Abstract
Background:
We aimed to value the usefulness of free to total prostate-specific antigen and Prostate-specific antigen (PSA) density for prostate cancer in the patients with PSA levels of 4.0 ng/ml or less.
Methods:
A total of 343 subjects with PSA levels of 4.0 ng/ml or less were biopsied. All patients were divided into four groups according to the PSA levels: 0 to 1.0 ng/ml, 1.1 to 2.0 ng/ml, 2.1 to 3.0 ng/ml, and 3.1 to 4.0 ng/ml. The reliability of cancer detection in relation to the f/t PSA ratio and PSAD were estimated.
Results:
Overall, 65 people were diagnosed with prostate cancer. The detection rate was 16.28%、17.17%, 21.82%, 25.00% in subjects with PSA levels of 0 to 1.0 ng/ml, 1.1 to 2.0 ng/ml, 2.1 to 3.0 ng/ml, and 3.1 to 4.0 ng/ml, respectively. The f/t PSA ratio was significantly lower in patients with prostate cancer and PSA levels of 2.1 to 4.0 ng/ml (P<0.05). The PSAD had no statistical significance between the two groups.
Conclusions:
Routine prostate biopsy should be undertaken if the f/t PSA ratio less than 15% with /without abnormal DRE/TRUS findings.
Keywords: Biopsy, Prostate cancer, Prostate-specific antigen, PSA ratio, PSAD
Introduction
Since first described in 1970 (1), prostate-specific antigen (PSA) was considered a useful tumor marker for detecting prostate cancer (PCa) and assessing treatment responses and follow-up among patients with PCa gradually. A PSA level of more than 4.0 ng/ml was considered to have predictive value for the prostate cancer (2, 3).
However, prostate cancers were not rare in patients with PSA level of 4.0 ng/ml or less and could be detected even in patients with PSA level lower than 0.5 ng/ml (4, 5). Overall, 82% of prostate cancers in younger men and 65% of cancers in older men would be missed with a PSA cutoff of 4.1ng/ml (6). Some investigators have suggested lowering the diagnostic threshold to increase the sensitivity of PSA in detecting prostate cancer (7–10). However, a lower threshold not only significantly increases the unnecessary biopsies but also cause the over diagnostic and the proportion of biopsies that identify clinically insignificant disease.
Therefore, we investigated the PSA density (PSAD) and free to total PSA ratio (f/t PSA) to assess a possible relationship with prostate cancer detection in the total PSA levels of 4.0 ng/ml or less.
Materials and Methods
Between April 1996 and December 2012, 2976 subjects, age 30 to 91 yr old, with PSA levels higher than 4.0ng/ml and/or abnormal findings on DRE or TRUS were biopsied in Chinese PLA General Hospital, China. All patients were provided written informed consent, and the study was approved by the institutional review boards of the hospital.
The abnormal DRE findings were defined as palpable induration, nodularity, irregularity, or asymmetry and the abnormal TRUS findings were defined as capsular irregularity, deformation, or existence of a hypoechoic region/nodule. TRUS were performed with Acuson Sequoia512 (Siemens Medical Solutions USA, Inc, Mountain View, California, USA) and IU22 (Philips Ultrasound, Bothell, Washington, USA) scanners. TRUS-guided sextant biopsies were performed, and, if TRUS or DRE revealed abnormal findings, we performed additional one to two biopsies in the suspicious areas.
We defined the inclusion criteria as follows: 1) All patients were biopsied for the first time; 2) Sextant biopsies was down, and, if TRUS or DRE revealed abnormal findings, we performed additional one to two biopsies in the suspicious areas; 3) PSA level was 4.0 ng/ml or less.
The exclusion criteria as follows:1) Patients with a present or past history of detecting prostate cancer or treatment for benign prostatic hyperplasia; 2)The pathology result lost and/or Gleason score was not available ;3) The pathology results proved to be non-prostate cancers.
A total of 343 patients were eligible for this study finally.
We stratified the patients into four groups with the PSA levels of 0.0~1.0 ng/ml、1.1~2.0 ng/ml、2.1~3.0 ng/ml and 3.1~4.0 ng/ml, and assessed the diagnostic significance of PSAD, f/t PSA, TRUS and DRE in prostate cancer detection. Meanwhile, we stratified the patients into five groups with the age of ≤49 years, 50~59 years, 60~69 years, 70~79years and ≥80years to assess the cancer detection rate according to age.
Analysis was performed using the Statistical Package for Social Sciences software program (SPSS for Windows Ver.11.5). We used the t or t’ test to compare cancer detection rates by PSAD and f/t PSA ratio. We also performed the chi-square test to compare cancer detection rates by age. We calculated the positive predictive value and 95% confidence interval (CI) for cancer detection stratified by PSAD and f/t PSA ratio. Receiver operating characteristic curves (ROC-curve) analyses were performed and areas under the curve were calculated. An area under the curve of 1.0 indicates a test with perfect discrimination between subjects with disease and those without disease, whereas an area under the curve of 0.5 indicates a test with no discriminatory power. For all analyses, a P-value<0.05 was considered statistically significant.
Results
Among the 343 patients, 65 prostate caners were detected and the detection rate was 19.0%. The diagnostic sensitivity of DRE and TRUS was 66.2% and 69.2%, and the specificity was 34.2% and 33.1% respectively. The diagnostic sensitivity and specificity of DRE combined TRUS were 49.2% and 59.7% respectively. The cancer detection rate was relevant low with the PSA levels of 1.0ng/ml or less, and it increased gradually as the PSA levels up. Table 1 shows the cancer detection rate of DRE and TRUS relative to PSA range.
Table 1: The cancer detection rate of DRE and TRUS relative to PSA range DRE: Digital Rectal Examination, TRUS: Transrectal Ultrasonography, PSA: Prostate-Specific Antigen, PCa: Prostate cancer.
| PSA range (ng/ml) | Abnormal Dre | Detection rate (%) | Abnormal Trus | Detection rate (%) | Abnormal Dre and trus | Detection rate (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| n | Pca | n | Pca | n | Pca | ||||
| 0.0–1.0 | 73 | 11 | 15.1 | 85 | 12 | 14.1 | 55 | 9 | 16.4 |
| 1.1–2.0 | 69 | 13 | 18.8 | 68 | 14 | 20.6 | 37 | 11 | 29.3 |
| 2.1–3.0 | 38 | 8 | 21.1 | 34 | 8 | 23.5 | 22 | 5 | 22.7 |
| 3.1–4.0 | 46 | 11 | 23.9 | 44 | 11 | 25.0 | 30 | 7 | 23.3 |
| total | 226 | 43 | 19.0 | 231 | 45 | 19.5 | 144 | 32 | 22.2 |
Among all the patents, the f/t PSA decreased gradually as the PSA level increased, and f/t PSA was lower in cancer group than in non-cancer group (P<0.05), but as we stratified them into four groups according to the PSA levels, we could find that the f/t PSA had no statistical significance (P>0.05) in PSA groups 0.0~1.0ng/ml and 1.1~2.0 ng/ml, and it had statistical significance (P<0.05) in PSA groups 2.1~3.0 ng/ml and 3.1~4.0 ng/ml. The PSAD was slightly higher in cancer groups (0.09±0.16ng/ml/cc) than in non-cancer groups (0.06±0.07ng/ml/cc), but there had no statistical significance.
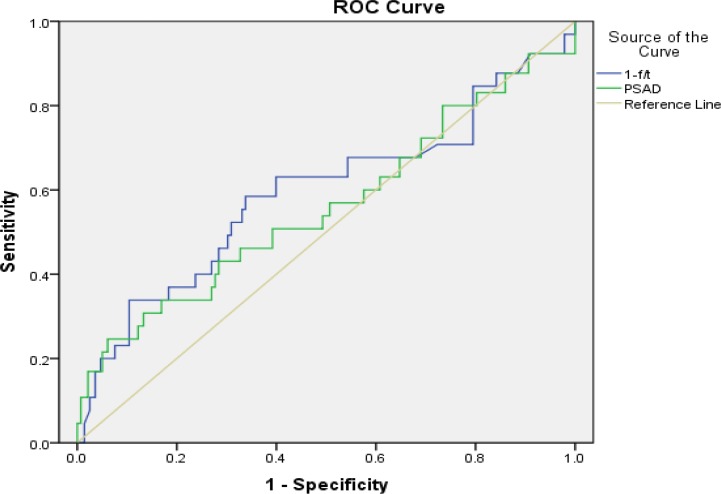
Table 2 shows the P value of f/t PSA and PSAD in differentiating cancers and non-cancers relative to PSA ranges. ROC curves for PSAD and f/t PSA ratio with PSA range 0.0–4.0 ng/ml showed in Fig. 1. The areas under the curves for PSAD and f/t PSA ratio in each PSA group showed in Table 3. In the 65 cancers, 31 with Gleason score≤6, 17 with Gleason score=7 and 17 with Gleason score≥8. Among the 31 patients with Gleason score≤6, 10 (32.26%) from PSA group 0.0–1.0 ng/ml, eight (25.81%) from PSA group 3.1–4.0 ng/ml ; among the 17 patients with Gleason score≥8, nine (52.94%) from PSA group 0.0–1.0 ng/ml, one (5.88%) from PSA group 3.1–4.0 ng/ml.
Table 2: The P value of f/t PSA and PSAD in differentiating cancers and non-cancers relative to PSA ranges.
| PSA range (ng/ml) | f/t PSA (S±x¯) | P Value | PSAD (S± x¯) | P Value | ||
|---|---|---|---|---|---|---|
| non-PCa | PCa | non-PCa | PCa | |||
| 0.0–1.0 | 0.35±0.17 | 0.34±0.13 | 0.802 | 0.02±0.01 | 0.02±0.02 | 0.515 |
| 1.1–2.0 | 0.25±0.11 | 0.20±0.10 | 0.098 | 0.06±0.05 | 0.13±0.27 | 0.391 |
| 2.1–3.0 | 0.22±0.06 | 0.15±0.06 | 0.001 | 0.10±0.14 | 0.09±0.05 | 0.830 |
| 3.1–4.0 | 0.20±0.10 | 0.14±0.06 | 0.032 | 0.10±0.07 | 0.16±0.18 | 0.259 |
| Total | 0.27±0.13 | 0.22±0.13 | 0.005 | 0.06±0.07 | 0.09±0.16 | 0.147 |
PSA: Prostate-Specific Antigen, f/t: Free To Total PSA Ratio, PSAD: Prostate-Specific Antigen Density, PCa: Prostate Cancer
Fig. 1: ROC curves for PSAD and f/t PSA ratio with PSA range 0.0–4.0ng/ml.
Table 3: Areas under the ROC curves for PSAD and f/t PSA ratio in each PSA group.
| PSA levels(ng/ml) | parameters | AUC-ROC(95% CI) |
|---|---|---|
| 1.0–4.0 | f/t PSA | 0.593 (0.507–0.679) |
| PSAD | 0.559 (0.472–0.645) | |
| 0.0–1.0 | f/t PSA | 0.406 (0.283–0.530) |
| PSAD | 0.515 (0.358–0.672) | |
| 1.1–2.0 | f/t PSA | 0.663 (0.518–0.809) |
| PSAD | 0.469 (0.286–0.651) | |
| 2.1–3.0 | f/t PSA | 0.813 (0.677–0.950) |
| PSAD | 0.440 (0.226–0.655) | |
| 3.1–4.0 | f/t PSA | 0.817 (0.709–0.925) |
| PSAD | 0.611 (0.449–0.773) |
PSA: Prostate-specific Antigen, f/t PSA: Free to Total PSA Ratio, PSAD: Prostate-specific Antigen Density, ROC: Receiver Operating Characteristic; AUC: Areas under the Curves
The Gleason score had no statistical significance in the four PSA groups. Table 4 shows the proportion of patients with different Gleason score in each PSA group.
Table 4: proportion of patients with different Gleason score in each PSA group.
| PSA(ng/ml) | Detection Rate (%) | ||
|---|---|---|---|
| Gleason≤6 | Gleason=7 | Gleason≥8 | |
| 0.0–1.0 | 10(32.26) | 4(23.53) | 9(52.94) |
| 1.1–2.0 | 8(25.81) | 2(11.76) | 5(29.41) |
| 2.1–3.0 | 5(16.13) | 5(29.41) | 2(11.76) |
| 3.1–4.0 | 8(25.81) | 6(35.29) | 1(5.88) |
| Total | 31 (100.00) | 17 (100.00) | 17 (100.00) |
PSA: Prostate Specific Antigen
The cancer detection rate increased as the PSA levels up, but there had no statistic significance among the age groups. Cancers could be detected in any age patients. Seven cancers were detected in the group of younger than 49 and the detection rate was 23.33%, 12 cancers were detected in the group of older than 80 and the detection rate was 20.69%. Table 5 shows the cancer detection rates of the five age groups relative to PSA ranges.
Table 5: The cancer detection rates of the five age groups relative to PSA ranges.
| PSA (ng/ml) age | 0.0–1.0 | Detection rate (%) | 1.1–2.0 | Detection rate (%) | 2.1–3.0 | Detection rate (%) | 3.1–4.0 | Detection rate (%) | Total | Detection rate (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Pca | n | Pca | n | Pca | n | Pca | n | Pca | ||||||
| ≤49 | 12 | 3 | 25.0 | 12 | 2 | 16.7 | 3 | 1 | 33.3 | 3 | 1 | 33.3 | 30 | 7 | 23.3 |
| 50–59 | 17 | 3 | 17.7 | 12 | 1 | 8.3 | 4 | 1 | 25.0 | 2 | 0 | 0.0 | 35 | 5 | 14.3 |
| 60–69 | 33 | 5 | 15.2 | 24 | 5 | 20.8 | 15 | 5 | 33.3 | 14 | 2 | 14.3 | 86 | 17 | 19.8 |
| 70–79 | 46 | 8 | 17.4 | 39 | 6 | 15.4 | 23 | 3 | 13.0 | 26 | 7 | 26.9 | 134 | 24 | 17.9 |
| ≥80 | 21 | 2 | 9.5 | 12 | 3 | 25.0 | 10 | 2 | 20.0 | 15 | 5 | 33.3 | 58 | 12 | 20.7 |
| total | 129 | 21 | 16.3 | 99 | 17 | 17.2 | 55 | 12 | 21.8 | 60 | 15 | 25.0 | 343 | 65 | 19.0 |
PSA: Prostate-specific Antigen, PCa: Prostate Cancer
Discussion
DRE was the principal method for prostate cancer screening before the advent of PSA testing. The positive predictive value (PPV) of a suspicious DRE was 5%, 14% and 29% in white men and 8%, 37% and 50% in black men with PSA of 0~1.0 ng/ml, 1.1~2.5 ng/ml and 2.6~4.0 ng/ml, respectively (8). TRUS has not been used in the first-line screening examination for prostate cancer because it lacks the ability to diagnose the prostate cancer in early stage, while the cancers with PSA levels of 4.0 ng/ml or less are mostly in the early stage (9, 11). The detection rate of abnormal findings on DRE and TRUS was 14.4% and 9.5% respectively with patients’ PSA levels of 4.0 ng/ml or less, and adding TRUS to DRE increased the detection rate of prostate cancer to 30.8% in the patients with PSA levels of 2.0 to 4.0ng/ml (7). The authors suggested that TRUS should be undertaken in patients with PSA levels of 2.0 to 4.0ng/ml and prostate biopsy should be undertaken in patients with abnormal findings on TRUS in this PSA range, and they also suggested that in patients with a PSA level of less than 2.0 ng/ml and slightly abnormal DRE findings did not need a routine biopsy (7).
In the current study, the cancer detection rate for DRE with PSA range of 0~1.0ng/ml, 1.1~2.0 ng/ml, 2.1~3.0 ng/ml and 3.1~4.0 ng/ml was 15.1%, 18.8%, 21.1% and 23.9% respectively, and for TRUS was 14.1%, 20.6%, 23.5% and 25.0% respectively. The detection rate for both methods was relatively low with the PSA level of 1.0 ng/ml of less, and it increased gradually with an increase in PSA levels.
Shröder et al. reported a 14% incidence of PSA in the range of 1.0 to 3.9 ng/ml, and 28% of these men had cancer (12). The morbidity of prostate cancer increased with an increase in PSA levels. This phenomenon could be found in patients with a PSA level lower than 4.0 ng/ml. Thompson et al. reported in the Prostate Cancer Prevention Trial (PCPT) that the incidence of prostate cancer was 15.2% in subjects with a PSA level lower than 4.0ng/ml or less, and the incidence was 6.6%, 10.1%, 17.0%, 23.9% and 26.9% in subjects with PSA level range of 0~0.5 ng/ml, 0.6~1.0 ng/ml, 1.1~2.0 ng/ml, 2.1~3.0 ng/ml and 3.1~4.0 ng/ml (13). In the current study, the cancer detection rate was 19.0%, which was a little more higher than Thompson’s finding 15.2%, and which maybe because of the patients in our study were all had positive findings with DRE or TRUS. At the same time, we found in our study that the cancer detection rate increased gradually with the PSA level increased and which in accordance with Thompson’s findings.
In patients whose cancer was detected in the PSA range of 2.0 to 4.0 ng/ml, the f/t PSA was significantly lower (14), and f/t PSA could provide increased specificity in cancer detection when PSA levels were less than 4.0ng/ml (15). Yet there also have some disparate opinions. Carlson et al. reported that f/t PSA was not predictive of prostate cancer when PSA level was less than 4.0 ng/ml (16). In current study, the f/t PSA was 0.22±0.13 in cancer group and 0.27±0.13 in non-cancer group, and there were statistical significance between the two groups (P<0.01), but when we stratified the subjects into four groups according to the PSA levels of 0.0~1.0ng/ml, 1.1~2.0 ng/ml, 2.1~3.0 ng/ml and 3.1~4.0 ng/ml, we could find that the f/t PSA had no statistical significance between cancer group and non-cancer group with the PSA levels of 2.0 ng/ml or less, while it had statistical significance (P<0.05) with the PSA range of 2.1 to 4.0 ng/ml. Use 0.15 as the cutoff of f/t PSA, the sensitivity and specificity for cancer detection was 64.3% and 84.8% respectively, the PPV and negative predictive value (NPV) was 46.9% and 85.7% respectively in subjects with the PSA range of 2.1 to 4.0 ng/ml.
The PSAD was a little higher in cancer group (0.06±0.03 ng/ml/cc) than in non-cancer group (0.04±0.06 ng/ml/cc), but there had no significance between the two groups (13). In current study, the PSAD was (0.09±0.16) ng/ml/cc in cancer group and (0.06±0.07) ng/ml/cc in non-cancer group, and there had no statistical significance between the two groups. Furthermore, there had no statistical significance between the two groups when stratified the subjects according to the PSA levels.
Men with a PSA level of 4.0 ng/mL or lower represent 14% of incident prostate cancer cases, and approximately 54% of patients with PSA levels of 4.0 ng/mL or lower at the time of diagnosis had low-risk cancers (17). Men with screen-detected cancer and with a PSA level of 4.0 ng/mL or lower were less likely to have high-grade tumors, and more than half were classified as having low-risk cancer and with lower Gleason scores (17).
The natural history of prostate cancer is to be progressing, and it is impossible to identify with certainty cancers that do not have the capacity to cause suffering or death during the lifetime of any given individual (5). In the United States and Europe, the proportion of men with prostate cancer and organ-confined disease was 84% to 87.9 % (18). Even prostate cancer found at PSA level of 4.0 ng/ml or less can be highly aggressive (13). For these prostate cancers, PSA cannot be the useful marker for detecting prostate cancer and assessing treatment responses and follow-up. Some patients with PSA level of 0.5ng/ml or less had a higher rate of seminal vesicle invasion, extracapsular tumor extension and regional lymph node metastasis than those with higher PSA levels (19), which was thought that the epithelial ells of these cancers lost expression of a PSA encoding gene (20).
Conclusion
The follow-up should be taken regularly for the patients with PSA levels of 2.1 ng/ml to 4.0ng/ml, and prostate biopsy should be taken if the f/t PSA less than 15%. For the patients with PSA levels of 2.0ng/ml or less, DRE, TRUS, f/t PSA and PSAD cannot diagnose the prostate cancer effectively. Maybe with the presence of new biomarkers like benign prostate specific antigen (BPSA), inactive prostate specific antigen (iPSA) and precursor of PSA, we can distinguish the prostate cancer more effectively.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
The authors declare that there is no conflict of interests.
References
- 1. Ablin RJ, Soanes WA, Bronson P, Witebsky E. (1970). Precipitating Antigens of the Normal Human Prostate. J Repro Fert, 22: 573– 574. [DOI] [PubMed] [Google Scholar]
- 2. Cooner WH, Mosley BR, Rutherford CL, Jr, Beard JH, Pond HS, Terry WJ, Igel TC, Kidd DD. (1990). Prostate cancer detection in a clinical urological practice by ultrasonography, digital rectal examination and prostate specific antigen. J Urol, 143( 6): 1146– 1152 [DOI] [PubMed] [Google Scholar]
- 3. Catalona WJ, Hudson MA, Scardino PT, Richie JP, Ahmann FR, Flanigan RC, deKernion JB, Ratliff TL, Kavoussi LR, Dalkin BL. (1994). Selection of optimal prostate specific antigen cutoffs for early detection of prostate cancer: receiver operating characteristic curves. J Urol, 152( 6 pt 1): 2037– 2042. [DOI] [PubMed] [Google Scholar]
- 4. Schröder FH, Hugosson J, Roobol MJ, et al. (2009). Screening and prostate-cancer mortality in a randomized European study. N Engl J Med, 360( 13): 1320– 1328. [DOI] [PubMed] [Google Scholar]
- 5. Andriole GL, Crawford ED, Grubb RL, et al. (2009). Mortality results from a randomized prostate-cancer screening trial. N Engl J Med, 360( 13): 1310– 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Punglia RS, D'Amico AV, Catalona WJ, Roehl KA, Kuntz KM. (2003). Effect of verification bias on screening for prostate cancer by measurement of prostate-specific antigen. N Engl J Med, 349: 335– 342. [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto T, Ito K, Ohi M, Kubota Y, Suzuki K, Fukabori Y, Kurokawa K, Yamanaka H. (2001). Diagnostic Significance of Digital Rectal Examination and Transrectal Ultrasonography in Men with Prostate-Specific Antigen Levels of 4 ng/ml or Less. Urology, 58 ( 6): 994– 998. [DOI] [PubMed] [Google Scholar]
- 8. Carvalhal GF, Smith DS, Mager DE, Ramos C, Catalona WJ. (1999). Digital rectal examination for detecting prostate cancer at prostate specific antigen levels of 4 ng/ml or less. J Urol, 161: 835– 839. [PubMed] [Google Scholar]
- 9. Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, deKernion JB, Ratliff TL, Kavoussi LR, Dalkin BL. (1994). Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multi-center clinical trial of 6,630 men. J Urol, 151: 1283– 1290. [DOI] [PubMed] [Google Scholar]
- 10. Gosselaar C, Roobol MJ, Schröder FH. (2005). Prevalence and characteristics of screen-detected prostate carcinomas at low prostate-specific antigen levels: aggressive or insignificant? BJU Int, 95( 2): 231– 237. [DOI] [PubMed] [Google Scholar]
- 11. Catalona WJ, Partin AW, Finlay JA, Chan DW, Rittenhouse HG, Wolfert RL, Woodrum DL. (1999). Use of percentage of free prostate-specific antigen to identify men at high risk of prostate cancer when PSA levels are 2.51 to 4 ng/ml and digital rectal examination is not suspicious for prostate cancer: an alternative model. Urology, 54( 2): 220– 224. [DOI] [PubMed] [Google Scholar]
- 12. Shröder FH, Damhuis RA, Kirkels WJ, De Koning HJ, Kranse R, Nus HG, Blijenberg BG. (1996). European randomized study of screening for prostate cancer—the Rotterdam pilot studies. Int J Cancer, 65 (2): 145. [DOI] [PubMed] [Google Scholar]
- 13. Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA., Jr (2004). Prevalence of prostate cancer among men with a prostate-specific antigen level≤4.0ng permilliliter. N Engl J Med, 350( 22): 2239– 2246. [DOI] [PubMed] [Google Scholar]
- 14. Kravchick S, Peled R, Dorfman D, Agulansky L, Ben-Dor D, Cytron S. (2005). Predictive Criteria for Prostate Cancer Detection in Men with Serum PSA Concentration of 2.0 to 4.0 ng/ml. Urology, 66 ( 3): 542– 546. [DOI] [PubMed] [Google Scholar]
- 15. Catalona WJ, Smith DS, Ornstein DK. (1997). Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/ml and benign prostate examination: enhancement of specificity with free PSA measurements. JAMA, 277( 18): 1452– 1455. [PubMed] [Google Scholar]
- 16. Carlson GD, Calvanese CB, Childs SJ. (1998). The appropriate lower limit for the percent free prostate-specific antigen reflex range. Urology, 52( 3): 450– 454. [DOI] [PubMed] [Google Scholar]
- 17. Shao YH, Albertsen PC, Roberts CB, Lin Y, Mehta AR, Stein MN, DiPaola RS, Lu-Yao GL. (2010). Risk Profiles and Treatment Patterns Among Men Diagnosed as Having Prostate Cancer and a Prostate-Specific Antigen Level Below 4.0 ng/ml. Arch Intern Med, 170( 14): 1256– 1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horninger W, Volgger H, Rogatsch H, Gschwendtner A, Bartsch G. (2001). Consideration of low PSA cut-off levels to optimize the detection of curable prostate cancer. Eur Urol, 39( Suppl 4): 43– 46. [DOI] [PubMed] [Google Scholar]
- 19. Berglund RK, Stephenson AJ, Cronin AM, Vickers AJ, Eastham JA, Klein EA, Guillonneau BD. (2009). Comparison of observed biochemical recurrence-free survival in patients with low PSA values undergoing radical prostatectomy and predictions of preoperative nomogram. Urology, 73( 5): 1098– 1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weir EG, Partin AW, Epstein JI. (2000). Correlation of serum prostate specific antigen and quantitative immunohistochemistry. J Urol, 163( 6): 1739– 42. [PubMed] [Google Scholar]