Abstract
The complex life cycle of the parasitic nematode Strongyloides stercoralis leads to either developmental arrest of infectious third-stage larvae (iL3) or growth to reproductive adults. In the free-living nematode Caenorhabditis elegans, analogous determination between dauer arrest and reproductive growth is governed by dafachronic acids (DAs), a class of steroid hormones that are ligands for the nuclear hormone receptor DAF-12. Biosynthesis of DAs requires the cytochrome P450 (CYP) DAF-9. We tested the hypothesis that DAs also regulate S. stercoralis development via DAF-12 signaling at three points. First, we found that 1 μM Δ7-DA stimulated 100% of post-parasitic first-stage larvae (L1s) to develop to free-living adults instead of iL3 at 37°C, while 69.4±12.0% (SD) of post-parasitic L1s developed to iL3 in controls. Second, we found that 1 μM Δ7-DA prevented post-free-living iL3 arrest and stimulated 85.2±16.9% of larvae to develop to free-living rhabditiform third- and fourth-stages, compared to 0% in the control. This induction required 24–48 hours of Δ7-DA exposure. Third, we found that the CYP inhibitor ketoconazole prevented iL3 feeding in host-like conditions, with only 5.6±2.9% of iL3 feeding in 40 μM ketoconazole, compared to 98.8±0.4% in the positive control. This inhibition was partially rescued by Δ7-DA, with 71.2±16.4% of iL3 feeding in 400 nM Δ7-DA and 35 μM ketoconazole, providing the first evidence of endogenous DA production in S. stercoralis. We then characterized the 26 CYP-encoding genes in S. stercoralis and identified a homolog with sequence and developmental regulation similar to DAF-9. Overall, these data demonstrate that DAF-12 signaling regulates S. stercoralis development, showing that in the post-parasitic generation, loss of DAF-12 signaling favors iL3 arrest, while increased DAF-12 signaling favors reproductive development; that in the post-free-living generation, absence of DAF-12 signaling is crucial for iL3 arrest; and that endogenous DA production regulates iL3 activation.
Author Summary
Strongyloides stercoralis is a parasitic nematode that infects hundreds of millions of people worldwide. The infectious form of S. stercoralis is a developmentally arrested third-stage larva (iL3); once inside the host, the iL3 activates and develops into an adult parasitic female. First-stage larvae (L1) excreted in the host feces have two routes of development: either directly to iL3 or indirectly to free-living adults. The molecular mechanisms controlling iL3 developmental arrest and activation, and the switch regulating post-parasitic L1 development, are poorly understood. The free-living nematode Caenorhabditis elegans has a developmentally arrested stage, morphologically similar to iL3, called dauer. Dauer formation is prevented by endogenous production of a class of steroid hormones called dafachronic acids (DAs), which are synthesized by a cytochrome P450. We demonstrated that in S. stercoralis, administering DA can both stimulate post-parasitic L1 to develop to free-living adults instead of iL3 as well as prevent iL3 developmental arrest. Additionally, blocking cytochrome P450 function prevents iL3 activation in a host-like environment, suggesting endogenous DA production. We also characterized the developmental expression of cytochrome P450s present in the S. stercoralis genome. Together, our data demonstrate that DA regulates S. stercoralis iL3 arrest and activation and the post-parasitic developmental switch.
Introduction
Strongyloides stercoralis is a parasitic nematode that infects both humans and dogs and is the causative agent of strongyloidiasis, which predominately afflicts socio-economically disadvantaged people in developing countries [1–3]. While chronic strongyloidiasis is often asymptomatic or accompanied by low-grade gastrointestinal symptoms, S. stercoralis infection in immunocompromised or corticosteroid-treated patients can progress to hyperinfection and disseminated strongyloidiasis, which can be fatal [4,5]. Understanding the mechanisms regulating the development of S. stercoralis may lead to improved diagnostic, control, and treatment strategies.
Similar to many nematodes, including the free-living nematode Caenorhabditis elegans, S. stercoralis has crucial points in its life cycle, where the organism is either fated towards reproductive adulthood or developmental arrest (Fig 1). Female post-parasitic first-stage larvae excreted in the feces of an infected host can undertake two possible routes of development: a homogonic route leading directly to developmentally arrested infectious third-stage larvae (iL3) or a heterogonic route leading to free-living adults [6]. This developmental switch is analogous to the switch between dauer arrest and reproductive development made by first-stage C. elegans larvae [7], with dauer arrest favored at high temperatures, low food abundance, and high population density, which is signaled by rising titers of constitutively-produced ascaroside pheromones [7–9]. S. stercoralis also exercises strict developmental controls in the post-free-living generation, where larvae invariably mature into non-feeding iL3; however, upon entering a permissive host, third-stage larvae resume feeding and development, eventually maturing into parthenogenetic parasitic females in the intestinal lumen [3,10,11]. Similarly, C. elegans dauer larvae resume feeding and develop to reproductive adults when environmental conditions improve [7]. Parallels between S. stercoralis iL3 and C. elegans dauer larvae extend beyond these functional similarities and include shared morphological features, including a long, radially constricted filariform pharynx, a plugged buccal cavity, and a stress-resistant cuticle [12–14]. While pathways regulating dauer arrest, and to a lesser extent dauer exit, have been well-studied in C. elegans [15], the developmental controls regulating S. stercoralis iL3 formation and activation have only recently been examined [16–20]. The "dauer hypothesis" predicts that similar mechanisms govern iL3 and dauer development [14,21]; however, given that C. elegans and S. stercoralis are members of two different nematode clades [22], with parasitism thought to have arisen independently in each [23], it is entirely plausible that different signaling mechanisms could regulate formation of S. stercoralis iL3 and C. elegans dauer larvae [14,24].
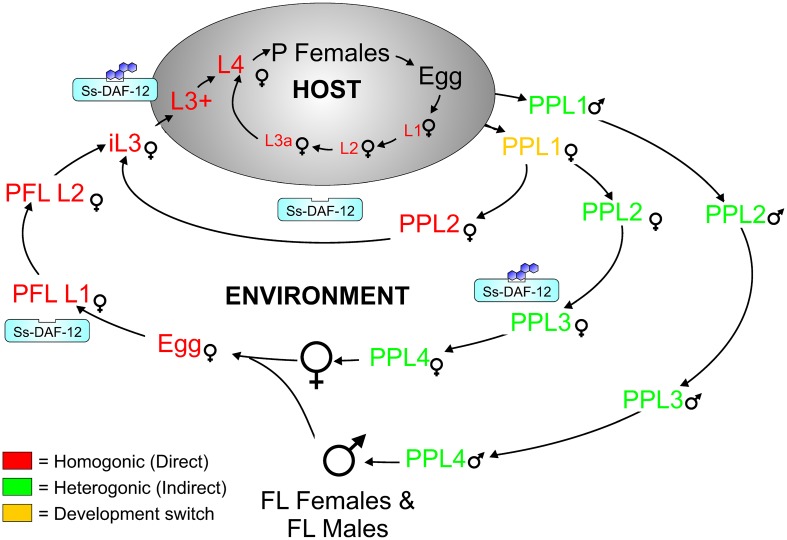
Fig 1. Hypothesized regulation of the Strongyloides stercoralis life cycle by the nuclear hormone receptor DAF-12.
The S. stercoralis parasitic female (P Female) produces larval progeny by mitotic parthenogenesis, and these progeny have several possible developmental fates. A female post-parasitic first-stage larva (PP L1) can either precociously develop inside the host to an autoinfective third-stage larva (L3a), which develops to a second-generation parasitic female, or be passed in the feces to develop outside the host by one of two routes: a homogonic route directly to a developmentally arrested infectious third-stage larva (iL3), which is favored at host-like temperatures (e.g., 37°C), or a heterogonic route to a free-living adult female (FL Female), which is favored at lower temperatures (e.g., 22°C). We hypothesize that this developmental checkpoint is regulated by dafachronic acid ligands for the nuclear hormone receptor Ss-DAF-12, with liganded Ss-DAF-12 favoring heterogonic development. Larval progeny of the single free-living generation of females and males invariably form iL3, and this developmental arrest is hypothesized to be governed by the absence of Ss-DAF-12 signaling. Once inside a host, the third-stage larva resumes development and feeding, resulting in a form designated the L3+. We hypothesize that resumption of development by iL3 entering the host and maturation to the P Female requires an increase in signaling by Ss-DAF-12, stimulated by increased biosynthesis of its steroid ligand.
In C. elegans, one of the primary mechanisms regulating the determination between reproductive development and dauer arrest involves a class of endogenous steroid hormones known as dafachronic acids (DAs) [25]. Under conditions promoting reproductive growth and development, DAs are abundant and bind the nuclear hormone receptor Ce-DAF-12, which controls a network of genes that carry out these functions. Conversely, conditions favoring dauer arrest lead to a paucity of DAs and to Ce-DAF-12 functioning as a co-repressor, thereby instituting a genetic program for developmental arrest [26]. This mutually exclusive developmental switch occurs in the first-stage larvae (L1) and must be reinforced to prevent development of worms with both dauer and adult attributes, which would be detrimental to the organism. When an L1 encounters favorable conditions, environmental cues—transduced by upstream cyclic guanosine monophosphate (cGMP) signaling, and then by parallel insulin/insulin-like growth factor (IIS) and dauer transforming growth factor β (TGFβ) signaling pathways [8,15]—trigger DA production in neuroendocrine XXX cells in the head of the developing larva [27]. The initial small quantity of DAs produced by XXX cells promotes further DA production throughout the hypodermis via a Ce-DAF-12-mediated positive feedback loop of DA synthesis, thereby ensuring the worm commits to reproductive development [28–30]. In C. elegans, the key enzyme in endogenous DA biosynthesis is the cytochrome P450 Ce-DAF-9 [28,31,32], which is reflected in a significant increase in Ce-daf-9 transcripts during reproductive development and dauer exit [29,33].
Similar to steroids in other animals, C. elegans DAs are derived from cholesterol [25], which is first modified by the Rieske-like oxygenase Ce-DAF-36 [34], and subsequently by the 3-hydroxysteroid dehydrogenase Ce-DHS-16 [35], before the final redox reaction is carried out by the hydroxylase Ce-DAF-9 in partnership with the NADPH-cytochrome P450 reductase Ce-EMB-8 [35]. Careful biochemical work originally described DAs as the Ce-DAF-12 ligands, the most potent of which is Δ7-DA [25]. More recent work examining metabolites of DAs has described Δ1,7-DA and 3α-OH-Δ7-DA as additional ligands of Ce-DAF-12 [36]. When DAs are absent, the co-repressor Ce-DIN-1 blocks Ce-DAF-12 activity [25,37]. When DAs are present, they bind Ce-DAF-12 and reverse Ce-DIN-1 repression [25], allowing Ce-DAF-12 to simultaneously increase the transcription of Ce-let-7 microRNA family members that block dauer-formation pathways [38–41] and initiate a reproductive developmental program that promotes the aerobic catabolism of fatty acids for growth [42].
In parasitic nematodes, the mechanisms controlling iL3 arrest and activation as well as homogonic and heterogonic development in Strongyloides spp. are less well-understood [43]. The developmental checkpoint regulating homogonic versus heterogonic development in female post-parasitic L1 is regulated by both strain genetics and temperature, with commitment occurring early in L1 development in S. stercoralis [44] and the closely related Strongyloides ratti [45]. In the S. stercoralis UPD strain used in this study, >95% of larvae develop via the heterogonic route; other isolates range from fully homogonic to fully heterogonic in their development [46,47]. Similarly, frequencies of homogonic and heterogonic development vary among geographical isolates and over the course of infection in S. ratti [48]. Temperature also regulates the developmental switch between homogonic and heterogonic pathways in Strongyloides spp. In S. stercoralis, temperatures below 34°C signal post-parasitic L1 to take the heterogonic pathway, while temperatures similar to that of the host, 34°C or above, result in development directly to iL3 [44]. Moreover, the two amphidial neurons ASF and ASI regulate the developmental switch between these two routes; when both neurons are inactivated, the vast majority of post-parasitic L1 develop homogonically even at temperatures below 34°C [49]. This is similar to the regulation of the developmental switch in C. elegans L1 by the analogous ADF and ASI amphidial neurons [50]. However, no specific cellular signal transduction pathway has been implicated in regulating this developmental switch in Strongyloides spp.
In S. stercoralis and closely related parasitic nematodes, including S. ratti and Strongyloides papillosus, progeny of the single generation of free-living male and female adults invariably form developmentally arrested iL3, which are all genetically female—thus leading to a strictly female parasitic generation [6]. However, this post-free-living developmental fate is not shared by all members of the Strongyloides genus, as Strongyloides planiceps can produce a limited number of free-living generations of males and females [51], and the evolutionarily more distant Parastrongyloides trichosuri can produce apparently unlimited generations of free-living males and females [52]. While the formation of P. trichosuri iL3 is mediated by a constitutively secreted pheromone [53], similar to C. elegans dauer pheromone [54,55], this does not appear to be the case for S. stercoralis because iL3 form in the post-free-living generation regardless of population density. However, S. stercoralis iL3 arrest does require reduced IIS [17]. Interestingly, application of exogenous Δ7-DA to the post-free-living generation of S. stercoralis and S. papillosus prevents iL3 arrest, resulting in rhabditiform L3 and L4 in S. stercoralis [56] and a second generation of fecund free-living females in S. papillosus [57], thus suggesting that iL3 arrest may be the result, in part, of diminished DAF-12 signaling. However, the duration of exposure to DA necessary to induce these phenotypes has been unknown.
Of the developmental checkpoints, factors regulating iL3 activation in S. stercoralis and other parasitic nematodes are perhaps the best studied. S. stercoralis iL3 exhibit positive chemotaxis and thermotaxis towards a variety of molecules indicative of a host [58], including carbon dioxide [59], sodium chloride [60], urocanic acid [61], and host body temperature [62], with many of these responses mediated by amphidial neurons. Upon entering a permissive host, iL3 quickly resume feeding and development, a process that is mediated in part by ASJ amphidial neurons [63]. Resumption of feeding is accompanied by modulation of insulin-like peptide transcripts [19,20], while inhibition of IIS prevents iL3 feeding [18]. Furthermore, increases in cGMP signaling and DA signaling, by exogenous application of these compounds, trigger iL3 feeding [20,56]. However, to our knowledge, it remains unknown whether S. stercoralis, or any other parasitic nematodes, produces endogenous ligands for DAF-12.
In this study using S. stercoralis, we demonstrate that DA modulates the post-parasitic switch regulating the decision between reproductive development and iL3 arrest, with increased DAF-12 signaling favoring reproductive development. Furthermore, we demonstrate that in the post-free-living generation, exposure to DA also effects a shift from iL3 arrest, favoring formation of reproductively developing larvae. In the majority of worms, this commitment to reproductive development occurs within 24–48 hours of DA exposure. We also provide the first evidence for endogenous biosynthesis of Ss-DAF-12 ligand, as a blockade of iL3 activation by a chemical inhibitor of cytochrome P450s is partially overridden by administration of DA.
Methods
The UPD strain of S. stercoralis, originally isolated from naturally infected dogs in 1976, was maintained and cultured as previously described [64,65].
Ethics statement
S. stercoralis was maintained in purpose-bred, prednisone-treated mix breed dogs and in purpose-bred Mongolian gerbils according to protocols 802593 and 804883 approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC). All IACUC protocols, as well as routine husbandry care of the animals, were conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Developmental switching of S. stercoralis post-parasitic larvae by Δ7-DA
Post-parasitic L1 of S. stercoralis that were unexposed to environmental cues affecting development were harvested from the intestines of experimentally infected gerbils at necropsy as follows. Tum/Mon strain Meriones unguiculatus (Mongolian gerbils) were experimentally infected with 3,000 iL3 and euthanized 21 days later by CO2 asphyxiation in accordance with standards established by the American Veterinary Medical Association. The intestines from individual animals were placed in Dulbecco's Modified Eagle's Medium (DMEM), supplemented with 1 mg/ml gentamicin sulfate, with either 1 μM (25S)-Δ7-DA (CAS 949004-12-0) or ethanol carrier (0.1% ethanol) at 37°C. Post-parasitic L1 were picked from the intestinal debris using a stereomicroscope with a 37°C heated stage. Post-parasitic L1 harvested in DMEM with Δ7-DA were transferred to a 35 x 10 mm nematode growth medium (NGM) plate containing 3 ml of agar and spotted with 300 μl of E. coli OP50 containing 10 μM Δ7-DA, resulting in a final concentration of 1 μM Δ7-DA (0.1% ethanol), and incubated at 37°C. Post-parasitic L1 harvested in DMEM with ethanol carrier were transferred to NGM plates spotted with E. coli OP50 containing ethanol carrier and incubated at either 37°C or 22°C. The developmental stage of the developing larvae was recorded for all three conditions at both 24 and 48 hours post-plating. Five biological replicates were performed, and the mean percentages of larvae developing to either filariform iL3 or rhabditiform L3-Adult forms, with the standard deviation, were calculated. Proportions of worms in each developmental class as functions of temperature and presence of Δ7-DA were analyzed by 2-way ANOVA with post hoc comparisons of selected frequencies by the Bonferroni test.
Induction of rhabditiform post-free-living L3-L4 forms by Δ7-DA: Assessing dose dependency
Petri dishes (35 x 10 mm) containing 3 ml of NGM agar were seeded with 300 μl of a suspension of E. coli OP50 in LB broth containing Δ7-DA at concentrations ranging from 331 nM to 10 μM. Assuming uniform dispersal of the compound in the agar, this resulted in NGM/OP50 plates containing 33.1 nM to 1 μM Δ7-DA. Control plates containing 0.1% ethanol, the maximal concentration of Δ7-DA carrier to which the worms were exposed, were made by spotting plates with OP50 suspensions containing 1% ethanol. Semi-synchronous populations of S. stercoralis eggs were prepared on experimental and control plates by transferring 20–30 gravid free-living S. stercoralis females to each plate and allowing them to oviposit for three hours at room temperature. At the end of this interval, egg-laying worms were removed from the plates, which then contained cohorts of 50–200 eggs. Plates with eggs were then sealed with Parafilm and incubated for 72 hours at 22°C. Following this incubation, developing worms were classed as rhabditiform L1-L2, filariform iL3, or rhabditiform L3-L4. Mean percentages of worms in each developmental class from three experimental replicates, with standard deviations, were plotted as a function of Δ7-DA concentration. The EC50 for induction of rhabditiform L3-L4 forms was calculated by non-linear regression of frequency in the L3-L4 developmental class on log-transformed Δ7-DA concentrations.
Kinetics of rhabditiform L3-L4 induction by Δ7-DA: ascertaining a discrete, early triggering event vs. the requirement for continuous exposure
We established triplicate semi-synchronous cultures of post-free-living S. stercoralis larvae in the same range of Δ7-DA concentrations as described above for the dose-response assessment. At intervals of 24 and 48 hours in culture at 22°C, worms from one of the triplicate cultures at each Δ7-DA concentration were washed off the plate with M9 buffer and then subjected to two additional washes in 10 volumes of M9 buffer; subsequently, the worms were re-plated on non-DA-treated plates and cultured for the balance of the standard 72-hour culture period at 22°C. Worms cultured for 72 hours in the presence of Δ7-DA constituted the continuously exposed controls. At the end of the 72-hour incubation, developing S. stercoralis larvae were classed as rhabditiform L1-L2, filariform iL3, or rhabditiform L3-L4 as before. Mean percentages, and standard deviations, of worms in the L3-L4 class in each concentration of Δ7-DA were calculated for three experimental replicates. Effects of Δ7-DA exposure duration and concentration were analyzed by 2-way ANOVA with post hoc pairwise comparisons of frequencies by the Bonferroni test.
S. stercoralis iL3 in vitro activation
In vitro activation of S. stercoralis iL3 was performed as previously described [18,20,63] with the following adaptations. All conditions were supplemented with antibiotics (final concentrations: 100 U/ml penicillin, 10 μg/ml streptomycin, and 12.5 μg/ml tetracycline). iL3 were isolated from seven-day-old charcoal coprocultures (incubated at 25°C) by the Baermann technique at 27–29°C. iL3 were subsequently washed twice in deionized water and incubated in M9 buffer [66], supplemented with antibiotics, for three hours at room temperature before distribution amongst the different conditions.
Experiments examining inhibition of iL3 activation with ketoconazole (CAS 65277-42-1; Sigma) were carried out in DMEM supplemented with L-glutamine, 4.5 g/L glucose, and sodium pyruvate (Corning). This medium with the indicated supplements supports resumption of feeding by a majority of S. stercoralis iL3 at 37°C without additional host-like factors [20]. A 10 mM stock solution of ketoconazole in dimethyl sulfoxide (DMSO) was used for the experimental conditions, which included varying concentrations of ketoconazole (10 μM, 20 μM, 30 μM, 40 μM, and 60 μM) in DMEM, each with 0.8% DMSO. The negative control was M9 buffer, and the positive control was DMEM, each with 0.8% DMSO.
Experiments to ascertain rescue of ketoconazole-mediated inhibition of iL3 activation by Δ7-DA were carried out in DMEM. A 1 mM stock solution of Δ7-DA in ethanol was used for the experimental conditions, which included varying concentrations of Δ7-DA (50 nM, 200 nM, 400 nM, and 800 nM) in DMEM with 35 μM ketoconazole; each condition contained 0.35% DMSO and 0.2% ethanol. The positive controls were DMEM and DMEM supplemented with 800 nM Δ7-DA, while the negative control was M9 buffer; each condition contained 0.35% DMSO and 0.2% ethanol.
Each condition consisted of three wells in a 96-well plate, with approximately 100 iL3 in 100 μl total volume in each well. iL3 were incubated at 37°C in 5% CO2 in air for 21 hours; subsequently, 2 μl of fluorescein isothiocyanate (FITC) (CAS 3326-32-7; Sigma) dissolved in N,N-dimethylformamide (DMF) (CAS 68-12-2; Sigma) at 20 mg/ml and incubated for ≥ one month was added to each well, and the cultures were incubated an additional three hours at 37°C and 5% CO2 in air (24 hours total). Pre-incubation of FITC solutions was empirically determined to decrease binding of FITC to iL3 cuticles relative to that seen with fresh dilutions of the dye in DMF. iL3 for each condition were pooled and washed five times in 14 ml of M9 buffer, with centrifugation at 75 x g for five minutes at 20°C. iL3 were then mounted on glass slides with grease-edged cover-slips and viewed by fluorescence microscopy using an SZX12 stereomicroscope (Olympus) equipped with an X-Cite 120LED illuminator (Lumen Dynamics). Only live iL3 (indicated by movement) with FITC in the pharynx were scored as "positive" for feeding. Apart from loss of the buccal plug and resumption of feeding, no further development of the iL3 occurs in this system. Dead worms, indicated by lack of movement and/or whole-body FITC staining, were excluded from the feeding analysis, and the percentage of dead worms was determined from the total number of worms present. At least 200 iL3 were counted for each condition. Four biological replicates were performed, and the mean percentage of iL3 feeding in each condition, with the standard deviation, was plotted. Coefficients of correlations between ketoconazole concentration and iL3 feeding and between Δ7-DA concentration and iL3 feeding in the presence of 35 μM ketoconazole were computed by the nonparametric Spearman method. IC50 for ketoconazole inhibition of iL3 feeding and EC50 for Δ7-DA rescue of iL3 feeding in the presence of 35 μM ketoconazole were calculated by non-linear regression of iL3 feeding frequency on concentrations of ketoconazole and Δ7-DA, respectively.
Identification of S. stercoralis cytochrome P450s
To identify S. stercoralis homologs of cytochrome P450-encoding genes, we performed reciprocal BLAST searches of the S. stercoralis genome v.2.0.4 (available: ftp://ftp.sanger.ac.uk/pub/project/pathogens/HGI/) with C. elegans cytochrome P450 protein sequences (WormBase release WS245) using Geneious v.6.1.8 (Biomatters Ltd.); BLAST hits were manually annotated using RNAseq data viewed with the Integrative Genomics Viewer v.2.0.34 (available: www.broadinstitute.org/igv/) and Geneious [19,20]. Using mapped and de novo assembled RNAseq data, coding sequences were manually corrected to derive full-length coding sequences (S1 Data) and putative protein sequences (S2 Data). Putative cytochrome P450-encoding genes were named, or renamed from those previously described [19], using the standard convention [67]. These genes were identified and related using a combination of reverse-BLAST searches, a protein-identity matrix, and a ClustalW-generated protein alignment (S3 Data) and neighbor-joining phylogenetic tree, with 1000 iterations of boot-strapping, utilizing metazoan cytochrome P450 protein sequences, including C. elegans, Caenorhabditis briggsae, and Homo sapiens. All of these analyses were performed with Geneious.
Transcript abundances for each of the S. stercoralis cytochrome P450-encoding genes were determined as previously described [19,20], with the following adaptations. RNAseq raw reads, derived from polyadenylated RNA libraries, for iL3 (ArrayExpress accession number E-MTAB-2192; http://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-2192/) [20] as well as in vivo activated L3 (L3+), parasitic females, post-parasitic L1, post-parasitic L3 enriched for females, free-living females, and post-free-living L1 (ArrayExpress accession number E-MTAB-1164; http://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-1164) [19], were mapped to the S. stercoralis v.2.0.4 genome contigs using Tophat2 v.2.0.13 [68], which utilized Bowtie2 v2.2.4 [69] and Samtools v0.1.18 [70], and previously established parameters [20]. Normalized transcript abundances for cytochrome P450-encoding genes (S4 Data), calculated as fragments per kilobase of coding exon per million fragments mapped (FPKM) with paired-end reads counting as single sampling events, and 95% confidence intervals were determined using Cuffdiff v.2.0.2 [71].
Data analysis
For all experiments, statistical analyses were carried out and plots were created with Prism version 5.03 (GraphPad Software, Inc.). Statistical probabilities P < 0.05 were considered significant.
Results
Exogenous Δ7-DA regulates the developmental fate of post-parasitic larvae of S. stercoralis
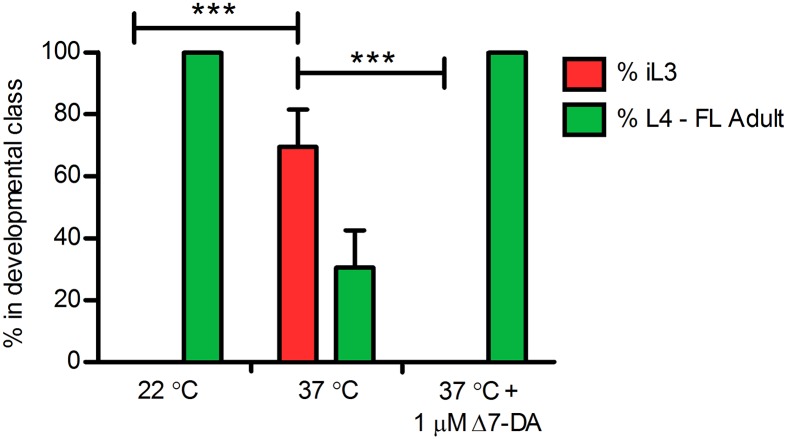
Based on its role in promoting continuous reproductive development in C. elegans [26], we hypothesized that Δ7-DA or a similar Ss-DAF-12 ligand promotes heterogonic development by post-parasitic larvae of S. stercoralis (Fig 1). Conversely, we hypothesized that down-regulation of Ss-DAF-12 ligand(s) should drive direct development of post-parasitic L1 to iL3. We tested these hypotheses by administering Δ7-DA to cultures of post-parasitic L1 developing at 37°C, where direct development predominates. As expected, control larvae reared at 22°C developed almost exclusively to free-living adults. However, a mean of 69.4 ± 12.0% (standard deviation) of control larvae reared at 37°C developed directly to iL3, with the remaining minority developing to free-living adults. In contrast to controls reared at 37°C, larvae reared at this temperature in the presence of 1 μM Δ7-DA developed exclusively to free-living adults (Fig 2). These results demonstrate that Ss-DAF-12 signaling regulates switching between homogonic and heterogonic developmental alternatives in post-parasitic larvae of S. stercoralis. Moreover, the ability of Δ7-DA to override the high temperature signal to post-parasitic L1 indicates that Ss-DAF-12 signaling operates downstream of mechanisms transducing temperature cues to the larvae from the environment.
Fig 2. Δ7-Dafachronic acid regulates the developmental switch controlling the homogonic or heterogonic fates of post-parasitic female larvae of Strongyloides stercoralis.
The frequency distribution of homogonically (red bar) and heterogonically (green bars) developing S. stercoralis post-parasitic females in cultures maintained at 22°C or at 37°C in the presence or absence of 1 μM Δ7-dafachronic acid (Δ7-DA) was plotted. Data are based on counts of worms, explanted to culture as hatchling first-stage larvae from the intestines of experimentally infected gerbils, scored for development at 24 and 48 hours of culture and assigned to one of two developmental classes representing larvae developing by the homogonic route to infectious third-stage larvae (iL3) or larvae developing via the heterogonic route to free-living females via rhabditiform second, third, and fourth larval stages (L4-FL Adult). Post-parasitic males were excluded from the analysis, as males only develop heterogonically. The bar height represents the mean of five biological replicates and the error bar +1 standard deviation. Brackets indicate statistical comparisons of iL3 frequency at 37°C to frequencies at both 22°C and 37°C with 1 μM Δ7-DA; *** indicates P < 0.001.
Δ7-DA induces a time-dependent development of free-living fourth-stage larvae among the progeny of free-living males and females
Under normal circumstances, the progeny of free-living male and female S. stercoralis develop exclusively to iL3 [3]. Just as absence of DA synthesis promotes dauer arrest in C. elegans [25], we hypothesized that the invariant pattern of iL3 formation that takes place in the post-free-living generation of S. stercoralis results from absence of Ss-DAF-12 ligands (Fig 1). In support, we previously demonstrated that exogenous Δ7-DA can induce a portion of post-free-living S. stercoralis larvae to bypass iL3 developmental arrest, developing instead to rhabditiform L3-L4 [56]. Moreover, similar concentrations of Δ7-DA are known to induce post-free-living larvae of S. papillosus to develop to reproductively competent second-generation free-living females [57]. Thus, we sought to achieve a more precise dose-response profile of Δ7-DA induction of development to advanced rhabditiform larvae or adults by post-free living S. stercoralis larvae; additionally, we sought to ascertain whether this induction occurs in a discrete time interval, or conversely, whether Δ7-DA is required throughout the period of post free-living larval development in order to effect this switch in developmental fate.
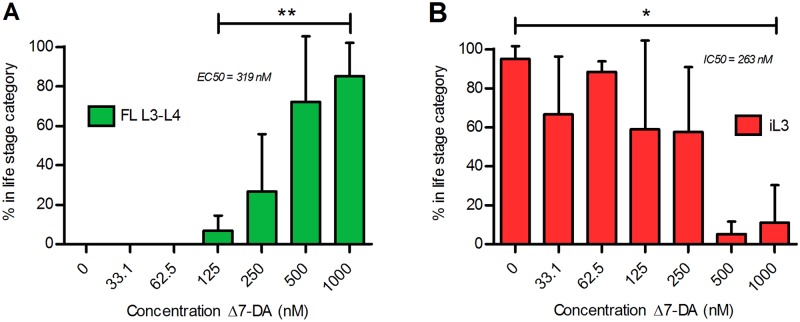
We first cultured semi-synchronous populations of post-free-living S. stercoralis larvae for 72 hours at 22°C on NGM agar plates with lawns of E. coli OP50 and fecal bacteria and with Δ7-DA at concentrations ranging from 0 to 1 μM and then assessed the degree of larval development. In the range of 125 nM to 1 μM, Δ7-DA brought about a dose-dependent increase in the frequency of larvae developing to rhabditiform L3 and L4 (Fig 3A). The EC50 for this response was 318 nM, which is comparable to the EC50 of 147 nM for activation of the Ss-DAF-12 ligand-binding domain by Δ7-DA in a cell-based reporter assay [56]. As proportions of rhabditiform L3 and L4 increased in response to increasing Δ7-DA concentration, proportions of larvae developing to the iL3 declined (Fig 3B). Proportions of larvae remaining as L1 and L2 remained roughly constant at all concentrations of Δ7-DA (S1 Fig).
Fig 3. Exogenous Δ7-dafachronic acid promotes formation of Strongyloides stercoralis second-generation free-living larvae and blocks the formation of infective third-stage larvae.
Post-free-living larvae of S. stercoralis developing synchronously in agar plate cultures were exposed to increasing concentrations of Δ7-dafachronic acid (Δ7-DA). A) Frequency of development to rhabditiform post-free-living third- and fourth-stage larvae (FL L3-L4) as a function of Δ7-DA concentration. The sample denoted 0 nM Δ7-DA is the ethanol control. ** Positive correlation of FL L3-L4 frequency with Δ7-DA concentration is significant (P = 0.001; R2 = 0.902). B) Frequency of development to infective third-stage larvae (iL3) as a function of Δ7-DA concentration, with 0 nM Δ7-DA as the ethanol control. Negative correlation of iL3 frequency with Δ7-DA concentration is significant (P = 0.0135; R2 = 0.736). In both panels, bar height represents the mean of three biological replicates; error bars represent +1 standard deviation.
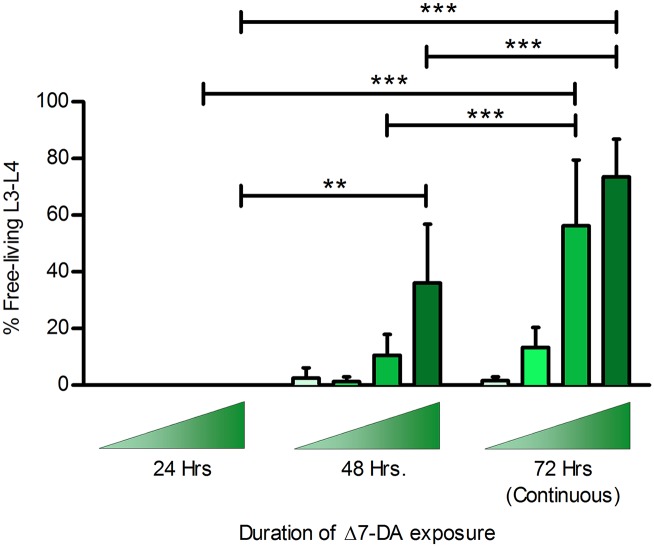
To ascertain discrete developmental triggering by Δ7-DA, we initiated semi-synchronous plate cultures of post-free-living S. stercoralis larvae in the presence of increasing concentrations of Δ7-DA. We then washed cohorts of larvae out of the compound at 24 and 48 hours of culture, re-plated them on non-DA-treated plates, and continued culture at 22°C for the balance of the 72-hour culture period. At 72 hours of culture, we compared the frequency of development to rhabditiform L3-L4 in these transiently exposed worms to that of larvae developing for 72 hours at 22°C under continuous exposure to Δ7-DA. None of the larvae exposed to Δ7-DA for the first 24 hours of culture developed to rhabditiform L3-L4 (Fig 4). However, a significant proportion of larvae exposed to Δ7-DA for the first 48 hours developed to rhabditiform L3 and L4. Proportions of larvae undergoing the developmental switch increased with increasing concentration of Δ7-DA. Thus, it appears that between 24 and 48 hours of development at 22°C, a significant proportion of larvae exposed to exogenous Δ7-DA commit to bypassing the iL3 and developing instead to rhabditiform L3-L4. Despite these kinetic refinements, post-free-living S. stercoralis larvae failed to develop to sexually mature free-living adults, as S. papillosus larvae do when treated with similar levels of Δ7-DA [57].
Fig 4. Formation of second-generation free-living larvae in Strongyloides stercoralis requires 24–48 hours of exposure to Δ7-dafachronic acid.
Frequency of development to second-generation free-living rhabditiform L3 and L4 (FL L3-L4) in S. stercoralis was plotted as a function of duration of exposure to increasing concentrations of Δ7-dafachronic acid (Δ7-DA). Ascending shaded triangles indicate that developmental frequencies were determined at Δ7-DA concentrations of 125 nM (lightest), 250 nM, 500 nM, and 1,000 nM (darkest). Bar height represents the mean of three biological replicates; error bars represent +1 standard deviation. The overall effects of Δ7-DA exposure duration and concentration were significant, P < 0.0001. For all possible pairwise statistical comparisons of FL L3-L4 frequency with DA exposure durations of 24, 48, and 72 hours, ** P < 0.01; *** P < 0.001.
Suppression of feeding by the cytochrome P450 inhibitor ketoconazole in iL3 of S. stercoralis is reversed by Δ7-DA
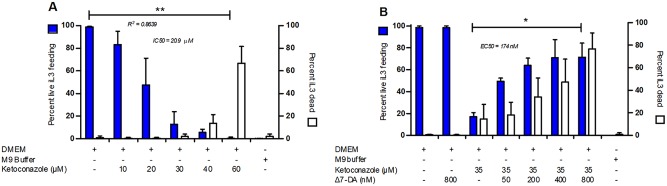
If we consider the iL3 of S. stercoralis to be the developmental equivalent of C. elegans dauer larvae, then resumption of development by these infectious larvae during the infective process would be the equivalent of dauer recovery [14]. Based on the role of Δ7-DA in promoting continuous development in C. elegans [25,72], we hypothesized that biosynthesis of this or other Ss-DAF-12 ligand(s) is necessary for resumption of development by S. stercoralis iL3 at the time of infection (Fig 1). In C. elegans, the cytochrome P450 encoded by Ce-daf-9 is required for biosynthesis of Δ7-DA from its precursor lathosterone [25,35]. Extending this analogy to S. stercoralis, we hypothesized that cytochrome P450 activity is required for endogenous biosynthesis of DA(s) or related Ss-DAF-12 ligand(s) and that inhibition of this activity would block resumption of development by iL3 at the time of infection. To test this, we asked whether the cytochrome P450 inhibitor ketoconazole could inhibit resumption of development by S. stercoralis iL3 under host-like in vitro culture conditions. Ketoconazole suppressed resumption of feeding by S. stercoralis iL3 cultured under permissive conditions. The positive control (DMEM) cultures, which reflect host-like biochemical conditions, supported resumption of feeding by a mean of 98.8 ± 0.4% (mean and standard deviation) of the iL3 (Fig 5A). The inhibitory effect of ketoconazole administered to iL3 in DMEM cultures was dose-dependent and maximal at 40 μM and higher, with a mean of 5.6 ± 2.9% of iL3 feeding at 40 μM. In the M9 buffer negative control, 0.1 ± 0.3% of iL3 were feeding.
Fig 5. Ketoconazole inhibits developmental activation of Strongyloides stercoralis iL3 in host-like culture conditions; this inhibition is rescued by Δ7-dafachronic acid.
Frequency of feeding by cultured larvae, as reflected by ingestion of fluorescein isothiocyanate (FITC), was used as an index of developmental activation of infectious third-stage larvae (iL3). Larval mortality was scored as the percentage of larvae classed as non-motile and therefore “dead.” (A) Frequency of iL3 feeding and larval mortality as a function of ketoconazole concentration in Dulbecco’s modified Eagle’s Medium (DMEM), a permissive culture medium. DMEM without ketoconazole was the positive control. M9 buffer, a non-permissive medium, was used without ketoconazole as the negative control. ** Negative correlation of iL3 feeding and ketoconazole concentration (bracketed) is significant (P = 0.0028; Spearman r = -1.000). (B) Frequency of iL3 feeding and larval mortality as a function of Δ7-dafachronic acid (Δ7-DA) concentration in DMEM cultures containing an inhibitory concentration of ketoconazole (35 μM). Cultures of larvae in DMEM alone or in DMEM with 800 nM Δ7-DA were positive controls. Cultures of larvae in M9 buffer constituted the negative control. * Positive correlation of iL3 feeding in 35 μM ketoconazole and Δ7-DA concentration (bracketed) is significant (P = 0.0167; Spearman r = 1.000). In both panels, bar heights represent the mean percentage of iL3 feeding (blue bars) or the mean percentage of dead iL3 (white bars) in four biological replicates. Error bars indicate +1 standard deviation.
Ketoconazole broadly inhibits cytochrome P450s [73], and so its suppression of feeding by developing S. stercoralis iL3 could result from inhibition of multiple such enzymes, including the ortholog of C. elegans DAF-9. To address this uncertainty, we asked what proportion of the observed suppression of feeding by ketoconazole could be attributable to depletion of Δ7-DA or a similar developmental regulatory steroid from the worms. To this end, we cultured iL3 in permissive medium (DMEM) containing either 35 μM ketoconazole alone or in 35 μM ketoconazole supplemented with increasing concentrations of Δ7-DA. In DMEM with 35 μM ketoconazole, Δ7-DA restored feeding responses among cultured iL3 in a dose-dependent fashion to a maximum of 71.2 ± 16.4% feeding in 400 nM Δ7-DA, compared to 17.1 ± 3.8% feeding in DMEM cultures with 35 μM ketoconazole alone (Fig 5B). This represents a 4-fold increase in feeding over worms cultured in 35 μM ketoconazole alone and accounts for approximately two-thirds of the feeding response seen in non-ketoconazole-treated controls. Mortality, as reflected by the proportion of non-motile (scored as dead) worms, increased as a function of Δ7-DA concentration in the presence of 35 μM ketoconazole (Fig 5B). At concentrations of 200 nM Δ7-DA and higher, the frequency of this mortality was higher than that observed at any concentration of Δ7-DA alone or at comparable levels of ketoconazole (Fig 5A), suggesting a synergistic toxic interaction between ketoconazole and Δ7-DA in this range of concentrations.
The S. stercoralis genome contains 26 cytochrome P450-encoding genes
Our findings that the CYP inhibitor ketoconazole suppresses resumption of feeding by iL3 under host-like culture conditions and that this effect can be partially rescued by Δ7-DA suggest that biosynthesis of a steroidal ligand of Ss-DAF-12 is necessary for this developmental step. The key enzyme for DA biosynthesis in C. elegans is the cytochrome P450 DAF-9, which is homologous to the human CYP27A1 that produces bile acids [25]. In order to identify potential homologs of cytochrome P450-encoding genes in S. stercoralis, including a potential DAF-9 ortholog, we performed reciprocal BLAST searches, followed by manual annotation and correction of the hits to derive putative cytochrome P450 protein sequences (S2 Data). This resulted in the determination of 26 cytochrome P450-encoding genes in S. stercoralis, which were grouped by family and subfamily by standard cytochrome P450 nomenclature [67]. Of the 26 cytochrome P450s, there were 2 subfamilies with several members which, due to their spatial proximity in the genome and sequence similarity, likely arose as a result of tandem gene duplications. These are the CYP3A subfamily, which contains seven genes, and the CYP29A family, which consists of nine.
Since C. elegans daf-9 transcripts are up-regulated during reproductive growth and development as well as during dauer exit [28,31,33], we examined the transcript abundance regulation for each of the cytochrome P450 homologs during the S. stercoralis life cycle (S5 Data and S2 Fig). Congruent with the hypothesis that iL3 activation is mediated by a cytochrome P450, we identified several genes that had an increase in transcript abundance from iL3 to in vivo activated L3 –members of the 3A subfamily: Ss-cyp3a23 and Ss-cyp3a27; members of the 29A subfamily: Ss-cyp29a6, Ss-cyp29a13, Ss-cyp29a25, and Ss-cyp29a26; Ss-cyp33e2; and Ss-cyp22a9. We identified Ss-cyp22a9, previously identified as Ss-cyp-9 [19], as the homolog with the closest similarity to Ce-daf-9, based on both its phylogenetic relation to Ce-DAF-9 (S3 Fig) and its increased transcript abundance in developing larvae and during iL3 activation (S2 Fig). Confirmation of Ss-CYP22A9 as the ortholog of Ce-DAF-9 awaits future functional studies.
Discussion
In this study, we hypothesized that DA signaling in S. stercoralis, through the nuclear hormone receptor DAF-12, would stimulate reproductive growth and development, while decreased DAF-12 activity, resulting from a reduction in DA, would cause developmental arrest. We interrogated several developmental checkpoints in S. stercoralis to determine whether DA signaling regulates the parasite's developmental program. Our data suggest that DA regulation of DAF-12 signaling plays an important role in the development of iL3 in this pathogen.
While hookworm, filarial worm, and ascarid larvae all constitutively develop to iL3, similar to dauer constitutive (daf-c) mutants in C. elegans [13], post-parasitic larvae from Strongyloides spp. can form a non-obligatory free-living generation of male and female adult worms. However, the molecular mechanisms regulating the developmental switch in the post-parasitic L1 that controls homogonic and heterogonic development have remained elusive. The strain of S. stercoralis used in this study, the UPD strain, almost exclusively develops via the heterogonic route, whereby post-parasitic female larvae develop to a single free-living generation of adult worms. Other S. stercoralis isolates have post-parasitic females that develop predominantly via the homogonic route directly to iL3, which are always female, or via a mix of heterogonic and homogonic development [46]. In this study, we demonstrated that developmentally uncommitted female post-parasitic L1 maturing at an elevated temperature, where iL3 arrest normally predominates, may instead be stimulated with exogenous Δ7-DA to develop to free-living adults via the heterogonic pathway (Fig 2). These data not only establish a role for Ss-DAF-12 in regulating the post-parasitic L1 checkpoint, but also demonstrate that signaling through this nuclear receptor lies downstream of the pathway sensing thermal cues. This is consistent with findings in C. elegans, where AFD thermosensitive neurons transduce information through cGMP signaling [74], which lies upstream of DAF-12 signaling [26]. Future studies comparing the effect of DA on post-parasitic L1 development in other S. stercoralis strains that are genetically predisposed to homogonic development may shed additional light on the requirement for DAF-12 signaling in heterogonic development and the influence of additional signaling pathways on this checkpoint.
S. stercoralis post-free-living larvae invariably undergo developmental arrest as iL3 in physiological conditions; however, another Strongyloides spp., S. planiceps, can form several successive generations of free-living adults [43,51]. We hypothesized that addition of DA would stimulate S. stercoralis post-free-living L1 to complete a second free-living generation, similar to that observed with S. papillosus [57], which also has a single free-living generation in physiological conditions. We found that rearing S. stercoralis post-free-living larvae in the presence of Δ7-DA suppressed iL3 formation and favored development of rhabditiform L3-L4 (Fig 3), which were morphologically similar to worms undergoing free-living development. While increasing concentrations of Δ7-DA increased the proportion of larvae developing to rhabditiform L3-L4, no free-living adult females were observed. We hypothesize that this difference from the result observed in S. papillosus may be due to a requirement in S. stercoralis for additional stimulatory factors or the possibility that Δ7-DA is not the endogenous ligand for S. stercoralis DAF-12.
We also sought to determine the length of exposure to Δ7-DA required to stimulate S. stercoralis post-free-living larvae to develop into rhabditiform L3 and L4. Based on work with C. elegans, where a small quantity of endogenously-produced ligand results in a DAF-12-mediated amplification loop [28], we expected that only a brief pulse of Δ7-DA would be required to prevent iL3 arrest. However, we found that post-free-living larvae required 24–48 hours of exposure to Δ7-DA (Fig 4). This suggests that repressive mechanisms during iL3 arrest actively inhibit DAF-12 function, a phenomenon that could result from: production of DAF-12 antagonists; a decrease in DA precursors by the action of an S. stercoralis strm-1 homolog [75], which is supported by an increase in Ss-strm-1 transcripts in iL3 [19]; metabolism of DAs into inactive compounds, potentially by other cytochrome P450s that are up-regulated in iL3 (S2 Fig); or the possibility that Δ7-DA is only a weak agonist for Ss-DAF-12. We assume that such repressive mechanisms or pharmacokinetic differences are not found in S. papillosus, where comparable levels of exogenously applied Δ7-DA elicited formation of reproductively competent second-generation free-living females [57].
Δ7-DA causes S. stercoralis iL3 to resume feeding, which is a hallmark of activation, and initiates a developmental program similar to activation in a permissive host [20,56]. Consequently, we hypothesized that S. stercoralis endogenously produces DAs or similar Ss-DAF-12 ligands during iL3 activation. In C. elegans, the final biosynthetic step in the production of Δ7-DA is performed by the cytochrome P450 Ce-DAF-9, and we hypothesized that a similar enzyme in S. stercoralis produces endogenous Ss-DAF-12 ligands. In order to test this hypothesis, we used ketoconazole, which is a cytochrome P450 CYP3A family inhibitor at nanomolar concentrations and a broad-spectrum P450 inhibitor at micromolar concentrations [73], to broadly inhibit cytochrome P450 function in S. stercoralis iL3. We found that ketoconazole does indeed inhibit feeding in S. stercoralis iL3 in a dose-dependent fashion in the micromolar range (Fig 5A), consistent with the synthesis of an endogenous steroid hormone during activation. Inhibition of iL3 feeding by ketoconazole in hookworm species suggests that DA signaling may also be important in iL3 activation in Clade V parasitic nematodes [56,76], which are more closely related to C. elegans [23]. However, in S. stercoralis, this ketoconazole-mediated inhibition may be due to a block in the function of a DAF-9 homolog or of one/several of the other cytochrome P450s in the worm. Thus, we sought to determine the extent to which this phenotype could be attributable to the loss of DA by attempting to rescue the ketoconazole-inhibited iL3 by adding back Δ7-DA. We found that approximately two-thirds of the iL3 feeding could be restored by Δ7-DA (Fig 5B), providing evidence that ketoconzaole-mediated iL3 inhibition is due to suppressed production of DA or related Ss-DAF-12 ligand(s). Together, these data strongly suggest that S. stercoralis synthesizes Ss-DAF-12 ligands that promote the developmental activation of iL3.
When performing the Δ7-DA rescue experiments, we also noted an increase in iL3 mortality that corresponded with increasing concentrations of Δ7-DA in the presence of ketoconazole (Fig 5B). Since ketoconazole and carrier solution concentrations remained constant, we could only attribute this mortality to an interaction between Δ7-DA and ketoconazole. This synergism suggests ketoconazole may also be blocking the metabolism of exogenous Δ7-DA by inhibition of other cytochrome P450s, allowing toxic levels of the compound or partially metabolized intermediates to accumulate in the worms. This phenomenon might be comparable to the synergism of pyrethrin insecticides when paired with the cytochrome P450 inhibitor piperonyl butoxide [77]. This synergistic effect might be exploited in the potential development of DAF-12 ligands as anthelmintics.
The data we report here support the hypothesis that endogenous production of DA in S. stercoralis promotes free-living development and iL3 activation, while repression of DAF-12 signaling promotes and maintains iL3 arrest. However, formal proof of this hypothesis awaits discovery and characterization of the natural ligands of Ss-DAF-12. Since S. stercoralis and C. elegans are phylogenetically distant, as members of separate clades where parasitism is thought to have evolved independently [23], the biosynthesis of steroid hormones may be different in these species. Biochemical- and in vitro-based studies, similar to those used to identify Δ7-DA in C. elegans [25,56], are called for to identify endogenous DAs in S. stercoralis. Furthermore, additional studies to elucidate the biosynthesis of natural Ss-DAF-12 ligands are a significant priority, as this biosynthetic pathway may constitute a chemotherapeutic target in S. stercoralis and other parasitic nematodes. Our phylogenetic study of the cytochrome P450s in S. stercoralis (S3 Fig) and of regulation of their transcripts throughout the life cycle (S2 Fig) provide a logical starting point for such biosynthetic experiments. Based upon these findings, we hypothesize that Ss-cyp22a9 is the functional homolog of Ce-daf-9 in the parasite. Provided constructs encoding this gene can be optimized for expression in mammalian cells, this hypothesis should be testable using modifications of proven cell-based assay methods [56].
In conclusion, our demonstration that a developmental blockade in S. stercoralis iL3 by ketoconazole can be rescued by Δ7-DA (Fig 2) constitutes the first functional evidence of DAF-12 signaling stimulated by endogenous synthesis of its ligand in a parasitic nematode. Furthermore, our findings that induction of rhabditiform L3 and L4 in the post-free-living generation by Δ7-DA (Figs 3, 4 and 5) and that homogonic to heterogonic development in post-parasitic L1 is shifted by administration of Δ7-DA (Fig 5) support the hypotheses we frame in this paper about ligation states of Ss-DAF-12 and the overall function of Ss-DAF-12 signaling during the S. stercoralis life cycle (Fig 1). The fact that this crucial developmental regulatory signaling pathway can be manipulated by exogenous administration of a steroid ligand, in this case a heterologous one from C. elegans [25], raises the possibility that Ss-DAF-12 signaling may represent a new chemotherapeutic target in S. stercoralis. This potential for a new class of anthelmintics likely extends to a diverse array of parasitic nematodes, as DAF-12 is conserved in all members of the Strongyloididae investigated to date [57,78] and in hookworms [56,79]. Regarding the latter group of parasitic nematodes, which are phylogenetically diverged from Strongyloides spp, the biological activity of the DAs has also been confirmed in Ancylostoma caninum [56]. Given the wide range of existing drugs targeting nuclear receptors [80], we propose that the potential of DAF-12 signaling in parasitic nematodes be actively investigated as a novel chemotherapeutic target.
Supporting Information
S. stercoralis post-parasitic larvae were hatched out onto plates with concentrations of Δ7-dafachronic acid (Δ7-DA) ranging from 33.1 nM to 1000 nM, as well as an ethanol carrier control. Regardless of Δ7-DA concentration, the percentage of remaining first-stage and second-stage larvae (L1-L2) after 72 hours of culture at 22°C remained roughly the same, with the maximum percentage at 125 nM (34.2 ± 38.3%) and the minimum at 1000 nM (3.7 ± 6.4%). The bar height represents the mean of five biological replicates and the error bar +1 standard deviation.
(TIF)
S. stercoralis cytochrome P450 (cyp)-encoding genes were identified in the genome, manually annotated, and named according to the family and subfamily. Both the Ss-cyp3a and Ss-cyp29a families appeared to have several members resulting from tandem gene duplication events. An S. stercoralis homolog of Caenorhabditis elegans daf-9, Ss-cyp22a9, was also identified. Mean transcript abundances, calculated as fragments per kilobase of coding exon per million fragments mapped (FPKM), were determined for the following developmental stages: gravid free-living females (FL Female), post-free-living first-stage larvae (PFL L1), infectious third-stage larvae (iL3), in vivo activated third-stage larvae (L3+), gravid parasitic females (P Female), homogonically developing post-parasitic first-stage larvae (PP L1), and homogonically developing post-parasitic approximately third-stage larvae enriched for females (PP L3). Error bars represent 95% confidence intervals.
(TIF)
A neighbor-joining phylogenetic tree, with 1000 iterations of boot-strapping, was constructed using cytochrome P450 protein sequences from the following species: Ascaris suum (As), Brugia malayi (Bm), Bursaphelenchus xylophilus (Bx), Caenorhabditis briggsae (Cb), Caenorhabditis elegans (Ce), Drosophila melanogaster (Dm), Homo sapiens (Hs), Hydra vulgaris (Hv), Loa loa (Ll), Mus musculus (Mm), Pristionchus pacificus (Pp), Strongylocentrotus purpuratus (Sp), Strongyloides ratti (Sr), S. stercoralis (Ss), Takifugu rubripes (Tr), and Xenopus laevis (Xl). The S. stercoralis CYP22A9 putative protein grouped with C. elegans DAF-9 and related nematode cytochrome P450s.
(TIF)
(FASTA)
(FASTA)
(NEX)
(GFF3)
(XLSX)
Acknowledgments
We are grateful for technical assistance by members of the Lok lab and the Hollins University Biology Department. A special thanks to Kristina Lewis for critical reading of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Institutes of Health grants R01-AI050668 and R33 AI 105856 (JBL), and R01DK067158 (SAK and DJM); the Robert A. Welch Foundation (grant I-1558 to SAK and grant I-1275 to DJM); Hollins University (JDS); the Virginia Foundation for Independent Colleges (MMYA); and the Howard Hughes Medical Institute (DJM). The NIH Resource-related Research Grant RR02512 to Dr. Mark Haskins provided research materials for the study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, et al. (2006) Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367: 1521–1532. [DOI] [PubMed] [Google Scholar]
- 2. Schar F, Trostdorf U, Giardina F, Khieu V, Muth S, et al. (2013) Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl Trop Dis 7: e2288 10.1371/journal.pntd.0002288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schad GA (1989) Morphology and life history of Strongyloides stercoralis In: Grove DI, editor. Strongyloidiasis a major roundworm infection of man. London: Taylor and Francis; pp. 85–104. [Google Scholar]
- 4. Igra-Siegman Y, Kapila R, Sen P, Kaminski ZC, Louria DB (1981) Syndrome of hyperinfection with Strongyloides stercoralis . Rev Infect Dis 3: 397–407. [DOI] [PubMed] [Google Scholar]
- 5. Toledo R, Munoz-Antoli C, Esteban JG (2015) Strongyloidiasis with emphasis on human infections and its different clinical forms. Adv Parasitol 88: 165–241. 10.1016/bs.apar.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 6. Viney ME, Lok JB (2007) Strongyloides spp. WormBook: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cassada RC, Russell RL (1975) The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans . Dev Biol 46: 326–342. [DOI] [PubMed] [Google Scholar]
- 8. Hu PJ (2007) Dauer. WormBook: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Golden JW, Riddle DL (1984) The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol 102: 368–378. [DOI] [PubMed] [Google Scholar]
- 10. Crook M, Thompson FJ, Grant WN, Viney ME (2005) daf-7 and the development of Strongyloides ratti and Parastrongyloides trichosuri . Mol Biochem Parasitol 139: 213–223. [DOI] [PubMed] [Google Scholar]
- 11. Hammond MP, Robinson RD (1994) Chromosome complement, gametogenesis, and development of Strongyloides stercoralis . J Parasitol 80: 689–695. [PubMed] [Google Scholar]
- 12. Albert PS, Riddle DL (1988) Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev Biol 126: 270–293. [DOI] [PubMed] [Google Scholar]
- 13. Hotez P, Hawdon J, Schad GA (1993) Hookworm larval infectivity, arrest and amphiparatenesis: the Caenorhabditis elegans Daf-c paradigm. Parasitol Today 9: 23–26. [DOI] [PubMed] [Google Scholar]
- 14. Crook M (2014) The dauer hypothesis and the evolution of parasitism: 20 years on and still going strong. Int J Parasitol 44: 1–8. 10.1016/j.ijpara.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fielenbach N, Antebi A (2008) C. elegans dauer formation and the molecular basis of plasticity. Genes Dev 22: 2149–2165. 10.1101/gad.1701508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Massey HC Jr., Bhopale MK, Li X, Castelletto M, Lok JB (2006) The fork head transcription factor FKTF-1b from Strongyloides stercoralis restores DAF-16 developmental function to mutant Caenorhabditis elegans . Int J Parasitol 36: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castelletto ML, Massey HC Jr., Lok JB (2009) Morphogenesis of Strongyloides stercoralis infective larvae requires the DAF-16 ortholog FKTF-1. PLoS Pathog 5: e1000370 10.1371/journal.ppat.1000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stoltzfus JD, Massey HC Jr., Nolan TJ, Griffith SD, Lok JB (2012) Strongyloides stercoralis age-1: a potential regulator of infective larval development in a parasitic nematode. PLoS ONE 7: e38587 10.1371/journal.pone.0038587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stoltzfus JD, Minot S, Berriman M, Nolan TJ, Lok JB (2012) RNAseq analysis of the parasitic nematode Strongyloides stercoralis reveals divergent regulation of canonical dauer pathways. PLoS Negl Trop Dis 6: e1854 10.1371/journal.pntd.0001854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stoltzfus JD, Bart SM, Lok JB (2014) cGMP and NHR Signaling Co-regulate Expression of Insulin-Like Peptides and Developmental Activation of Infective Larvae in Strongyloides stercoralis . PLoS Pathog 10: e1004235 10.1371/journal.ppat.1004235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rogers WP, Sommerville RI (1963) The infective stage of nematode parasites and its significance in parasitism. Adv Parasitol 1: 109–177. [DOI] [PubMed] [Google Scholar]
- 22. von Megen HHB, van den Elsen SJJ, Holterman MHM, Karssen G, Mooijman PJW, et al. (2009) A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology 11: 927–950. [Google Scholar]
- 23. Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, et al. (1998) A molecular evolutionary framework for the phylum Nematoda. Nature 392: 71–75. [DOI] [PubMed] [Google Scholar]
- 24. Viney ME (2009) How did parasitic worms evolve? Bioessays 31: 496–499. 10.1002/bies.200900010 [DOI] [PubMed] [Google Scholar]
- 25. Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, et al. (2006) Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans . Cell 124: 1209–1223. [DOI] [PubMed] [Google Scholar]
- 26. Antebi A (2015) Nuclear receptor signal transduction in C. elegans. WormBook: 1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ohkura K, Suzuki N, Ishihara T, Katsura I (2003) SDF-9, a protein tyrosine phosphatase-like molecule, regulates the L3/dauer developmental decision through hormonal signaling in C. elegans . Development 130: 3237–3248. [DOI] [PubMed] [Google Scholar]
- 28. Schaedel ON, Gerisch B, Antebi A, Sternberg PW (2012) Hormonal signal amplification mediates environmental conditions during development and controls an irreversible commitment to adulthood. PLoS Biol 10: e1001306 10.1371/journal.pbio.1001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gerisch B, Antebi A (2004) Hormonal signals produced by DAF-9/cytochrome P450 regulate C. elegans dauer diapause in response to environmental cues. Development 131: 1765–1776. [DOI] [PubMed] [Google Scholar]
- 30. Mak HY, Ruvkun G (2004) Intercellular signaling of reproductive development by the C. elegans DAF-9 cytochrome P450. Development 131: 1777–1786. [DOI] [PubMed] [Google Scholar]
- 31. Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A (2001) A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev Cell 1: 841–851. [DOI] [PubMed] [Google Scholar]
- 32. Jia K, Albert PS, Riddle DL (2002) DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development 129: 221–231. [DOI] [PubMed] [Google Scholar]
- 33. Wang J, Kim SK (2003) Global analysis of dauer gene expression in Caenorhabditis elegans . Development 130: 1621–1634. [DOI] [PubMed] [Google Scholar]
- 34. Rottiers V, Motola DL, Gerisch B, Cummins CL, Nishiwaki K, et al. (2006) Hormonal control of C. elegans dauer formation and life span by a Rieske-like oxygenase. Dev Cell 10: 473–482. [DOI] [PubMed] [Google Scholar]
- 35. Wollam J, Magner DB, Magomedova L, Rass E, Shen Y, et al. (2012) A novel 3-hydroxysteroid dehydrogenase that regulates reproductive development and longevity. PLoS Biol 10: e1001305 10.1371/journal.pbio.1001305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mahanti P, Bose N, Bethke A, Judkins JC, Wollam J, et al. (2014) Comparative metabolomics reveals endogenous ligands of DAF-12, a nuclear hormone receptor, regulating C. elegans development and lifespan. Cell Metab 19: 73–83. 10.1016/j.cmet.2013.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ludewig AH, Kober-Eisermann C, Weitzel C, Bethke A, Neubert K, et al. (2004) A novel nuclear receptor/coregulator complex controls C. elegans lipid metabolism, larval development, and aging. Genes Dev 18: 2120–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bethke A, Fielenbach N, Wang Z, Mangelsdorf DJ, Antebi A (2009) Nuclear hormone receptor regulation of microRNAs controls developmental progression. Science 324: 95–98. 10.1126/science.1164899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hammell CM, Karp X, Ambros V (2009) A feedback circuit involving let-7-family miRNAs and DAF-12 integrates environmental signals and developmental timing in Caenorhabditis elegans . Proc Natl Acad Sci U S A 106: 18668–18673. 10.1073/pnas.0908131106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hochbaum D, Zhang Y, Stuckenholz C, Labhart P, Alexiadis V, et al. (2011) DAF-12 regulates a connected network of genes to ensure robust developmental decisions. PLoS Genet 7: e1002179 10.1371/journal.pgen.1002179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shen Y, Wollam J, Magner D, Karalay O, Antebi A (2012) A steroid receptor-microRNA switch regulates life span in response to signals from the gonad. Science 338: 1472–1476. 10.1126/science.1228967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Z, Stoltzfus J, You YJ, Ranjit N, Tang H, et al. (2015) The nuclear receptor DAF-12 regulates nutrient metabolism and reproductive growth in nematodes. PLoS Genet 11: e1005027 10.1371/journal.pgen.1005027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Viney ME, Lok JB (2015) The biology of Strongyloides spp . WormBook: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nolan TJ, Brenes M, Ashton FT, Zhu X, Forbes WM, et al. (2004) The amphidial neuron pair ALD controls the temperature-sensitive choice of alternative developmental pathways in the parasitic nematode, Strongyloides stercoralis . Parasitology 129: 753–759. [DOI] [PubMed] [Google Scholar]
- 45. Viney ME (1996) Developmental switching in the parasitic nematode Strongyloides ratti . Proc Biol Sci 263: 201–208. [DOI] [PubMed] [Google Scholar]
- 46. Faust EC, Kagy ES (1933) Experimental studies on human and Primate Species of Strongyloides I. The variability and instability of types. Am J Trop Med Hyg Jan: 47–65. [Google Scholar]
- 47. Viney ME, Brown M, Omoding NE, Bailey JW, Gardner MP, et al. (2004) Why does HIV infection not lead to disseminated strongyloidiasis? J Infect Dis 190: 2175–2180. [DOI] [PubMed] [Google Scholar]
- 48. Viney ME, Matthews BE, Walliker D (1992) On the biological and biochemical nature of cloned populations of Strongyloides ratti . J Helminthol 66: 45–52. [DOI] [PubMed] [Google Scholar]
- 49. Ashton FT, Bhopale VM, Holt D, Smith G, Schad GA (1998) Developmental switching in the parasitic nematode Strongyloides stercoralis is controlled by the ASF and ASI amphidial neurons. J Parasitol 84: 691–695. [PubMed] [Google Scholar]
- 50. Bargmann CI, Horvitz HR (1991) Control of larval development by chemosensory neurons in Caenorhabditis elegans . Science 251: 1243–1246. [DOI] [PubMed] [Google Scholar]
- 51. Yamada M, Matsuda S, Nakazawa M, Arizono N (1991) Species-specific differences in heterogonic development of serially transferred free-living generations of Strongyloides planiceps and Strongyloides stercoralis. J Parasitol 77: 592–594. [PubMed] [Google Scholar]
- 52. Grant WN, Stasiuk S, Newton-Howes J, Ralston M, Bisset SA, et al. (2006) Parastrongyloides trichosuri, a nematode parasite of mammals that is uniquely suited to genetic analysis. Int J Parasitol 36: 453–466. [DOI] [PubMed] [Google Scholar]
- 53. Stasiuk SJ, Scott MJ, Grant WN (2012) Developmental plasticity and the evolution of parasitism in an unusual nematode, Parastrongyloides trichosuri . Evodevo 3: 1 10.1186/2041-9139-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Butcher RA, Fujita M, Schroeder FC, Clardy J (2007) Small-molecule pheromones that control dauer development in Caenorhabditis elegans . Nat Chem Biol 3: 420–422. [DOI] [PubMed] [Google Scholar]
- 55. Ludewig AH, Schroeder FC (2013) Ascaroside signaling in C. elegans . WormBook: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Z, Zhou XE, Motola DL, Gao X, Suino-Powell K, et al. (2009) Identification of the nuclear receptor DAF-12 as a therapeutic target in parasitic nematodes. Proc Natl Acad Sci U S A 106: 9138–9143. 10.1073/pnas.0904064106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ogawa A, Streit A, Antebi A, Sommer RJ (2009) A conserved endocrine mechanism controls the formation of dauer and infective larvae in nematodes. Curr Biol 19: 67–71. 10.1016/j.cub.2008.11.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Castelletto ML, Gang SS, Okubo RP, Tselikova AA, Nolan TJ, et al. (2014) Diverse host-seeking behaviors of skin-penetrating nematodes. PLoS Pathog 10: e1004305 10.1371/journal.ppat.1004305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sciacca J, Forbes WM, Ashton FT, Lombardini E, Gamble HR, et al. (2002) Response to carbon dioxide by the infective larvae of three species of parasitic nematodes. Parasitol Int 51: 53–62. [DOI] [PubMed] [Google Scholar]
- 60. Forbes WM, Ashton FT, Boston R, Zhu X, Schad GA (2004) Chemoattraction and chemorepulsion of Strongyloides stercoralis infective larvae on a sodium chloride gradient is mediated by amphidial neuron pairs ASE and ASH, respectively. Vet Parasitol 120: 189–198. [DOI] [PubMed] [Google Scholar]
- 61. Safer D, Brenes M, Dunipace S, Schad G (2007) Urocanic acid is a major chemoattractant for the skin-penetrating parasitic nematode Strongyloides stercoralis . Proc Natl Acad Sci U S A 104: 1627–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lopez PM, Boston R, Ashton FT, Schad GA (2000) The neurons of class ALD mediate thermotaxis in the parasitic nematode, Strongyloides stercoralis . Int J Parasitol 30: 1115–1121. [DOI] [PubMed] [Google Scholar]
- 63. Ashton FT, Zhu X, Boston R, Lok JB, Schad GA (2007) Strongyloides stercoralis: Amphidial neuron pair ASJ triggers significant resumption of development by infective larvae under host-mimicking in vitro conditions. Exp Parasitol 115: 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schad GA, Hellman ME, Muncey DW (1984) Strongyloides stercoralis: hyperinfection in immunosuppressed dogs. Exp Parasitol 57: 287–296. [DOI] [PubMed] [Google Scholar]
- 65. Lok JB (2007) Strongyloides stercoralis: a model for translational research on parasitic nematode biology. WormBook: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stiernagle T (2006) Maintenance of C. elegans . WormBook: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, et al. (2004) Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14: 1–18. [DOI] [PubMed] [Google Scholar]
- 68. Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, et al. (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, et al. (2013) Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol 31: 46–53. 10.1038/nbt.2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee SS, Schroeder FC (2012) Steroids as central regulators of organismal development and lifespan. PLoS Biol 10: e1001307 10.1371/journal.pbio.1001307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Eagling VA, Tjia JF, Back DJ (1998) Differential selectivity of cytochrome P450 inhibitors against probe substrates in human and rat liver microsomes. Br J Clin Pharmacol 45: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y (1996) Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans . Neuron 17: 707–718. [DOI] [PubMed] [Google Scholar]
- 75. Hannich JT, Entchev EV, Mende F, Boytchev H, Martin R, et al. (2009) Methylation of the sterol nucleus by STRM-1 regulates dauer larva formation in Caenorhabditis elegans . Dev Cell 16: 833–843. 10.1016/j.devcel.2009.04.012 [DOI] [PubMed] [Google Scholar]
- 76. Huang SC, Chan DT, Smyth DJ, Ball G, Gounaris K, et al. (2010) Activation of Nippostrongylus brasiliensis infective larvae is regulated by a pathway distinct from the hookworm Ancylostoma caninum . Int J Parasitol 40: 1619–1628. 10.1016/j.ijpara.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 77. Jones D, Ed. (1998) Piperonyl Butoxide: The Insecticide Synergist: Academic Press. [Google Scholar]
- 78. Hunt VL, Tsai IJ, Coghlan A, Reid AJ, Holroyd N, et al. (2015) The Genomic Basis of Parasitism in the Strongyloides Clade of Nematodes. Nat Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhi X, Zhou XE, Melcher K, Motola DL, Gelmedin V, et al. (2012) Structural conservation of ligand binding reveals a bile acid-like signaling pathway in nematodes. J Biol Chem 287: 4894–4903. 10.1074/jbc.M111.315242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Evans RM, Mangelsdorf DJ (2014) Nuclear Receptors, RXR, and the Big Bang. Cell 157: 255–266. 10.1016/j.cell.2014.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S. stercoralis post-parasitic larvae were hatched out onto plates with concentrations of Δ7-dafachronic acid (Δ7-DA) ranging from 33.1 nM to 1000 nM, as well as an ethanol carrier control. Regardless of Δ7-DA concentration, the percentage of remaining first-stage and second-stage larvae (L1-L2) after 72 hours of culture at 22°C remained roughly the same, with the maximum percentage at 125 nM (34.2 ± 38.3%) and the minimum at 1000 nM (3.7 ± 6.4%). The bar height represents the mean of five biological replicates and the error bar +1 standard deviation.
(TIF)
S. stercoralis cytochrome P450 (cyp)-encoding genes were identified in the genome, manually annotated, and named according to the family and subfamily. Both the Ss-cyp3a and Ss-cyp29a families appeared to have several members resulting from tandem gene duplication events. An S. stercoralis homolog of Caenorhabditis elegans daf-9, Ss-cyp22a9, was also identified. Mean transcript abundances, calculated as fragments per kilobase of coding exon per million fragments mapped (FPKM), were determined for the following developmental stages: gravid free-living females (FL Female), post-free-living first-stage larvae (PFL L1), infectious third-stage larvae (iL3), in vivo activated third-stage larvae (L3+), gravid parasitic females (P Female), homogonically developing post-parasitic first-stage larvae (PP L1), and homogonically developing post-parasitic approximately third-stage larvae enriched for females (PP L3). Error bars represent 95% confidence intervals.
(TIF)
A neighbor-joining phylogenetic tree, with 1000 iterations of boot-strapping, was constructed using cytochrome P450 protein sequences from the following species: Ascaris suum (As), Brugia malayi (Bm), Bursaphelenchus xylophilus (Bx), Caenorhabditis briggsae (Cb), Caenorhabditis elegans (Ce), Drosophila melanogaster (Dm), Homo sapiens (Hs), Hydra vulgaris (Hv), Loa loa (Ll), Mus musculus (Mm), Pristionchus pacificus (Pp), Strongylocentrotus purpuratus (Sp), Strongyloides ratti (Sr), S. stercoralis (Ss), Takifugu rubripes (Tr), and Xenopus laevis (Xl). The S. stercoralis CYP22A9 putative protein grouped with C. elegans DAF-9 and related nematode cytochrome P450s.
(TIF)
(FASTA)
(FASTA)
(NEX)
(GFF3)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.