Abstract
Background:
Fascioliasis, caused by Fasciola hepatica and F. gigantica, is one of the most important plant and water borne disease in Iran. This study aimed to determine the prevalence of fascioliasis and intestinal helminthes in inhabitants of rural areas of Boyer-Ahmad in Kohgiluyeh and Boyer-Ahmad province in Southwest of Iran.
Methods:
Stool samples (1025) were collected from inhabitant of 50 randomly selected villages in Boyer-Ahmad Township. Samples were evaluated with modified Telemann and formalin-ethyl acetate methods. Blood samples of Fasciola positive cases were assessed with ELISA and Western blotting. DNA was extracted from Fasciola eggs from stool of positive individuals and evaluated by molecular (PCR) method and the PCR products were sequenced and analyzed.
Results:
Of the 1025 participants, 473 (46.1%) were male and 552 (53.9%) were female. The mean age of the subjects was 20.25 (±15.86) years. Fasciola eggs were detected in stools of two cases (0.19%). Blood samples were obtained from the Fasciola positive cases and their infection was further confirmed by ELISA and Western blotting. Molecular analysis revealed that both cases are infected with F. hepatica.
Furthermore, seven of participants (0.68%) were found to be infected with H. nana, 4 cases (0.39%) with E. vermicularis, and one case (0.09%) with Trichuris trichiura.
Conclusion:
Findings of this study showed that Boyer-Ahmad district is one of the endemic areas of human fascioliasis in Iran. The study also documented that the rate of helminthic infections in rural areas of the district has drastically declined over the past years.
Keywords: Fascioliasis, Prevalence, Helminthes infection, Iran
Introduction
More than a billion people worldwide are infected by intestinal helminthes (1). The main species that infect humans are Ascaris lumbricoides, Trichuris trichiura and hookworms (Necator americanus and Ancylostoma duodenale) (1).
Intestinal helminthes are widely distributed in developing countries, where control measures are often difficult to implement. Intestinal helminthes induce a wide range of symptoms including diarrhea, abdominal pain, general malaise and weakness and impair the nutritional status of infected individuals.
More than intestinal helminthes, fascioliasis is a serious health and veterinary problem in few countries of the world, including Iran (2). While animal fascioliasis is common in livestock in most areas of Iran, human fascioliasis is also a health problem in few areas of the country (3–4). Human fascioliasis has emerged as a serious problem in the Northern Province of Guilan in Iran during the past decades. This province experienced two outbreaks of human fascioliasis in 1987, affecting more than 10,000 people, and in 1997, affecting several thousands of people (3). Cases of human fascioliasis have also been reported from other provinces of Iran including Kohgiluyeh and Boyer-Ahmad in the southwest of the country (4). Our recent study revealed a seroprevalence rate of 1.86% for human fascioliasis in this province (4). In north of the country, Ashrafi et al. reported a prevalence of 0.4% and 1.2% for human fascioliasis in rural areas by parasitological and serological methods, respectively (5).
The current study was conducted to assess the prevalence and intensity of fascioliasis and intestinal helminthes in rural areas of Boyer-Ahmad district, southwest Iran.
Materials and Methods
Study area
This cross-sectional study was conducted between June 2014 and December 2014, in Boyer-Ahmad Township in Kohgiluyeh and Boyer-Ahmad province, Southwest Iran. The province has geographical coordinates between latitudes 30-9° to 31-27° N. and longitudes 49–55° to 51-42° E. It has a moderate and cool climate. Boyer-Ahmad Township is located in a cold region and has a cold winters and temperate summers. Snow and rainfall (mean annual rainfall of 600 mm) are plentiful in this region. Ground covered with pastures, including wild pistachio, tulips and oak forests. Climate and socio-economic conditions make the people to depend more on animal husbandry and agriculture for their livelihood. Local people traditionally consume high levels of wild freshwater plants such as Nasturtium microphyllum (local name Bakaloo), Mentha logifolia (local name, Pooneh) and spearmint. Moderate temperatures and large pasture for ruminants provide appropriate conditions for transmission and establishment of parasitic helminthes, including cystic echinococcosis and fascioliasis in this area (4, 6).
Sample collecting
Approval for the study was obtained from the Institutional Ethics Committees of the Shiraz University of Medical Sciences. All participants were counseled about the study and they were requested to provide informed consent. Confidentiality of the details of the participants was guaranteed.
Stool samples were collected from 1025 villagers (with 84816 populations) in 50 randomly selected villages in Boyer-Ahmad Township. Sample size was calculated based on the population living in the study area and also the prevalence of helminthic infection reported in previous study (7). A questionnaire was used to obtain demographic information as well as information on participants’ education, occupation, sanitation and water supply facilities, and eating of freshwater vegetables. Stool samples were evaluated with formol-ethyl acetate sedimentation technique and the sedimentary materials were examined by conventional light microscope for helminthes’ ova. Formol-ethyl acetate technique was performed by dissolving one gram of stool sample in 7 ml of 10% formalin and passing through a pad of four layers of clearing gauze. Then, three ml of ethyl acetate was added to the sample and gently mixed and centrifuged at 800 g for one minute. The supernatant was discarded and the sediment was used to prepare direct smear slide.
Modified Telemann’s method was used for detecting of Fasciola eggs in the stool samples. Briefly, three grams of stool was dissolved in 10 ml of normal saline in a 15 ml tube and the solution was passed through a four layers of gauze. Sample was centrifuges, 800 g for one minute, and supernatant was discarded. The sediment was mixed with 5 ml of 15% hydrochloric acid. Then, 5 ml of ethyl acetate was added and the tube was gently shaken and centrifuged at 800 g for one minute. The supernatant was discarded and the sediment was used to prepare a direct smear slide. Slides were examined under the light microscope, using 10X and 40X magnifications. Intensity of infection was measured by Stoll methods. Eggs were counted, and the intensity of infection derived by determining the eggs per gram of feces (EPG).
DNA extraction and polymerase chain reaction (PCR) amplification
DNA was extracted from eggs of Fasciola collected from stool of positive cases of fascioliasis, using a DNA extraction kit (QIAamp DNA Stool Mini Kit, QIAGEN), based on manufacturer’s instructions. PCR was performed as described before (8). PCR primer sets were used for amplifying fragments of the CO1 (Mitochondrial) gene of Fasciola. PCR was done using F: Ita8 (5′-ACGTTGGATCATAAGCGTGT-3′) and R: Ita9 (5′- CCTCATCCAACATAACCTCT-3′) primers. After PCR amplification, an approximately 485 bp PCR product representing a fragment of the CO1 gene was amplified.
PCR product was excised from 1.5% agarose gel and purified with a DNA Gel Extraction Kit (Bioneer's AccuPrep Gel Purification Kit), according to the manufacturer’s instructions. Purified PCR product was sequenced, using the same primers as described for the amplification process. The sequences were aligned and compared with those of existing sequences related to Fasciola spp. available in the GenBank, using the BLAST program of GenBank.
Serological evaluation by ELISA and western blotting
Sera were collected form Fasciola positive cases and evaluated by both ELISA and Western blotting, as previously described (9).
Statistical analysis
Data were entered into SPSS for Windows (Release 18). The chi-square test was used to examine relationships between variables. The level of significance was set at 5%.
Results
From 1025 participants, 473 (46.1%) were male and 552 cases (53.9%) were female. Mean age of the participants was 20.25 (±15.86) year (range 1–89 year). Most of the subjects (38.63%) were in age group of 1–10 years.
More than 60% of the cases used to consume aquatic plants including Nasturtium microphyllum (local name Bakaloo), Mentha logifolia (local name, Pooneh) and spearmint. The overall prevalence of intestinal helminthic infections was 1.35% (14 out of 1025 cases). Hymenolepis nana was the predominant intestinal parasite (0.68%, 7 cases) followed by Enterobius vermicularis (0.39%, 4 cases), and Trichuris trichiura (0.09%, 1 case). The most important finding of this study was the detection of Fasciola eggs in stool of two cases in the area, where sporadic cases of human fascioliasis have been previously reported. Accordingly, the prevalence rate of human fascioliasis was found to be 0.19% in the area. To rule out the possibility of spurious infection, stool samples were collected from the positive cases after 3 days and the Fasciola eggs were again detected in the stool samples. Both cases of fascioliasis were female (age 13 and 28) and both cases used to consume aquatic plants including Nasturtium microphyllum (local name Bakaloo). Moreover, clinical signs and symptoms of both cases were suggestive of fascioliasis.
Multiple helminthic infections (H. nana and E. vermicularis) were seen in two cases. Age group of 1–10 years had the most prevalence rate of helminthic infection. Intensity of infection was measured by Stoll methods. Mean of EPG (egg per gram) for Fasciola was 50 eggs and for H. nana and E. vermicularis were 1371 and 350 respectively. Serum sample was taken from the two fascioliasis cases and evaluated by ELISA and western blotting. Both cases were positive by both serological methods.
DNA was extracted from the eggs of Fasciola, collected from stool of fascioliasis cases, and PCR amplified. PCR product revealed a band of about 485 bp corresponding to Fasciola spp. PCR product was sequenced and aligned with sequences of CO1 gene of Fasciola in the GenBank. Sequences of CO1 of F. hepatica of this study were deposited in GenBank (accession numbers: KT722984 and KT853031).
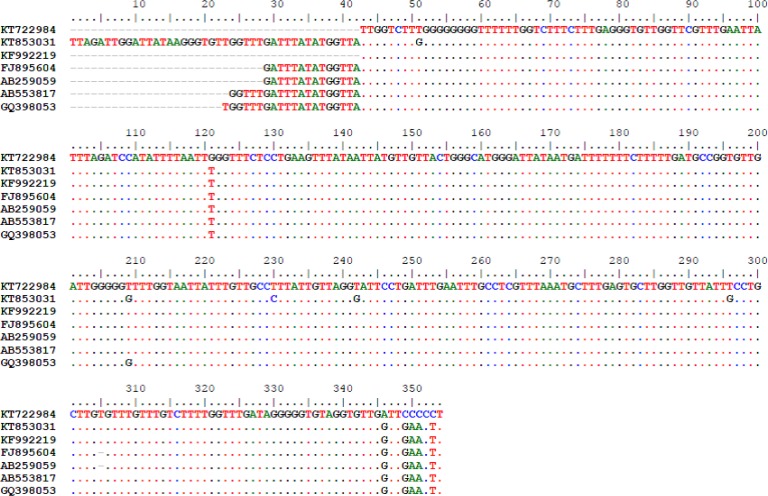
Sequence analysis of mitochondrial (CO1) gene of two fasciolid egg’s specimens revealed that both cases are F. hepatica. Both cases had 99% identity with those of F. hepatica which we previously isolated and reported, (Accession No. KF992219), from a goat’s liver in the area (7). Fig. 1 shows the alignment of sequences of our current human Fasciola cases with other sequences in the GenBank including one of our previously reported animal fascioliasis case (KF992219).
Fig. 1: Alignment of CO1 sequences of F. hepatica eggs isolated from human cases (KT722984 and KT853031), with F. hepatica of goat from Kohgiluyeh and Boyer-Ahmad province, Iran (KF992219), F. hepatica of human from North of Iran (FJ895604) and Japan (AB259059), F. hepatica of sheep from Egypt (AB553817) and F. hepatica of cattle from Iran (GQ398053).
Alignment of sequences of the new cases with other sequences in the GenBank revealed that the current isolates have a few nucleotide differences in few positions of the sequences.
Discussion
Control of helminthic infections is possible because of the availability of practical intervention approaches. For proper implementation of control strategies, understanding where at-risk populations live is essential. The current study aimed to find out the status of intestinal helminthes and fascioliasis in rural areas of Boyer-Ahmad district in southwest of Iran.
Our results demonstrated a low prevalence of helminthic infection in this area and this is in line with other studies, which have been carried out in Iran in recent years.
During the last decades, a significant decrease has been seen in the prevalence of intestinal helminthiasis in Iran (3). While prevalence of H. nana and E. vermicularis are still relatively high, the prevalence of important soil transmitted helminthes such as A. lumbricoides and Strongyloides stercoralis fall below 0.5% in the country (3).
In accordance with the above-mentioned point, there appears to have been a marked decline in prevalence and in absolute numbers of intestinal helminthes in several other Asian countries. This is mostly because of national control activities, together with social and economic development (1). Factors which contribute to a significant decrease in intensity and prevalence of intestinal helminthes in a given area are include; human factors such improved toileting behavior of children, awareness of disease transmission, improvement of individual hygiene, and environmental factors such as improvement in sanitary conditions of the environment, access to better drinking water sources, construction of toilets in rural areas, and sanitary disposal of human excreta.
Most of these contributing factors in prevalence of helminthic infection have drastically improved in Iran in recent years, not only in urban but also in rural areas. Support for this view comes from the recent studies that have been done in different areas of the country.
The prevalence of intestinal parasitic infection among the students of South Khorasan Province, eastern Iran, was evaluated by Taheri et al., where they found intestinal helminthes, apart from H. nana, in only 0.55% of the studied population (10). Ashtiani et al., evaluated the prevalence of parasitic infections in children referred to Tehran medical centers during 18 years, 1991–2008. H. nana was the most prevalent helminthes in their study. The prevalence of intestinal parasites was dropped from 8% to 1% between the first 10 years in comparison with the next 8 years of the study (11).
Only 0.028% of people in Karaj city, next to Tehran, were infected with Taenia proglottids and 0.03% with S. stercoralis larvae (12).
In our study, no cases of A. lumbricoides, hook-worms or S. stercoralis were found among the studied population. This is in keeping with previous study conducted by Mohammad et al. in the area, where they did not find any case of Ascaris, hook-worms or S. stercoralis in the area (7).
Prevalence of enterobiasis in our study is low. This is mainly because the Scotch tape method, which is the most reliable method for diagnosing of pinworm infection, has not been used in this study.
Our key finding in this study was the detection of human fascioliasis cases in the area, by finding the Fasciola eggs in stools of the infected subjects in coprological surveys. A prevalence rate of about 0.2% was found for human fascioliasis in the area, where sporadic cases of fascioliasis have been previously reported.
Human fascioliasis is a serious health problem in Iran (3). The disease is more common in north of the country where several thousands of people were affected in two outbreaks of human fascioliasis in 1987 and 1997 (2–3). Cases of human fascioliasis have also been reported from other provinces of Iran including Yasuj district in Kohgiluyeh and Boyer-Ahmad province (4). This district seems to be emerging as a new focus of human fascioliasis in southwest of the country. A seroprevalence study of human fascioliasis in the region revealed that 1.8% of the populations are seropositive for fascioliasis (3).
The level of endemicity of human fascioliasis in our study is lower than those reported in north areas of the country. Study of Ashrafi et al., about the endemicity of human fascioliasis in rural communities of Bandar-E-Anzali district in north of Iran, revealed the prevalence of 0.4% and 1.2%, using coprological and serological methods, respectively (5). Molecular analysis has not been performed for the human cases in their study.
In our study sequence analysis of mitochondrial (CO1) gene of two fasciolid egg’s specimens obtained from humans stools, revealed 99% identity with those of F. hepatica which we previously isolated and reported from a goat liver in the area (8). Several studies about the molecular characterization of Fasciola spp. isolated form animals have been done in different areas of Iran, (8, 13–17). However, just a few such studies have been performed about fasciolid specimens obtained from human. In a study by Sharifiyazdi et al., sequence analysis of ribosomal (ITS1) and mitochondrial (CO1 and ND1) genes of two fasciolid adult specimens obtained from humans in Guilan province, Northern Iran, were evaluated and confirmed both cases as F. hepatica with 100% homology with a human case of fascioliasis from Japan (18).
Conclusion
A relatively low prevalence of intestinal helminthes was found among the villagers in rural areas of Boyer-Ahmad Township. This indicates that the rate of helminthic infections in rural areas of the district has drastically declined over the past years. Moreover, findings of this study showed that Boyer-Ahmad district is one of the endemic areas of human fascioliasis and prevention measurements should be considered for proper control of the disease in the area.
Ethical Considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
The results described in this paper were part of MSc thesis of Ghasem Hosseini. The study was financially supported by the office of vice-chancellor for research of Shiraz University of Medical Sciences (Grant No. 6887). The authors declare that there is no conflict of interests.
References
- 1. de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. (2003). Soil-transmitted helminth infections: updating the global picture. Trends Parasitol, 19 ( 12): 547– 51. [DOI] [PubMed] [Google Scholar]
- 2. Mas-Coma S, Valero MA, Bargues MD. (2014). Fascioliasis. Adv Exp Med Biol, 766: 77– 114. [DOI] [PubMed] [Google Scholar]
- 3. Rokni MB. (2008). The present status of human helminthic diseases in Iran. Ann Trop Med Parasitol, 102 (4): 283– 95. [DOI] [PubMed] [Google Scholar]
- 4. Sarkari B, Ghobakhloo N, Moshfea A, Eilami O. (2012). Seroprevalence of human fasciolosis in a new-emerging focus of fasciolosis in Yasuj district, southwest of Iran. Iran J Parasitol, 7 (2): 15– 20. [PMC free article] [PubMed] [Google Scholar]
- 5. Ashrafi K, Saadat F, O'Neill S, et al. (2015). The endemicity of human fascioliasis in Guilan province, northern Iran: the baseline for implementation of control strategies. Iran J Public Health, 44 (4): 501– 11. [PMC free article] [PubMed] [Google Scholar]
- 6. Sarkari B, Sadjjadi SM, Beheshtian MM, Aghaee M, Sedaghat F. (2010). Human cystic echinococcosis in Yasuj District in Southwest of Iran: an epidemiological study of seroprevalence and surgical cases over a ten-year period. Zoonoses Public Health, 57 (2): 146– 50. [DOI] [PubMed] [Google Scholar]
- 7. Mohammad K, Zalie MR, Sirous S, Masjedi Mr. (1995). Intestinal parasites in Iran. Iran J Public Health, 24 (3–4): 9– 26. [Google Scholar]
- 8. Shafiei R, Sarkari B, Sadjjadi SM, Mowlavi GR, Moshfe A. (2014). Molecular and morphological characterization of Fasciola spp. isolated from different host species in a newly emerging focus of human fascioliasis in Iran. Vet Med Int, 405740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shafiei R, Sarkari B, Sadjjadi SM. (2015). Performance of a 27 KDa Fasciola hepatica antigen in the diagnosis of human fascioliasis. J Lab Physicians, 7 (1): 17 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taheri F, Namakin K, Zarban A, Sharifzadeh G. (2011). Intestinal parasitic infection among school shildren in South Khorasan Province, Iran. J Res Health Sci, 11( 1): 45– 50. [PubMed] [Google Scholar]
- 11. Ashtiani MT, Monajemzadeh M, Saghi B, et al. (2011). Prevalence of intestinal parasites among children referred to Children's Medical Center during 18 years (1991–2008), Tehran, Iran. Ann Trop Med Parasitol, 105( 7): 507– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nasiri V, Esmailnia K, Karim G, Nasir M, Akhavan O. (2009). Intestinal parasitic infections among inhabitants of Karaj City, Tehran province, Iran in 2006–2008. Korean J Parasitol, 47( 3): 265– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shahbazi A, Akbarimoghaddam M, Izadi S, Ghazanchaii A, Jalali N, Bazmani A. (2011). Identification and genetic variation of Fasciola species from Tabriz, north-Western Iran. Iran J Parasitol, 6 (3): 52– 9. [PMC free article] [PubMed] [Google Scholar]
- 14. Moazeni M, Sharifiyazdi H, Izadpanah A. (2012). Characterization of Fasciola hepatica genotypes from cattle and sheep in Iran using cytochrome C oxidase gene (CO1). Parasitol Res, 110 (6): 2379– 84. [DOI] [PubMed] [Google Scholar]
- 15. Amor N, Halajian A, Farjallah S, Merella P, Said K, Ben Slimane B. (2011). Molecular characterization of Fasciola spp. from the endemic area of northern Iran based on nuclear ribosomal DNA sequences. Exp Parasitol, 128 (3): 196– 204. [DOI] [PubMed] [Google Scholar]
- 16. Ashrafi K, Valero MA, Panova M, Periago MV, Massoud J, Mas-Coma S. (2006). Phenotypic analysis of adults of Fasciola hepatica, Fasciola gigantica and intermediate forms from the endemic region of Gilan, Iran. Parasitol Int, 55 (4): 249– 60. [DOI] [PubMed] [Google Scholar]
- 17. Shafiei R, Sarkari B, Moshfe A. (2013). A consistent PCR-RFLP assay based on its-2 ribosomal DNA for differentiation of Fasciola species. Iran J Basic Med Sci, 16( 12): 1266– 9. [PMC free article] [PubMed] [Google Scholar]
- 18. Sharifiyazdi H, Moazeni M, Rabbani F. (2012). Molecular characterization of human Fasciola samples in Gilan province, Northern Iran on the basis of DNA sequences of ribosomal and mitochondrial DNA genes. Comp Clin Pathol, 21 (5): 889– 894. [Google Scholar]