Abstract
Ambiguine I isonitrile (AmbI) obtained from the cultured cyanobacterium Fischerella ambigua was identified as a potent NF-κB inhibitor (IC50=30 nM). The cytotoxic effect was evaluated in both HT-29 colon cancer cell line (EC50=4.35 μM) and MCF-7 breast cancer cell line (EC50=1.7 μM) using the SRB assay. In the cells treated with AmbI, an increased population of cells was detected in sub G1-phase. The apoptotic effect was associated with block in G1-phase of the cell cycle in treated cells; however, cell death was induced independently of caspase-7. The NF-κB expression of p50 and p65 units were also examined in treated cells and compared with the positive control, rocaglamide (IC50=75 nM). Moreover, the expression of mediators of the NF-κB pathway such as kinase IKKκ was studied at increasing concentrations of AmbI. The down stream effect of NF-κB inhibition and the effect on the expression of TNF-α induced ICAM-1 was evaluated. Thus, the dose-dependent and time-dependent effect of AmbI on MCF-7 cells was examined in an attempt to investigate its potential mechanism of action on inducing apoptosis.
Keywords: Ambiguine I isonitrile, MCF-7, caspase-independent, apoptosis, mitochondria, ROS, NF-κB
1. INTRODUCTION
The transcription factor NF-κB regulates the expression of important regulatory factors involved in the proliferation of cancer cells. NF-κB prevents cancer cells from entering apoptosis, and contributes to progression of certain cancers.1 Upon phosphorylation of IκB by the IκB kinase (IKK), NF-κB is released and translocated to the nucleus2 where it exerts its effects binding to the consensus region, activating the target genes involved in cell adhesion and metastasis of MCF-7 breast cancer cells. Tumor necrosis factor-α (TNF-α) induces the activation of NF-κB and plays a pivotal role in the metastasis of MCF-7 estrogen-sensitive breast cancer cells.1, 2 Hence, mediators in the NF-κB pathway represents potential targets for inhibiting the proliferation of tumor cells. Studying and identifying specific inhibitors of the NF-κB activating pathway provide a strategy for identifying new potential anticancer agents with specific anticancer effects. The structurally complex indole alkaloid, Ambiguine I isonitrile (AmbI) was previously obtained from the cyanobacterium Fischerella ambigua.3 In this study, AmbI showed potent NF-κB inhibition and also exhibited antiproliferative effects. The apoptotic effect was analyzed by cell flow cytometry and expression of the NF-κB mediators p65, p50, and IKKκ were analyzed by immunoblot analysis. The impact on the mitochondria was also studied. Thus, we hypothesized that AmbI induced cell cycle arrest and apoptosis in treated hormone-dependent MCF-7 breast cancer cells and possibly prevented metastasis of tumor cells.
2. MATERIALS AND METHODS
2.1 Ambiguine I isonitrile
AmbI was obtained as previously described.3
2.2 Cell culture
HeLa, MCF-7, and HT-29 cancer cell lines were obtained from American Type Culture Collection. Cells were cultured in Dubelcco’s modified Eagle’s medium (DMEM) and Roswell Park Memorial Institute medium (RPMI-1640), containing 10% fetal bovine calf serum and 10% Antibiotic-Antimycotic (Gibco, Rockville, MD). The cells were kept at 37ºC and in an atmosphere with 5% CO2.
2.3 NF-κB assay
The NF-κB assay was carried out according to established protocol.4 In brief, a nuclear extract was prepared from HeLa cells. The Transcription Assay System (Pierce Biotechnology, Rockford, IL) was used to evaluate the binding affinity to a biotinylated consensus sequence of the NF-κB subunit p65. Luminescence was detected using Fluostar Optima plate reader (BMG Labtech Inc, Durham, NC). Rocaglamide was used as a positive control.
2.4 SRB- cytotoxicity assay
The MCF-7 and HT-29 cell suspensions were plated in a 96-well plate and incubated with AmbI for 72h at 37ºC and in an atmosphere with 5% CO2. Cells were fixed using 20% trichloroacetic acid (TCA) for 30 min, followed by staining using suforhodamine B (SRB) (0.4%) for 30 min at room temperature.5 SRB was removed by washing with 3x acetic acid. After adding 200 μM Trisbase solution to each well, the 96-well plates were placed on a shaker for 5 min. Absorbance reading at wavelength 515 nm was performed using Fluostar Optima plate reader (BMG Labtech Inc, Durham, NC). Paclitaxel was used as a positive control.
2.5 Immunoblotting
Immunoblot was performed following a previous published protocol.6 MCF-7 cells were treated at different concentrations of AmbI (0.008, 0.016, 0.4, 2.0 and 10 μM) for 3 h. The cells were lysed using PhosphoSafe Lysis Buffer (Novagen), and the lysates were analyzed by western blot analysis with primary (1:1000) and secondary antibodies (1:2000). Protein concentration in the lysate was determined using a Bradford protein assay kit with an albumin standard (Thermo Scientific). Absorbance was measured using Fluostar Optima plate reader (BMG Labtech Inc, Durham, NC). Equal amounts of protein (20 μg) were loaded together with LDS sample loading buffer (Invitrogen) and resolved using Nu-PAGE 10% SDS-PAGE Bis-Tris gels together with SeeBlue® Plus2 Pre-Stained Standard (Invitrogen).
Electrophoresis was performed using SDS-PAGE buffer in a Nu-PAGE XCell SureLock Module from Invitrogen. Proteins were transferred to a polyvinyldiene fluoride (PVDF) membrane using transfer buffer. The blots were blocked at room temperature using non-fat milk and probed using primary antibodies against each target protein using BSA in TBS-T overnight. Conjugated antibodies were detected using Chemiluminescent substrates Supersignal Femto kit from Thermo Scientific and relative band densities were determined.
2.6 Cell cycle assay
The MCF-7 cells were seeded into 10 cm dishes and 24h later treated using different concentrations of AmbI. After 24h of incubations, cells were harvested and pelleted by centrifugation, washed with PBS and fixed in ice-cold 70% ethanol. DNA was stained with 10 μg/mL Propidium Iodine (PI) in a reaction solution containing 1 mM EDTA and 100 μg/mL RNase A. Fluorescence emitted from the PI-DNA complex was quantified using BD FACS Canto II (Biosciences, San Jose, CA) at 488 nm.
2.7 XTT Cell Proliferation assay
The intracellular levels of nicotinamide adenine dinucleotide phosphate [NAD(P)H] were assessed using a modified version of a previously published protocol by Nakamura et al. 2003.7 The HT-29 colon cancer cells were seeded (1x104cells/well) in a 96-well plate and allowed to attach overnight. The cells were treated with AmbI (1.3 nM-100 μM) and incubated at 37°C and 5% CO2. Mitochondrial function was indirectly determined by measuring the formation of formazan dye produced by metabolically active cells at one-hour intervals over an 11-hour period. A volume of 100 μL of XTT and 1-methoxy-5-methylphenazidium methyl sulfate (1-methoxy PMS) in RPMI media was added to each well to yield final concentrations of 0.25 mM for XTT and 0.01 μM for 1-methoxy PMS. The absorbance was measured at 485 nm and NAD(P)H depletion was calculated. Staurosporine (20 μM) was used as a positive control. Each sample was tested in triplicate and in two independent experiments.
2.8 Reactive Oxygen Species (ROS) assay
The assay was performed following a previously described procedure.4 The intracellular levels of ROS generated by AmbI were measured using a fluorescent probe, 2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA). MCF-7 cells were seeded in a 96-well plate, treated with AmbI (0.0014-1.4 μM), daunomycin (0.0014-1.4 μM), or DMSO control, followed by 5h incubation at 37ºC with 5% CO2. Subsequently, cells were incubated with H2O2 (1.25 mM) and FeSO4 (0.2 mM) for 30 min at 37ºC. Afterward, the fluorescent probe DCFH-DA was added to determine intracellular ROS. Fluorescence was measured using FLUOstar Optima fluorescence plate reader (BMG Lab technologies GmbH, Inc, Durham, NC USA) at an excitation wavelength of 485 nm and emission wavelength of 530 nm. All treatments were performed in triplicate and are representative of at least two different experiments.
3. RESULTS AND DISCUSSION
AmbI has previously been shown to exhibit antibacterial activity against Mycobacterium tuberculosis as well as antifungal activity.3, 8 AmbI was evaluated in our ongoing screening program aimed at identifying natural products from aquatic and terrestrial organisms that interfere with pathways upregulated in cancer cells. AmbI was identified as a strong NF-κB inhibitor (IC50=30 nM) with comparable potency to the positive control, rocaglamide (IC50=75 nM).
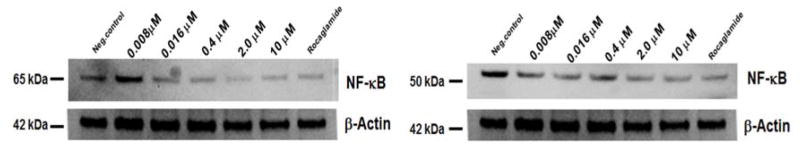
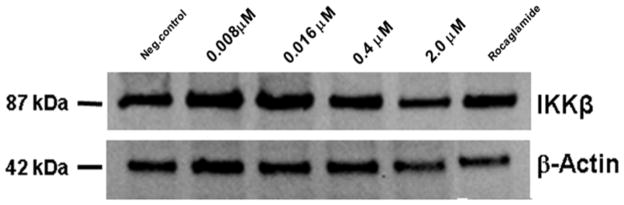
It has been reported that TNF-α induces the up-regulation of genes involved in cell adhesion in estrogen–positive breast cancer cells.9 The activation of the NF-κB cell signaling pathway, triggered by TNF-α, is responsible for tumor progression, cell growth in the metastatic stages of inflammatory breast cancer, and moreover promotes resistance to certain tumorigenic agents and chemotherapy.2 AmbI was also found to have antiproliferative activity in MCF-7 breast cancer cell line using the SRB assay (EC50= 1.7 μM). Furthermore, immunoblot analysis was performed to confirm the inhibitory effect of AmbI on the mediators of the NF-κB pathway (Fig. 1 and 2). The results from the immunoblot analysis showed that both subunits of NF-κB, p65 and p50, were down-regulated in a concentration-dependent manner (Fig. 1). Expression levels of IKKβ were tested at different concentration levels of AmbI (0.01–10 μM). The inhibitory effect was compared to the positive control rocaglamide (10 nM) (Fig. 2) and the western blots and protein expression confirmed p65 NF-κB inhibition.
Figure 1.
Expression of NF-κB p65 and p50 subunits. Transcription factor NF-κB was down-regulated in treated MCF-7 breast-cancer cells when compared with the negative control.
Figure 2.
The expression of kinase IKKκ in MCF-7 cells. The kinase IKKκ was down-regulated in a concentration-dependent manner. Upstream IKK kinases regulate NF-κB activation.
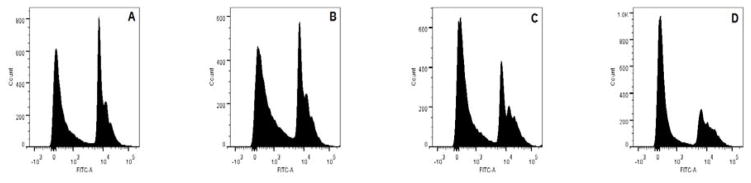
To further investigate the apoptotic effects, cell-cycle analysis was performed using different concentrations of AmbI (0.08 μM, 0.4μM, and 2 μM). Cells treated at the lowest concentration had undergone apoptosis, and 37% of the cells were in sub-G1 phase followed by 71% and 72% at higher doses, respectively (Fig. 3). This suggested that apoptosis was induced in treated cells and that the cell cycle was blocked in G1-phase, similar to results reported for other apoptosis-inducing agents of natural origin.8,10 Thus, AmbI induced apoptosis in a concentration-dependent manner in MCF-7 cells.
Figure 3.
Concentration-dependent induction of DNA fragmentation and apoptosis in estrogen-dependent MCF-7 breast cancer cells treated with AmbI for 15 h. The increasing concentration of AmbI led to an increased cell population in sub-G1 phase. A. At the concentration of 0.0016 μM, 50.8 % of cells were found in sub G1-phase, 26.4% were found in G1-phase and 15.9% were in G2-phase. B. At 0.008 μM, 55.0 % were in sub G1-phase, 24.9% were in G1-phase and 15.9% were in G2-phase. C. At the concentration of 0.4 μM, 60.8 % were in sub G1-phase, 15.8% were in G1-phase and 15.2% were in G2-phase. D. At 2 μM 66.1 % were in sub G1-phase, 12.7% were in G1-phase and 14.8% were in G2-phase.
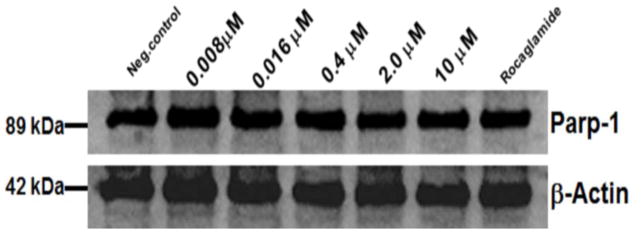
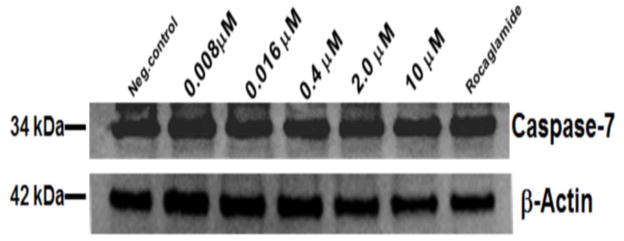
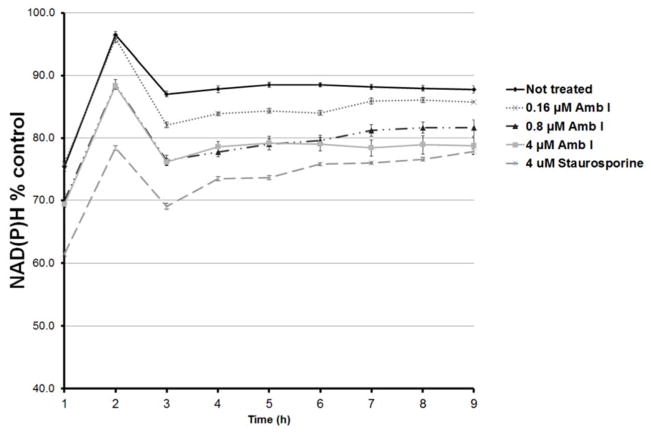
To further investigate the mechanism of action through which AmbI exhibited its cytotoxic effect, it was hypothesized that treated MCF-7 cells undergo caspase-mediated apoptosis. Thus, western blot analysis was performed to evaluate the effect on apoptotic proteins, procaspase-7 andpoly-ADP-polymerase (PARP-1) (Fig. 4 and 5). However, the protein expression in treated MCF-7 cells suggested that treatment with AmbI for 3h did not significantly affect the levels of procaspase-7 in treated cells. Similar findings were detected for levels of downstream protein, PARP-1. Thus, our findings suggested that treatment with AmbI induced and mediated apoptosis through a caspase-independent pathway in MCF-7 cells. Similar findings have been previously published for other drug leads.11–13 We also tested the activity of cytokine TNF-α and found that AmbI exerted NF-κB inhibitory activity, hence possibly acting in concert with p53 and allowing the p53 anti-tumor effect to trigger apoptosis in MCF-7 breast cancer cells. According to a previous study, compensatory caspase-activation might be mediated by the mitochondria.11 Mitochondria is the largest source of NAD(P)H. The effect of AmbI on the mitochondria and on the levels of NAD(P)H was examined. NAD(P)H levels were found to have decreased in treated cells, suggesting that the formation of NAD(P)H was affected in the mitochondria. The real-time XTT assay showed that the levels of NAD(P)H decreased after 3h of treatment (Fig. 6). Furthermore, a moderate concentration-dependent increment of ROS was detected after 5h in treated cells, and produced as a consequence of the damage caused to the electron transfer in the mitochondria (Fig. 7). Together these findings strongly suggested that apoptosis by AmbI was induced through a non-caspase dependent pathway in MCF-7 cells. It appears that AmbI induced apoptosis through a different mechanism of action, where the mitochondrial dysfunction appears to play a key role in preceding apoptosis. The presented data shows that caspase activity may not be essential for apoptosis in MCF-7 cells, and that compensatory pathways appear to be mediated through the mitochondria. Previous reports14–16, suggested that calpains were the major proteases triggering programmed cell death in treated cells through non-caspase dependent pathway. This mechanism could possibly be mediated by the release of Ca2+ from the endoplasmatic reticulum as previously described by Mathiasen and co-workers.15 However, no previous studies have found an association between the loss of the mitochondrial transmembrane potential and the release of Ca2+ in MCF-7 cells, thus the study of alternative caspase-independent pathway that trigger cells death remain to be explored in future studies.
Figure 4.
Western blot analysis of Parp-1 expression in treated MCF-7 breast cancer cells. No significant difference in the expression of Parp-1 was found in MCF-7 treated cells at different levels of AmbI.
Figure 5.
Western blot analysis of caspase-7 expression in treated MCF-7 breast cancer cells. No significant difference in expression of caspase-7 was found when treated at different levels of AmbI.
Figure 6.
Mitochondrial function in MCF-7 cells measured using the real-time XTT assay. Levels of NAD(P)H were monitored over a period of 12 h. Increasing concentrations of AmbI decreased the amount of NAD(P)H observed and the changes were significant when compared to the non-treated cells. Different concentrations of AmbI (0.16– 4μM) were also compared with the effects of the positive control staurosporine (4μM).
Figure 7.
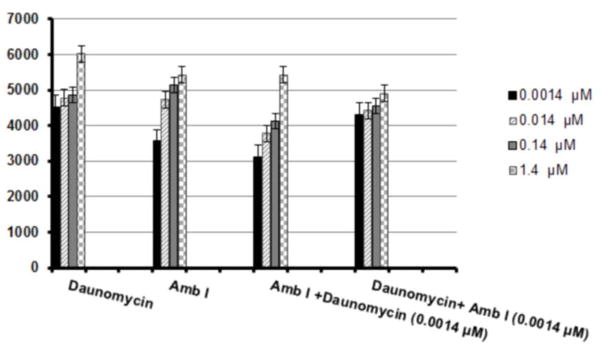
Intracellular levels of reactive oxygen species (ROS) formed in MCF-7 cancer cells after 5 h of treatment with daunomycin and AmbI. The cells were treated at four different concentration levels and the formation of ROS was detected with fluorescent probe DCFH-DA. Although the ROS inducing effect was not as potent for AmbI as the highest dose of daunomycin (1.4 μM), a synergistic effect was observed when hormone-dependent breast cancer MCF-7 cells were treated with the combination of daunomycin and the lowest concentration of AmbI (0.0014 μM).
Recent findings have shown that pharmacological inhibition of NF-κB causes cells to undergo TNF-α dependent ROS-mediated programmed cell death in antigen presenting cells.18, 19 Consequently, the effect of AmbI on the formation of ROS in MCF-7 cell was also evaluated. Although minor concentration dependent increases of ROS were detected in AmbI treated cells, the results presented suggested that the MCF-7 cancer cells are possibly more susceptible to oxidative stress, and thus targeting the redox state of estrogen sensitive MCF-7 cancer cells is a possible avenue to induce cell death. The evaluation of the intracellular levels of ROS and possible synergistic effect obtained from the combination of two pharmacological agents was calculated following the previously presented method by Chou-Talalay.20 Although the ROS inducing effects were not significantly higher (p>0.05) for the combination treatment (Fig. 7), the results suggested that synergistic effect was displayed between NF-κB inhibiting agents and DNA-intercalating agents, such as daunomycin, to mediate ROS inducing activity through independent pathways. A combination index CI<1 showed that AmbI at a concentration of 0.0014 μM synergized with increasing concentrations of daunomycin. Treatment with AmbI at the concentration of 0.0014 μM, in combination with a low dose of daunomycin (0.0014 μM) increased the amount of ROS produced from 23% to 53%, in treated MCF-7 cells. The synergistic effects suggested that combination treatments employing NF-κB inhibiting agents may increase the efficacy of existing treatments. These data leads to valuable insight in the development of more effective chemotherapy regimens. Thus, identifying anticancer agents that increase the susceptibility of cancer cells to undergo programmed cell death and induce ROS mediated apoptosis are a strategic approach to find new chemotherapeutic agents that sensitize cell for current for drug therapies.
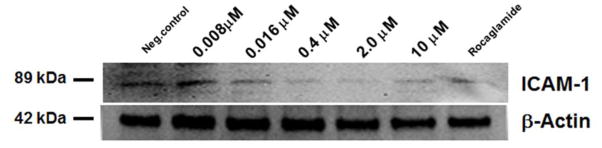
Cellular adhesion molecules are involved in the metastasis of cancer. Higher levels of intracellular cell adhesion protein (ICAM-1) have been detected in the serum of patients with malignant diseases e.g. breast cancer and gastrointestinal cancer.21 This suggests that cellular adhesion molecules are involved in the progression of the disease. It has also previously been reported that TNF-α induced the up-regulation of genes involved in cell adhesion in MCF-7 cancer cell line.22 Herein we investigated AmbI downstream effect of NF-κB inhibition on intracellular ICAM-1 (Fig. 8). The immunoblot analysis of ICAM-1 was tested in TNF-α treated cells at different concentrations of AmbI. The results showed that AmbI and suppression of NF-κB-dependent transcriptional activation had an effect on TNF-α induced ICAM-1 expression. Similarly, the inhibited expression of ICAM-1 has been reported in HT-29 colon cancer cells when treated with anti-inflammatory agents and selective COX-2 inhibitor celecoxib, suggesting that this treatment might have chemopreventive effects particularly against colorectal cancer.23, 24 Agents that block ICAM-1, such as AmbI, can have protective effects against cancer development and interfere in the migration and invasion of cancer cells. Blocking of this membrane bound protein might represent a new targeted anticancer therapy that only affect cancer cells, since the adhesion molecule ICAM-1 is an immunoglobulin membrane bound glycoprotein that is not expressed in normal epithelial tissue and appears to play a significant role in the angiogenesis, and vascularization of tumors.25
Figure 8.
Intracellular levels of ICAM-1 in TNF-α treated MCF-7 cancer cells. Cells were treated at five different concentration levels of AmbI. Down-regulation of ICAM-1 expression was observed with increasing concentration of AmbI.
MCF-7 breast cancer cells exhibit reduced sensitivity to a variety of anti-cancer drugs, due to lack of apoptosis inducing caspase-3 mediation and activity. Thus, further understanding of the mechanism of action of AmbI on estrogen sensitive MCF-7 cells could reveal and identify significant targets that initiate cell-death in chemoresistant cells. Our findings also suggested that NF-κB inhibition displayed a significant role in promoting cell death in treated MCF-7 estrogen sensitive breast cancer cells. Furthermore, this implied that AmbI promoted the effect of standard chemotherapeutic agents in the treatment of hormone-dependent breast cancer, particularly in chemoresistant types, by interfering with the NF-κB pathway. Chemical derivatives of ambiguine-type compounds have previously been successfully attempted,17 suggesting that in future studies synthetic derivatives might lead to insight into the structure-activity relationship of these indole alkaloids and the mechanism of NF-κB inhibition as potential new agents for the treatment of breast cancer.
4. CONCLUSION
In summary, the study showed that the indole alkaloids from cyanobacteria, such as AmbI, show promising antiproliferative activity against estrogen-sensitive breast cancer cells. AmbI display potent NF-κB inhibiting activity. When tested using estrogen-sensitive MCF-7 cells, treatment with AmbI prompted mitochondrial dysfunction and induced increased levels of ROS. This was followed by cell death through a caspase-independent pathway. The results of the present study, therefore, suggest that AmbI could potentially be used as a chemotherapeutic agent for tumors resistant to chemotherapeutic agents dependent on the classical caspase cascade.
Acknowledgments
We greatly acknowledge the financial support from the program project grant P01 CA125066 and P01 CA125066-S2 from the National Cancer Institute, NIH, Bethesda, MD to carry out this work. The authors would like to thank Drs. Mark E. Drew and Dawn Walker for facilitating the use of the BD FACS Canto II instrument.
Footnotes
CONFLICT OF INTEREST
No conflict of interest was disclosed by the authors.
Contributor Information
Ulyana Muñoz Acuña, Division of Pharmacy Practice and Administration and Division of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, The Ohio State University, 500 W. 12th Avenue, Columbus, OH 43210.
Jiachen Zi, Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, 833 S. Wood St., Chicago, IL 60612.
Jimmy Orjala, Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, 833 S. Wood St., Chicago, IL 60612.
Esperanza J. Carcache de Blanco, Division of Pharmacy Practice and Administration and Division of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, The Ohio State University, 500 W. 12thAvenue, Columbus, OH 43210
References
- 1.Darnell JE., Jr Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2(10):740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 2.Lerebours F, Vacher S, Andrieu C, Espie M, Marty M, Lidereau R, Bieche I. NF-kappaB genes have a major role in inflammatory breast cancer. BMC Cancer. 2008;8:e41. doi: 10.1186/1471-2407-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mo S, Krunic A, Chlipala GE, Orjala J. Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J Nat Prod. 2009;72(5):894–899. doi: 10.1021/np800751j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JA, Lau EK, Pan L, Carcache de Blanco EJ. NF-kappaB inhibitors from Brucea javanica exhibiting intracellular effects on reactive oxygen species. Anticancer Res. 2010;30(9):e3295–3300. [PMC free article] [PubMed] [Google Scholar]
- 5.Pan L, Kardono LB, Riswan S, Chai H, Carcache de Blanco EJ, Pannell CM, Soejarto DD, McCloud TG, Newman DJ, Kinghorn AD. Isolation and characterization of minor analogues of silvestrol and other constituents from a large-scale re-collection of Aglaia foveolata. J Nat Prod. 2010;73(11):1873–1878. doi: 10.1021/np100503q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muñoz Acuña U, Wittwer J, Ayers S, Pearce CJ, Oberlies NH, Carcache de Blanco EJ. Effects of (5Z)-7- oxozeaenol on the oxidative pathway of cancer cells. Anticancer Res. 2012;32(7):2665–2671. [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura J, Asakura S, Hester SD, de Murcia G, Caldecott KW, Swenberg JA. Quantitation of DNA single strand break repair in base excision repair deficient cells in real time. Nucleic Acids Res. 2003;31(17):e104. doi: 10.1093/nar/gng105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raveh A, Carmeli S. Antimicrobial ambiguines from the cyanobacterium Fischerella sp. collected in Israel. J Nat Prod. 2007;70(2):e196–201. doi: 10.1021/np060495r. [DOI] [PubMed] [Google Scholar]
- 9.Deng Y, Balunas MJ, Kim JA, Lantvit DD, Chin YW, Chai H, Sugiarso S, Kardono LB, Fong HH, Pezzuto JM, Swanson SM, de Blanco EJ, Kinghorn AD. Bioactive 5,6-dihydro-alpha-pyrone derivatives from Hyptis brevipes. J Nat Prod. 2009;72(6):1165–1169. doi: 10.1021/np9001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou CC, Wu YC, Wang YF, Chou MJ, Kuo SJ, Chen DR. Capsaicin-induced apoptosis in human breast cancer MCF-7 cells through caspase-independent pathway. Oncol Rep. 2009;21(3):665–671. [PubMed] [Google Scholar]
- 11.Zheng TS, Hunot S, Kuida K, Momoi T, Srinivasan A, Nicholson DW, Lazebnik Y, Flavell RA. Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nat Med. 2000;6(11):1241–1247. doi: 10.1038/81343. [DOI] [PubMed] [Google Scholar]
- 12.Mooney LM, Al-Sakkaf KA, Brown BL, Dobson PR. Apoptotic mechanisms in T47D and MCF-7 human breast cancer cells. Br J Cancer. 2002;87(8):909–917. doi: 10.1038/sj.bjc.6600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Teruya K, Eto H, Shirahata S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS One. 2011;6(11):e27441. doi: 10.1371/journal.pone.0027441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathiasen IS, Lademann U, Jäättelä M. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res. 1999;5919:4848–4856. [PubMed] [Google Scholar]
- 15.Mathiasen IS, Sergeev IN, Bastholm L, Elling F, Norman AW, Jäättelä M. Calcium and calpain as key mediators of apoptosis-like death induced by vitamin D compounds in breast cancer cells. J Biol Chem. 2002;277(34):30738–30745. doi: 10.1074/jbc.M201558200. [DOI] [PubMed] [Google Scholar]
- 16.Mathiasen IS, Jäättelä M. Triggering caspase-independent cell death to combat cancer. Trends Mol Med. 2002;8(5):212–220. doi: 10.1016/s1471-4914(02)02328-6. [DOI] [PubMed] [Google Scholar]
- 17.Shenvi RA, O'Malley DP, Baran PS. Chemoselectivity: the mother of invention in total synthesis. Acc Chem Res. 2009;42(4):530–541. doi: 10.1021/ar800182r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilstra JS, Gaddy DF, Zhao J, Davé SH, Niedernhofer LJ, Plevy SE, Robbins PD. Pharmacologic IKK/NF-κB inhibition causes antigen presenting cells to undergo TNFα dependent ROS-mediated programmed cell death. Sci Rep. 2014;4:3631–3641. doi: 10.1038/srep03631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai Y, Rahmani M, Dent P, Grant S. Blockade of histone deacetylase inhibitor-induced RelA/p65 acetylation and NF-kappaB activation potentiates apoptosis in leukemia cells through a process mediated by oxidative damage, XIAP downregulation, and c-Jun N-terminal kinase 1 activation. Mol Cell Biol. 2005;25:5429–5444. doi: 10.1128/MCB.25.13.5429-5444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Banks RE, Gearing AJ, Hemingway IK, Norfolk DR, Perren TJ, Selby PJ. Circulating intercellular adhesion molecule-1 (ICAM-1), E-selectin and vascular cell adhesion molecule-1 (VCAM-1) in human malignancies. Br J Cancer. 1993;68(1):122–124. doi: 10.1038/bjc.1993.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin Y, Chen X, Shu Y. Gene expression of the invasive phenotype of TNF-alpha-treated MCF-7 cells. Biomed Pharmacother. 2009;63(6):421–428. doi: 10.1016/j.biopha.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Dianzani C, Brucato L, Gallicchio M, Rosa AC, Collino M, Fantozzi R. Celecoxib modulates adhesion of HT-29 colon cancer cells to vascular endothelial cells by inhibiting ICAM-1 and VCAM-1 expression. Br J Pharmacol. 2008;153(6):1153–1161. doi: 10.1038/sj.bjp.0707636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallicchio M, Rosa AC, Dianzani C, Brucato L, Benetti E, Collino M, Fantozzi R. Celecoxib decreases expression of the adhesion molecules ICAM-1 and VCAM-1 in a colon cancer cell line (HT29) Br J Pharmacol. 2008;153(5):870–878. doi: 10.1038/sj.bjp.0707634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park JS, Kim KM, Kim MH, Chang HJ, Baek MK, Kim SM, Jung YD. Resveratrol inhibits tumor cell adhesion to endothelial cells by blocking ICAM-1 expression. Anticancer Res. 2009;29(1):355–362. [PubMed] [Google Scholar]