in vitro and in vivo investigation of toxicity. Nanotoxicology. 2007;1:118–129. [Google Scholar]
- 144.Fraczek A, Menaszek E, Paluszkiewicz C, Blazewicz M. Comparative in vivo biocompatibility study of single- and multi-wall carbon nanotubes. Acta Biomater. 2008;4:1593–1602. doi: 10.1016/j.actbio.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 145.Kostarelos K, Lacerda L, Pastorin G, Wu W, Wieckowski S, Luangsivilay J, Godefroy S, Pantarotto D, Briand JP, Muller S, Prato M, Bianco A. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat Nanotechnol. 2007;2:108–113. doi: 10.1038/nnano.2006.209. [DOI] [PubMed] [Google Scholar]
- 146.Kam NWS, Liu ZA, Dai HJ. Carbon nanotubes as intracellular transporters for proteins and DNA: An investigation of the uptake mechanism and pathway. Angew Chem, Int Ed. 2006;45:577–581. doi: 10.1002/anie.200503389. [DOI] [PubMed] [Google Scholar]
- 147.Yang ST, Guo W, Lin Y, Deng XY, Wang HF, Sun HF, Liu YF, Wang X, Wang W, Chen M, Huang YP, Sun YP. Biodistribution of pristine single-walled carbon nanotubes in vivo. J Phys Chem C. 2007;111:17761–17764. [Google Scholar]
- 148.Yang ST, Wang X, Jia G, Gu YQ, Wang TC, Nie HY, Ge CC, Wang HF, Liu YF. Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicol Lett. 2008;181:182–189. doi: 10.1016/j.toxlet.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 149.Wick P, Manser P, Limbach LK, ttlaff-Weglikowska U, Krumeich F, Roth S, Stark WJ, Bruinink A. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett. 2007;168:121–131. doi: 10.1016/j.toxlet.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 150.Mercer RR, Scabilloni J, Wang L, Kisin E, Murray AR, Schwegler-Berry D, Shvedova AA, Castranova V. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Physiol: Lung Cell Mol Physiol. 2008;294:L87–L97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- 151.Warheit DB, Borm PJ, Hennes C, Lademann J. Testing strategies to establish the safety of nanomaterials: conclusions of an ECETOC workshop. Inhalation Toxicol. 2007;19:631–643. doi: 10.1080/08958370701353080. [DOI] [PubMed] [Google Scholar]
- 152.Lovric J, Bazzi HS, Cuie Y, Fortin GR, Winnik FM, Maysinger D. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J Mol Med. 2005;83:377–385. doi: 10.1007/s00109-004-0629-x. [DOI] [PubMed] [Google Scholar]
- 153.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Delivery Rev. 2009;61:428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mailander V, Landfester K. Interaction of nanoparticles with cells. Biomacromolecules. 2009;10:2379–2400. doi: 10.1021/bm900266r. [DOI] [PubMed] [Google Scholar]
- 155.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 156.Johnston HJ, Hutchison G, Christensen FM, Peters S, Hankin S, Stone V. A review of the in vivo and in vitro toxicity of silver and gold particulates: Particle attributes and biological mechanisms responsible for the observed toxicity. Crit Rev Toxicol. 2010;40:328–346. doi: 10.3109/10408440903453074. [DOI] [PubMed] [Google Scholar]
- 157.Yang RH, Chang LW, Wu JP, Tsai MH, Wang HJ, Kuo YC, Yeh TK, Yang CS, Lin P. Persistent tissue kinetics and redistribution of nanoparticles, quantum dot 705, in mice: ICP-MS quantitative assessment. Environ Health Perspect. 2007;115:1339–1343. doi: 10.1289/ehp.10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meng H, Chen Z, Xing GM, Yuan H, Chen CY, Zhao F, Zhang CC, Zhao YL. Ultrahigh reactivity provokes nanotoxicity: Explanation of oral toxicity of nano-copper particles. Toxicol Lett. 2007;175:102–110. doi: 10.1016/j.toxlet.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 159.Seaton A, Donaldson K. Nanoscience, nanotoxicology, and the need to think small. Lancet. 2005;365:923–924. doi: 10.1016/S0140-6736(05)71061-8. [DOI] [PubMed] [Google Scholar]
- 160.Donaldson K, Stone V, Tran CL, Kreyling W, Borm PJA. Nanotoxicology. Occup Environ Med. 2004;61:727–728. doi: 10.1136/oem.2004.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen Z, Meng HA, Xing GM, Chen CY, Zhao YL, Jia GA, Wang TC, Yuan H, Ye C, Zhao F, Chai ZF, Zhu CF, Fang XH, Ma BC, Wan LJ. Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett. 2006;163:109–120. doi: 10.1016/j.toxlet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 162.Hillyer JF, Albrecht RM. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J Pharm Sci. 2001;90:1927–1936. doi: 10.1002/jps.1143. [DOI] [PubMed] [Google Scholar]
- 163.Sonavane G, Tomoda K, Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf, B. 2008;66:274–280. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 164.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJAM, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 165.Chen YS, Hung YC, Liau I, Huang GS. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 2009;4:858–864. doi: 10.1007/s11671-009-9334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Parng C. In vivo zebrafish assays for toxicity testing. Curr Opin Drug Discovery Dev. 2005;8:100–106. [PubMed] [Google Scholar]
- 167.Bar-Ilan O, Albrecht RM, Fako VE, Furgeson DY. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small. 2009;5:1897–1910. doi: 10.1002/smll.200801716. [DOI] [PubMed] [Google Scholar]
- 168.Barbier O, Jacquillet G, Tauc M, Cougnon M, Poujeol P. Effect of heavy metals on, and handling by, the kidney. Nephron Physiol. 2005;99:105–110. doi: 10.1159/000083981. [DOI] [PubMed] [Google Scholar]
- 169.Shiohara A, Hoshino A, Hanaki K, Suzuki K, Yamamoto K. On the cyto-toxicity caused by quantum dots. Microbiol Immunol. 2004;48:669–675. doi: 10.1111/j.1348-0421.2004.tb03478.x. [DOI] [PubMed] [Google Scholar]
- 170.Song Y, Li X, Du X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur Respir J. 2009;34:559–567. doi: 10.1183/09031936.00178308. [DOI] [PubMed] [Google Scholar]
- 171.Kreuter J. Nanoparticles—a historical perspective. Int J Pharm. 2007;331:1–10. doi: 10.1016/j.ijpharm.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 172.Vauthier C, Labarre D, Ponchel G. Design aspects of poly(alkylcyanoacrylate) nanoparticles for drug delivery. J Drug Targeting. 2007;15:641–663. doi: 10.1080/10611860701603372. [DOI] [PubMed] [Google Scholar]
- 173.Abeylath SC, Turos E. Glycosylated polyacrylate nanoparticles by emulsion polymerization. Carbohydr Polym. 2007;70:32–37. doi: 10.1016/j.carbpol.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lherm C, Muller RH, Puisieux F, Couvreur P. Alkylcyanoacrylate drug carriers. 2. Cytotoxicity of cyanoacrylate nanoparticles with different alkyl chain-length, Int. J Pharm. 1992;84:13–22. [Google Scholar]
- 175.Ren H, Huang X. Polyacrylate nanoparticles: toxicity or new nanomedicine? Eur Respir J. 2010;36:218–221. doi: 10.1183/09031936.00022410. [DOI] [PubMed] [Google Scholar]
- 176.Semete B, Booysen L, Lemmer Y, Kalombo L, Katata L, Verschoor J, Swai HS. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine. 2010;6:662–671. doi: 10.1016/j.nano.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 177.Panyam J, Dali MA, Sahoo SK, Ma WX, Chakravarthi SS, Amidon GL, Levy RJ, Labhasetwar V. Polymer degradation and in vitro release of a model protein from poly(DL-lactide-CO-glycolide) nano- and microparticles. J Controlled Release. 2003;92:173–187. doi: 10.1016/s0168-3659(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 178.Cho MJ, Cho WS, Choi M, Kim SJ, Han BS, Kim SH, Kim HO, Sheen YY, Jeong JY. The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. Toxicol Lett. 2009;189:177–183. doi: 10.1016/j.toxlet.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 179.Kaewamatawong T, Kawamura N, Okajima M, Sawada M, Morita T, Shimada A. Acute pulmonary toxicity caused by exposure to colloidal silica: Particle size dependent pathological changes in mice. Toxicol Pathol. 2005;33:743–749. doi: 10.1080/01926230500416302. [DOI] [PubMed] [Google Scholar]
- 180.Chen HW, Su SF, Chien CT, Lin WH, Yu SL, Chou CC, Chen JJW, Yang PC. Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. FASEB J. 2006;20:2393–+. doi: 10.1096/fj.06-6485fje. [DOI] [PubMed] [Google Scholar]
- 181.Drobne D, Jemec A, Tkalec ZP. In vivo screening to determine hazards of nanoparticles: Nanosized TiO2. Environ Pollut. 2009;157:1157–1164. doi: 10.1016/j.envpol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 182.Park EJ, Bae E, Yi J, Kim Y, Choi K, Lee SH, Yoon J, Lee BC, Park K. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ Toxicol Pharmacol. 2010;30:162–168. doi: 10.1016/j.etap.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 183.Zhang YB, Chen W, Zhang J, Liu J, Chen GP, Pope C. In vitro and in vivo toxicity of CdTe nanoparticles. J Nanosci Nanotechnol. 2007;7:497–503. doi: 10.1166/jnn.2007.125. [DOI] [PubMed] [Google Scholar]
- 184.Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol. 2001;175:191–199. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- 185.Amrite AC, Kompella UB. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J Pharm Pharmacol. 2005;57:1555–1563. doi: 10.1211/jpp.57.12.0005. [DOI] [PubMed] [Google Scholar]
- 186.Gou ML, Zheng XL, Men K, Zhang J, Zheng L, Wang XH, Luo F, Zhao YL, Zhao X, Wei YQ, Qian ZY. poly(epsilon-caprolactone)/poly(ethylene glycol)/poly(epsilon-caprolactone) nanoparticles: preparation, characterization, and application in doxorubicin delivery. J Phys Chem B. 2009;113:12928–12933. doi: 10.1021/jp905781g. [DOI] [PubMed] [Google Scholar]
- 187.Verma A, Stellacci F. Effect of surface properties on nanoparticle–cell interactions. Small. 2010;6:12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 188.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lu Z, Qiao Y, Zheng XT, Chan-Park MB, Li CM. Effect of particle shape on phagocytosis of CdTe quantum dot-cystine composites. MedChemComm. 2010;1:84–86. [Google Scholar]
- 190.Doshi N, Mitragotri S. Needle-shaped polymeric particles induce transient disruption of cell membranes. J R Soc, Interface. 2010;7:S403–S410. doi: 10.1098/rsif.2010.0134.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ferrari M. Nanogeometry: Beyond drug delivery. Nat Nanotechnol. 2008;3:131–132. doi: 10.1038/nnano.2008.46. [DOI] [PubMed] [Google Scholar]
- 192.Radomski A, Jurasz P, onso-Escolano D, Drews M, Morandi M, Malinski T, Radomski MW. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146:882–893. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Park KH, Chhowalla M, Iqbal Z, Sesti F. Single-walled carbon nanotubes are a new class of ion channel blockers. J Biol Chem. 2003;278:50212–50216. doi: 10.1074/jbc.M310216200. [DOI] [PubMed] [Google Scholar]
- 194.Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GAM, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 195.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 196.Al Faraj A, Bessaad A, Cieslar K, Lacroix G, Canet-Soulas E, Cremillieux Y. Long-term follow-up of lung biodistribution and effect of instilled SWCNTs using multiscale imaging techniques. Nanotechnology. 2010;21 doi: 10.1088/0957-4484/21/17/175103. [DOI] [PubMed] [Google Scholar]
- 197.Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7 doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Murphy FA, Poland CA, Duffin R, Al-Jamal KT, Ali-Boucetta H, Nunes A, Byrne F, Prina-Mello A, Volkov Y, Li S, Mather SJ, Bianco A, Prato M, Macnee W, Wallace WA, Kostarelos K, Donaldson K. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am J Pathol. 2011;178:2587–2600. doi: 10.1016/j.ajpath.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gurr JR, Wang ASS, Chen CH, Jan KY. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology. 2005;213:66–73. doi: 10.1016/j.tox.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 200.Petkovic J, Zegura B, Stevanovic M, Drnovsek N, Uskokovic D, Novak S, Filipic M. DNA damage and alterations in expression of DNA damage responsive genes induced by TiO2 nanoparticles in human hepatoma HepG2 cells. Nanotoxicology. 2011;5:341–353. doi: 10.3109/17435390.2010.507316. [DOI] [PubMed] [Google Scholar]
- 201.Ispas C, Andreescu D, Patel A, Goia DV, Andreescu S, Wallace KN. Toxicity and developmental defects of different sizes and shape nickel nanoparticles in zebrafish. Environ Sci Technol. 2009;43:6349–6356. doi: 10.1021/es9010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 203.Hamilton RF, Wu NQ, Porter D, Buford M, Wolfarth M, Holian A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part Fibre Toxicol. 2009;6 doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hartig SM, Greene RR, Dikov MM, Prokop A, Davidson JM. Multifunctional nanoparticulate polyelectrolyte complexes. Pharm Res. 2007;24:2353–2369. doi: 10.1007/s11095-007-9459-1. [DOI] [PubMed] [Google Scholar]
- 205.Lee MK, Lim SJ, Kim CK. Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Biomaterials. 2007;28:2137–2146. doi: 10.1016/j.biomaterials.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 206.Schaffazick SR, Pohlmann AR, Dalla-Costa T, Guterres SS. Freeze-drying polymeric colloidal suspensions: Nanocapsules, nanospheres and nanodispersion. A comparative study. Eur J Pharm Biopharm. 2003;56:501–505. doi: 10.1016/s0939-6411(03)00139-5. [DOI] [PubMed] [Google Scholar]
- 207.Aaron JS, Greene AC, Kotula PG, Bachand GD, Timlin JA. Advanced optical imaging reveals the dependence of particle geometry on interactions between CdSe quantum dots and immune cells. Small. 2011;7:334–341. doi: 10.1002/smll.201001619. [DOI] [PubMed] [Google Scholar]
- 208.Alkilany AM, Nagaria PK, Wyatt MD, Murphy CJ. Cation exchange on the surface of gold nanorods with a polymerizable surfactant: Polymerization, stability, and toxicity evaluation. Langmuir. 2010;26:9328–9333. doi: 10.1021/la100253k. [DOI] [PubMed] [Google Scholar]
- 209.Andelman T, Gordonov S, Busto G, Moghe PV, Riman RE. Synthesis and cytotoxicity of Y2O3 nanoparticles of various morphologies. Nanoscale Res Lett. 2010;5:263–273. doi: 10.1007/s11671-009-9445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hsiao IL, Huang YJ. Effects of various physicochemical characteristics on the toxicities of ZnO and TiO2 nanoparticles toward human lung epithelial cells. Sci Total Environ. 2011;409:1219–1228. doi: 10.1016/j.scitotenv.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 211.Peng X, Palma S, Fisher NS, Wong SS. Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquat Toxicol. 2011;102:186–196. doi: 10.1016/j.aquatox.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 212.Sun YN, Wang CD, Zhang XM, Ren L, Tian XH. Shape dependence of gold nanoparticles on in vivo acute toxicological effects and biodistribution. J Nanosci Nanotechnol. 2011;11:1210–1216. doi: 10.1166/jnn.2011.3094. [DOI] [PubMed] [Google Scholar]
- 213.Yang DP, Cui DX. Advances and prospects of gold nanorods. Chem–Asian J. 2008;3:2010–2022. doi: 10.1002/asia.200800195. [DOI] [PubMed] [Google Scholar]
- 214.Mercer RR, Scabilloni J, Wang L, Kisin E, Murray AR, Schwegler-Berry D, Shvedova AA, Castranova V. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Physiol: Lung Cell Mol Physiol. 2008;294:L87–L97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- 215.Vevers WF, Jha AN. Genotoxic and cytotoxic potential of titanium dioxide (TiO2) nanoparticles on fish cells in vitro. Ecotoxicology. 2008;17:410–420. doi: 10.1007/s10646-008-0226-9. [DOI] [PubMed] [Google Scholar]
- 216.Jung H, Choi H. Kinetics and mechanism of heterogeneous catalytic ozonation of para-chlorobenzoic acid in the presence of nanosize ZnO. 2007:213–217. [Google Scholar]
- 217.Su F, Lv L, Fang YL, Liu T, Cooper AI, Xiu SZ. Thermally reduced ruthenium nanoparticles as a highly active heterogeneous catalyst for hydrogenation of monoaromatics. J Am Chem Soc. 2007;129:14213–14223. doi: 10.1021/ja072697v. [DOI] [PubMed] [Google Scholar]
- 218.Mortensen LJ, Oberdörster G, Pentland AP, DeLouise LA. In vivo skin penetration of quantum dot nanoparticles in the murine model: The effect of U.V.R. Nano Lett. 2008;8:2779–2787. doi: 10.1021/nl801323y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hoshino A, Fujioka K, Oku T, Suga M, Sasaki YF, Ohta T, Yasuhara M, Suzuki K, Yamamoto K. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 2004;4:2163–2169. [Google Scholar]
- 220.Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjugate Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 221.Saxena RK, Williams W, Mcgee JK, Daniels MJ, Boykin E, Gilmour MI. Enhanced in vitro and in vivo toxicity of polydispersed acid-functionalized single-wall carbon nanotubes. Nanotoxicology. 2007;1:291–300. [Google Scholar]
- 222.Pietroiusti A, Massimiani M, Fenoglio I, Colonna M, Valentini F, Palleschi G, Camaioni A, Magrini A, Siracusa G, Bergamaschi A, Sgambato A, Campagnolo L. Low doses of pristine and oxidized single-wall carbon nanotubes affect mammalian embryonic development. ACS Nano. 2011;5:4624–4633. doi: 10.1021/nn200372g. [DOI] [PubMed] [Google Scholar]
- 223.Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood–brain barrier integrity and permeability. J Drug Targeting. 2004;12:635–641. doi: 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- 224.Kohli AK, Alpar HO. Potential use of nanoparticles for transcutaneous vaccine delivery: effect of particle size and charge. Int J Pharm. 2004;275:13–17. doi: 10.1016/j.ijpharm.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 225.Bhattacharjee S, de Haan LHJ, Evers NM, Jiang X, Marcelis ATM, Zuilhof H, Rietjens IMCM, Alink GM. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part Fibre Toxicol. 2010:7. doi: 10.1186/1743-8977-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Heiden TCK, Dengler E, Kao WJ, Heideman W, Peterson RE. Developmental toxicity of low generation PAMAM dendrimers in zebrafish. Toxicol Appl Pharmacol. 2007;225:70–79. doi: 10.1016/j.taap.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Malik N, Evagorou EG, Duncan R. Dendrimer-platinate: a novel approach to cancer chemotherapy. Anti-Cancer Drugs. 1999;10:767–776. [PubMed] [Google Scholar]
- 228.Harper S, Usenko C, Hutchison JE, Maddux BLS, Tanguay RL. In vivo biodistribution and toxicity depends on nanomaterial composition, size, surface functionalisation and route of exposure. J Exp Nanosci. 2008;3:195–206. [Google Scholar]
- 229.Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem. 2008;27:1972–1978. doi: 10.1897/08-002.1. [DOI] [PubMed] [Google Scholar]
- 230.Chen JY, Dong X, Zhao J, Tang GP. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J Appl Toxicol. 2009;29:330–337. doi: 10.1002/jat.1414. [DOI] [PubMed] [Google Scholar]
- 231.Kirchner C, Liedl T, Kudera S, Pellegrino T, Javier AM, Gaub HE, Stozle S, Fertig N, Parak WJ. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005;5:331–338. doi: 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- 232.Moon HS, Guo DD, Song HH, Kim IY, Jin HL, Kim YK, Chung CS, Choi YJ, Lee HK, Cho CS. Regulation of adipocyte differentiation by PEGylated all-trans retinoic acid: reduced cytotoxicity and attenuated lipid accumulation. J Nutr Biochem. 2007;18:322–331. doi: 10.1016/j.jnutbio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 233.Morris MC, Gros E, Aldrian-Herrada G, Choob M, Archdeacon J, Heitz F, Divita G. A non-covalent peptide-based carrier for in vivo delivery of DNA mimics. Nucleic Acids Res. 2007:35. doi: 10.1093/nar/gkm053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Delivery Rev. 2003;55:403–419. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 235.Kato T, Yashiro T, Murata Y, Herbert DC, Oshikawa K, Bando M, Ohno S, Sugiyama Y. Evidence that exogenous substances can be phagocytized by alveolar epithelial cells and transported into blood capillaries. Cell Tissue Res. 2003;311:47–51. doi: 10.1007/s00441-002-0647-3. [DOI] [PubMed] [Google Scholar]
- 236.Weissleder R, Stark DD, Engelstad BL, Bacon BR, Compton CC, White DL, Jacobs P, Lewis J. Superparamagnetic iron oxide: pharmacokinetics and toxicity. Am J Roentgenol. 1989;152:167–173. doi: 10.2214/ajr.152.1.167. [DOI] [PubMed] [Google Scholar]
- 237.Lacava ZGM, Azevedo RB, Lacava LM, Martins EV, Garcia VAP, Rébula CA, Lemos APC, Sousa MH, Morais PC, Tourinho FA, Da Silva MF. Toxic effects of ionic magnetic fluids in mice. J Magn Magn Mater. 1999;194:90–95. [Google Scholar]
- 238.Lacava ZGM, Azevedo RB, Martins EV, Lacava LM, Freitas MLL, Garcia VAP, Rébula CA, Lemos APC, Sousa MH, Tourinho FA, Da Silva MF, Morais PC. Biological effects of magnetic fluids: toxicity studies. J Magn Magn Mater. 1999;201:431–434. [Google Scholar]
- 239.Schulze E, Ferrucci JR, Poss K, Lapointe L. Cellular uptake and trafficking of prototypical magnetic iron oxide label in vitro. Invest Radiol. 1995;30:604–610. doi: 10.1097/00004424-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 240.Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Guo G, Liu W, Liang J, He Z, Xu H, Yang X. Probing the cytotoxicity of CdSe quantum dots with surface modification. Mater Lett. 2007;61:1641–1644. [Google Scholar]
- 242.Su Y, He Y, Lu H, Sai L, Li Q, Li W, Wang L, Shen P, Huang Q, Fan C. The cytotoxicity of cadmium based, aqueous phase-synthesized, quantum dots and its modulation by surface coating. Biomaterials. 2009;30:19–25. doi: 10.1016/j.biomaterials.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 243.Zhao MX, Xia Q, Feng XD, Zhu XH, Mao ZW, Ji LN, Wang K. Synthesis, biocompatibility and cell labeling of l-arginine-functional β-cyclodextrin-modified quantum dot probes. Biomaterials. 2010;31:4401–4408. doi: 10.1016/j.biomaterials.2010.01.114. [DOI] [PubMed] [Google Scholar]
- 244.Chen F, Gerion D. Fluorescent CdSe/ZnS nanocrystal–peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Lett. 2004;4:1827–1832. [Google Scholar]
- 245.Mancini MC, Kairdolf BA, Smith AM, Nie S. Oxidative quenching and degradation of polymer-encapsulated quantum dots: New insights into the long-term fate and toxicity of nanocrystals in vivo. J Am Chem Soc. 2008;130:10836–10837. doi: 10.1021/ja8040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Geys J, Nemmar A, Verbeken E, Smolders E, Ratoi M, Hoylaerts MF, Nemery B, Hoet PHM. Acute toxicity and prothrombotic effects of quantum dots: impact of surface charge. Environ Health Perspect. 2008;116:1607–1613. doi: 10.1289/ehp.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kim S, Fisher B, Eisler HJ, Bawendi M. Type-II quantum dots: CdTe/CdSe(core/shell) and CdSe/ZnTe(core/shell) heterostructures. J Am Chem Soc. 2003;125:11466–11467. doi: 10.1021/ja0361749. [DOI] [PubMed] [Google Scholar]
- 248.Bang J, Chon B, Won N, Nam J, Joo T, Kim S. Spectral switching of type-II quantum dots by charging. J Phys Chem C. 2009;113:6320–6323. [Google Scholar]
- 249.Blackman B, Battaglia DM, Mishima TD, Johnson MB, Peng X. Control of the morphology of complex semiconductor nanocrystals with a type II heterojunction, dots vs. peanuts, by thermal cycling. Chem Mater. 2007;19:3815–3821. [Google Scholar]
- 250.Dorfs D, Franzi T, Osovsky R, Brumer M, Lifshitz E, Klar TA, Eychmüller A. Type-I and type-II nanoscale heterostructures based on CdTe nanocrystals: A comparative study. Small. 2008;4:1148–1152. doi: 10.1002/smll.200800287. [DOI] [PubMed] [Google Scholar]
- 251.Zgaren I, Sellami K, Jaziri S. Size-dependent optical properties in II–VI quantum-dot/quantum-well heterostructures. Sensor Lett. 2009;7:967–971. [Google Scholar]
- 252.Deng D, Chen X, Zhang J, Liu F, Cao J, Gu Y. Aqueous synthesis of PbS quantum dots for noninvasive near-infrared fluorescence imaging in a mouse model. 2010 [Google Scholar]
- 253.Kuo TR, Lee CF, Lin SJ, Dong CY, Chen CC, Tan HY. Studies of intracorneal distribution and cytotoxicity of quantum dots: Risk assessment of eye exposure. Chem Res Toxicol. 2010;24:253–261. doi: 10.1021/tx100376n. [DOI] [PubMed] [Google Scholar]
- 254.Oostendorp M, Douma K, Wagenaar A, Slenter JMGM, Hackeng TM, Van Zandvoort MAMJ, Post MJ, Backes WH. Molecular magnetic resonance imaging of myocardial angiogenesis after acute myocardial infarction. Circulation. 2010;121:775–783. doi: 10.1161/CIRCULATIONAHA.109.889451. [DOI] [PubMed] [Google Scholar]
- 255.Sharma P, Singh A, Brown SC, Bengtsson N, Walter GA, Grobmyer SR, Iwakuma N, Santra S, Scott EW, Moudgil BM. Multimodal nanoparticulate bioimaging contrast agents. Methods Mol Biol (Clifton, NJ) 2010;624:67–81. doi: 10.1007/978-1-60761-609-2_5. [DOI] [PubMed] [Google Scholar]
- 256.Sun D, Yang K, Zheng G, Li Z, Cao Y. Study on effect of peptide-conjugated near-infrared fluorescent quantum dots on the clone formation, proliferation, apoptosis, and tumorigenicity ability of human buccal squamous cell carcinoma cell line BcaCD885. Int J Nanomed. 2010;5:401–405. doi: 10.2147/ijn.s10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mahmoudi M, Lynch I, Ejtehadi R, Monopoli M, Laurent S. Protein–nanoparticle interactions: possibilities and limitations. Chem Rev. 2010 doi: 10.1021/cr100440g. in press. [DOI] [PubMed] [Google Scholar]
- 258.Mahmoudi M, Laurent S, Azadmanesh K, Journeay WS. Effect of nanoparticles on the cell life cycle. Chem Rev. 2011 doi: 10.1021/cr1003166. in press. [DOI] [PubMed] [Google Scholar]
- 259.Mahmoudi M, Milani AS, Stroeve P. Surface architecture of superparamagnetic iron oxide nanoparticles for application in drug delivery and their biological response: a review. Int J Biomed Nanosci Nanotechnol. 2010;1:164–201. [Google Scholar]
- 260.Mahmoudi M, Sardari S, Shokrgozar MA, Laurent S, Stroeve P. Interaction of superparamagnetic iron oxide nanoparticles with human transferrin: Irreversible changes in human transferrin conformation. Nanoscale. 2011;3:1127–1138. doi: 10.1039/c0nr00733a. [DOI] [PubMed] [Google Scholar]
- 261.Amiri H, Mahmoudi M, Lascialfari A. Superparamagnetic colloidal nanocrystal clusters coated with polyethylene glycol fumarate: A possible novel theranostic agent. Nanoscale. 3:1022–1030. doi: 10.1039/c0nr00603c. [DOI] [PubMed] [Google Scholar]
- 262.Berry CC, Wells S, Charles S, Curtis ASG. Dextran and albumin derivatised iron oxide nanoparticles: Influence on fibroblasts in vitro. Biomaterials. 2003;24:4551–4557. doi: 10.1016/s0142-9612(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 263.Xie J, Xu C, Kohler N, Hou Y, Sun S. Controlled PEGylation of monodisperse Fe3O4 nanoparticles for reduced non-specific uptake by macrophage cells. Adv Mater. 2007;19:3163–3166. [Google Scholar]
- 264.Cho WS, Cho M, Jeong J, Choi M, Cho HY, Han BS, Kim SH, Kim HO, Lim YT, Chung BH. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol Appl Pharmacol. 2009;236:16–24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 265.Hu Y, Xie J, Tong YW, Wang CH. Effect of PEG conformation and particle size on the cellular uptake efficiency of nanoparticles with the HepG2 cells. J Controlled Release. 2007;118:7–17. doi: 10.1016/j.jconrel.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 266.Lacerda L, Soundararajan A, Singh R, Pastorin G, Al-Jamal KT, Turton J, Frederik P, Herrero MA, Li S, Bao A, Emfietzoglou D, Mather S, Phillips WT, Prato M, Bianco A, Goins B, Kostarelos K. Dynamic imaging of functionalized multi-walled carbon nanotube systemic circulation and urinary excretion. Adv Mater. 2008;20:225–230. [Google Scholar]
- 267.Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano–bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 268.Verma A, Uzun O, Hu Y, Han HS, Watson N, Chen S, Irvine DJ, Stellacci F. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat Mater. 2008;7:588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lin YS, Haynes CL. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J Am Chem Soc. 2010;132:4834–4842. doi: 10.1021/ja910846q. [DOI] [PubMed] [Google Scholar]
- 270.De Angelis F, Pujia A, Falcone C, Iaccino E, Palmieri C, Liberale C, Mecarini F, Candeloro P, Luberto L, De Laurentiis A, Das G, Scala G, Di Fabrizio E. Water soluble nanoporous nanoparticle for in vivo targeted drug delivery and controlled release in B cells tumor context. Nanoscale. 2010;2:2230–2236. doi: 10.1039/c0nr00161a. [DOI] [PubMed] [Google Scholar]
- 271.Park JH, Gu L, Von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater. 2009;8:331–336. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chen D, Li L, Tang F, Qi S. Facile and scalable synthesis of tailored silica “nanorattle” structures. Adv Mater. 2009;21:3804–3807. [Google Scholar]
- 273.Li L, Tang F, Liu H, Liu T, Hao N, Chen D, Teng X, He J. In vivo delivery of silica nanorattle encapsulated docetaxel for liver cancer therapy with low toxicity and high efficacy. ACS Nano. 2010;11:6874–6882. doi: 10.1021/nn100918a. [DOI] [PubMed] [Google Scholar]
- 274.Erogbogbo F, Yong K, Roy I, Hu R, Law W, Zhao W, Ding H, Wu F, Kumar R, Swihart MT, Prasad PN. In vivo targeted cancer imaging, sentinel lymph node mapping and multi-channel imaging with biocompatible silicon nanocrystals. ACS Nano. 2011;5:413–423. doi: 10.1021/nn1018945. [DOI] [PubMed] [Google Scholar]
- 275.Sayes CM, Marchione AA, Reed KL, Warheit DB. Comparative pulmonary toxicity assessments of C60 water suspensions in rats: few differences in fullerene toxicity in vivo in contrast to in vitro profiles. Nano Lett. 2007;8:2399–2406. doi: 10.1021/nl0710710. [DOI] [PubMed] [Google Scholar]
- 276.Mu CJ, LaVan DA, Langer RS, Zetter BR. Self-assembled gold nanoparticle molecular probes for detecting proteolytic activity in vivo. ACS Nano. 2010;3:1511–1520. doi: 10.1021/nn9017334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Eghtedari N, Liopo AV, Copland JA, Oraevsky AA, Motamedi M. Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nano Lett. 2009;9:287–291. doi: 10.1021/nl802915q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Bhang SH, Won N, Lee TJ, Jin H, Nam J, Park J, Chung H, Park H, Sung Y, Hahn SK, Kim Y, Kim S. Hyaluronic acid quantum dot conjugates for in vivo lymphatic vessel imaging. ACS Nano. 2009;3:1389–1398. doi: 10.1021/nn900138d. [DOI] [PubMed] [Google Scholar]
- 279.Diagaradjane P, Deorukhkar A, Gelovani JG, Maru DM, Krishnan M. Gadolinium chloride augments tumor-specific imaging of targeted quantum dots in vivo. ACS Nano. 2010;4:4131–4141. doi: 10.1021/nn901919w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ajima K, Murakami T, Mizoguchi Y, Tsuchida K, Ichihashi T, Iijima S, Yudasaka M. Enhancement of in vivo anticancer effects of cisplatin by incorporation inside single-wall carbon nanohorns. ACS Nano. 2008;2:2057–2064. doi: 10.1021/nn800395t. [DOI] [PubMed] [Google Scholar]
- 281.Miyawaki J, Matsumura S, Yuge R, Murakami T, Sato S, Tomida A, Tsuruo T, Ichihashi T, Fujinami T, Irie H, Tsuchida K, Iijima S, Shiba K, Yudasaka M. Biodistribution and ultrastructural localization of single-walled carbon nanohorns determined in vivo with embedded Gd2O3 labels. ACS Nano. 2009;3:1399–1406. doi: 10.1021/nn9004846. [DOI] [PubMed] [Google Scholar]
- 282.Altinolu EI, Russin TJ, Kaiser JM, Barth BM, Eklund PC, Kester M, Adair JH. Near-infrared emitting fluorophore-doped calcium phosphate nanoparticles for in vivo imaging of human breast cancer. ACS Nano. 2008;2:2075–2084. doi: 10.1021/nn800448r. [DOI] [PubMed] [Google Scholar]
- 283.Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XH. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano. 2007;1:133–143. doi: 10.1021/nn700048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.George S, Xia T, Rallo R, Zhao Y, Ji Z, Lin S, Wang X, Zhang H, France B, Schoenfeld D, Damoiseaux R, Liu R, Bradley KA, Cohen Y, Nel AE. Use of a high-throughput screening approach coupled with in vivo zebrafish embryo screening to develop hazard ranking for engineered nanomaterials. ACS Nano. 2011;5:1805–1817. doi: 10.1021/nn102734s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Jiang JK, Oberdorster G, Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res. 2009;11:77–89. [Google Scholar]
- 286.Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol. 2003;21:1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]


