Abstract
Introduction
HIV-1 prevention programs targeting HIV-1 serodiscordant couples need to identify couples that are likely to become pregnant to facilitate discussions about methods to minimize HIV-1 risk during pregnancy attempts (i.e. safer conception) or effective contraception when pregnancy is unintended. A clinical prediction tool could be used to identify HIV-1 serodiscordant couples with a high likelihood of pregnancy within one year.
Methods
Using standardized clinical prediction methods, we developed and validated a tool to identify heterosexual East African HIV-1 serodiscordant couples with an increased likelihood of becoming pregnant in the next year. Datasets were from three prospectively followed cohorts, including nearly 7,000 couples from Kenya and Uganda participating in HIV-1 prevention trials and delivery projects.
Results
The final score encompassed the age of the woman, woman’s number of children living, partnership duration, having had condomless sex in the past month, and non-use of an effective contraceptive. The area under the curve (AUC) for the probability of the score to correctly predict pregnancy was 0.74 (95% CI 0.72–0.76). Scores ≥7 predicted a pregnancy incidence of >17% per year and captured 78% of the pregnancies. Internal and external validation confirmed the predictive ability of the score.
Discussion
A pregnancy likelihood score encompassing basic demographic, clinical and behavioral factors defined African HIV-1 serodiscordant couples with high one-year pregnancy incidence rates. This tool could be used to engage African HIV-1 serodiscordant couples in counseling discussions about fertility intentions in order to offer services for safer conception or contraception that align with their reproductive goals.
Introduction
Pregnancy and the birth of healthy children are important aspirations for many couples, including those affected by HIV-1. For HIV-1 serodiscordant couples (i.e. couples in which one partner is HIV-1 infected and the other is not), the risk of HIV-1 transmission is heightened during pregnancy and the period leading up to pregnancy.[1–3] Despite the risk for HIV-1 transmission that accompanies pregnancy attempts—specifically, times when couples reduce or entirely forgo condom use—the achievement of fertility goals is paramount for couples; indeed, pregnancy rates among HIV-1 serodiscordant couples are similar to the general population.[4, 5] For HIV-1 serodiscordant couples with fertility goals, an early strategy is to engage them in discussion about their fertility desires and timing so that appropriate recommendations about safer conception services or effective contraception can be made. Key safer conception interventions for low resource settings include antiretroviral therapy (ART) use by the HIV-1 infected partner, pre-exposure prophylaxis (PrEP) for the HIV-1 uninfected partner and condomless sex limited to periods with peak fertility.[6, 7] Additional interventions, including fertility screening, diagnostic testing with treatment for genital infections, and vaginal self-insemination can further reduce risk and are consistent with a harm reduction approach.[8, 9]
Half of all new HIV-1 infections in sub-Saharan Africa are estimated to occur in stable heterosexual relationships, making HIV-1 serodiscordant couples a priority target population for HIV-1 prevention interventions [10, 11]. While strategies exist to minimize HIV-1 risk for HIV-1 serodiscordant couples planning pregnancy, a challenge lies in identifying couples who may soon become pregnant in order to initiate discussions about fertility desires. Timely counseling can help direct couples towards safer conception interventions (in the event that pregnancy is desired) or contraception (if pregnancy is not immediately desired). Worldwide, 40% of pregnancies are estimated to be unintended [12]. Public health systems may benefit from a simple tool that uses easy-to-capture information to identify couples likely to become pregnant. [13]
Clinical prediction tools have been developed to aid providers in identifying persons at risk for clinical outcomes, including, in reproductive health, pregnant women at risk of operative delivery and preeclampsia.[14, 15] However, a tool has not been developed to identify couples who are most likely to become pregnant. We used standardized clinical prediction methods to generate and validate a simple tool to identify heterosexual East African HIV-1 serodiscordant couples with increased likelihood of becoming pregnant in the next one year.[16, 17]
Methods
Data from three prospectively followed cohorts of East African heterosexual HIV-1 serodiscordant couples were used to derive and externally validate a pregnancy prediction model. Couples included those with HIV-1 infected women at risk of HIV-1 transmission to male partners as well as those with uninfected women at risk of HIV-1 acquisition. Participants were ≥18 and sexually active and HIV-1 infected partners were not using ART at enrollment. Interviewer-administered standardized questionnaires were used to obtain information about demographics, medical history and symptoms, sexual behavior and contraceptive use. Across studies, participants received comprehensive HIV-1 prevention services including individual and couples counseling, free condoms and treatment of sexually transmitted infections (STI) at all visits.
In the Partners PrEP Study (derivation cohort), pregnancy testing was conducted on a monthly basis for HIV-1 uninfected women and as clinically indicated during quarterly study visits for HIV-1 infected women. This cohort consisted of 4747 HIV-1 serodiscordant couples from 9 sites in Kenya and Uganda who were participating in a randomized clinical trial of daily oral pre-exposure prophylaxis (PrEP) for HIV-1 prevention [18, 19]. For HIV-1 uninfected women, routine visit procedures included HIV-1 testing, study drug dispensing, and adherence counseling; blinded study drug was withheld during pregnancy and breastfeeding. HIV-1 infected women underwent 6-monthly CD4 count and HIV-1 RNA testing.
In the Partners in Prevention HSV/HIV Transmission Study (external validation cohort), pregnancy testing was conducted quarterly for the 3408 women from 14 sites in 7 East and southern African countries who were participating in this randomized trial of daily acyclovir to prevent HIV-1 transmission from partners dually-infected with HSV-2 and HIV-1 [20, 21]. HIV-1 infected partners completed monthly study visits for study drug dispensation and adherence counseling and HIV-1 uninfected partners completed quarterly study visits for HIV-1 testing. To validate the pregnancy prediction model with this cohort, data were restricted to the 1760 couples from Kenyan and Ugandan sites.
In the ongoing Partners Demonstration Project (external validation cohort), pregnancy testing is conducted for all women at enrollment and as clinically indicated during quarterly follow-up visits. Information on fertility intention is collected for all participants through a standardized interviewer-administered questionnaire asking if the participant desires another child in the future and when he/she would like to have a future child. In this implementation science-driven delivery project of PrEP as a “bridge” to ART use, 1013 high risk HIV-1 serodiscordant couples from 4 sites in Kenya and Uganda are followed for up to 24 months to assess their use of PrEP in a time-limited fashion until the HIV-1 infected partner initiates and sustains ART use [22]. To validate the pregnancy prediction model with this cohort, we included data through December 2014.
Score derivation
Using univariate Cox proportional hazards models, we identified enrollment demographic, medical, and sexual behavior characteristics from female and male partners of participants in the Partners PrEP Study that predicted the first occurrence of a new pregnancy using a p-value cutoff of 0.05. We limited consideration of possible predictors to baseline characteristics so that the final tool would use information that could be routinely captured during an initial clinic visit. Pregnancy was defined to begin on the self-reported date of last menstrual period (LMP), for most pregnancies, or for those without LMP data, by counting backwards from the date of delivery based using the reported gestational age that the pregnancy achieved. Follow-up time was censored after the occurrence of a woman’s first pregnancy and after one year in the study. Continuous variables were grouped into the most predictive categories using optimal cutpoints identified through signal detection receiver operating characteristic (ROC) curves.[23] Categories covering few integers were collapsed to ensure that the final scoring tool would be easy to apply in a clinic setting.
All factors identified as predictive in univariate analysis were combined into a multivariate Cox proportional hazards model and a fully stepwise sequence selection procedure was used to identify the most predictive combination of factors. When two co-linear factors were identified through univariate analysis, we chose the factor that would be most simple to obtain from a woman to ensure that the final model would be feasible to implement. The Akaike Information Criterion (AIC) was used to identify the most predictive model (with the lowest AIC). To derive the score value for each category within predictors, we divided each coefficient from the multivariate proportional hazards model by the lowest coefficient among all predictors and rounded to the nearest integer.
Once we identified the most predictive model and scores for each predictor category, we applied the score to each woman in the dataset and calculated her pregnancy likelihood score. Pregnancy incidence rates were calculated as the number of new pregnancies occurring within one year of follow-up divided by the total time accrued between enrollment and pregnancy or one year for women who did not become pregnant. Score categories were determined by collapsing adjacent score levels that had similar incidence rates. We used ROC analysis to calculate the area under the curve (AUC) with the score as the sole predictor of pregnancy.
Validation
We used a 10-fold cross validation technique to check for internal consistency of AUC within the derivation cohort. For external validation, we applied the score to enrollment data from participants from Kenyan and Ugandan sites in the Partners in Prevention HSV/HIV Transmission Study and the Partners Demonstration Project and calculated the pregnancy score for each woman. For each validation dataset, we calculated the AUC with the score as the sole predictor of pregnancy and pregnancy incidence rates for each category of the score. Women pregnant at enrollment were excluded and follow-up time was censored at one year in both external validation datasets.
Assessing fertility intentions could be a simple alternative method to predict upcoming pregnancy. However, it is unclear if this would be as predictive as a compilation of factors incorporated into one tool due to the frequency of unintended pregnancies and often changing pregnancy goals. Using data from the Partners Demonstration Project, we examined the predictive ability of fertility intentions by calculating the AUC for pregnancy prediction with a binary variable of immediate fertility intention (desiring a child within 1 year) as the sole predictor.
All analyses were conducted using SAS 9.4 (Cary, NC) and public domain ROC5 (Department of Veteran’s Affairs and the National Institute of Aging of the United States of America). Protocols for each study were approved by the University of Washington Human Subjects Division and ethics review committees for each of the study sites. Participants provided written informed consent.
Results
Participant characteristics
In the Partners PrEP Study, 58.9% of couples had an HIV-1 infected woman, the median age of women was 31 (interquartile range [IQR]: 26–36), most couples had at least 1 child together, and the median duration of partnerships was 8 years (IQR: 4–15, Table 1). Women reported a median of 4 sex acts with their partner in the month prior to enrollment and approximately one-quarter reported at least one sex act with their partner that was unprotected by a condom. Of the 4,340 couples whose female partner was not pregnant at enrollment, 600 (13.8%) became pregnant during the first year of follow up and the pregnancy incidence was 15.0 (95% confidence interval [CI] 13.8–16.2) per 100 person-years.
Table 1. Characteristics of couples participating in studies used for score derivation and validation.
| Partners PrEP Study | Partners in Prevention HSV/HIV Transmission Study, Kenyan and Ugandan sites | Partners Demonstration Project | |
|---|---|---|---|
| Number of couples | 4340 | 1760 | 872 |
| % with HIV-1 infected women | 2555 (58.9%) | 1191 (67.7%) | 538 (61.7%) |
| Woman’s age, Median (IQR) | 31 (26–36) | 29 (25–35) | 27 (23–33) |
| Women’s number of children, Median (IQR) | 3 (2–5) | 2 (1–4) | 2 (1–3) |
| Couple’s number of children, Median (IQR) | 2 (1–4) | 1 (1–3) | 0 (0–2) |
| Partnership duration, years, Median (IQR) | 8 (4–15) | 6 (3–11) | 4 (1–8) |
| % Married or cohabiting | 4292 (98.9%) | 1717 (97.6%) | 851 (97.6%) |
| Years of school completed by the woman, Median (IQR) | 7 (3–8) | 8 (6–10) | 8 (6–11) |
| Sex acts between study partners, past month, Median (IQR)* | 4 (2–8) | 3 (2–6) | 5 (3–10) |
| % couples having at least 1 sex act without a condom, past month* | 1091 (25.2%) | 475 (27.0%) | 548 (62.8%) |
| % women reporting a non-study sexual partner, past month | 37 (0.9%) | 16 (1.0%) | 12 (1.4%) |
| % women using effective contraception (injectable, oral, IUD, implant, or surgical method) | 1776 (40.9%) | 338 (19.2%) | 273 (35.2%) |
| % women with an STI** | 433 (10.3%) | 157 (9.5%) | — |
| % of HIV-1 uninfected women with HSV-2 infection** | 1398 (80.3%) | 492 (88.8%) | — |
| % experiencing pregnancy during follow-up | 600 (13.8%) | 312 (17.7%) | 141 (16.2%) |
| One year pregnancy incidence rate per 100 person-years (95% CI) | 15.0 (13.8–16.2) | 19.8 (17.6–22.0) | 17.4 (14.0–20.7) |
*Based on the woman’s report.
**Neisseria Gonorrhoea, Chlamydia trachomatis,Trichonomas vaginalis, or syphilis; Baseline STI and HSV-2 testing was not conducted within the Partners Demonstration Project.
Score derivation model
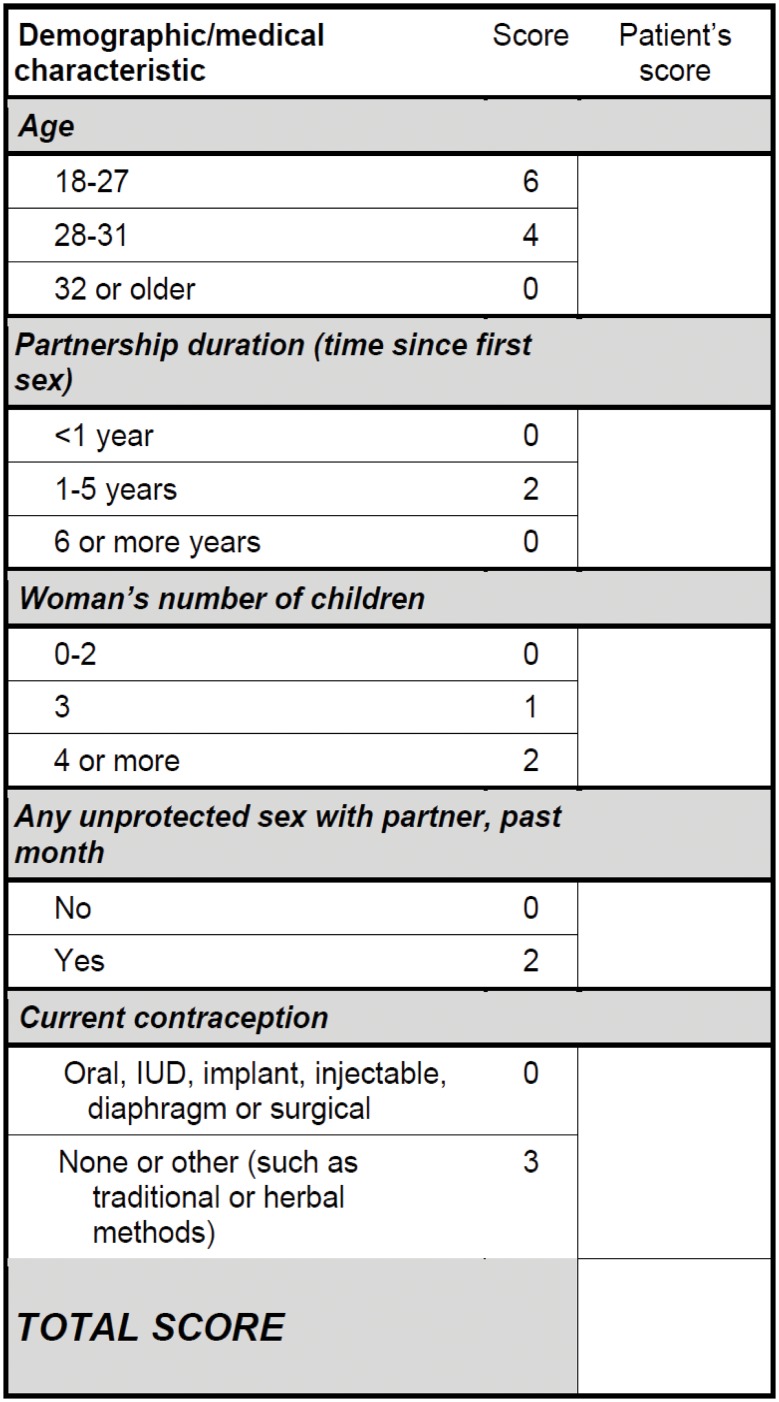
Univariate analysis identified multiple factors significantly associated with incident pregnancy within one year (Table 2). In a stepwise duration, woman’s number of children, having had sex unprotected by a condom in the past month, Cox proportional hazards multivariate model, five factors were retained for the final prediction model: woman’s age, partnership and non-use of an effective contraceptive. The highest value for an individual risk factor was 6 for women aged 18–27, with other factors scoring at lower values (Fig 1). Notably, HIV-1 status did not emerge as a key predictor.
Table 2. Predictors of pregnancy in the derivation cohort.
| Univariate models | Multivariate model | Stepwise multivariate model | ||||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | Regression coefficient | Score | |
| Age of woman (vs. ≥32) | ||||||||
| 18–27 | 4.13 (3.36–5.09) | <0.001 | 4.87 (3.58–6.62) | <0.001 | 4.09 (3.22–5.19) | <0.001 | 1.41 | 6 |
| 28–31 | 2.38 (1.85–3.06) | <0.001 | 2.71 (1.94–3.78) | <0.001 | 2.50 (1.93–3.24) | <0.001 | 0.92 | 4 |
| Age of male partner (vs. ≥28) | ||||||||
| 18–24 | 2.23 (1.72–2.90) | <0.001 | ||||||
| 25–27 | 1.95 (1.52–2.50) | <0.001 | ||||||
| Age difference (vs. male is 10 years older) | ||||||||
| Male is younger | 0.70 (0.52–0.95) | 0.023 | 1.10 (0.72–1.67) | 0.7 | ||||
| Same age | 0.55 (0.33–0.93) | 0.024 | 0.77 (0.41–1.45) | 0.4 | ||||
| Male is 1–5 years older | 0.86 (0.69–1.08) | 0.186 | 1.07 (0.80–1.43) | 0.7 | ||||
| Male is 6–10 years older | 0.98 (0.78–1.24) | 0.885 | 1.19 (0.88–1.60) | 0.3 | ||||
| Woman’s level of education, years (vs. ≥5 years) | ||||||||
| <1 year | 0.88 (0.68–1.14) | 0.341 | ||||||
| 1–4 years | 0.92 (0.74–1.14) | 0.451 | ||||||
| Partnership duration of 1–6 years (vs. <1 year or ≥6 years) | 2.31 (1.95–2.73) | <0.001 | 1.80 (1.41–2.30) | <0.001 | 1.58 (1.30–1.91) | <0.001 | 0.45 | 2 |
| Married or cohabiting (vs. no) | 1.36 (0.51–3.61) | 0.537 | ||||||
| Woman’s total number of children (vs. 0–2 children) | ||||||||
| 3 | 0.79 (0.63–0.98) | 0.035 | 1.42 (1.06–1.90) | 0.02 | 1.29 (1.02–1.62) | 0.0349 | 0.25 | 1 |
| ≥4 | 0.55 (0.45–0.66) | <.001 | 1.49 (1.11–2.00) | 0.008 | 1.56 (1.24–1.96) | 0.0002 | 0.44 | 2 |
| Number of children woman has with her male study partner (vs. 0–1) | ||||||||
| 2 | 0.93 (0.75–1.16) | 0.534 | ||||||
| ≥3 | 0.53 (0.44–0.65) | <.001 | ||||||
| Number of sex acts with male partner, past month | ||||||||
| None | 1.00 | |||||||
| 1–4 | 1.28 (0.73–2.72) | 0.39 | 1.54 (0.67–3.52) | 0.3 | ||||
| 5–10 | 1.68 (0.95–2.99) | 0.08 | 1.84 (0.80–4.25) | 0.2 | ||||
| 11–18 | 1.71 (0.93–3.13) | 0.84 | 1.90 (0.81–4.46) | 0.1 | ||||
| ≥19 | 2.63 (1.38–5.02) | 0.003 | 2.08 (0.81–5.29) | 0.1 | ||||
| Condom use frequency with male partner, past month (vs. 100% condom use) | ||||||||
| No sex with study partner | 0.76 (0.43–1.34) | 0.343 | ||||||
| No condom use | 1.63 (1.28–2.06) | <0.001 | ||||||
| Some condom use | 1.50 (1.21–1.87) | <0.001 | ||||||
| Unprotected sex with male partner, past month (vs. none) | 1.57 (1.32–1.87) | <0.001 | 1.45 (1.14–1.83) | 0.003 | 1.63 (1.36–1.95) | <0.001 | 0.49 | 2 |
| Additional partner(s), past month (vs. none) | 0.42 (0.11–1.61) | 0.203 | ||||||
| Male partner circumcised (vs. not circumcised) | ||||||||
| Fully circumcised | 0.93 (0.79–1.10) | 0.398 | ||||||
| Partially circumcised | 0.64 (0.15–2.62) | 0.531 | ||||||
| BV (vs. no BV) | 1.16 (0.95–1.42) | 0.152 | ||||||
| Woman has infection with gonorrhoea, chlamydia, trichomonas and/or syphilis | 1.19 (0.91–1.55) | 0.20 | ||||||
| Woman is HIV uninfected (vs. HIV-infected) | 0.61 (0.45–0.82) | <0.001 | 0.86 (0.64–1.15) | 0.3 | ||||
| HSV-2 uninfected (HIV uninfected women only) | ||||||||
| Indeterminate | 1.49 (0.76–2.91) | 0.248 | ||||||
| Positive | 0.61 (0.44–0.85) | 0.003 | ||||||
| No use of effective contraception (vs. use of oral, injectable, IUD, implant or surgical) | 1.82 (1.52–2.19) | <0.001 | 1.74 (1.38 (2.20) | <0.001 | 1.92 (1.59–2.32) | <0.001 | 0.65 | 3 |
Fig 1. Pregnancy Likelihood Scorecard.
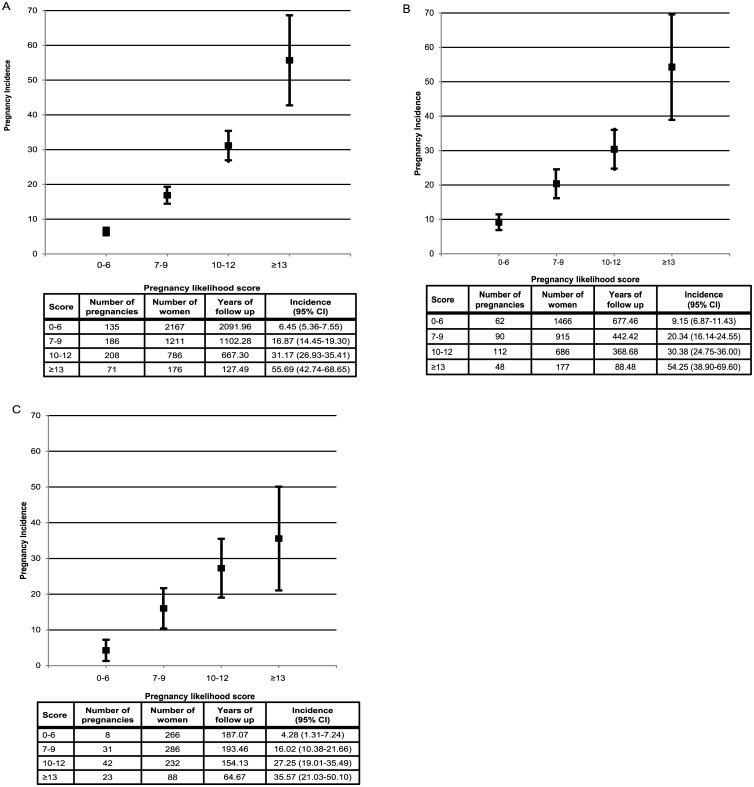
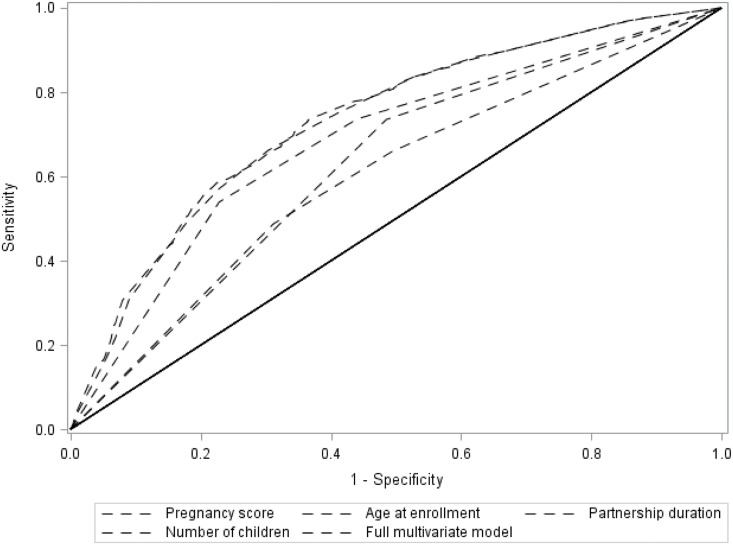
We applied the scores for each predictor to enrollment data from women in the Partners PrEP Study and calculated the pregnancy incidence for women in each level of the score. Half of the couples had a score of ≥7 and this score captured 78% of the pregnancies that occurred during follow-up; scores ≥13 predicted a pregnancy incidence >50% per year (Fig 2, panel A). The composite score had greater predictability than any of the individual factors alone (Fig 3). Among the categorical risk factors, having sex unprotected by a condom predicted only 33% of pregnancies. In ROC analysis, the score (as the sole predictor) and the stepwise multivariate model (with each predictor as a covariate) essentially overlapped, demonstrating that the score captured essentially all of the predictability of the multivariate model. The area under the curve (AUC) for the probability of the score to correctly predict pregnancy was 0.74 (95% CI 0.72–0.76). Ten-fold cross validation produced an average AUC of 0.75 (95% CI: 0.65–0.80), indicating the internal robustness of the prediction algorithm.
Fig 2. Pregnancy incidence rates by score among women in the A) Partners PrEP Study B) Partners in Prevention HSV/HIV Transmission Study at Kenyan and Ugandan sites and C) Partners Demonstration Project.
Fig 3. Receiver operating characteristic (ROC) curves for the pregnancy prediction score, individual continuous pregnancy predictors, and the multivariate model containing all predictors.
For external validation, we applied the score to Kenyan and Ugandan participants in the Partners in Prevention HSV/HIV Transmission Study and the Partners Demonstration Project. Characteristics of couples in the validation datasets were similar to the derivation cohort (Table 1) with trends towards younger ages, fewer children, and higher risk sexual behavior in the Partners Demonstration Project, which was designed to recruit couples at higher risk for HIV-1 transmission. [17, 24] The overall 12-month pregnancy incidence was 19.8 (95% CI 17.6–22.0) in the Partners in Prevention HSV/HIV Transmission Study and 17.4 (95% CI 14.0–20.7) in the Partners Demonstration Project.
In the Partners in Prevention HSV/HIV Transmission Study, 60.0% of the cohort had a pregnancy likelihood score ≥7 and couples in this range accounted for 250 (80.1%) of pregnancies that were experienced. In the Partners Demonstration Project, 69.5% of the cohort had a score ≥7 and couples in this range accounted for 92.3% of the pregnancies experienced. Thus, the score effectively identified a subset of each cohort with the highest pregnancy likelihood and the great majority of the pregnancies were experienced by this subset. When the score was applied to these external validation datasets, the AUC was 0.67 (95% CI 0.64–0.70) for the Partners in Prevention HSV/HIV Transmission Study and 0.67 (95% CI 0.63–0.71) for the Partners Demonstration Project.
The Partners Demonstration Project collected data at enrollment on fertility desires and intentions. Using immediate fertility intention as a sole predictor of pregnancy (indicated by saying “currently trying to get pregnant”) identified only 14% of pregnancies and the AUC was poor at 0.53 (95% CI 0.50–0.56); reporting immediate fertility intention or within the next 3 years accounted for 62.5% of the pregnancies, with an AUC of 0.60 (0.55–0.64).
Discussion
We developed a pregnancy prediction scoring tool that can be used in research and clinical settings to identify HIV-1 serodiscordant couples that are likely to become pregnant within one year. In the current era of HIV-1 prevention, where antiretroviral interventions are incorporated into combination prevention strategies that nearly eliminate transmission within HIV-1 serodiscordant partnerships, efficient and cost-effective approaches are needed to identify and target couples who are at highest risk of transmission, including those likely to become pregnant.[6, 22] Couples often lack opportunities to discuss pregnancy desires with their care providers and many shy away from introducing the topic due to cultural stigma and the past guidance for HIV infected women to avoid pregnancy.[25] Providers could use this tool to engage HIV-1 serodiscordant couples in discussions about their fertility intentions, to empower women and their partners to determine when/if they want to have children and to receive the appropriate counseling and care that matches these desires, such as safer conception services or the provision of effective contraception. Notably, the pregnancy score appeared to be a better predictor than reported fertility intention. Thus, the tool could be used in conjunction with routine assessment of fertility desires to identify couples that might benefit most from clinician-initiated discussion about couple and individual fertility goals and counseling on how to achieve those goals. Depending on the clinic setting and goals, lower or higher cutpoints could be used to focus attention on couples with moderate, high, or extremely high pregnancy likelihood and balance the size of the population targeted with clinician time available for counseling.
Clinical prediction tools are useful to identify novel cohorts for research on a specific outcome or in a public health setting to triage individuals towards an individually-tailored intervention. In the ongoing Partners Demonstration Project, the use of a validated scoring tool for HIV-1 transmission successfully identified a high risk cohort with age, sexual behavior, and plasma viral load characteristics indicative of much greater HIV-1 risk than our previous cohorts.[24] Thus, in a research setting, these types of tools are useful and feasible to implement. Further operational research is needed to determine the feasibility of using these types of tools in a public health clinic setting where patient burden is greater and provider time is limited.
These data were from East African heterosexual HIV-1 serodiscordant couples and our results are most applicable to HIV-1 serodiscordant couples in that context, with similar pregnancy rates and fertility intentions. Importantly, our cohort did not include women who do not know their partner’s HIV-1 status, a group at potential risk for HIV-1 and pregnancy, and in urgent need of interventions to reduce HIV-1 risk that are integrated with pregnancy planning. Also, our cohort included only HIV-1 serodiscordant couples but couples that are HIV-1 seroconcordant (positive or negative) can benefit from open discussion and counseling with providers about fertility desires, pregnancy planning and their HIV-1 risks. We also do not know that all pregnancies were fathered by the male partner enrolled in the study, but reports of partnerships with men aside from those in the study were few and limiting follow-up to one year after study enrollment reduces this limitation. A strength of our methods was the use of multiple distinct cohorts to rigorously validate the prediction model and trends in pregnancy rates across categories of the score.
Pregnancy planning, pre-conception care, and open discussions with partners and providers are important, especially in the context of HIV-1 infection, to optimize pre-pregnancy health and birth outcomes. [26] The integration of discussions about fertility intentions into HIV-1 programs is urgently needed, especially for HIV-1 serodiscordant couples struggling to understand serodiscordance and to find ways to preserve their relationship in the midst of the risk of HIV-1 transmission. Our scoring tool could provide such an opportunity, to identify couples who have a high chance of becoming pregnant and increase dialogue about their best options for meeting their immediate and long term fertility goals with the lowest possible HIV-1 risk.
Acknowledgments
We are grateful to all of the participants and the study teams for the Partners PrEP Study, the Partners in Prevention HSV/HIV Transmission Study, and the Partners Demonstration Project who made this study possible. The authors acknowledge the Director, KEMRI for support.
The Partners PrEP Study Team
University of Washington Coordinating Center: Connie Celum (principal investigator, protocol co-chair), Jared M. Baeten (medical director, protocol co-chair), Deborah Donnell (protocol statistician), Robert W. Coombs, Lisa Frenkel, Craig W. Hendrix, Jairam Lingappa, M. Juliana McElrath. Study sites and site principal investigators: Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University, University of Washington): Patrick Ndase, Elly Katabira; Kampala, Uganda (Makerere University): Elly Katabira, Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig Cohen; Mbale, Uganda (The AIDS Support Organization, CDC-Uganda): Jonathan Wangisi, James Campbell, Jordan Tappero; Nairobi, Kenya (University of Nairobi, University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Kenya Medical Research Institute, Nairobi, Kenya: Nelly Mugo; Tororo, Uganda (CDC-Uganda, The AIDS Support Organization): James Campbell, Jordan Tappero, Jonathan Wangisi.
The Partners in Prevention HSV/HIV Transmission Study Team
University of Washington Coordinating Center and Central Laboratories, Seattle, USA: Connie Celum (principal investigator), Anna Wald (protocol co-chair), Jairam Lingappa (medical director), Jared M. Baeten, Mary Campbell, Lawrence Corey, Robert W. Coombs, James P. Hughes, Amalia Magaret, M. Juliana McElrath, Rhoda Morrow, James I. Mullins. Study sites and site principal investigators: Cape Town, South Africa (University of Cape Town): David Coetzee; Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife, Edwin Were; Gaborone, Botswana (Botswana Harvard Partnership): Max Essex, Joseph Makhema; Kampala, Uganda (Infectious Disease Institute, Makerere University): Elly Katabira, Allan Ronald; Kigali, Rwanda (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Kayitesi Kayitenkore, Etienne Karita; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig Cohen; Kitwe, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, William Kanweka; Lusaka, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Bellington Vwalika; Moshi, Tanzania (Kilimanjaro Christian Medical College, Harvard University): Saidi Kapiga, Rachel Manongi; Nairobi, Kenya (University of Nairobi, University of Washington): Carey Farquhar, Grace John-Stewart, James Kiarie; Ndola, Zambia (Rwanda Zambia HIV Research Group, and Emory University): Susan Allen, Mubiana Inambao; Orange Farm, South Africa (Reproductive Health Research Unit, University of the Witwatersrand): Sinead Delany-Moretlwe, Helen Rees; Soweto, South Africa (Perinatal HIV Research Unit, University of the Witwatersrand): Guy de Bruyn, Glenda Gray, James McIntyre; Thika, Kenya (University of Nairobi, University of Washington): Nelly Mugo
The Partners Demonstration Project Team
University of Washington Coordinating Center: Jared Baeten (protocol chair), Connie Celum (protocol co-chair), Renee Heffron (director), Deborah Donnell (protocol statistician), Ruanne Barnabas, Harald Haugen, Lara Kidoguchi, Susan Morrison, Jennifer Morton, Andrew Mujugira, Caitlin Scoville, Bettina Shell-Duncan, Kathy Thomas. Study sites and site principal investigators: Kabwohe, Uganda (Kabwohe Clinical Research Center): Steven Asiimwe, Edna Tindimwebwa, Elioda Tumwesigye; Kampala, Uganda (Infectious Disease Institute, Makerere University): Elly Katabira, Nulu Bulya; Kisumu, Kenya (Kenya Medical Research Institute): Elizabeth Bukusi, Josephine Odoyo; Thika, Kenya (Kenya Medical Research Institute): Nelly Mugo, Kenneth Ngure; MGH/Harvard University: David Bangsberg, Jessica Haberer, Norma Ware, Monique Wyatt; Johns Hopkins University: Craig Hendrix, Mark Marzinke.
Data management was provided by DF/Net Research, Inc. (Seattle, USA) and site laboratory oversight was provided for the Partners PrEP Study and the Partners in Prevention HSV/HIV Transmission Study by Contract Laboratory Services (University of the Witwatersrand, Johannesburg, South Africa).
Data Availability
Data are from the Partners PrEP Study, the Partners in Prevention HSV/HIV Transmission Study, and the Partners Demonstration Project. Due to agreements with the human subjects committees at each institution involved in this study, the authors are unable to post the data. If readers wish to request data, the primary contact person is Dr. Jared Baeten, jbaeten@uw.edu He will be able to facilitate access to the data upon request.
Funding Statement
RH was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K99HD076679 and R00HD076679). Funding for the Partners HSV/HIV Transmission Study and the Partners PrEP Study was provided by the Bill and Melinda Gates Foundation (OPP26469 & OPP47674). The Partners Demonstration Project is funded by the Bill & Melinda Gates Foundation (OPP1056051), the National Institute of Mental Health of the US National Institutes of Health (R01 MH095507), and the United States Agency for International Development (AID-OAA-A-12-00023). This work is made possible by the generous support of the American people through USAID; the contents are the responsibility of the authors and do not necessarily reflect the views of USAID, NIH, or the United States Government.
References
- 1. Mugo NR, Heffron R, Donnell D, Wald A, Were EO, Rees H, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS 2011,25:1887–1895. 10.1097/QAD.0b013e32834a9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet 2005,366:1182–1188. [DOI] [PubMed] [Google Scholar]
- 3. Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med 2014,11:e1001608 10.1371/journal.pmed.1001608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ngure K, Heffron R, Mugo NR, Celum C, Cohen CR, Odoyo J, et al. Contraceptive method and pregnancy incidence among women in HIV-1-serodiscordant partnerships. AIDS 2012,26:513–518. 10.1097/QAD.0b013e32834f981c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guthrie BL, Choi RY, Bosire R, Kiarie JN, Mackelprang RD, Gatuguta A, et al. Predicting pregnancy in HIV-1-discordant couples. AIDS Behav 2010,14:1066–1071. 10.1007/s10461-010-9716-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 2011,25:2005–2008. 10.1097/QAD.0b013e32834a36d0 [DOI] [PubMed] [Google Scholar]
- 7. Schwartz SR, Bassett J, Sanne I, Phofa R, Yende N, Van Rie A. Implementation of a safer conception service for HIV-affected couples in South Africa. AIDS 2014,28 Suppl 3:S277–285. 10.1097/QAD.0000000000000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mmeje O, Cohen CR, Cohan D. Evaluating safer conception options for HIV-serodiscordant couples (HIV-infected female/HIV-uninfected male): a closer look at vaginal insemination. Infect Dis Obstet Gynecol 2012,2012:587651 10.1155/2012/587651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matthews LT, Mukherjee JS. Strategies for harm reduction among HIV-affected couples who want to conceive. AIDS Behav 2009,13 Suppl 1:5–11. 10.1007/s10461-009-9551-0 [DOI] [PubMed] [Google Scholar]
- 10. Dunkle KL, Stephenson R, Karita E, Chomba E, Kayitenkore K, Vwalika C, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet 2008,371:2183–2191. 10.1016/S0140-6736(08)60953-8 [DOI] [PubMed] [Google Scholar]
- 11. Piot P, Bartos M, Larson H, Zewdie D, Mane P. Coming to terms with complexity: a call to action for HIV prevention. Lancet 2008,372:845–859. 10.1016/S0140-6736(08)60888-0 [DOI] [PubMed] [Google Scholar]
- 12. Alkema L, Kantorova V, Menozzi C, Biddlecom A. National, regional, and global rates and trends in contraceptive prevalence and unmet need for family planning between 1990 and 2015: a systematic and comprehensive analysis. Lancet 2013,381:1642–1652. 10.1016/S0140-6736(12)62204-1 [DOI] [PubMed] [Google Scholar]
- 13. Sedgh G, Singh S, Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann 2014,45:301–314. 10.1111/j.1728-4465.2014.00393.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schuit E, Kwee A, Westerhuis M, Van Dessel H, Graziosi G, Van Lith J, et al. A clinical prediction model to assess the risk of operative delivery. BJOG 2012,119:915–923. 10.1111/j.1471-0528.2012.03334.x [DOI] [PubMed] [Google Scholar]
- 15. McDonald S, Wall J, Forbes K, Kingston D, Kehler H, Vekved M, et al. Development of a prenatal psychosocial screening tool for post-partum depression and anxiety. Paediatr Perinat Epidemiol 2012,26:316–327. 10.1111/j.1365-3016.2012.01286.x [DOI] [PubMed] [Google Scholar]
- 16. Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med 1985,313:793–799. [DOI] [PubMed] [Google Scholar]
- 17. Kahle EM, Hughes JP, Lingappa JR, John-Stewart G, Celum C, Nakku-Joloba E, et al. An empiric risk scoring tool for identifying high-risk heterosexual HIV-1-serodiscordant couples for targeted HIV-1 prevention. J Acquir Immune Defic Syndr 2013,62:339–347. 10.1097/QAI.0b013e31827e622d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mujugira A, Baeten JM, Donnell D, Ndase P, Mugo NR, Barnes L, et al. Characteristics of HIV-1 Serodiscordant Couples Enrolled in a Clinical Trial of Antiretroviral Pre-Exposure Prophylaxis for HIV-1 Prevention. PLoS ONE 2011,6:e25828 10.1371/journal.pone.0025828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012,367:399–410. 10.1056/NEJMoa1108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 2010,362:427–439. 10.1056/NEJMoa0904849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lingappa JR, Baeten JM, Wald A, Hughes JP, Thomas KK, Mujugira A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet 2010,375:824–833. 10.1016/S0140-6736(09)62038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baeten J, Heffron R, Kidoguchi L, Mugo N, Katabira E, Bukusi E, et al. Near elimination of HIV transmission in a demonstration project of PrEP and ART, Abstract 24 In: Conference on Retroviruses and Opportunistic Infections. Seattle, USA; 2015. [Google Scholar]
- 23. Kiernan M, Kraemer HC, Winkleby MA, King AC, Taylor CB. Do logistic regression and signal detection identify different subgroups at risk? Implications for the design of tailored interventions. Psychol Methods 2001,6:35–48. [DOI] [PubMed] [Google Scholar]
- 24.Irungu E, Heffron R, Mugo N, Ngure K, Katabira E, Bulya N, et al. Use of a Risk Scoring Tool to Identify Higher-risk HIV-1 Serodiscordant Couples for an Antiretroviral-based HIV-1 Prevention Intervention, Abstract OA28.02. In: HIV Research for Prevention (R4P). Cape Town, South Africa; 2014. [DOI] [PMC free article] [PubMed]
- 25. Centers for Disease Control. Recommendations for assisting in the prevention of perinatal transmission of human T-lymphotropic virus type III/lymphadenopathy-associated virus and acquired immunodeficiency syndrome. MMWR Morb Mortal Wkly Rep 1985,34:721–726, 731–722. [PubMed] [Google Scholar]
- 26. Ahmed S, Li Q, Liu L, Tsui AO. Maternal deaths averted by contraceptive use: an analysis of 172 countries. Lancet 2012,380:111–125. 10.1016/S0140-6736(12)60478-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are from the Partners PrEP Study, the Partners in Prevention HSV/HIV Transmission Study, and the Partners Demonstration Project. Due to agreements with the human subjects committees at each institution involved in this study, the authors are unable to post the data. If readers wish to request data, the primary contact person is Dr. Jared Baeten, jbaeten@uw.edu He will be able to facilitate access to the data upon request.