Abstract
We report a case of thrombotic thrombocytopenic purpura (TTP) that immediately followed symptomatic dengue virus infection in a pregnant lady. The patient developed dengue fever at 16 weeks of gestation, resulting in spontaneous abortion. Subsequently, fever reappeared with persistent thrombocytopenia and jaundice. Investigations revealed microangiopathic hemolysis; there was no evidence of disseminated intravascular coagulation. The TTP episode resolved after six cycles of therapeutic plasma exchange with fresh-frozen plasma. An ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 motif 13 repeats) activity assay, done during convalescence, showed normal activity. The patient had an uneventful second pregnancy and has remained free of TTP recurrence for more than 2 years now. We review the pathophysiological basis of TTP in dengue infection, and suggest that jaundice with disproportionate elevation of serum aspartate aminotransferase level in a patient with dengue should arouse the suspicion of TTP.
Introduction
Thrombocytopenia is a characteristic finding in patients with symptomatic dengue virus infection.1 Although its pathogenesis is attributed to dengue virus-induced bone marrow suppression and immune-mediated clearance of platelets,2,3 biochemical changes similar to that of thrombotic thrombocytopenic purpura (TTP) have also been described in dengue.4 However, clinically manifested TTP is seldom seen in dengue. Here, we report a case of overt TTP following dengue virus infection in a pregnant woman.
CASE REPORT
A 25-year-old woman, a primigravida, presented to us at 16 weeks of gestation for fever, body aches, and vomiting since 2 days. The fever was high grade, and she had had about five episodes of non-bilious vomiting. She had no bleeding manifestations. Physical examination revealed a conscious, febrile patient (temperature 103.4°F) with mild pallor and congestion of palpebral conjunctiva. There was no rash, petechiae, icterus, or lymphadenopathy. She was tachycardic with a pulse rate of 140 beats/minute, and her blood pressure was 116/70 mmHg. Examination of systems was unremarkable. She had been investigated at another facility where a blood test for dengue nonstructural protein 1 (NS1) antigen was found to be positive.
She was admitted with a provisional diagnosis of dengue fever in pregnancy. Her blood counts on the day of admission were hemoglobin 7.5 g/dL, total leukocyte count 9,200 cells/μL, and platelet count 130 × 103/μL. She was treated with oral acetaminophen 500 mg qid and intravenous crystalloids to maintain adequate fluid intake. On Day 2 of hospitalization, she complained of bleeding per vaginum and was transferred to the labor room in view of threatened abortion. One day later, the patient spontaneously expelled the dead fetus, and instrumental evacuation of retained products of conception was done.
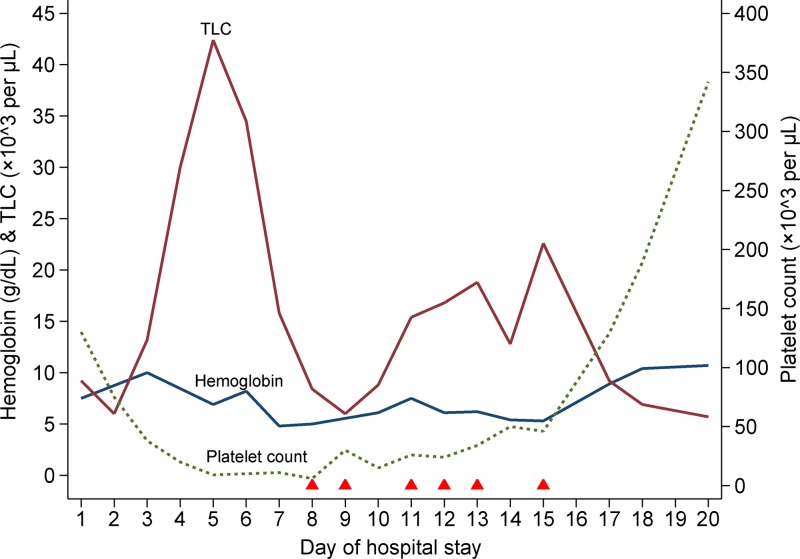
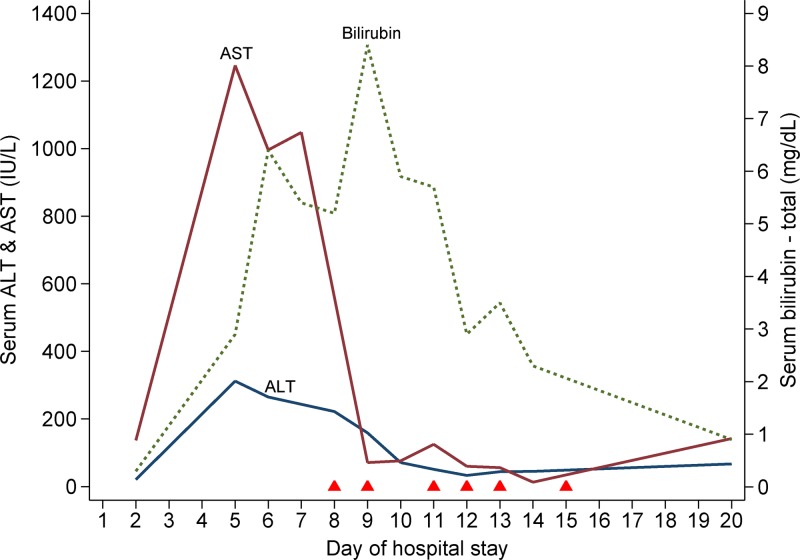
By this time, her platelet count had dropped progressively to 9 × 103/μL and the hemoglobin was 6.9 g/dL (Figure 1 ). She received packed red cell and platelet transfusions. Her fever, which had subsided by then, reappeared on Day 4 with spikes of 101°F–103°F. She had developed facial puffiness and pedal edema, and scleral icterus was also noted. A chest radiograph showed small pleural effusions bilaterally, and abdominal ultrasonography revealed moderate amounts of free fluid. There was a prominent derangement of liver function tests: total bilirubin 2.9 mg/dL (indirect fraction 70%), serum albumin 2.9 g/dL, aspartate aminotransferase (AST) 1,246 IU/L (upper limit: 40 IU/L), and alanine aminotransferase (ALT) 312 IU/L (upper limit: 45 IU/L) (Figure 2 ). Her blood counts showed marked leukocytosis (total white cell count 42,400 cells/μL). She became progressively disoriented and irritable. At this stage, we considered the possibilities of dengue-associated acute liver failure and postabortal sepsis, and initiated her on the treatment of hepatic encephalopathy and broad spectrum antibiotics. But, her sensorium worsened further, and she needed mechanical ventilation. A computed tomographic scan ruled out intracranial bleed and cerebral infarct.
Figure 1.
Serial changes in blood counts since hospital admission up to discharge and the relation to therapeutic plasma exchanges. The solid triangles along the x axis indicate the timing of therapeutic plasma exchange sessions. TLC = total leukocyte count.
Figure 2.
Serial changes in liver function test parameters since hospital admission up to discharge. The solid triangles along the x axis indicate the timing of therapeutic plasma exchange sessions. ALT = alanine aminotransferase; AST = aspartate aminotransferase.
A peripheral blood smear at this juncture revealed fragmented red cells; a few nucleated red cells (3/100 white blood cells) were also present. There was neutrophilic leukocytosis and severe thrombocytopenia. Serum lactate dehydrogenase (LDH) level was elevated (> 2,000 IU/L [upper limit: 200 IU/L]). The prothrombin time was repeatedly normal (14.7–18 seconds; control 13.5 seconds; international normalized ratio 1.1 to 1.4), and a test for fibrin degradation products was negative. Despite the supportive measures, platelet count and hemoglobin dropped further to 6 × 103/μL and 5.0 g/dL, respectively. Tests for hepatitis B surface antigen and IgM antibodies to hepatitis A and E viruses were negative. She also tested negative for human immunodeficiency virus (HIV). Blood cultures remained sterile. A direct Coombs test was negative, ruling out immune-mediated hemolysis.
Features of microangiopathic hemolysis, thrombocytopenia, fever, and altered sensorium without any evidence of disseminated intravascular coagulation (DIC) strongly suggested a diagnosis of TTP. She also developed ecchymotic skin lesions over the limbs. Following this, on Day 8, therapeutic plasma exchange with fresh-frozen plasma was started. A repeated test for dengue NS1 antigen and dengue IgG antibody (sample drawn on Day 12 of symptoms) returned positive. The patient received six plasma exchanges over the next 8 days. Her platelet counts gradually increased to 46 × 103/μL (Figure 1) on which plasma exchanges were stopped. Subsequently, the platelet count further rose to 342 × 103/μL, serum LDH level dropped to 739 IU/L, and the liver function tests normalized (Figure 2). Her sensorium also improved, and she was weaned off the ventilator and extubated on Day 11. Her hemoglobin recovered to 10.7 g/dL, and a repeat peripheral blood smear examination revealed no features of hemolysis. The later part of her hospital stay was punctuated by nosocomial sepsis (infected hematoma of right upper limb) which was presumptively treated with meropenem and clindamycin. She was discharged home on Day 20.
An ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 motif 13 repeats) activity assay (fluorescence resonance energy transfer assay; Mayo Medical Laboratories, Rochester, MN) was done about a month after discharge, and it showed normal activity (100%; reference value ≥ 70%). Tests for antinuclear and anti-cardiolipin antibodies were also negative. About 3 months after hospital discharge, the patient conceived again. She went on to have an uneventful pregnancy (with periodic monitoring of platelet count and hemoglobin), and delivered a healthy baby at term.
Discussion
This patient had TTP as evidenced by the presence of microangiopathic hemolysis, thrombocytopenia, fever, and altered sensorium. We could exclude alternative explanations such as DIC and HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome for the following reasons: there was no laboratory evidence of DIC except for thrombocytopenia and she did not have preeclampsia. Further, a prompt response to plasma exchange also supports a diagnosis of TTP. We could not estimate ADAMTS13 activity during the acute phase. Nevertheless, demonstration of ADAMTS13 deficiency is not essential to make a diagnosis of TTP, and about two-thirds of TTP patients would have normal ADAMTS13 activity.5 However, we estimated ADAMTS13 activity during convalescence to rule out inherited deficiency and to predict the risk of relapse. One component of the classical pentad of TTP that was missing in our patient was renal dysfunction. But, the pentad is observed in less than 10% of patients with TTP.5
Our patient had a documented symptomatic dengue virus infection. Detectable levels of IgG antibody in the acute phase generally suggest secondary dengue infection. But, IgG antibody is detectable in a considerable proportion of primary infections after Day 10 of symptoms. Hence, it is unclear whether our patient had a primary or secondary dengue infection. The timing of symptomatic dengue infection immediately preceding the onset of TTP raises the possibility of dengue infection triggering TTP. Infections are well-recognized triggers of TTP episodes. Even in those with inherited deficiency of ADAMTS13, majority of TTP episodes are triggered by preceding infections. In the French TTP registry, 41% had infections at diagnosis.6 Viruses such as HIV, human parvovirus B19, and hepatitis C virus are known to be associated with TTP.7–9
Dengue virus infection, however, is not a recognized trigger of TTP. Notwithstanding, pathophysiological similarities between TTP and dengue are well known.10 Increased levels of von Willebrand factor (vWF) including presence of abnormal vWF multimers and decreased levels of ADAMTS13 are described in severe dengue infection.4,11,12 These changes are the hallmark of TTP.13 Yet, until very recently, symptomatic TTP has not been reported in patients with dengue. The first and only published report so far is of a 45-year-old man from Brazil, who developed thrombotic microangiopathy following symptomatic dengue virus infection, which improved with 11 plasma exchanges.14 The patient had transient ADAMTS13 deficiency attributed to IgG autoantibodies against ADAMTS13. It has been observed that ADAMTS13 activity levels in severe dengue recover quickly,4 which might explain the normal ADAMTS13 activity seen in our patient during convalescence.
A clinically important question is when to suspect TTP in a patient with dengue. Jaundice, as such, is a rare physical sign in dengue. In a study of 644 Vietnamese adults with dengue, only 11 (< 2%) had jaundice.15 In almost all such instances, jaundice is attributable to liver involvement, and rarely dengue might cause acute liver failure.15 In dengue-associated liver involvement, serum AST level is slightly higher than ALT level, and prothrombin time is usually prolonged.15 We suggest that, when jaundice is accompanied by disproportionate elevation of serum AST level and persistent thrombocytopenia, as noted in this case, one should suspect TTP especially if the prothrombin time is normal.
Apart from infections, pregnancy is another recognized trigger for TTP. But, in the absence of preeclampsia, pregnancy-associated TTP typically manifests during the late second to early third trimester (24 ± 9 weeks).16 However, our patient developed TTP earlier than this, at 16 weeks of gestation. Moreover, pregnancy-associated TTP often recurs in subsequent pregnancies.17 For these reasons, pregnancy was unlikely the immediate trigger in our patient. However, it is possible that dengue virus infection in pregnancy acted as a two-hit phenomenon resulting in TTP.
Dengue in pregnancy per se is considered a high-risk clinical category.18 With the spread of dengue to previously non-endemic areas,19 dengue is increasingly becoming a disease of the adults rather than children. However, published information on dengue in pregnancy is sparse. Recent reports indicate that dengue in pregnancy is associated with poor fetal and maternal outcomes such as miscarriage, low birth weight, preterm birth, and maternal death.20–23 One should be cautious while generalizing the findings from individual case reports, but they are useful for generating hypotheses. This case report exemplifies the fact that dengue in pregnancy is a ripe setting for the development of TTP. Whether TTP could be a pathophysiologic mechanism responsible for the poor maternal and fetal outcomes in pregnant women with dengue, is an interesting possibility that merits further study.
ACKNOWLEDGMENTS
The American Committee on Clinical Tropical Medicine and Travelers' Health (ACCTMTH) assisted with publication expenses.
Footnotes
Authors' addresses: Surendran Deepanjali, Raghuramulu R. Naik, Sharada Mailankody, Sivamani Kalaimani, and Tamilarasu Kadhiravan, Department of Medicine, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India, E-mails: deepanjali.s@jipmer.edu.in, raghu2010jipmer@gmail.com, sharadajayaram27@gmail.com, kalaimani.sivamani@gmail.com, and kadhir@jipmer.edu.in.
References
- 1.Potts JA, Rothman AL. Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health. 2008;13:1328–1340. doi: 10.1111/j.1365-3156.2008.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Russa VF, Innis BL. Mechanisms of dengue virus-induced bone marrow suppression. Baillieres Clin Haematol. 1995;8:249–270. doi: 10.1016/s0950-3536(05)80240-9. [DOI] [PubMed] [Google Scholar]
- 3.Mitrakul C, Poshyachinda M, Futrakul P, Sangkawibha N, Ahandrik S. Hemostatic and platelet kinetic studies in dengue hemorrhagic fever. Am J Trop Med Hyg. 1977;26:975–984. doi: 10.4269/ajtmh.1977.26.975. [DOI] [PubMed] [Google Scholar]
- 4.Djamiatun K, van der Ven AJ, de Groot PG, Faradz SM, Hapsari D, Dolmans WM, Sebastian S, Fijnheer R, de Mast Q. Severe dengue is associated with consumption of von Willebrand factor and its cleaving enzyme ADAMTS-13. PLoS Negl Trop Dis. 2012;6:e1628. doi: 10.1371/journal.pntd.0001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George JN. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood. 2010;116:4060–4069. doi: 10.1182/blood-2010-07-271445. [DOI] [PubMed] [Google Scholar]
- 6.Morgand M, Buffet M, Busson M, Loiseau P, Malot S, Amokrane K, Fortier C, London J, Bonmarchand G, Wynckel A, Provôt F, Poullin P, Vanhille P, Presne C, Bordessoule D, Girault S, Delmas Y, Hamidou M, Mousson C, Vigneau C, Lautrette A, Pourrat J, Galicier L, Azoulay E, Pène F, Mira JP, Rondeau E, Ojeda-Uribe M, Charron D, Maury E, Guidet B, Veyradier A, Tamouza R, Coppo P, Thrombotic Microangiopathies Reference Center High prevalence of infectious events in thrombotic thrombocytopenic purpura and genetic relationship with toll-like receptor 9 polymorphisms: experience of the French Thrombotic Microangiopathies Reference Center. Transfusion. 2014;54:389–397. doi: 10.1111/trf.12263. [DOI] [PubMed] [Google Scholar]
- 7.Gunther K, Garizio D, Nesara P. ADAMTS13 activity and the presence of acquired inhibitors in human immunodeficiency virus-related thrombotic thrombocytopenic purpura. Transfusion. 2007;47:1710–1716. doi: 10.1111/j.1537-2995.2007.01346.x. [DOI] [PubMed] [Google Scholar]
- 8.Kok RH, Wolfhagen MJ, Klosters G. A syndrome resembling thrombotic thrombocytopenic purpura associated with human parvovirus B19 infection. Clin Infect Dis. 2001;32:311–312. doi: 10.1086/318481. [DOI] [PubMed] [Google Scholar]
- 9.Yagita M, Uemura M, Nakamura T, Kunitomi A, Matsumoto M, Fujimura Y. Development of ADAMTS13 inhibitor in a patient with hepatitis C virus-related liver cirrhosis causes thrombotic thrombocytopenic purpura. J Hepatol. 2005;42:420–421. doi: 10.1016/j.jhep.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Krishnamurti C, Kalayanarooj S, Cutting MA, Peat RA, Rothwell SW, Reid TJ, Green S, Nisalak A, Endy TP, Vaughn DW, Nimmannitya S, Innis BL. Mechanisms of hemorrhage in dengue without circulatory collapse. Am J Trop Med Hyg. 2001;65:840–847. doi: 10.4269/ajtmh.2001.65.840. [DOI] [PubMed] [Google Scholar]
- 11.Basuki PS. A glance at the von Willebrand factor in dengue virus infection. Southeast Asian J Trop Med Public Health. 2003;34:559–563. [PubMed] [Google Scholar]
- 12.Sosothikul D, Seksarn P, Pongsewalak S, Thisyakorn U, Lusher J. Activation of endothelial cells, coagulation and fibrinolysis in children with dengue virus infection. Thromb Haemost. 2007;97:627–634. [PubMed] [Google Scholar]
- 13.Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112:11–18. doi: 10.1182/blood-2008-02-078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi FC, Angerami RN, de Paula EV, Orsi FL, Shang D, del Guercio VM, Resende MR, Annichino-Bizzacchi JM, da Silva LJ, Zheng XL, Castro V. A novel association of acquired ADAMTS13 inhibitor and acute dengue virus infection. Transfusion. 2010;50:208–212. doi: 10.1111/j.1537-2995.2009.02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trung DT, Thao le TT, Hien TT, Hung NT, Vinh NN, Hien PT, Chinh NT, Simmons C, Wills B. Liver involvement associated with dengue infection in adults in Vietnam. Am J Trop Med Hyg. 2010;83:774–780. doi: 10.4269/ajtmh.2010.10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin JN, Jr, Bailey AP, Rehberg JF, Owens MT, Keiser SD, May WL. Thrombotic thrombocytopenic purpura in 166 pregnancies: 1955–2006. Am J Obstet Gynecol. 2008;199:98–104. doi: 10.1016/j.ajog.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Vesely SK, Li X, McMinn JR, Terrell DR, George JN. Pregnancy outcomes after recovery from thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Transfusion. 2004;44:1149–1158. doi: 10.1111/j.1537-2995.2004.03422.x. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization and the Special Programme for Research and Training in Tropical Diseases (TDR) Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. New edition. Geneva, Switzerland: WHO Press; 2009. pp. 1–147. [Google Scholar]
- 19.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machado CR, Machado ES, Rohloff RD, Azevedo M, Campos DP, de Oliveira RB, Brasil P. Is pregnancy associated with severe dengue? A review of data from the Rio de Janeiro surveillance information system. PLoS Negl Trop Dis. 2013;7:e2217. doi: 10.1371/journal.pntd.0002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adam I, Jumaa AM, Elbashir HM, Karsany MS. Maternal and perinatal outcomes of dengue in PortSudan, eastern Sudan. Virol J. 2010;7:153. doi: 10.1186/1743-422X-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman EE, Dallah F, Harville EW, Myers L, Buekens P, Breart G, Carles G. Symptomatic dengue infection during pregnancy and infant outcomes: a retrospective cohort study. PLoS Negl Trop Dis. 2014;8:e3226. doi: 10.1371/journal.pntd.0003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan PC, Soe MZ, Si Lay K, Wang SM, Sekaran SD, Omar SZ. Dengue infection and miscarriage: a prospective case control study. PLoS Negl Trop Dis. 2012;6:e1637. doi: 10.1371/journal.pntd.0001637. [DOI] [PMC free article] [PubMed] [Google Scholar]