Abstract
Seven new 17-norpimarane and (9βH)-17-norpimarane diterpenoids, icacinlactones A-G (1-7), were isolated from the tuber of Icacina trichantha. The structures were elucidated by spectroscopic and HRMS techniques, and the absolute configuration of 2 was determined by means of X-ray crystallographic analysis. Compounds 1-7, as well as four known related structures, were evaluated for cytotoxic activity against MDA-MB-435 (human melanoma cancer), MDA-MB-231 (human breast cancer), and OVCAR3 (human ovarian cancer) cell lines. Several of these natural products displayed significant cytotoxic activity, with humirianthenolide C being most active.
Icacina trichantha Oliv. (Icacinaceae) is a traditional herbal medicine used in Nigeria and other regions of western Africa.1 The tuber of this plant is often prescribed by herbalists for the treatment of food poisoning, constipation, and malaria;1 and the macerated tuber in alcohol is a common household first-aid medicine for emergency treatment of food poisoning.2 Recent studies on I. trichantha have revealed its antihyperglycemic, anticonvulsion, sedative, analgesic, and antimicrobial properties.3-5 In a previous study on this plant, five cytotoxic diterpenoids (17-hydroxyicacinol, icacinol, humirianthol, humirianthenolide C, and icacenone), belonging to the small subclasses of (9βH)-pimarane and (9βH)-17-norpimarane, were isolated.6 Further investigation now led to the purification of seven new 17-norpimaranes and (9βH)-17-norpimaranes. In the present report, the isolation, structural elucidation, and cytotoxic properties of these diterpenoids are described.
RESULTS AND DISCUSSION
The 80% aqueous MeOH extract of the tubers of I. trichantha was successively partitioned into petroleum ether, EtOAc, and n-BuOH fractions. Seven new diterpenoid lactones, icacinlactones A-G (1-7), were purified from the EtOAc and n-BuOH fractions.
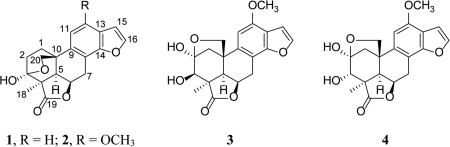
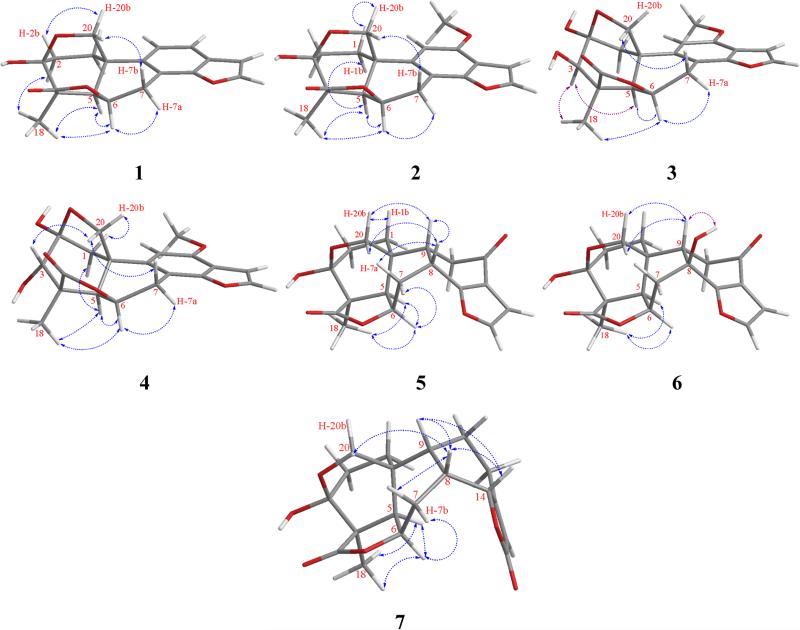
Icacinlactone A (1) was obtained as white powder. HRESIMS showed a quasi-molecular ion at m/z 327.1206 [M + H]+ (cacld for C19H19O5, 327.1232), suggesting a molecular formula of C19H18O5 with 11 indices of hydrogen deficiency when the 13C NMR spectroscopic data are taken into consideration. The IR absorption at 1750 cm−1 indicated the presence of a γ-lactone moiety. The 1H NMR spectrum displayed four olefinic protons at δH 7.75 (d, J = 2.2 Hz, H-16), 7.49 (dd, J = 0.9, 8.1 Hz, H-12), 7.23 (d, J = 8.2 Hz, H-11), and 6.81 (d, J = 2.2 Hz, H-15), and one methyl group at δH 1.44 (s, CH3-18) (Table 1). The 13C NMR spectrum exhibited 19 carbon signals corresponding to a methyl, four methylenes (including an oxygenated one), six methines (four olefinic and two aliphatic), a dioxygenated secondary carbon, an oxygenated tertiary carbon, a carbonyl carbon, and five quaternary carbons. (Table 3). All proton signals were assignable to their attached carbons through an HSQC experiment (Table 1). Based on the 1H-1H COSY data, four coupled spin systems corresponding to H-1 (δH 2.75, 1.90) / H-2 (δH 2.28, 2.05), H-5 (δH 2.37) / H-6 (δH 5.19) / H-7 (δH 4.22, 3.03), H-11 (δH 7.23) / H-12 (δH 7.49), and H-15 (δH 6.81) / H-16 (δH 7.75) were unambiguously established, allowing the assignment of the connectivities of these fragments (Figure 1). Interpretation of the HMBC data then led to the proposed structure of 1. It is noteworthy that the deshielded dioxygenated secondary carbon at δC 98.2 (C-3) displayed long-range correlations with both H-1 and H-2. On the other hand, the methylene protons (δH 4.12, 3.77) exhibited HMBC correlations with C-1 (δC 30.9), C-5 (δC 52.1), and C-10 (δC 36.2). At the same time, H-20a (δH 4.12) exhibited HMBC correlation with C-3 (δC 98.2). All the above evidence indicated the presence of a 3,20-epoxy bridge. Indeed, the chemical shift of C-3 (δC 98.2) was in agreement with a hemiketal structure. The other parts of the molecule were established as follows. The connection between the A- and B-rings was confirmed by HMBC correlations observed for C-10 and H-1a, H-2, H-5, and H-6. The γ-lactone moiety was established first by assigning the quaternary carbon at δC 50.9 to C-4 based on its HMBC correlations with H-2b and H-5. Other carbons in this part of the molecule, including C-3(δC 98.2), C-4 (δC 50.9), C-5 (δC 52.1), and C-19 (δC 181.7), all showed long-range correlations with CH3-18 (δH 1.44). Furthermore, the benzofuran part was proposed based on the observations of the following HMBC cross signals: H-11 with C-8 / C-13, H-12 with C-14 / C-15, and H-16 with C-13 / C-14. All available evidence thus led to the determination of the structure of 1 as depicted. The relative configuration of 1 was then assigned on the basis of NOESY analysis, in which the following key correlations were clearly observed: H-5 (δH 2.37) with H-6 (δH 5.19) and H-18 (δH 1.44), H-6 with H-7a (δH 4.22) and H-18, between H-18 and H-2a (δH 2.28), between H-2b (δH 2.05) and H-20b (δH 3.77), as well as between H-7b (δH 3.03) and H-20a (δH 4.12) (Figure 2). Consequently, the new 17-norpimarane (1) was elucidated to be 3β,20:14,16-diepoxy-3α-hydroxy-17-norpimar-8(9),11,13(14),15-tetraen-19,6β-olide, and given the trivial name icacinlactone A.
Table 1.
1H (400 MHz) NMR Spectroscopic Data for Compounds 1-4 (δ in ppm, J in Hz)a
| 1b |
2c |
3c |
4b |
|
|---|---|---|---|---|
| position | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) |
| 1 | 2.75, ddd (4.5, 12.4, 12.4) | 2.68, ddd (4.8, 12.1, 12.18) | 2.92, d (11.8) | 2.45, dd (0.8, 11.5) |
| 1.90, dddd (4.1, 4.2, 12.2, 12.4 ) | 1.88, dddd (4.2, 4.4, 12.2, 12.5) | 1.86, dd (1.5, 11.8) | 2.31, dd (1.6, 11.5) | |
| 2 | 2.28, ddd (4.5, 12.0, 14.0) | 2.21, md | ||
| 2.05, ddd (4.5, 12.1, 14.0) | - | - | ||
| 3 | - | - | 3.75, d (11.0) | 3.95, d (1.0) |
| 5 | 2.37, dd (2.2, 8.0) | 2.26, dd (2.2, 8.0)d | 2.24, dd (1.3, 5.8) | 2.19, dd (1.1, 5.8) |
| 6 | 5.19, ddd (5.7, 8.0, 9.7) | 5.11, ddd (6.0, 8.1, 9.7) | 5.17, ddd/br quintet-like (5.1, 5.1, 10.2) | 5.21, ddd/br quintet-like (4.9, 5.7, 10.2) |
| 7 | 4.22, dd (9.7, 17.3) | 4.15, dd (9.9, 17.2) | 4.05, dd (9.9, 17.4) | 4.03, dd (9.9, 17.3) |
| 3.03, dd (5.6, 17.2) | 2.98, dd (5.9, 17.1) | 2.98, dd (4.8, 17.4) | 2.93, dd (4.7, 17.3) | |
| 11 | 7.23, d (8.2) | 6.57, s | 6.52, s | 6.65, s |
| 12 | 7.49, dd (0.9, 8.1) | - | - | - |
| 15 | 6.81, d (2.2) | 6.83, d (2.2) | 6.84, d (2.2) | 6.84, d (2.2) |
| 16 | 7.75, d (2.2) | 7.53, d (2.2) | 7.52, d (2.2) | 7.64, d (2.2) |
| 18 | 1.44, s | 1.44, s | 1.60, s | 2.14, s |
| 20 | 4.12, dd (3.7, 9.6) | 4.14, dd (3.7, 9.7) | 4.25, dd (1.5, 9.0) | 4.18, dd (1.6, 8.6) |
| 3.77, dd (2.2, 9.6) | 3.82, dd (2.2, 9.7) | 3.76, dd (1.5, 9.0) | 3.54, dd (1.5, 8.6) | |
| OCH3 | - | 3.92, s | 3.91, s | 3.94, s |
| 2-OH | - | - | 4.29, s | - |
| 3-OH | - | - | 5.01, d (10.6) | - |
For CH2, the deshielded signal was assigned as Ha, and the shielded signal as Hb.
Data measured in methanol-d4.
Data measured in CDCl3.
Signal was partially obscured.
Table 3.
13C (100 MHz) NMR Spectroscopic Data for Compounds 1-7 (δ in ppm)
| 1a |
2b |
3b |
4a |
5a |
6a |
7b |
|
|---|---|---|---|---|---|---|---|
| position | δC, type | δC, type | δC, type | δC, type | δC, type | δC, type | δC, type |
| 1 | 30.9, CH2 | 29.7, CH2 | 40.3, CH2 | 37.9, CH2 | 31.0, CH2 | 31.4, CH2 | 30.4, CH2 |
| 2 | 28.4, CH2 | 26.2, CH2 | 103.9, C | 108.0, C | 29.4, CH2 | 29.5, CH2 | 27.1, CH2 |
| 3 | 98.2, C | 96.9, C | 81.3, CH | 75.3, CH | 97.8, C | 97.3, C | 96.7, C |
| 4 | 50.9, C | 49.1, C | 44.2, C | 48.8, C | 53.3, C | 52.3, C | 51.5, C |
| 5 | 52.1, CH | 51.4, CH | 54.5, CH | 52.8, CH | 45.5, CH | 47.0, CH | 45.7, CH |
| 6 | 75.9, CH | 75.0, CH | 74.7, CH | 76.4, CH | 75.8, CH | 75.1, CH | 74.8, CH |
| 7 | 26.9, CH2 | 25.7, CH2 | 24.7, CH2 | 25.7, CH2 | 28.0, CH2 | 35.4, CH2 | 19.4, CH2 |
| 8 | 118.2, C | 109.6, C | 109.2, C | 110.7, C | 28.8, CH | 69.2, C | 32.8, CH |
| 9 | 135.0, C | 134.3, C | 133.8, C | 136.5, C | 39.4, CH | 47.6, CH | 37.2, CH |
| 10 | 36.2, C | 35.5, C | 48.0, C | 48.8, C | 33.5, C | 34.6, C | 33.1, C |
| 11 | 120.4, CH | 100.1, CH | 98.7, CH | 99.9, CH | 35.2, CH2 | 38.8, CH2 | 20.9, CH2 |
| 12 | 120.4, CH | 152.3, C | 152.5, C | 153.8, C | 195.2, C | 194.9, C | 26.4, CH2 |
| 13 | 127.9, C | 116.7, C | 117.0, C | 117.9, C | 120.9, C | 121.0, C | 167.1, C |
| 14 | 154.4, C | 154.2, C | 154.1, C | 155.6, C | 172.5, C | 169.9, C | 82.4, CH |
| 15 | 107.7, CH | 104.3, CH | 104.4, CH | 105.2, CH | 107.1, CH | 106.9, CH | 114.6, CH |
| 16 | 146.8, CH | 144.0, CH | 143.9, CH | 145.2, CH | 145.3, CH | 146.1, CH | 173.0, C |
| 18 | 20.0, CH3 | 20.0, CH3 | 22.7, CH3 | 20.9, CH3 | 17.4, CH3 | 18.2, CH3 | 17.6, CH3 |
| 19 | 181.7, C | 180.6, C | 180.1, C | 181.9, C | 180.8, C | 180.8, C | 179.6, C |
| 20 | 72.6, CH2 | 71.2, CH2 | 72.2, CH2 | 72.2, CH2 | 73.9, CH2 | 75.3, CH2 | 73.0, CH2 |
| OCH3 | - | 55.7, CH3 | 55.7, CH3 | 56.2, CH3 | - | - | - |
Data measured in methanol-d4.
Data measured in CDCl3.
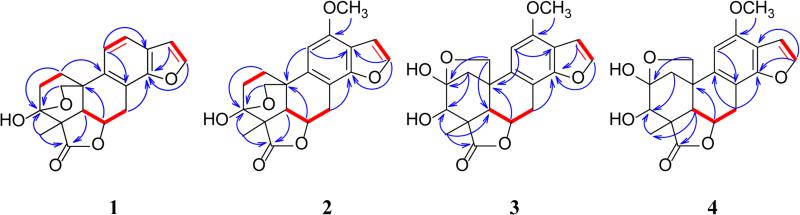
Figure 1.
1H-1H COSY and selected HMBC correlations for 1-7
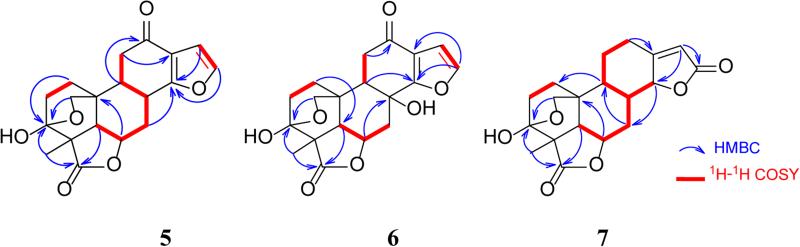
Figure 2.
Selected NOESY correlations for 1-7
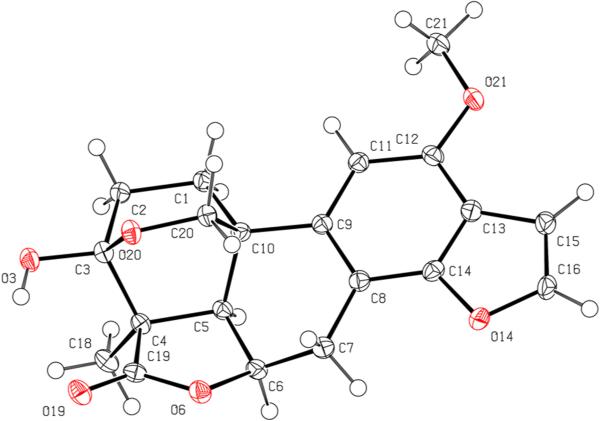
Icacinlactone B (2) was obtained as white powder. The molecular formula C20H20O6 was deduced from 13C NMR spectroscopic data and HRESIMS data (m/z 357.1309 [M +H]+, calcd for C20H21O6, 357.1338), implying 11 indices of hydrogen deficiency. Similar to 1, the IR absorption at 1752 cm−1 suggested the presence of a γ-lactone moiety. A comparison of the 1H and 13C NMR data revealed that 2 was a methoxy derivative of 1 (Tables 1 and 3). The location of the OCH3 group (δH 3.92, δC 55.7) at C-12 (δC 152.3) was determined by the HMBC correlation between the methoxy protons and C-12 (Figure 1). Indeed, in comparison with 1, significant shielding of C-8 (−8.6 ppm), C-11 (−20.3 ppm), and C-13 (−11.2 ppm), with a concomitant deshielding of C-12 (+31.9 ppm), supported the assignment based upon the resonance and inductive effects of the 12-OCH3 subsituent. The relative configuration of 2 was then proposed on the basis of NOESY analysis (Figure 2). Finally, crystals of 2 were available from a mixture of MeOH and EtOAc, and its absolute configuration was determined by means of X-ray diffraction analysis assisted by a CCD area detector using a CuKα X-ray source (Figure 3). The structure of the new 17-norpimarane (2) was thus elucidated to be (3S, 4R, 5R, 6R, 10R)-3β,20:14,16-diepoxy-3α-hydroxy-12-methoxy-17-norpimar-8(9),11,13(14),15-tetraen-19,6β-olide, and given the trivial name icacinlactone B.
Figure 3.
ORTEP representation of 2
Icacinlactone C (3) was obtained as white powder. It has a molecular formula of C20H20O7 as determined by 13C NMR spectroscopic data and HRESIMS data (m/z 395.1088 [M + Na]+; calcd for C20H20O7Na, 395.1107). The presence of a γ-lactone moiety was indicated by the IR absorption at 1750 cm−1. The 1H NMR spectrum displayed two olefinic doublets at δH 7.52 (J = 2.2 Hz, H-16), and 6.84 (J = 2.2 Hz, H-15), an olefinic singlet at δH 6.52 (H-11), a methoxy singlet at δH 3.91, and a methyl singlet at δH 1.60 (CH3-18). The corresponding carbons were identified by the HSQC analysis (Table 3). Among the proton signals, two D2O-exchangable signals at δH 4.29 (s) and δH 5.01 (d, J = 10.6 Hz) were assigned to 2-OH and 3-OH, respectively, on the basis of HMBC correlation data. A comparison between the 13C NMR data of 2 and 3 revealed high similarity, the major difference being detected in the A-ring (Table 3). In contrast to 1 and 2, in which the CH3-18 protons had long-range coupling with the C-3 hemiketal carbon (δC > 96), the CH3-18 of 3 correlated to an oxygenated methine (δC 81.3), that was assigned to C-3. Instead, H-1a (δH 2.92) and H-20a (δH 4.25) displayed long-range correlations with the hemiketal carbon at δC 103.9 (C-2), suggesting the presence of an oxygen bridge between C-2 and C-20. The relative configuration of 3 was proposed on the basis of NOESY analysis. Thus, correlations from CH3-18 (δH 1.60) to H-5 (δH 2.24) and H-6 (δH 5.17), from H-6 to H-5, H-7a (δH 4.05), and CH3-18, as well as between H-20a (δH 4.25) and H-7b (δH 2.98), indicated the α-orientation of H-5, H-6, and 4-CH3, and the β-orientation of the 2,20-epoxy bridge (Figure 2). Owning to partial overlapping of the H-3 (δH 3.75) and H-20b (δH 3.76) signals in CDCl3, unambiguous assignment of NOESY cross peaks was not possible. By changing to the methanol-d4 solvent, however, the H-3 signal was well resolved at δH 3.78. Irradiation of this signal resulted in signal enhancement of 5α-H (δH 2.35) and 4α-CH3 (δH 1.58), indicating an α-orientation of H-3. The above evidence led to the proposed structure of the new 17-norpimarane (3) as 2β,20:14,16-diepoxy-3β-hydroxy-12-methoxy-17-norpimar-8(9),11,13(14),15-tetraen-19,6β-olide (icacinlactone C).
Icacinlactone D (4) was obtained as white powder. It has the same molecular formula (C20H20O7) as that of 3 according to the 13C NMR spectroscopic data and HRESIMS data (395.1082 [M + Na]+ (calcd for C20H20O7Na, 395.1107). Its 13C NMR data (Table 3) were similar to those of 3, with major difference observed for the 13C NMR chemical shifts of C-2 (+4.1 ppm) and C-3 (−6.0 ppm), indicating a possible configurational variation. The NOESY experiment failed when methanol-d4 was used as solvent due to partial overlapping of the H-3 (δH 3.95, d, J = 1.0 Hz) and OCH3 (δH 3.94) signals. In a mixed solvent containing CDCl3-methanol-d4 (100:1), however, the H-3 signal could be observed at δH 4.14 and the following NOESY correlations were recorded: H-5 (δH 2.20) with H-1b (δH 2.39) and H-18 (δH 1.41); H-6 (δH 5.12) with H-5, H-7a (δH 4.03), and H-18; H-3 with H-20a (δH 4.23); H-20a with H-7b (δH 2.93); as well as H-20b with H-1a (δH 2.46). These NOESY results supported the assignment of the relative configuration of 4 as shown, differing from 3 only at C-3. The structure of compound 4 was thus elucidated as the new 17-norpimarane, 2β,20:14,16-diepoxy-3α-hydroxy-12-methoxy-17-norpimar-8(9),11,13(14),15-tetraen-19,6β-olide, and given a trivial name icacinlactone D.
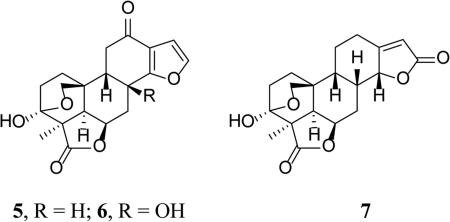
Icacinlactone E (5) was obtained as white powder. A molecular formula of C19H20O6 possessing 10 indices of hydrogen deficiency was deduced from the 13C NMR spectroscopic data and the quasi-molecular ion peak at m/z 345.1302 [M + H]+ (calcd for C19H21O6, 345.1338) in its HRESIMS. The IR spectrum displayed two strong absorptions at 1761 cm−1 and 1660 cm−1, assignable to γ-lactone and conjugated carbonyl moieties, respectively. The 1H NMR spectrum revealed two olefinic doublets at δH 7.53 (J = 2.0 Hz, H-16) and 6.65 (J = 2.0 Hz, H-15), and a methyl singlet at δH 1.36 (CH3-18) (Table 2). The 13C NMR and DEPT spectra contained nineteen carbon signals, including a methyl, five methlyenes, six methines, a dioxygenated secondary carbon, an oxygenated tertiary carbon, two carbonyl carbons, and three quaternary carbons. The 1H-1H COSY spectrum (Figure 1) displayed three spin systems as evidenced by the following correlations: between H-1b (δH 1.65) and H-2a (δH 2.10); between H-5 (δH 2.32) and H-6 (δH 4.79); between H-6 and H-7b (δH 1.94); between H-7 (δH 2.54, 1.94) and H-8 (δH 3.26), between H-9 (δH 2.20) and H-11 (δH 2.59, 2.40), as well as between H-15 (δH 6.65) and H-16 (δH 7.53). The connectivities of C-1/C-2, C-5/C-6/C-7/C-8/C-9/C-11, and C-15/C-16 were established with the aid of HSQC results. In the 13C NMR spectrum, the presence of a carbonyl carbon at δC 180.8 (C-19), a dioxygenated secondary carbon at δC 97.8 (C-3), an oxygenated methine carbon at δC 75.8 (C-6), an oxygenated methylene carbon at δC 73.9 (C-20), as well as a methyl at δC 17.4 (C-18), indicated 5 possessed a 19,6-γ-lactone and a 3,20-epoxy bridge as in 1 and 2 (Figure 1). The carbonyl carbon at δC 195.2 was assigned to C-12 since both H-9 (δH 2.20) and H-11 (δH 2.59 and 2.40) correlated to it in the HMBC spectrum. The assignments of C-13 (δC 120.9) and C-14 (δC 172.5) were supported by the following HMBC correlations: between H-7a (δH 2.54) and C-14, between H-11b (δH 2.40) and C-13, and between H-8 (δH 3.26) and C-13 / C-14. On the other hand, the proton signals at δH 6.65 and 7.53 correlated with each other in the 1H-1H COSY, and both exhibited HMBC correlations with C-14, leading to the assignments of H-15 (δH 6.65) / C-15 (δC 107.1) and H-16 (δH 7.53) / C-16 (δC 145.3), as well as confirming the connectivity of C-14 and C-16 via an oxygen atom. The structure of 5 was therefore determined as shown, and the relative configuration could be deduced from the NOESY data (Figure 2). Consequently, the structure of compound 5 was elucidated as the new (9βH)-17-norpimarane, 3β,20:14,16-diepoxy-3α-hydroxy-12-oxo-(9βH)-17-norpimar-13(14),15-dien-19,6β-olide, and given a trivial name icacinlactone E.
Table 2.
1H (400 MHz) NMR Spectroscopic Data for Compounds 5-7 (δ in ppm, J in Hz)a
| 5b |
6b |
7c |
|
|---|---|---|---|
| position | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) |
| 1 | 1.81-1.88, md | 1.82, br d (8.6)d | 1.75, dddd (3.3, 3.3, 10.3, 10.3) |
| 1.65, ddd/dt-like (6.6, 11.7, 11.7) | 1.67, ddd (3.5, 9.5, 12.8) | 1.60-1.67, md | |
| 2 | 2.10, ddd (6.2, 12.0, 12.8) | 2.03-2.08, md | 1.95-2.01, md |
| 1.81-1.88, md | 1.82, br d (8.6)d | ||
| 5 | 2.32, dd (1.3, 5.4) | 2.03-2.04, md | 1.98-2.01, md |
| 6 | 4.79, ddd/br t-like (0.8, 5.3, 5.4) | 4.71-4.77, md | 4.67, ddd/br t-like (0.9, 5.0, 5.2) |
| 7 | 2.54, ddd (1.1, 5.2, 16.1) | 2.56-2.64, md | 2.20-2.25, m |
| 1.94, ddd (4.1, 12.9, 16.2) | 1.23-1.29, m | ||
| 8 | 3.26, ddd/dt-like (4.8, 4.8, 12.8)d | - | 2.73-2.80, m |
| 9 | 2.20, ddd/dt-like (4.2, 4.2, 13.9) | 2.04-2.08, md | 1.57-1.62, md |
| 11 | 2.59, dd (13.9, 17.0) | 2.81, dd (4.8, 16.9) | 1.82-1.83, m |
| 2.40, dd (4.1, 17.0) | 2.52-2.62, md | 1.32-1.40, md | |
| 12 | 2.89, ddd (1.5, 4.6, 14.4) | ||
| - | - | 2.27-2.35, m | |
| 14 | - | - | 4.88, dd (1.0, 6.3) |
| 15 | 6.65, d (2.0) | 6.66, d (2.0) | 5.81, s |
| 16 | 7.53, d (2.0) | 7.63, d (2.0) | - |
| 18 | 1.36, s | 1.29, s | 1.33, s |
| 20 | 4.39, dd (3.4, 9.6) | 4.71-4.77, md | 4.42, dd (3.6, 9.6) |
| 3.65, dd (1.7, 9.5) | 3.67, dd (1.9, 10.1) | 3.65, dd (1.6, 9.5) |
For CH2, the deshielded signal was assigned as Ha, and the shielded signal as Hb.
Data measured in methanol-d4.
Data measured in CDCl3.
Signal was partially obscured.
Icacinlactone F (6) was obtained as white powder. The 13C NMR spectroscopic data and the HRESIMS result (m/z 361.1278 [M + H]+; calcd for C19H21O7, 361.1287) indicated a molecular formula of C19H20O7 with 10 indices of hydrogen deficiency. The spectroscopic data (Tables 2 and 3, and Figure 1) of 6 were similar to those of 5 except for the presence of an additional hydroxy group, which could be assigned to C-8 based on 1H-1H COSY, HSQC, and HMBC data. Furthermore, when compared to 5, the chemical shifts of C-8 (δC 69.2), C-7 (δC 35.4), and C-9 (δC 47.6) in 6 were deshielded by +40.4 ppm, +7.4 ppm, and +8.2 ppm, respectively. Such chemical shift differences were consistent with the α- and β-substituent effects of the 8-OH group. To determine the relative configuration of compound 6, a selective 1D-NOESY experiment in CDCl3 was performed. Thus, selective irradiation of H-9 (δH 2.04) and H-18 (δH 1.23) resulted in the signal enhancement of H-20 (δH 4.40 and 3.78) and H-5 (δH 1.72) / H-6 (δH 4.59), respectively, revealing the same configuration as 5, i.e. 3α-OH, 4α-CH3, 5α-H, 6α-H, 9β-H, and 3β,20-epxoy. On the other hand, selective 1D-NOE irradiation (in DMSO-d6) on 8-OH (δH 5.93) enhanced the signal of 9β-H (δH 1.96), indicating an 8β-OH orientation. The structure of compound 6 was therefore determined as the new (9βH)-17-norpimarane, 3β,20:14,16-diepoxy-3α,8β-dihydroxy-12-oxo-(9βH)-17-norpimar-13(14),15-dien-19,6β-olide, and given a trivial name icacinlactone F.
Icacinlactone G (7) was obtained as white powder. The molecular formula of C19H22O6 was established from 13C NMR spectroscopic data and HRESIMS data (m/z 347.1472 [M +H]+, calcd for C19H23O6, 347.1495), indicating nine indices of hydrogen deficiency. The presence of a γ-lactone moiety was indicated by the strong IR absorption at 1739 cm−1. Its NMR data (Tables 2, and 3) resembled those of 5 and 6. In the 13C NMR spectrum, the presence of a carbonyl carbon at δC 179.6 (C-19), a dioxygenated secondary carbon at δC 96.7 (C-3), an oxygenated methine carbon at δC 74.8 (C-6), an oxygenated methylene carbon at δC 73.0 (C-20), as well as a methyl at δC 17.6 (C-18), indicated the presence of the 19,6-γ-lactone moiety and the 3,20-epoxy bridge, which could be confirmed by HMBC analysis. Through 1H-1H COSY analysis, the connection from C-1 (δC 30.4) through C-12 (δC 26.4), and between C-8 (δC 32.8) and C-14 (δC 82.4), could be readily established with the aid of HSQC and further confirmed by the HMBC results (Figure 1). The rest of the unassigned groups, i.e. an olefinic methine at δC 114.6, an olefinic quaternary carbon at δC 167.1, and a carbonyl carbon at δC 173.0, were analyzed by the HMBC data. Thus, both H-12 (δH 2.89 and 2.28-2.35) and H-8 (δH 2.73-2.80) correlated to the olefinic quaternary carbon at δC 167.1 (C-13); the olefinic proton (δH 5.81, which was attached to C-15 at δC 114.6) correlated to both C-14 (δC 82.4) and the carbonyl carbon at δC 173.0 (C-16). These findings led to the assignment of an α,β-unsaturated γ-lactone moiety. Compound 7 is therefore a (9βH)-17-norpimarane dilactone. The relative configuration was then assigned on the basis of the NOESY analysis which revealed the following correlations: H-18 with both H-5 and H-6; H-5 with H-6; H-6 with H-7b; H-8 with H-7a, H-9, H-14, and H-20a; as well as H-9 with H-14. Thus, the structure of 7 was elucidated as the new (9βH)-17-norpimarane, 3β,20-epoxy-3α-hydroxy-(9βH)-17-norpimar-13(15)-en-19,6β:16,14α-diolide, and given a trivial name icacinlactone G.
Icacinlactons A-G (1-7), together with icacinol, humirianthol, humirianthenolide C, and icacenone, were evaluated for cytotoxic activity in MDA-MB-435 (human melanoma cancer), MDA-MB-231 (human breast cancer), and OVCAR3 (human ovarian cancer) cell lines (Table 4). Besides icacinol, humirianthol, humirianthenolide C, and icacenone, whose cytotoxic activity in MDA-MB-435 cells has been previously reported,6 icacinlactone F (6) displayed moderate cytotoxic activity in the MDA-MB-435 cells (IC50 6.16 μM). In the MDA-MB-231 cells, icacinol (IC50 7.30 μM), humirianthol (IC50 3.74 μM), humirianthenolide C (IC50 0.67 μM), and icacinlactone F (6) (IC50 8.94 μM) were active. On the other hand, icacinol (IC50 7.55 μM), humirianthol (IC50 4.12 μM), and humirianthenolide C (IC50 1.05 μM) were also active against OVCAR3 cells. Among the tested compounds, humirianthenolide C was most potent against all three cancer cell lines.
Table 4.
Cytotoxic Activity (IC50 μM) in Cancer Cell Lines
| IC50 (μM) |
|||
|---|---|---|---|
| compound | MDA-MB-435 | MDA-MB-231 | OVCAR3 |
| Icacinol | 1.25* | 7.30 | 7.55 |
| Humirianthol | 1.65* | 3.74 | 4.12 |
| Humirianthenolide C | 0.66* | 0.67 | 1.05 |
| Icacenone | 6.44* | 10.85 | 18.71 |
| Icacinlactone A (1) | > 20 | > 20 | 17.76 |
| Icacinlactone B (2) | > 20 | > 20 | > 20 |
| Icacinlactone C (3) | > 20 | > 20 | > 20 |
| Icacinlactone D (4) | > 20 | > 20 | > 20 |
| Icacinlactone E (5) | > 20 | > 20 | > 20 |
| Icacinlactone F (6) | 6.16 | 8.94 | 10.50 |
| Icacinlactone G (7) | > 20 | > 20 | > 20 |
| Vinblastine | 0.49 nM | 8.78 nM | 1.82 nM |
cited for comparison6
A few (9βH)-17-norpimaranes and (9βH)-pimaranes have been found to occur in the genera of Icacina (I. trichantha, I. claessensis, I. mannii, and I. guesfeldtii)6-9 and Humirianthera (syn. Casimirella) (H. rupestris, and H. ampla)10-12, both belonging to the Icacinaceae family. These icacinaceae (9βH)-pimarane derivatives are characterized by the presence of a 19,6β-γ-lactone moiety and a 3β,20-epoxy bridge. Compounds 3 and 4 are the first examples of this type of structure bearing a 2β,20-epoxy group. On the other hand, 1-4 are structurally unique with the absence of both 9-H and C-17, and the formation of benzofuran rings in the molecules. Biologically, several (9βH)-pimarane lactone isolated from Casimirella spp. have been reported to show cytotoxic activity against the A2780 human ovarian cancer cell line (IC50 1.7 - 6.1 μM).12
In summary, icacinlactones A-G (1-7) are new pimarane-type diterpenoids obtained from the tuber of I. trichantha. They belong to the small subclasses of 17-norpimarane and (9βH)-17-norpimarane. While the known structures of icacinol, humirianthol, humirianthenolide C, and icacenone exhibited cytotoxicity, the new icacinlactone F (6) was moderately active against MDA-MB-435, MDA-MB-231, and OVCAR3 cancer cell lines. Among all test compounds, humirianthenolide C was most active, showing an IC50 of 0.7 μM in MDA-MB-435 and MDA-MB-231 cells.
EXPERIMENTAL SECTION
General Experimental Procedures
The melting points were measured on a Thomas-Hoover capillary melting point apparatus (Arthur H. Thomas Company, Philadelphia, PA., U.S.A.). Optical rotations at the sodium D line were measured with a Perkin-Elmer 241 digital polarimeter using quartz cell with a path length of 100 mm at room temperature. Concentrations (c) are given in g/100mL. IR spectra were measured on a Jasco Fourier Transform IR Spectrometer (FT-IR model 410) loaded with an OMNIC software. NMR spectra were recorded on a Bruker DPX-400 spectrometer. All chemical shifts were quoted on the δ scale in ppm using residual solvent as the internal standard (DMSO-d6: 2.49 ppm for 1H NMR, 39.5 ppm for 13C NMR; CDCl3: 7.24 ppm for 1H NMR, 77.0 ppm for 13C NMR; methanol-d4: 3.30 ppm for 1H NMR, 49.90 ppm for 13C NMR). Coupling constants (J) are reported in Hz. For HPLC purification, a C18 semi-preparative HPLC column (Phenomenex C18 column, 250 × 10 mm, 5 μm) and a Shimadzu UFLC system were used. HRESIMS were measured on a Shimadzu LCMS-IT-TOF Mass Spectrometry. X-ray diffraction experiment was carried out on a Bruker Kappa APEXII DUO diffractometer with a CCD area detector using CuKα X-ray source. The absolute configuration of icacinlactone B (2) was determined with a Flack parameter of 0.00(3). The space group was P212121 (No. 19) with four molecules in the unit cell. The data have been deposited at the Cambridge Crystallographic Data Centre. CCDC 1039297 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; deposit@ccdc.cam.ac.uk). Human melanoma cancer cells MDA-MB-435, human breast cancer cells MDA-MB-231, and human ovarian cancer cells OVCAR3 were purchased from the American Type Culture Collection (Manassas, VA). Molecular models in figure 2 were generated by Chem3D Pr12.0 using MM2 force field calculation.
Plant Material
Fresh tubers of Icacina trichantha Oliv. were collected in June, 2011 from the Orba village in Nsukka of the Enugu State, Nigeria, and authenticated by Prof. B.O. Olorede of the Botany Department, University of Abuja, Nigeria, and Mr. A. Ozioko, botanist at the BDCP laboratories, Nsukka, Nigeria. A voucher specimen (UNN/FVM 456) was deposited in the pharmacology laboratory at the University of Nigeria, Nsukka, Nigeria.
Extraction and Isolation
The powdered tuber of I. trichantha (1.5 kg) was extracted with 80% aqueous MeOH by percolation to yield 166 g of dry crude extract. The crude extract was partitioned into petroleum ether-soluble (11 g), EtOAc-soluble (17 g), n-BuOH-soluble (15 g), and H2O-soluble (128 g) fractions. The EtOAc fraction (17 g) was separated into 88 sub-fractions on a silica gel column (5 × 60 cm) eluted with mixtures of petroleum ether and EtOAc (from 100:0 to 0:100 v/v; 600 mL each). 17-Hydroxyicacinol, icacinol, humirianthol, and humirianthenolide C were previously isolated from sub-fractions 19, 47-53, and 58.6 In the present study, sub-fractions 17-18 were combined and applied to a semi-preparative HPLC, eluted with MeOH-H2O (75:25 v/v; 3.5 mL/min) to afford icacinlactone A (1, 12 mg, tR = 7.10 min, soluble in MeOH). Combination of sub-fractions 24-25 was further separated by HPLC (MeOH-H2O, 75:25 v/v; 3.5 mL/min) to yield icacinlactone B (2, 50 mg, tR = 8.41 min, soluble in CHCl3 but sparingly soluble in MeOH). Sub-fractions 36-42 were further separated into five fractions by a Sephadex LH-20 chromatography (10 mm × 1 m, bed volume of 80 mL, MeOH). The 4th and 5th fractions were further purified by a semi-preparative HPLC (MeOH-H2O, 60:40 v/v; 3.5 mL/min) to afford icacinlactone C (3, 3.27 mg, tR = 14.17 min, soluble in both CHCl3 and MeOH). Subfraction 43 was applied to a semi-preparative HPLC, eluted with MeOH-H2O (60:40 v/v; 3.5 mL/min), to afford icacinlactone D (4, 3.6 mg, tR = 12.10 min, soluble in MeOH). A whiter precipitate obtained from subfractions 47-53, containing mostly icacinol, was further purified by a semi-preparative HPLC (MeOH-H2O, 35:65 v/v, 3.5 mL/min) to yield a crop of icacinlactone E (5, 9 mg, tR = 12.03 min, soluble in MeOH). Icacinlactone F (6, 1.71 mg, tR = 6.57 min, soluble in MeOH and CHCl3) was purified from a precipitate from subfractions 47-53, by HPLC separation (MeOH-H2O, 35:65 v/v; 3.5 mL/min). The white precipitate was shown to be a mixture of icacinol, humirianthol, and 6. The n-butanol-soluble fraction (19 g) was fractionated on a macroporous resin column (4 × 30 cm) eluted with aqueous MeOH (from 5% to 80%, 800 mL each) to obtain a crop of icacenone. From a precipitate containing mostly icacenone, icacinlactone G (7, 1.2 mg, tR = 8.19 min, soluble in CHCl3) was purified by semi-preparative HPLC (MeOH-H2O, 35:65 v/v, 3.5 mL/min).
Icacinlactone A (1)
white powder; mp 185 - 186 °C; ; IR (film) νmax 3495, 2930, 2874, 1750, 1303, 1229, 1155, 1125, 1110, 1050, 1017, 991, 934, 896, 873, 815, 741 cm−1; 1H NMR (methanol-d4, 400 MHz), see Table 1; 13C NMR (methanol-d4, 100 MHz), see Table 3; (+)-HRESIMS m/z 327.1206 [M + H]+ (calcd for C19H19O5, 327.1232).
Icacinlactone B (2)
white powder; mp 207 - 208 °C; ; IR (film) νmax 3494, 2937, 2875, 1752, 1623, 1604, 1497, 1465, 1401, 1375, 1332, 1178, 1151, 1110, 1101, 1041, 1017, 994, 951, 935, 897, 876, 831, 782, 768, 736, 695 cm−1; 1H NMR (CDCl3, 400 MHz), see Table 1; 13C NMR (CDCl3, 100 MHz), see Table 3; (+)-HRESIMS m/z 357.1309 [M + H]+ (calcd for C20H21O6, 357.1338).
Icacinlactone C (3)
white powder; mp 235 - 236 °C; ; IR (film) νmax 3448, 2969, 2875, 1750, 1625, 1604, 1499, 1454, 1403, 1375, 1316, 1284, 1185, 1152, 1122, 1088, 1029, 1006, 976, 903, 827, 806, 774, 759, 688 cm−1; 1H NMR (CDCl3, 400 MHz), see Table 1; 13C NMR (CDCl3, 100 MHz), see Table 3; 1H NMR (methanol-d4, 400 MHz) δ 7.65 (1H, d, J = 2.2 Hz, H-16), 6.84 (1H, d, J = 2.2 Hz, H-15), 6.66 (1H, s, H-11), 5.27 (1H, ddd/br quintet-like, J = 5.0, 5.0, 10.2 Hz, H-6), 4.22 (1H, dd, J = 1.5, 8.8 Hz, H-20a), 4.04 (1H, dd, J = 9.9, 17.3 Hz, H-7a), 3.94 (3H, s, OCH3), 3.78 (1H, s, H-3), 3.66 (1H, dd, J = 1.5, 8.8 Hz, H-20b), 2.94 (1H, dd, J = 4.7, 17.3 Hz, H-7b), 2.81 (1H, d, J = 11.8 Hz, H-1a), 2.35 (1H, dd, J = 1.4, 5.8 Hz, H-5), 1.99 (1H, dd, J = 1.5, 11.7 Hz, H-1b), 1.58 (3H, s, H-18). (+)-HRESIMS m/z 395.1088 [M + Na]+ (calcd for C20H20O7Na, 395.1107).
Icacinlactone D (4)
white powder; mp 240 - 241 °C; ; IR (film) νmax 3411, 2916, 1767, 1625, 1604, 1452, 1402, 1374, 1336, 1297, 1279, 1186, 1151, 1123, 1088, 1025, 999, 960, 903, 835, 767, 740, 681 cm−1; 1H NMR (methanol-d4, 400 MHz), see Table 1; 13C NMR (methanol-d4, 100 MHz), see Table 3; 1H NMR (CDCl3- methanol-d4 100 : 1, 400 MHz) δH 7.51 (1H, d, J = 2.1 Hz, H-16), 6.82 (1H, d, J = 2.2 Hz, H-15), 6.53 (1H, s, H-11), 5.12 (1H, ddd/br quintet-like, J = 5.2, 5.3, 10.2 Hz, H-6), 4.23 (1H, br d, J = 8.8 Hz, H-20a), 4.14 (1H, s, H-3), 4.03 (1H, dd, J = 9.9, 17.4 Hz, H-7a), 3.91 (3H, s, OCH3), 3.65 (1H, br d, J = 8.3 Hz, H-20b), 2.93 (1H, dd, J = 4.8, 17.3 Hz, H-7b), 2.46 (1H, d, J = 11.6 Hz, H-1a), 2.39 (1H, d, J = 11.5 Hz, H-1b), 2.20 (1H, br d, J = 5.7 Hz, H-5), 1.41 (3H, s, H-18). (+)-HRESIMS m/z 395.1082 [M + Na]+ (calcd for C20H20O7Na, 395.1107).
Icacinlactone E(5)
white powder; mp 231 - 232 °C; ; IR (film) νmax 3495, 2936, 2879, 1761, 1670, 1454, 1290, 1215, 1197, 1125, 1098, 1074, 1040, 1024, 1003, 981, 942, 904, 741 cm−1; 1H NMR (methanol-d4, 400 MHz), see Table 2; 13C NMR (methanol-d4, 100 MHz), see Table 3; (+)-HRESIMS m/z 345.1302 [M + H]+ (calcd for C19H21O6, 345.1338).
Icacinlactone F(6)
white powder; ; IR (film) νmax 3406, 2932, 1756, 1676, 1451, 1305, 1263, 1232, 1199, 1128, 1070, 1039, 946, 923, 873, 742 cm−1; 1H NMR (methanol-d4, 400 MHz), see Table 2; 13C NMR (methanol-d4, 100 MHz), see Table 3; 1H NMR (CDCl3, 400 MHz) δH 7.47 (1H, d, J = 2.0 Hz, H-16), 6.69 (1H, d, J = 2.0 Hz, H-15), 4.59 (1H, ddd, J = 1.0, 7.1, 15.0 Hz, H-6), 4.40 (1H, ddd, J = 3.9, 5.6, 9.8 Hz, H-20a), 3.78 (1H, br d, J = 10.0 Hz, H-20b), 3.00 (1H, dd, J = 5.4, 16.7 Hz, H-11a), 2.97 (1H, dd, J = 7.1, 14.8 Hz, H-7a), 2.56 (1H, dd, J = 5.4, 17.0 Hz, H-11b), 2.47 (1H, ddd, J = 3.1, 7.0, 14.5 Hz, H-7b), 2.04 (1H, dd, J = 1.2, 5.6 Hz, H-9), 1.72 (1H, ddd/dt-like, J = 2.4, 2.7, 6.7 Hz, H-5), 1.23 (3H, s, H-18). 1H NMR (DMSO-d6, 400 MHz, multiplicities of some signals were not clear due to poor peak shape) δH 7.81 (1H, d, J = 1.9 Hz, H-16), 6.65 (1H, d, J = 1.9 Hz, H-15), 5.93 (1H, d, J = 2.1 Hz, 8-OH), 5.57 (1H, s, 3-OH), 4.63 (1H, m, H-6), 4.56 (1H, m, H-20a), 3.49 (1H, br d, J = 10.1 Hz, H-20b), 1.96 (1H, br d, J = 5.7 Hz, H-9), 1.91 (1H, m, H-5), 1.65 (2H, m, H-1a and 2b), 1.52 (1H, m, H-1b), 1.16 (3H, s, H-18). (+)-HRESIMS m/z 361.1278 [M + H]+ (calcd for C19H21O7, 361.1287).
Icacinlactone G(7)
white powder; ; IR (film) νmax 3436, 2918, 2850, 1739, 1648, 1463, 1367, 1303, 1237, 1197, 1178, 1120, 1077, 1038, 996, 919, 884, 733, 684, 651 cm−1; 1H NMR (CDCl3, 400 MHz), see Table 2; 13C NMR (CDCl3, 100 MHz), see Table 3; (+)-HRESIMS m/z 347.1472 [M + H]+ (calcd for C19H23O6, 347.1495).
Cytotoxicity Assays
The cell line was propagated at 37°C in 5% CO2 in RPMI 1640 medium, supplemented with fetal bovine serum (10%), penicillin (100 units/mL), and streptomycin (100 μg/mL). Cells in log phase growth were harvested by trypsinization followed by two washings to remove all traces of enzyme. A total of 5,000 cells were seeded per well of a 96-well clear, flat-bottom plate (Microtest 96®, Falcon) and incubated overnight (37°C in 5% CO2). Samples dissolved in DMSO were then diluted and added to the appropriate wells (concentrations: 20 μM, 4 μM, 0.8 μM, 0.16 μM, 0.032 μM; total volume: 100 μL; DMSO: 0.5%). The cells were incubated in the presence of test substance for 72 h at 37°C and evaluated for viability with a commercial absorbance assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega Corp, Madison, WI) that measured viable cells. IC50 values are expressed in μM relative to the solvent (DMSO) control. Vinblastine was used for positive control.
Supplementary Material
ACKNOWLEDGMENTS
M. Monday Onakpa acknowledges the Institute of International Education of the United States, Department of State's Bureau for Education and Cultural Affairs, for an award from the Fulbright Junior Scholar Development Exchange Program, Grantee ID No. 15120356, to conduct research at the UIC.
Footnotes
The authors declare no competing financial interest.
Supporting Information
IR, 1D and 2D NMR spectra data for compounds 1-7. This material is available free of charge via Internet at http://pubs.acs.org.
REFERENCES
- 1.Asuzu IU, Abubakar II. Phytother. Res. 1995;9:21–25. [Google Scholar]
- 2.Asuzu IU, Ugwueze EE. J. Ethnopharmaco. 1990;28:151–156. doi: 10.1016/0378-8741(90)90024-n. [DOI] [PubMed] [Google Scholar]
- 3.Onakpa MM, Asuzu IU. Asian Pac. J. Trop. Biomed. 2013;3:628–633. doi: 10.1016/S2221-1691(13)60127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Timothy O, Idu M. Int. J. Med. Arom. Plants. 2011;1:184–188. [Google Scholar]
- 5.Asuzu IU, Egwu OK. Phytomedicine. 1998;5:35–39. doi: 10.1016/S0944-7113(98)80057-3. [DOI] [PubMed] [Google Scholar]
- 6.Onakpa MM, Zhao M, Goedecke T, Chen W-L, Che CT, Santarsiero BD, Swanson SM, Asuzu IU. Chem. Biodivers. 2014;11:1914–1922. doi: 10.1002/cbdv.201400151. [DOI] [PubMed] [Google Scholar]
- 7.On'okoko P, Vanhaelen M, Vanhaelen-Fastre R, Declercq JP, Van Meerssche M. Tetrahedron. 1985;41:745–748. [Google Scholar]
- 8.On'Okoko P, Vanhaelen M, Vanhaelen-Fastre R, Declercq JP, Van Meerssche M. Phytochemistry. 1985;24:2452–2453. [Google Scholar]
- 9.On'okoto P, Vanhaelen M. Phytochemistry. 1980;19:303–305. [Google Scholar]
- 10.Zoghbi MDGB, Roque NF, Gottlieb HE. Phytochemistry. 1981;20:1669–1673. [Google Scholar]
- 11.Graebner IB, Mostardeiro MA, Ethur EM, Burrow RA, Dessoy ECS, Morel AF. Phytochemistry. 2000;53:955–959. doi: 10.1016/s0031-9422(99)00585-3. [DOI] [PubMed] [Google Scholar]
- 12.Adou E, Williams RB, Schilling JK, Malone S, Meyer J, Wisse JH, Frederik D, Koese D, Werkhoven MCM, Snipes CE, Werk TL, Kingston DGI. Bioorg. Med. Chem. 2005;13:6009–6014. doi: 10.1016/j.bmc.2005.07.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.