Abstract
We performed bimonthly mosquito larval collections during 1 year, in an agricultural settlement in the Brazilian Amazon, as well as an analysis of malaria incidence in neighboring houses. Water collections located at forest fringes were more commonly positive for Anopheles darlingi larvae and Kulldorff spatial analysis pinpointed significant larval clusters at sites directly beneath forest fringes, which were called larval “hotspots.” Remote sensing identified 43 “potential” hotspots. Sampling of these areas revealed an 85.7% positivity rate for A. darlingi larvae. Malaria was correlated with shorter distances to potential hotpots and settlers living within 400 m of potential hotspots had a 2.60 higher risk of malaria. Recently arrived settlers, usually located closer to the tip of the triangularly shaped deforestation imprints of side roads, may be more exposed to malaria due to their proximity to the forest fringe. As deforestation progresses, transmission decreases. However, forest remnants inside deforested areas conferred an increased risk of malaria. We propose a model for explaining frontier malaria in the Amazon: because of adaptation of A. darlingi to the forest fringe ecotone, humans are exposed to an increased transmission risk when in proximity to these areas, especially when small dams are created on naturally running water collections.
Introduction
In the 1960s and 1970s, the Amazon frontier began expanding with the introduction of large-scale agricultural colonization projects.1 The reemergence of malaria in this setting has been referred to as frontier malaria.2 Deforestation has been linked to a rise in the prevalence of frontier malaria.3–5 Increased densities of Anopheles darlingi Root (Diptera: Culicidae) adults, the most important vector in the Amazon,6,7 have been demonstrated in areas with human presence and deforestation.8,9 The species is markedly dependent on water habitats for its survival and propagation and it has been proposed that deforestation and environmental changes imposed by human presence could increase A. darlingi breeding and malaria transmission.10 Deforestation creates an interface between two ecosystems and high risk areas of malaria morbidity appear to involve the forest fringes.1,11,12 We have demonstrated increased A. darlingi larval densities at forested-deforested transitions of a small river and proposed that deforestation may affect A. darlingi binomics by creating a new and unique ecosystem: the “forest fringe ecosystem.”13 In this article, initially we performed a “macrospatial analysis” to compare larval samplings at water collections located in forested, deforested, and forest fringe areas (“transitional water collections”) in a typical side road of a frontier colonization project. We showed transitional water collections are found to harbor greater number of A. darlingi larvae than forested or deforested habitats. Water bodies in dryland agricultural zones are basically represented by free running or dammed streams, typically less than 4 m wide but which intermingle with populated areas for dozens of kilometers. Fine scale “microspatial analysis” (every few meters) was performed to verify that larvae were not evenly distributed along their perimeters. As previously demonstrated in a temporary river in the same study area,13 we pinpoint larval clusters in small (< 20 m) areas of fish ponds and streams, usually under the primary (old-growth) forest trees neighboring the deforested areas. These so called “transitional larval hotspots,” were readily identifiable by remote sensing and it is demonstrated that the highly focal distribution of malaria is determined by proximity to these “potential hotspots.”
In the second part of this article, we combine remote sensing and field data to compare malaria incidence in recently deforested areas of the agricultural settlement (< 1–2 years) with areas that have been colonized for longer periods. We hypothesized that deforestation could affect malaria transmission by modifying the distance between houses and potential hotspots: homes of newly arrived settlers tended to be closer to hotspots and thus have greater malaria incidence. Meanwhile, houses located in thoroughly deforested areas would be associated with low malaria incidences. However, deforestation proved to be a complex and nonuniform process, both temporally and spatially. Leftovers of old growth forest frequently became intermingled with fields of locally cultivated crops. These leftovers are referred to, in this article, as “forest remnants.” Their potential to harbor transitional hotspots and boost malaria transmission within otherwise entirely deforested areas was studied in detail. In the final part of this article, we propose a transmission model based on the proximity to the forest fringe that categorizes frontier malaria into zones. Our transmission model, which suggests that promotion of malaria transmission in the initial stages of deforestation is a transient phenomenon, soon replaced by reduced transmission under extensive deforestation and establishment of farming and pastures, is compared with the former hypothesis stating that deforestation invariably promotes increased malaria transmission.8,10
Materials and Methods
Study site, climate, deforestation pattern statistics, and population dynamics.
The study was conducted within an agricultural frontier settlement of Rorainópolis, 300 km south of Boa Vista, the capital of the province of Roraima, in the northern Brazilian Amazon. The climate is tropical wet-dry (Am; groupings of Köppen–Geiger–Pohl's climatic types) and has two distinct seasons; a well-defined 6-month-long dry period lasts from November to April, and the wet season from August to March, with 55–60% of the precipitation occurring from May to July.14 Climate and ecoregional characteristics of Roraima have also been previously reported.15,16
Most settlements in the Anauá region of southern Roraima are recent (< 10 years of the collection period) and are composed of multiple side roads that run perpendicular to a main road or feeding artery, forming a characteristic fishbone pattern. Side road 19 is a typical secondary road, branching perpendicularly from the main road for 18.8 km into the rainforest, which entirely surrounds the area. The study site has been well characterized.17–19 The first 1.9 km of the side road lack houses because they are the back of larger land lots bordering the main road. From then on, at either side of the road, 75 houses were located. Distances and standard deviations (SD) between each house and the side road averaged 52.5 m (38), with a minimum of 9 m and a maximum of 242 m, but only three houses (4%) were spaced more than 100 m from the road. Houses were separated from each other at mean intervals of 229 m (SD = 174). Each house would usually be permanently inhabited by one family core, but four houses (5.3%) were inhabited less than 6 months a year and were excluded from quantitative malaria correlation studies. Five houses were inhabited for 7–10 months per year. Questionnaires were applied to 66 families (92.9%) to know their time of arrival; 5 families (7.1%) could not be contacted during the study period, but information was gathered from neighboring families.
The construction of side roads, performed from the main road into the forest, may take months or years to be completed and arriving settlers usually move to their lands only when the roads have reached them, which means that newcomers are usually further away from the main road, that is, the beginning of the side road, than settlers that arrived earlier. A 2-tailed Bonferroni adjusted Spearman rank correlation showed a significant negative association between the time of arrival of a family, in years, and the distance of their land lot to the beginning of Side road 19, in km (r = −0.46; P < 0.001), that is, the shorter the arrival time, the further away from the main road the settler would be.
Remote sensing was used to identify and quantitate the amount of deforestation. Four-band 2.4 m/pixel resolution satellite images, obtained on October 2003 by Quickbird 2 Digital Globe™, Longmont, CO), a commercial earth observation satellite, were used. Georeferenced maps were produced with Mapsource™ (Garmin Inc., Schaffhausen Switzerland). The position of houses and the perimeters of water collections were confirmed with Garmin™ 72 devices. Images are currently available through Google Earth™ (Google Inc., Mountain View, CA; http://www.google.com/earth/).
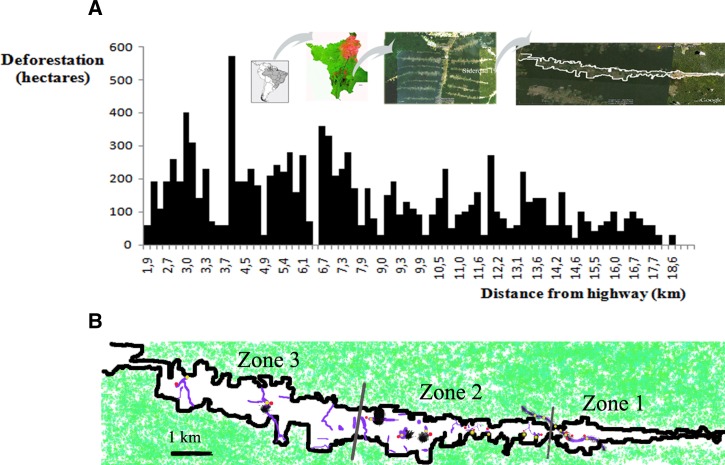
Each year, the forest is progressively cleared, always during the dry seasons, in an outward direction, perpendicularly to the main axis of each side road. Deforestation tended to be more extensive in land lots that had been occupied longer, closer to the beginning of the side road (Figure 1A ), which results in a roughly fin-tipped isosceles triangular configuration. In areas occupied earlier (for > 3 years), deforestation would have progressed up to 1,800 m into the forest and in land lots where settlers have recently arrived (< 1–2 years), the fringe was located closer to the house, only 130–250 m. A two-tailed Bonferroni adjusted Spearman rank correlation was significant between the time of arrival of a family in years and the amount of deforestation (in hectares) that had occurred in a particular land lot (r = 0.37; P < 0.01). Similarly, two-tailed Bonferroni adjusted Spearman rank correlation showed a significant negative association between the distance between a house and the forest fringe with the distance of the house to the beginning of the side road, that is, the further away from the main road, the closer houses would be to the forest fringe (P << 0.0001). The rate at which deforestation progressed was studied with simple regressions: a significant (P < 0.001) association was found for the linear equation y = a + bx, where y = deforested area, in hectares, and x = number of years since arrival. Parameter estimates were a = 4.386 and b = 1.551, which means that in 1 year a mean of 5.9 hectares would be deforested, 7.5 hectares in 2 years, and 9.0 in 3, that is, a mean of 1.551 hectares per year from the second year onward. A similar significant (P < 0.001) simple regression was performed for calculating the distance the forest fringe would be set back from the side road, retrieving a = 146.206 and b = 51.712: after 1 year of colonization, the forest would be located a mean of 197 m from the side road, and thereafter a mean of 51.7 m further per year.
Figure 1.
(A) Relationship between deforestation, as of August 2003, in hectares of forest in land lots at both sides of the side road, as related to the distance to the beginning of Side road 19. Inset: from left to right: South America and Brazil, Roraima Province, Anauá Colonization Project in southern Roraima and Side road 19 (outlined). (B) Detail of Side road 19, demonstrating the roughly triangular outline of the deforested areas. Deforestation was divided into three deforestation zones for the purposes of this article, as explained in the text.
Remote sensing, terrain topography, hydrology, and pond building statistics.
Satellite imagery was able to delineate the old growth forest and its fringes, identify and classify water collections (streams, small rivers, or rivers), distinguish low (< 2 m, deforested < 2 years earlier) vegetation from high shrub plants (> 2 m, usually deforested 2–5 years earlier), identify crops, houses, secondary forests (i.e., vegetation older than 10–15 years, with canopy heights of 5–15 m high), and forest remnants (forests with little evidence of human exploitation and dense canopies, > 15 m high). Primary rainforest is characterized by a full ceiling canopy and several layers of understory. The ground floor is clear of vegetation because the full canopy allows very little light, necessary for plant growth, to penetrate. The area has no large or medium-sized (> 20–100 m wide) rivers. There are no swamps in the locality and flooding is limited to the margins of the only small temporary river (5–15 m wide), the Azul River, during the wet season. A detailed description of the Azul River has been reported.13 Another two categories of naturally occurring water collections were distinguished: small streams (< 1 m wide) and large streams (2–4 m wide). The width of the water collections mentioned was defined as those encountered during the wet–dry transition period (November). Smaller streams were not systematically studied. Approximately 30 large streams were identified through cross-sectional surveys. Forest streams were not visible on satellite imagery, but streams in deforested zones were readily identifiable due to lack of overhead foliage. The sites where streams in deforested zones crossed into forested areas (“transitional hotspots”) were also easily located on satellite imagery. Of the 30 large streams, 19 (63.3%) were chosen for year-round sampling, among which were 8 forest streams and 11 transitional streams. Another 10 (33.3%) streams were chosen for single-shot sampling during the dry season.
Fish ponds were built for creating readily available water collections, which are used for bathing, washing, recreation, and aquaculture. At the time of study, no fishes had yet been artificially introduced in any pond; however, small naturally occurring fish of various species were present. Fish species were not determined. These blockages were created by raising wooden and earth barriers, usually 1–2 m high. Twenty ponds were identified in the side road, all of which were visible by satellite imagery. All ponds were located on streams and no man-made damming was built on the Azul River, presumably due to its size, requiring greater construction abilities. Ponds were numbered eastward from 1 to 20 and sampled throughout the year.
The construction of fish ponds was performed erratically, by settlers, but was linked to deforestation. An analysis of variance (ANOVA) using the presence or absence of a pond within 500 m of a house as the categorical predictor showed that ponds were associated with a significantly higher deforested area (F(1,73) = 13.20; P < 0.001) in a land lot (mean of 11.66 hectares, standard error [SE] = 1.09), as compared with land lots without ponds; mean of 6 hectares (SE = 1.07). Another ANOVA, showed that houses without nearby (< 400 m) ponds in Side road 19 (N = 38) were a mean of 12.6 km from the main road (SD = 5.4), whereas houses with ponds (N = 37) were a mean of 7.7 km (SD = 3.0), differing significantly (F(1,73) = 23.19; P << 0.0001).
Forest remnants will be defined, for the purposes of this article, as a remaining area of old growth forest (> 10 m2) located inside the deforested area of the side road, that is, surrounded on at least three cardinal directions by deforested areas. Remnants were classified either as “forest peninsulas” or “forest islands.” Forest peninsulas are defined as projections of old growth forest into the deforested colonization area that remain linked to the main body of the forest, but are bordered on three sides by deforested areas. Forest islands are defined as remnants of old growth forest that have lost their link with the main body of the old growth forest and are separated from it by at least 20 m. A projection of forest was considered a peninsula when it encroached on the deforested area at least 60% more than neighboring areas.
Macrospatial entomological studies: larval sampling, larval habitat parameters analyzed and perimeter classification.
Studies were conducted during bimestrial collecting excursions from August 2003 to July 2004, using a standard 0.5-L dipper (Bioquip Co., Gardena, CA),20 as previously described.13 It has been previously noted that Nyssorhynchus larvae are distributed on the margins of water collections and are rarely found free in the water body.21 Only the perimeters of the water collections were sampled. The type of overhead vegetation and average scene luminance were determined for each studied larval habitat. The distance to the nearest permanently inhabited house was estimated with global positioning system (GPS) mapping. Luminance was categorized as high (> 100 cd/m2, typically 400–500 cd/m2) or low (< 100 cd/m2, typically < 10 cd/m2) measuring the amount of reflected light at the sites where larvae were retrieved, as measured by a reflected light meter (Nikon™, Nikon Corp., Tokyo, Japan).13
For the purposes of the macrospatial analysis in this article, “forest fringes” are defined by the last row of standing old growth forest trees, either dead or with foliage, bordering a deforested area.
Human malaria data.
Malaria in the area is unstable and hypoendemic, predominantly due to Plasmodium vivax (Chaves and Rodrigues 2000). Morbidity data were collected from the Rorainópolis Municipal Health Service, based on symptomatic thick-smear-positive cases. Detailed entomological and epidemiological methods are described elsewhere.22 Every 1–2 weeks, community health agents would periodically perform thick blood smears in residents presenting nonspecific acute febrile symptoms and their household contacts. Active thick-blood-smear surveillance of the entire population (symptomatic and asymptomatic) was also performed in September 2003 and January 2004. Malaria morbidity data were available retrospectively from January 2002 to July 2003 and prospectively from August 2003 to July 2004, totaling 2.5 years of observation. These results were not confirmed with polymerase chain reaction. Households, where each case was reported, were georeferenced for spatial analysis. Informed consent was obtained from adult participants and from parents or legal guardians of minors and the procedures used in this article were approved by the ethics committee of the Oswaldo Cruz Foundation (Fiocruz) (Protocol 05/03).
Statistical and microspatial data analysis.
ANOVA, Spearman rank correlation, dependent sample t test, odds ratios (OR), χ2, and simple linear regressions were used for numerous analyses, using StatSoft® Inc. (2011) Statistica data analysis software system, version 12, at significance levels of 0.05. Mean number of larvae per site was compared with Wilcoxon's matched pairs test, using collection months as the pairing variable. A χ2 test using the number of larvae at a deforested water perimeter, as compared with a forested control site (expected frequencies), was performed. Fisher's exact test was used for comparing the number of larvae before and after deforestation for establishing manioc plantations and extending a cattle ranch in three ponds.
During exploratory sampling studies, an uneven spatial distribution of A. darlingi larvae soon became apparent. For this reason, during 1 year, we mapped the position of A. darlingi larvae along the perimeter of water collections. “Transitional hotspots” were defined as the perimeter of these water collections, less than 20 m long, located immediately under the forest fringes. Transitional hotspots were typically low luminance habitats. When a pond possessed both deforested and forested perimeters, it would be considered to have more than one “larval habitat” and these were designated by letters: “S” (sunlit) for the deforested habitat and “F” (forest) for the low luminance habitat under the forest. A single body of water more than 20 m wide crossing into the forest would be considered as having two transitional hotspots, one on each bank. A large stream that crossed into a deforested field more than 20 m wide and then back to the forest would be considered as harboring two transitional hotspots, one on each forested-deforested transition. A deforested stream and Pond 20 had more complex perimeter vegetation and were given their own lettering system, as explained below.
Since the resolution of available GPS devices was below that required for spatial mapping of dips in the ponds, the approximate location of each sample was determined by GPS coordinates taken every 20 m. The arbitrarily fixed reference point was considered 0 m, and the distances of that point to samples taken along the pond circumference were determined. One sample was taken at every 2 or 3 m. Cartesian coordinates were determined transforming the data into a linear metered Cartesian model and imported into SatScan version 9.1, a spatial statistical analysis software. A purely spatial scan, the Kulldorff statistical analysis, was used to test whether larvae were randomly distributed along the water margins and to identify significant spatial clusters, if present.23 A more detailed explanation of this procedure has been published.19
Results
Macrospatial, quantitative, and temporal patterns of A. darlingi larval distribution: transitional water collections, especially ponds, harbor higher and more seasonally stable numbers of A. darlingi larvae.
During the period of collection, over 8,233 dips were made, retrieving 1,254 specimens, comprising 12 species. In total, 313 larvae of A. darlingi were found in 23 (59%) of the 39 water collections.
Of the eight sampled old growth forest water collections, all of which were streams, six (75%) were found positive at least once. In the 13 deforested water collections sampled, all of which were ponds, only one (7.7%) (Pond number 7) was found positive on any occasion. Despite extensive sampling, no A. darlingi larvae were retrieved from ponds numbered 1, 2, 3, 4, 5, 6, 11, 14, 15, 17, 18, and 19, all of which had entirely sunlit margins, with only occasional low bordering shrub plants. The other seven ponds (numbered 8, 9, 10, 12, 13, 16, and 20) were all located on forested-deforested transitions and will be referred to, from now on, as “transitional ponds.” All seven transitional ponds studied (100%) were positive on at least one occasion. Of the 11 transitional streams sampled year-round, 9 (81.8%) were positive on at least one occasion and 2 (18.2%) were negative.
The number of A. darlingi larvae per 1,000 dips retrieved in each type of water collection was compared in Table 1. The eight positive ponds (7, 8, 9, 10, 12, 13, 16, and 20) accounted for 83.4% (N = 261) of the total. An ANOVA using these categories showed a significant difference between mean A. darlingi larval densities between transitional ponds and the other three types of water collections (F(3,35) = 40.55; P << 0.0001), but not among the members of the three categories. Deforested water collections (N = 13) had a mean of 4.2 larvae per 1,000 dips (SE = 12.4) during the collection period, whereas old growth forest water collections (N = 8) had a mean of 8.5 larvae per 1,000 dips (SE = 19.2) and transitional water collections (N = 18) 60.4 larvae/1,000 dips (SE = 76.3). The difference was primarily due to increased larval densities in ponds, as compared with streams: positive transitional ponds (N = 7) had significantly more larvae than positive transitional streams (N = 9) by ANOVA (F(1,14) = 41.92; P << 0.0001), with 140.7 larvae/1,000 dips (SE = 62.0), as compared with 7.2 larvae/1,000 dips (SE = 2.3), respectively. The mean number of larvae per site was significantly higher among all sampled ponds, as compared with streams, using Wilcoxon's matched pairs test (P < 0.05), with collection months as the pairing variable.
Table 1.
Mean Anopheles darlingi larval densities/1,000 dips retrieved per type of habitat in each collection period
| Type of positive larval habitat | August (SD) | November (SD) | January (SD) | March (SD) | May (SD) | July (SD) |
|---|---|---|---|---|---|---|
| 1. Forest streams* (N = 6) | 0 (0) | 0 (0) | 5,68 (16) | 39,58 (99) | 6,25 (17) | 0 (0) |
| 2. Deforested ponds† (N = 1) | 6,84 (24) | 6,84 (24) | 0 (0) | 6,84 (24) | 0 (0) | 0 (0) |
| 3. Transitional ponds (N = 7) | 164,02 (189) | 143,44 (113) | 155,99 (142) | 127,78 (215) | 179,08 (202) | 73,81 (66) |
| 4. Transitional streams‡ (N = 9) | 5,56 (84) | 8,59 (14) | 3,33 (12) | 26,67 (12) | 12,22 (13) | 0 (0) |
SD = standard deviation.
Sites that were negative in all collections were not included. The number of positive sites of each type is indicated in the first column.
Only six forest streams are shown; two forest streams were negative on all occasions.
Only Pond number 7 is shown; another 12 deforested ponds were negative on all occasions.
Only nine positive transitional streams are shown; another two transitional streams were negative on all occasions.
Using a three category classification for the vegetation cover (0 = old growth forest water collection, 1 = transitional water collection, and 2 = deforested water collection), an ANOVA of the number of times A. darlingi larvae were retrieved during the six collecting periods were significantly greater in transitional water collection, as compared with deforested water collection (F(2,36) = 5.97; P < 0.01). In forested habitats (N = 8), A. darlingi larvae were retrieved a mean of 1.62 (SE = 1.60) times per site, during the collection period, whereas in transitional water collections (N = 18) they were positive a mean of 2.5 times (SE = 2.31) and deforested water collections (N = 13) 0.31 times (SE = 0.75). The only type of water collection positive year-round was the transitional ponds and all streams were devoid of larvae during the wet season.
Potential larval hotspots may be identifiable by satellite imagery: remote sensing may predict locations of A. darlingi foci.
In total, 43 potential transitional hotspots were identified by satellite imagery in Side road 19 (Supplemental Table 1). The water collections of 18 (41.8%) of these transitional hotspots received year-round sampling (six times): 11 were located in streams and 7 were located in ponds (8, 9, 10, 12, 13, 16, and 20). Another 10 (23.3%) potential hotspots were sampled only once, during the dry season, which means a total of 65.1% potential hotspots were sampled at least once. Anopheles darlingi larvae were found in 85.7% of potential hotspots. Two (11.1%) hotspots sampled year-round, both large streams that had been blocked by raised earth during the construction of the side road, were found negative on all occasions. Also two hotspots sampled a single time, both large streams at forest fringes, were negative for A. darlingi larvae.
Anopheles darlingi larvae are unevenly distributed along the perimeter of positive water collections: microspatial analysis suggests clustering at hotspots.
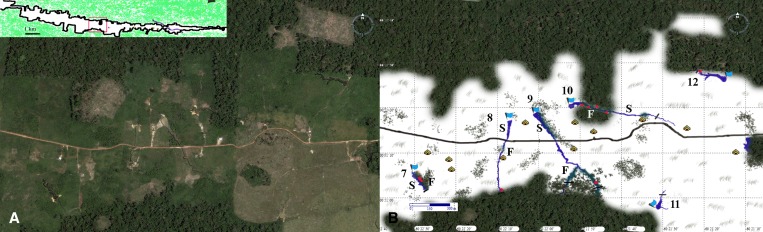
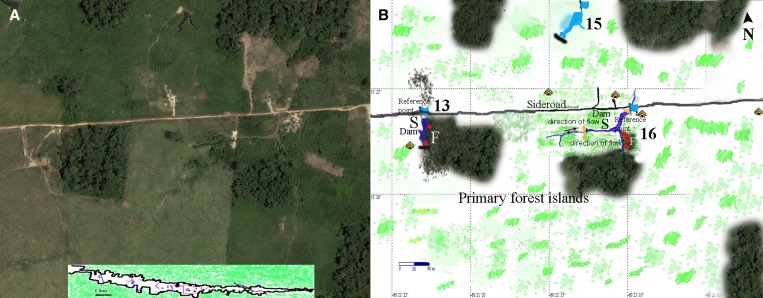
Spatial studies were performed on the eight positive ponds, as well as the deforested stream. Ponds 7, 8, 9, 10, and 12 are illustrated in Figure 2 . Ponds 13 and 16 are shown in Figure 3 . The old growth forest bordering the margins of Ponds 13 and 16 is part of forest islands, one with 1.7 hectares and the other 2.8 hectares. Larval clusters identified through Kulldorf spatial analysis are listed in Supplemental Table 2. Within the 9 water collections, covering a total distance of approximately 5,300 m, we identified 13 locations that harbored clusters (“cluster sites”); some clusters were encountered more than once during the 6 collection periods, totaling 29 larval clusters (significant or not), of which 16 were statistically significant. Clusters were found always in low luminance sites in 10–20 m long sites, which means that almost all larvae could be retrieved within only 360 m of the total perimeter (6.7% of the total distance sampled). The locations of most clusters, 81.8% (N = 9), were directly below old growth forest fringes. Of the remaining four clusters, one cluster was located under shrub plants, in Pond 7, where lush (approximately 2.0 m high) Cecropia sp. created a low luminance habitat. The other three clusters were also found below tall (> 2 m) shrub plants: one in Pond 9, where high shrub plants created a continuous low luminosity larval habitat, and two in the cattlefield stream. The latter is depicted in detail in Supplemental Figure 1.
Figure 2.
(A) Satellite view of Side road 19 showing six ponds (7–12), image from Google Earth® (http://earth.google.com/). (B) Schematic view of the same area. Flags indicate the starting point of samples and a black strike indicates the ending point. Samples were taken on both sides of the larval habitat from the starting point to the ending point. Dots indicate the approximate locations where clusters were identified in any of the six collecting excursions. Pond 10 crosses a typical forest peninsula. Pond 11 had entirely deforested margins and was negative on all occasions for Anopheles darling larvae. “S” = sunlit areas of the water collection; “F” = shaded areas.
Figure 3.
(A) Satellite view of three ponds (13, 15, and 16). (B) Schematic view of the same area. Ponds 13 and 16 are in proximity with old growth forest islands. Pond 13 borders a cattle ranch. The locations of houses are indicated (all had a high number of malaria cases) as well as the approximate site of occurrence of Anopheles darlingi larval clusters (dots). Flags indicate the starting point of samples and the black strikes the ending points. Pond 15 had entirely deforested margins and was negative on all occasions for A. darling larvae. “S” = sunlit areas of the water collection; “F” = shaded areas.
Out of the 13 identified cluster locations of A. darlingi larvae, most of the clusters found (8; 61.5%) were encountered under old growth forest. Three (23.0%) had both old growth and secondary forested margins and two others (15.3%) had only secondary forest. We verified a higher overall number of larvae (N = 142) in the old growth forest sites (a mean of 28.4 larvae/site/year), as compared with the secondary forest sites (N = 25) (mean of 8.3 larvae/site/year), but the difference was not statistically significant by Student's t test (P = 0.09).
Malaria incidence is closely linked with the house-to-potential-hotspot distances.
In total, 186 malaria cases of P. vivax malaria were reported. Of these cases, 13 (6.9%) occurred less than 50 days after the first episode. These cases were included in the study, since Plasmodium drug resistance has not been reported in the area and treatment failures are known to be a rare occurrence. Only two Plasmodium falciparum cases were encountered and none of Plasmodium malariae.
An ANOVA using a binomial categorical predictor describing the presence or absence of malaria cases in houses and the house-to-hotspot distance was significant (F(1,69) = 10,66; P < 0.005): houses with no malaria cases (N = 30) were associated with a mean house-to-hotspot distance of 640 m (SE = 106), whereas houses with malaria cases (N = 41) were associated with a mean distance of only 297 m (SE = 287); the upper 95% confidence interval (CI) for houses with malaria cases was 387 m and the lower 95% CI for houses without cases was 422 m. A mean of 3.41 malaria cases (SE = 0.58) were found in houses < 400 m of potential hotspots, whereas only 1.03 (SE = 0.72) cases were found in houses further away. We performed an ANOVA using the binomial categorical predictor “presence” or “absence” of a potential hotspot within 400 m, and the difference between total number of malaria cases was significant (F(1,69) = 5.90; P < 0.05). Of the 41 houses with malaria cases, 20 (48.7%) were within 400 m of positive ponds, another 13 (31.7%) were within 400 m of other types of hotspots on streams. About 95.1% of malaria positive houses (N = 39) were within 830 m away from the nearest hotspot. The two remaining houses were around 1–1.3 km away. Of houses with no malaria cases, 58.8% were located more than 500 m from these sites.
Table 2 summarizes OR calculations, χ2 statistic, and one-tailed significance levels for malaria in houses within 400 m of potential hotspots; ponds (either deforested or transitional); only transitional ponds; any kind of shaded water collection (either streams, ponds, or other small collections); any kind of forest remnant; forest peninsulas; and at various distances from forest fringes. The relative risk of malaria for settlers living within 400 m of a potential hotspots was 2.60 (95% CI = 1.40–4.81; P < 0.005) times higher than that of those living further away. Regarding transitional ponds, two-tailed Spearman rank correlation showed significant results between the total number of malaria cases per house and the distance to these ponds (r = −0.66; P < 0.01; N = 16). However, when any kind of pond was analyzed, either deforested or transitional, the relative risk of malaria for settlers living within 400 m was 1.08 (95% CI = 0.71–1.63; P = 0.72), which means that ponds per se did not induce an increased risk. The same way as increasing the distance to the beginning of the side road decreases the mean distance between the side road and the forest, it can be expected that the side road-to-potential-hotspot distance will also be negatively correlated: increasing the distance from the beginning of the side road decreases the distance between the side road and potential hotspots (F(1,41) = 58.70; P << 0.0001). Parameter estimates for the linear equation y = a + bx, where y = the distance in kilometer between the potential hotspot and the side road, and x = the distance to the beginning of the side road, were a = 0.457 and b = −0.026, which means that at 0 km, one would expect a 457 m distance between a potential hotspot at the forest fringe and the side road; decreasing approximately 26 m for every 1 km. A house in a land lot located at the 3rd km of Side road 19 would be a mean of 379 m away from a potential hotspot, whereas one at 10.2 km would be only approximately 191 m away.
Table 2.
Pearson's χ2 values, significance levels, and OR determinations for malaria risk factors in Side road 19
| Parameter | χ2 | P value | OR (95% CI) |
|---|---|---|---|
| < 400 m from nearest A. darling–positive fish pond | 14.403 | 0.0001 | 8.68 (2.59–29.14) |
| < 400 m of nearest potential hotspot | 13.992 | 0.0002 | 6.66 (2.36–18.79) |
| < 400 m of nearest forest remnant | 9.261 | 0.0023 | 4.86 (1.69–13.95) |
| < 400 m of any shaded water | 3.231 | 0.0723 | 2.49 (0.91–6.81) |
| < 400 m of any fish pond | 0.129 | 0.7198 | 1.18 (0.48–2.94) |
| < 400 m of a forest tongue | 0.011 | 0.9162 | 1.05 (0.42–2.61) |
| < 130 m from forest | 0.037 | 0.8473 | 0.91 (0.37–2.28) |
| < 160 m from forest | 0.135 | 0.7134 | 1.19 (0.46–3.08) |
| < 190 m from forest | 0.072 | 0.7882 | 0.87 (0.31–2.40) |
| < 50 m from forest | 0.974 | 0.3236 | 0.54 (0.15–1.87) |
CI = confidence interval; OR = odds ratio.
Only three risk factors were significant: proximity to Anopheles darlingi positive fish ponds, proximity to potential hotspots, and proximity to forest remnants.
The continuous parameter “house-to-potential hotspot distance,” in kilometer, was significantly associated with the total number of malaria cases per house by two-tailed Bonferroni adjusted Spearman rank correlation (r = −0.46; P < 0.001), as well as simple regression (F(1,69) = 8.44; P < 0.005). Parameter estimates for the y = ax + b equation, where x = house-to-potential-hotspot distance, in kilometer, and y = total number of malaria cases in 2.5 years of observation, were a = 3.954 and b = −2.866, which means that houses located 100 m (0.1 km) from a transitional hotspot would have a mean of 1.467 cases of malaria per year (3.668 cases during the 2.5 years), those at 500 m (0.5 km) would have 1.088 cases per year (2.521 cases in 2.5 years), and those located > 1 km would have less than 0.435 cases (1.088 cases in 2.5 years).
We conclude that initial deforestation promotes malaria incidence because of human presence and proximity of settlers with forest fringes and hotspots, but ongoing deforestation would increment the house-to-potential hotspot distance, therefore, increasing the isolation of dwellings and decreasing malaria incidence.
Slash-and-burn clearing of the forest edge: numbers of A. darlingi larvae decrease when the overlying forest is cleared.
Ponds 13, 16, and 20 were subjected to deforestation on their southern sides in February and March 2003, during the dry season. The numbers of larvae retrieved in each collection are shown in Table 3. A fine-scale schematic representation of the deforestation in Pond 20 is depicted in Supplemental Figure 2. Significant Fisher's exact test two-sided statistics for all three data sets, which remain significant even if Bonferroni correction for multiple testing is applied, suggest that deforestation decreases A. darlingi larval abundance.
Table 3.
Total number of Anopheles darlingi larvae collected from three ponds, during three collecting periods before deforestation and three periods after slash-and-burn clearing, as compared with areas in the same pond where the primary forest was not damaged (controls)
| Larval count (collection period) | Pond 13 (%) | Control area 13 (%) | Pond 16 (%) | Control area 16 (%) | Pond 20 (D) (%) | Control area 20 (E) (%) |
|---|---|---|---|---|---|---|
| Before deforestation (August 03–January 04) | 6 (31.6) | 13 (68.4) | 10 (23.3) | 33 (76.7) | 14 (63.6) | 8 (36.4) |
| After deforestation (March 04–July 04) | 0 (0) | 20 (100) | 2 (3.7) | 52 (96.3) | 2 (16.7) | 10 (83.3) |
| Fisher's exact test P value | 0.008 | 0.005 | 0.013 | |||
Fisher's exact 2-sided test significance values for each set of data are reported.
Forest remnants decrease house-to-potential-hotspot distances and are associated with increased malaria incidence.
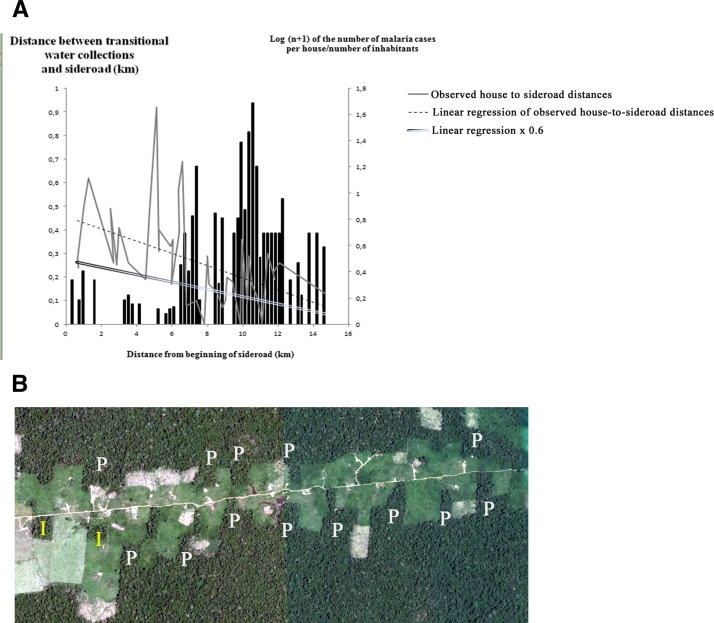
The linear equation describing the house-to-transitional-water-collection distances is depicted in Figure 4 , as well as the 0.6 cutoff values for defining a forest peninsula. For graphical representation, the position of the first inhabited house was considered 0 km. In the study area, 12 peninsulas and 3 old growth forest islands were identified, totaling 15 forest remnants, of which 5 (33.3%) were associated with transitional hotspots. Forest remnants decrease the distance of neighboring houses and the forest fringe. An ANOVA using the presence or absence of forest remnants within 400 m of a house and the distance to the nearest potential hotspot showed a significant difference (F(1,73) = 59.43; P << 0.001): whereas houses near remnants (N = 51) had a mean distance of only 250 m to the nearest transitional hotspot (SE = 177) and houses distant from remnants (N = 24) had a mean distance of 912 m (SE = 560). Forest peninsulas and islands were also shown to decrease the side road-to-transitional-water-collection distance (the dependent variable), as verified by an ANOVA using the presence or absence of forest remnants within 400 m as the categorical predictor (F(1,73) = 59.43; P << 0.0001).
Figure 4.
(A) Dispersion graph showing the true hotspot-side road distances (continuous grey line). The predicted hotspot-side road distances obtained by linear regression with a = 0.457 and b = −0.026 (dotted line) and 60% of these predicted distance values (double line). Forest projections were considered peninsulas when they encroached the deforested field > 60% of the predicted distance to the side road. The portions of the grey line below the double line were considered as representing the locations of forest peninsulas, but could also correspond to the location of old growth forest islands. The log (n + 1) of the ratio between the number of malaria cases per house and the number of occupants in each house is depicted in black columns. (B) Satellite view of the 7th–11th km of Side road 19, denoting the multitude of forest peninsulas (P) and forest islands (I).
In Figure 4, it is shown that the log (n + 1) of the ratio between the number of malaria cases per house and the number of occupants in each house, that is, the number of malaria cases per occupant, is graphically correlated with the occurrence of forest peninsulas and forest islands. A greater number of malaria cases per inhabitant was found after 6.78 km, where the side road-to-transitional water collection distance fell to approximately 80 m. However, no cases of malaria were found at the final 1.1 km of the side road. The presence or absence of forest remnants within 400 m of the house was associated with the number of malaria cases through ANOVA (F(1,69) = 4.95; P < 0.05), whereas houses near remnants (N = 49) had a mean number of 1.34 malaria cases per year (SE = 0.23) and houses distant from remnants (N = 22) had a mean number of 0.42 malaria cases per year (SE = 0.34). The OR for the presence of malaria in houses within 400 m of forest remnants is shown in Table 2.
Discussion
Frontier malaria from an entomological perspective: the “forest fringe model” predicts that behavioral and biological factors determine clustering of A. darlingi larvae at ecosystem transitions.
In this article, we have provided compelling evidence that the dead zone is a non-homogenous habitat for A. darlingi: larvae are preferentially encountered in areas with decreased luminosity, in contrast to “sunlit” portions of the same water collection. The preference for “shaded” water collections has been previously noted.24,25 The preference of A. darlingi for sites with lower luminosity may help explain the absence of the species in deforested areas, as well as in the largely treeless savanna in Suriname,26 English Guyana,27 and in northern Roraima.16,28 The species reappears in patches of forests and alluvial forests.16 The preference for lower luminosity has been suggested to be a behavioral characteristic of the forest-adapted ovipositing female.13 Until a better understanding of forest fringes is available, it maybe preferable that studies use the notion of luminance measured at the perimeter of each water margin rather than the more usual term “shade.” It is believed female Anophelines oviposit during twilight, when the distinction between shaded and unshaded sites is not possible because indirect sunlight is scattered in the upper atmosphere in multiple directions. During twilight, the amount of incident light, at the larval habitat, would be determined by the amount of steradians (solid angles) of unobstructed sky. Luminance better describes the amount of reflected light on the water surface of collections, as may be perceived by mosquitoes. We caution against the use of “percent of shade covering” on the totality of a given water collection. This measure may be biologically useless since larvae are limited to the dead zones, giving inconsistent results.
In this study, we verified the following entomologic findings: 1) A. darlingi larvae were not found in habitats with entirely deforested margins, positivity rates and larval densities were higher in transitional water collections; 2) larvae were clustered at forest fringes and absent where perimeters were deforested; 3) when the overlying forest is cleared, larval clusters disappear; 4) ponds harbor larvae in higher densities than streams, that are maintained during the wet season; 5) larvae were recovered in water collections bordered by forest remnants in high densities and also under tall grass and secondary forest; 6) larval hotspots may be remotely identified by searching for sites where forest fringes intercept water collections.
The well described “edge effect,” in the ecological literature, refers to the tendency for greater diversity of life in the region where two adjacent ecosystems overlap such as forest/grassland or land/water. At the boundary of overlapping ecosystems, species from both of these environments may coexist, as well as unique species that may be specially adapted to the conditions of the ecotone. In forest fringes, we have demonstrated an increased occurrence of Anopheline larval numbers, species diversity,18 as well as parasite infestation of larvae by trophonts of Tetrahymenidae,29 which were associated with larval habitats with unusually high larval densities. In particular, A. darlingi may have uniquely adapted to the forest ecotone of forested-deforested transitions due to its greater anthropophily, as compared with other Anopheline species.13 Deane and others30 observed that, during droughts, A. darlingi larvae become limited to foci that are almost always located at forest fringes. It has also been proposed that A. darlingi adults may also prefer to fly close to the edge of the forest, rather than disperse over the adjacent open ground.31
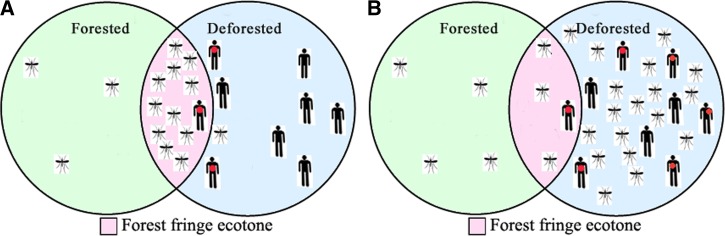
Based on clustering of larvae at deforestation transitions of a temporary river, we have advocated a “forest fringe model.”13 The model proposes that three factors that cooccur at forest fringes contribute to increase breeding of A. darlingi: availability of human blood meals, stable surface water, and low luminance water perimeters. The forest fringe model is summarized in Figure 5A : an increased vector density is found at the fringes of primary forests, determined by augmented A. darlingi larval colonization, as compared with forested or deforested zones. Because gravid females are either attracted to low luminance sites or repelled by high luminance water collections, the mosquito appears to have poorly adapted to both the deforested or urban environments created by man. We have so far failed to identify any other biochemical or physical factors that may be at play. The disappearance of larvae, after clearing of forest fringes, while numbers remain in untouched areas of the same water collection, suggest that luminance is a major factor. This is reported for the first time in this article. Humans, the preferred vertebrate host, rarely settle inside the primary forest ecosystem, but by positioning their dwellings near the forest fringe, they may reinforce the mosquitoes' blood feeding and oviposition cycles. The occurrence of A. darlingi larvae in proximity to human dwellings has been well described.10,18 Humans sleeping in proximity to the fringe are exposed to both increased vector bites and bites from recently oviposited (epidemiologically older) females, increasing malaria risk. This creates highly focal transmission zones because mosquitoes insinuate little into deforested areas, leaving most of the human population in deforested areas unaffected. In accordance with our data, Moutinho and others32 have demonstrated higher A. darlingi biting activity at the end of a side road, in less modified environments, where deforestation was still ongoing, as compared with a site with more degradation closer to the beginning of the side road. Malaria epidemiology data supporting the model are discussed below.
Figure 5.
(A) Forest fringe transmission model for explaining frontier malaria incidence in dry-land agricultural settlements in the Amazon: Anopheles darlingi breeding is limited to the old growth forest environment, but deforestation creates an unstationary forest fringe, where larval numbers are profoundly increased. Proximity to the fringe exposes humans to an increased risk of malaria infection (dots), while most of the population, within the deforested zone, remains unaffected. (B) Schematic representation of the traditional malaria transmission model, where A. darlingi breeding and malaria transmission are increased at deforested areas.
The preference for low luminosity and proximity to humans may be only secondary factors in the model, since standing water appears to be the key ecotone element bridging the neighboring ecosystems. In our study area, for example, low malaria incidence was verified at the final 1.1 km of the deforestation triangle, despite close proximity of dwellings to forest fringes. Water is a vital asset for survival of both humans and mosquitoes. Although running water is common inside the forest, where streams are numerous, turbulent flow and strong currents are almost ubiquitous, generated by heavy daily raining, most pronounced during the rainy season. Larval mortality may be induced by stage height variations, associated with high current speeds, current strengths, and turbulent flow, a phenomenon that has been referred to as the “flush-out theory.”19 Although stable larval habitats may be relatively rare in unmodified forest ecosystems, we have not performed systematic sampling for demonstrating this. Vittor and others33 has reported retrieving A. darlingi larvae in uninhabited and lightly inhabited areas. Deep, stable, crystal clear water collections, the preferred habitat of A. darlingi larvae,34 are relatively rare in dryland environments. Tree trunks fallen on river beds, after damaging gallery forests during slash-and-burn clearing of fields, as well as increased erosion, may cause obstructions to water flow, creating temporary pools with decreased stage height variations after each downpour, as well as current strength and laminar flow velocity, thus avoiding turbulence and enabling larval survival. We have referred to this as the “microdam effect.”13 Although not systematically studied, microdams appear to be more numerous at forest fringes than in forested or deforested stretches of rivers or streams. Microdams have been shown to increase the breeding season of A. darlingi larvae, with a marked influence on larval densities and malaria incidence in the proximities.
However, the most important anthropogenic environmental modification on A. darlingi binomics appears to be the deliberate creation of artificial barriers on otherwise free flowing streams, generating permanent desiccation-resistant and current-free habitats.22 An important commodity for humans, who prefer establishing houses as close to waterways as possible, many settlers will favor building ponds as a means of increasing their storage of usable water. In recent years, there has also been increased interest in aquaculture and in creation of larger water collections. By establishing stable water collections and choosing to live in their close proximity, the inherently social vertebrates inadvertently create ideal conditions for A. darlingi breeding: abundant blood meals and stable ovipositon sites. Ponds harbored significantly higher densities than other types of water collections, and were the only sites positive year-round, whereas all streams were negative during the wet season. Anopheles darlingi appears to have adapted well to fish farms, despite predation by fish larvae.35–37
Pond building requires community effort, political influence, or individual resources. Ponds were associated with areas with greater deforestation and no ponds were found in the forest. To verify the distribution of ponds in other side roads, besides Side road 19, we randomly selected six triangular shaped side roads in the Anauá Colonization Project and determined the number of remotely visible ponds within the last 5 km of each side road, as well as the number in the contiguous 5 km closer to the main road. A paired Student's t test demonstrated a significant difference (t = 7.05; df = 5; P < 0.001); in the last 5 km, there were a mean of 0.33 ponds/km (SD = 0.24), whereas in the 5 km closer to the artery there were a mean of 0.77 ponds/km (SD = 0.20). Data suggest that recently arrived settlers are less likely to have built ponds, as compared with settlers that have arrived earlier. Therefore, fish ponds are associated with areas that have undergone greater deforestation.
In summary, the forest fringe model states that human presence could promote three basic changes on the ecosystem, which are, in order of importance for A. darlingi binomics: 1) increase the number of dammed water collections by blocking streams, which creates stable water collections that prolong the breeding season and decreases flushing and mortality of larvae following downpours; 2) reduce the number of shaded larval habitats by deforestation, in a given geographical area, possibly resulting in concentration of larvae and, in particular, gravid or parous females, at the remaining shaded sites; 3) human presence by itself increases the availability of blood meals on the preferred host, increasing mosquito breeding.
To our knowledge, our study also contains the first description of increased breeding of A. darlingi at forest remnants. Shrub and cropland have also been proposed to increase breeding of A. darlingi.10
Frontier malaria from an epidemiological perspective: spatial and temporal distribution of malaria matches the forest fringe model.
The most important conclusion in this study was that malaria incidence was determined by spatial distribution of A. darlingi larval habitats. Limited flight dispersal of the species in deforested landscapes may have made this association possible. We have reported that the number of adult females decreases exponentially with the distance from the larval habitat: whereas 29% of mosquitoes emerging from a given larval site fly 400 m, only 4.5% will fly 1,000 m.38 In this article, the following observations regarding malaria epidemiology are proposed: 1) malaria incidence is highly focal and incidence is closely linked with the proximity to high risk areas, represented by potential hotspots and transitional ponds; 2) during initial deforestation, proximity to the forest fringe increases malaria transmission risk; 3) ongoing deforestation decreases the risk of transmission by increasing the distance between houses and the forest fringe; 4) forest remnants are related with a higher malaria transmission risk because they decrease larval habitat–human dwelling distances.
Foci of increased malaria transmission, comprising clusters of homesteads at high transmission intensity, have been observed around the globe,39–41 being consistently identified when fine-scale spatial studies of malaria epidemiology are performed.42 Very focal high-risk areas of malaria transmission in the Peruvian Amazon were mentioned to be in close proximity to secondary forest-covered larval habitats in a riverine community.43
The association of proximity to the fringe and malaria has been previously mentioned in the Machadinho Colonization Project, in Rondônia State,44 where it was observed that settlers living at distances > 50 m from the forest had an OR of 0.4043 of being infected with malaria, that is, there was an OR of 2.47 and a 60% increased rise in malaria odds for settlers living closer to the forest fringe. Malaria has also been associated with forest-related activities,11 such as land clearing, which appear to increase the contact with forest fringes and A. darlingi. Malaria was also been associated with areas of partial shade on forest fringes1; edge density (total length of the forest edge divided by land area)45; fish pond density46; and forest-deforested transitions of a temporary river.22 In Side road 19, proximity of houses with transitional water collections was the single most significant factor that explained malaria incidence. In this study area, it is believed that malaria transmission is self-sustained within the side road, since migration is limited and regression models showed no relation between malaria incidences and the number of overnight visits to town or activities that involved sleeping outdoors, such as fishing or hunting, when settlers were exposed to a high number of mosquito bites.22 Although malaria near free running rivers occurs only during the dry season, as expected by the dry-season-limited breeding of A. darlingi at these sites, near fish ponds, cases occur year-round.22 We therefore believe that ponds may play a role in maintaining the transmission cycle. The importance of asymptomatic cases in this setting remains to be determined.
In the governmental agricultural frontiers created in Brazil, settlers usually establish their homes near the side road, before beginning deforestation of their rectangular shaped land lots, which means that their homes, initially in a high-risk area, are progressively further away from the forest fringe. With time, ongoing deforestation clears large areas of land, surface water collections are stripped of perimeter vegetation, and the distance between human dwellings and larval hotspots increases progressively. According to the forest-fringe model, this should result in decreased malaria incidence. In agricultural frontier zones, it is well known that malaria transmission, after an initial rise, tends to decrease over time, stabilizing at endemic levels that are considerably lower than the initial epidemic magnitudes.1–3,47,48 We refer to this phenomenon as “third stage deforestation.” It has been previously mentioned that the long-term effect of land clearing appears to be to increase the distance of humans to forest edges and thus decrease malaria risk49 and Sawyer and Silveira50 have suggested that older colonization projects are less hospitable to A. darlingi.
Singer and de Castro3 have reported that in thoroughly deforested colonization projects, highly focal-increased transmission areas remained near protected forest reserves and close to the Machadinho River (> 40 m wide). It has also been noted that malaria risk increases with the extent of forest surrounding a subject's house,51 that proximity to conservation areas increase malaria risk and conservation efforts to decrease deforestation in places where people are already settled might inadvertently increase the number of malaria cases.52
Since a round-trip is necessary for breeding (larval-habitat-to-human-dwelling and back again), it seems reasonable that distances over 400 m are unlikely to be spanned. Our studies suggest that deforestation shares both contributory and protective effects against malaria, depending on what time frame is considered. Pond building, which is variably associated with deforestation, may be a fundamental element that sustains endemic levels of deforestation-related malaria over time. However, ponds would increase malaria transmission only if their margins are bordered by old growth forest.
Other factors that have been mentioned to be devoid of statistical significance for describing malaria epidemiology in this study site are the following: lack of resistance of incoming settlers13; social factors, such as poor living and housing conditions, education; and behavioral factors, such as bathing in streams.22
Methodological limitations: light spectrum imagery, wind speed, lack of replication, and preference for old growth instead of secondary forest.
Visible light spectrum remote satellite imagery is only useful in deforested sites, has limited use in fully identifying forest streams and may underestimate the real number of water collections under old growth forest. Using satellite visible light spectrum imagery to quantify streams is only possible in deforested sites and will underestimate the real number of water collections under old growth forest: we therefore may have underestimated the number of streams at the end of the side road. This would have little effect if larval and mosquito densities are low inside the forest. The remote sensing technique used was also judged of limited sensibility for identifying large streams when tall secondary vegetation was present and secondary vegetation was bluntly classified in categories. Plants were not routinely speciated or their shade effects compared. In this study, due to the small scales involved, we did not consider the effect of edge density on malaria incidence. Wind speed or direction was not systematically measured in this study, but values from 12 to 18 km/hour were usually encountered, as measured visually by the Beaufort scale, and could influence mosquito flight and dispersal.42,53 Flight dispersal studies were not performed in Side road 19 and it cannot be ascertained if the mosquito will breed in transitional ponds that are over 400 m away from human dwellings due to lack of variance: only one pond in this study site was located at such a distance.
It should also be noted that our data were derived from a single triangularly shaped side road in the common fishbone-like frontier zone colonization architecture and lack replicas. Also, fishbone colonies frequently have variable imprints and some side roads may be more rectangular than triangularly shaped. Also, different colonies may become intersected with each other or a side road can be transversed by a large river, creating a new epidemiological setting. The simple model presented here may become inapplicable and is only intended as a framework for guiding future studies.
Our data linking malaria with forest fringes were limited to linear distances to the nearest potential hotspot. However, in more densely inhabited sites or in areas with greater forest fragmentation, more complex spatial statistics may be preferable.
The working hypothesis that deforestation increases malaria fails to account for spatial and temporal case distributions and may have been biased by underscoring the importance of fish farming ponds.
There has been a growing debate on the effect of deforestation on A. darlingi binomics since Vittor and others8 reported increased A. darlingi landing catches, as well as larval occurrence,10 in deforested areas. They have proposed that the existence of volatile compounds in deforested areas could provide olfactory cues for A. darlingi, favoring its oviposition behavior. However, regarding A. darlingi biology, the hypothesis fails to account for the following observations: 1) larvae are unevenly distributed and increased presence of larvae is found under primary forest fringes, whereas larvae are rarely found at thoroughly deforested sites, as observed in this study; 2) larvae that were once found at forest fringes disappear after clearing the overlying forest margins; 3) higher A. darlingi biting activity has been reported at less modified areas of a side road, in contrast to a more degraded site 6 km closer to the main road.32
If deforestation should increase A. darlingi breeding, it would imply that malaria transmission should be increased at deforested sites, not only rising soon after deforestation but also continue rising thereafter (or at least remaining at higher levels), unless efficient control strategies were being actively practiced. Malaria would also be expected to be evenly distributed within deforested areas (Figure 5B). The following observations on malaria epidemiology would be unaccounted for 1) minimal malaria transmission is found when dense forest is rapidly cut and cleared, leaving large open areas, despite large-scale influx of nonresistant workers54; 2) malaria transmission in agricultural frontier settlements tend to decrease over time and increasing deforestation has been linked to a decrease, rather than increase, in malaria transmission risk45,55; 3) malaria presents a highly focal distribution within deforested areas, rather than being widespread; 4) Hahn and others56 failed to verify increased incidence of malaria in areas with current deforestation at the municipality level. A link between percent deforestation and malaria incidence has remained notoriously elusive, despite years of accumulated epidemiological data: for example, by analyzing data from the Brazilian Malaria Epidemiological Vigilance Information System (SIVEP-Malaria), one may conclude that an association between the number of malaria cases in the nine Brazilian Amazonian States, from 2000 to 2011, and the percentage of deforestation in each state yields a nonsignificant negative correlation (R = −0.45; P = 0.26). It may also be verified that the Brazilian state with the largest deforested area and highest active deforestation, Mato Grosso, had one of the greatest reductions in malaria incidence, 86% from 2000 to 2011. In Acre, the only state where the number of cases increased in the period, malaria incidence in each municipality had a nonsignificant negative correlation with both percent deforestation (R = −0.22; P = 0.31) and active deforestation (r = −0.2; P = 0.37). The degree of ongoing deforestation of a municipality is correlated with the percentage of deforestation that has already occurred. It appears that the contributory effect of new deforestation may be partly counterbalanced by the presumably protective effect of past deforestation, leading to inconclusive statistical results. It is also notorious that, in intensely degraded forests, such as the Brazilian Atlantic Forest, with only 7% of the original cover still standing, locally transmitted malaria has rarely been reported since the 1960s. Although, malaria was endemic throughout the country in the past, in the Atlantic coastline region, where 70% of the country's population is concentrated, locally transmitted malaria now correspond to less than 0.5% of total extra-Amazonian incidence, because almost all cases are actually imported from the Amazon, where only 13% of the population has settled.57 Local transmission in the Atlantic Forest region may therefore be considered as a very rare occurrence, although A. darlingi has been retrieved throughout the entire region and presumed responsible for the few outbreaks that have occurred.58
Increased A. darlingi human-biting activity was reported in areas of the Iquitos-Nauta road that have undergone deforestation, which was interpreted as implying that deforestation was a causal factor for A. darlingi presence and malaria risk.8 We suggest that this diverging result may be due to study bias generated by fish farming. Adult density reflects proximity to larval habitats and larval densities at these sites. The data reported by Vittor and others8 control only for the distance to the nearest water collection, rather than fish pond density (the most important larval habitat reported in their study area), which could have biased the results if density was greater in deforested areas than in forested areas. The introduction of fish farming in the early 1990s and the increasing importance of this activity on the Iquitos-Nauta road led to a very high pond density at the time of study, reaching over 30 ponds/km2, as compared with Side road 19 (maximum of 6/km2). By 2004, approximately 314 ponds were harvested by 195 different families along the road, totaling 253 hectares of water surface.59 Simpson (2006)60 reported that households closer to fish ponds had a higher number of self-reported malaria episodes and Giroux and others46 elegantly associated fish pond density with an increased risk of malaria transmission. These data suggest that fish pond density, rather than vegetation, may be responsible for the increased adult biting captures. Settlers in the Iquitos-Nauta road usually build fish ponds by intercepting water courses, commonly (> 70%) with the use of tractors, resulting in a concentration of the majority of fish ponds in more accessible areas,59 within 50–500 m of the Iquitos-Nauta road. Meanwhile, satellite imagery shows that the forest fringe was roughly found, at the time of study, at a mean of 400–800 m from the road, and ponds were rarely found in forested areas.
Fish farms with large circumferences (> 100 m) in deforested areas were reported as the most positive larval habitat by Vittor and others10 and this was interpreted as implying that deforestation was a causal factor for A. darlingi larval presence and malaria risk. We believe that the spatial association of fish ponds in the Iquitos-Nauta road with areas with greater deforestation may account for the finding of decreased primary forest cover in surrounding 1 × 1 km grids around positive larval habitats. However, differences between study sites regarding the time-period since deforestation may also have played a role. Although Side road 19 represents a typical “recently” (< 5–10 years) deforested side road of a Brazilian agricultural projects, the construction of the Iquitos-Nauta road began more than 30 years before the time of study and secondary growth (5–15 m high and > 15 years old) was abundant, including as perimeter vegetation of fish ponds. Although decreased malaria in older colonization areas suggests that secondary growth is not as important as primary forest fringes as habitats, we lack systematic data on water collections with bordering tall (> 2 m), secondary vegetation for comparison and suggest that more studies are required to clarify this issue. It is possible that higher adult mosquito densities in areas closer to the highway along with a higher density of human dwellings may have accounted for the smaller positivity rates of water collections found within forested areas.
Using health district–specific malaria incidence data from Mâncio Lima, Brazil, in 2006, higher malaria risk in areas with deforestation within the preceding 5–10 years was interpreted by Olson and others5 as an indicator that deforestation is a causal factor for malaria incidence. However, instead of a landscape-related tendency, the increased malaria incidence in 2006 appears to have been the peak of a major malaria epidemic that had reached the municipality in 2005 and spread concomitantly over seven other counties of northern Acre, totaling over 80,000 cases, 15,524 (19.1%) in Mâncio Lima.61 Epicentered in the neighboring town of Cruzeiro do Sul the epidemic began in 2003–2004, where it has been suggested that fish ponds played a major role in transmission.62 Fish farming was extensively performed in the town outskirts of Mâncio Lima from 2003 onward and over 36 large ponds/km2 could be encountered by 2006. The peri-urban areas of Mâncio Lima were the areas where most fish ponds were installed and some were as close as 50–100 m from the main street, where they border a swamp and a conservation area. In 2006, the peri-urban area harbored a mean fish pond density of approximately 74.2 (SD ± 68.4) per district, whereas other districts and the Moa river chain had only a mean of 26 (SD ± 7.84) ponds. After the 2006 epidemic, malaria cases continued at much higher baselines than before. We have performed field studies in the area in 2011 and 2012, and perceived that the number of georeferenced malaria cases decreased exponentially with increasing distance from ponds, approximating zero at > 500 m.61 Any conclusions linking deforestation with malaria must first make an in depth consideration of fish pond density. The importance of fish ponds in malaria epidemiology may have been vastly underestimated in the entomological literature.
The complex link between deforestation and malaria may only become clearer with the use of much finer scales than are currently available with most malaria surveillance systems such as the Brazilian SIVEP-Malaria. Data must be gathered at a subhealth district level, distinguishing morbidity in areas with initial deforestation from past deforestation.
Our data refine the profile of frontier malaria, suggesting that increased malaria transmission in initial stages of deforestation is a transient phenomenon that is replaced, within a few years, as crops and pasture lands are established, by reduced transmission. Our views are decidedly at odds with the claims of Vittor and others, where deforestation is invariably linked with increased malaria transmission.
Implications for control: increasing distances between larval habitats and human dwellings may considerably improve malaria control.
Although focal malaria transmission make malaria more difficult to control,63,64 we suggest that remote sensing may allow ready identification of larval hotspots by seeking intersections of water collections and old growth forests, identifying possible A. darlingi habitats. The sensitivity and sensibilities of the method remain to be ascertained. Targeting houses near transitional water collections may allow better employment of available resources. Vector control measures are likely to be more effective if intensified on foci of increased transmission,65 allowing spatially directed use of insecticide-impregnated bednets and residual spraying. Temporary rivers and streams appear to be epidemiologically important as larval habitats only during the dry season, when added control measures should be undertaken.13,22
Deforestation can either be performed preceding colonization or forest clearers and settlers can be offered protection by bednets, residual spraying, and/or malaria prophylaxis. Although we do not favor tropical rainforest deforestation, in colonization projects that are already underway, decreasing malaria incidence may help decrease evasion of settlers and abandonment of already deforested areas. Control programs may benefit from environmentally directed strategies, such as removing residual forests inside the colonization projects, as well as increasing the length-to-width ratio of land lots, which accelerates the process of creating safe house-to-forest distances. Clearing old growth forest at perimeters of ponds and avoiding damming at forest fringes may also mitigate malaria transmission by reducing the number of favorable aquatic habitats, and therefore emergence of host-seeking mosquitoes. Measures to avoid erosion and fallen tree trunks on river beds should also be sought. These strategies also increase the amount of time required for vectors to locate oviposition sites, which prolongs the gonotrophic cycle duration66 and decreases the contact of parous (older) females and humans, which has been suggested to be more efficient than larvicide application.67 Accordingly, we have shown, in the same study area, that secondary to the greater abundancy of water collections during the wet season, the gonotrophic cycle duration was shorter (2.19 days) than in the dry season (2.43 days). Despite this, malaria incidence decreased seasonally in the wet season, presumably secondary to droplet-induced adult mortality due to heavy raining.19
Although more studies are necessary for verifying the ideal distance, we can initially recommend that, in agricultural sites of southern Roraima, such as Side road 19, houses should not be located at less than 400–500 m from the nearest transitional water collection. Distances may have to be increased if swamps or river margins are present, which may create habitats with higher larval densities. It can be estimated that approximately 25% of mosquitoes emerging from a given larval habitat will fly 450 m. However, dispersion studies may be better performed locally, taking into account population density and environmental factors. We also point out that either dry or non-inhabited “buffer zones” encircling conservation areas within urban centers should be considered, to avoid the establishment of ecotone breeding. Most importantly, urgent entomologically guided supervision of fish farming practices is needed in malaria-endemic areas of the Amazon. Deforestation of land lots must precede damming of water collections for creating fish ponds and regulatory agencies should discourage damming in forested areas, forest fringes, or forest remnants. Preservation of gallery forests may also be important, to avoid erosion, but houses should be located at a safe distance from these sites. A multi-intervention program, possibly encompassing the use of larvicides, spatially directed distribution of bednets, and/or antimalarial drug prophylaxis, as well as screening strategies and localized house spraying, could have important implications on malaria epidemiology.
A spatially based modification of the classic frontier malaria cycle for dry land fishbone-like agricultural settlements.
Sawyer and Sawyer47 have proposed an elegant socioeconomically centered and sequential three-stage model of frontier malaria transmission: a first epidemic phase starts with the occupation of a settlement, being replaced, after a few years, by a second transitional phase characterized by significant decreases in transmission. A third and last phase (endemic) starts approximately 8 years after the inception of the settlement project, and registers much lower infection rates. The model describes a colonization project as a uniform and homogeneous block, where settlers synchronously arrive and are exposed to similar risks of transmission. The model fails to account for focal transmission, arrival of settlers in waves of migration, rather than concomitantly, and ignores the roughly triangularly shaped outlines of side roads, a hallmark of disparities in deforestation rates. We therefore advocate a new, entomologically based, and spatially oriented malaria model for fishbone-like frontier areas. Each side road represents epidemiological units, which may be linked by the main road, where zones correspond to diverging stages of deforestation that can be simultaneous found and are at constant interplay. Using sequential k-means analysis, three developmental clusters (k = 3) were considered to be simultaneously present in Side road 19. Clusters differed significantly by the distance to the main road (F(2,72) = 74.51; P << 0.0001), the deforested area (F(2,72) = 148.42; P << 0.0001), and the presence of dams (F(2,72) = 11.11; P << 0.0001). They were named “Deforestation Zones” 1, 2, and 3 and are depicted in Figure 1B. In Zone 1, during the initial phases of colonization (Stage 1), as occurs at the end of the side road, the forest fringe is located close to houses; damming of water courses has usually not been performed, and malaria incidence is low, despite human presence and abundant shaded streams. This may be the condition found in most indigenous communities in the Amazon. In Zone 2, typically in central portions of an advancing side road, ponds have been built, forest remnants are plentiful and the proximity of human dwellings to transitional water collections, especially dammed sites, combine to increase malaria transmission risk: all three contributing factors, that is, humans, ponds, and proximity to the forest fringe are present. In the more proximal parts of a side road, which represent the oldest portions (Zone 3), forest remnants have been removed and deforestation is more extensive, resulting in an increased distance between transitional water collections and houses, and therefore decreased malaria incidence. In Side road 19, the distance of houses to transitional water collections differed significantly among deforestation zones (F(2,72) = 11.17; P << 0.0001) by ANOVA. Zones 2 and 3 were associated with less distance, mean 156 m (SE = 81) and 430 m (SE = 90), respectively, whereas Zone 1 had a mean of 715 m (SE = 80 m). In Zones 2 and 3, the house-to-transitional-water collection distance was positively correlated with the amount of deforested hectares per land lot (R = 0.38; P < 0.01; N = 47). The increase was basically due to removal of forest remnants in areas of older deforestation, which means that the nearest transitional water collection will be only located at the forest fringes, at the back of the land lots. As deforestation progresses, all areas will reach third stage deforestation and the deforestation triangle will assume a roughly rectangular shape. It remains to be determined if a Stage 4 exists, driven by secondary growth, a few years after deforestation, when A. darlingi may reappear in greater numbers.
This frontier zone transmission model may provide a useful template that can help understand malaria incidence in similarly structured agricultural frontier zones. Our studies suggest that the single most important determinant of malaria incidence may be the proximity to transitional sites, and thus the forest fringe or forest-covered ponds. The settlers in Side road 19 were mostly poor immigrants from dry rice farming cultures in northeast Brazil and had little experience with pond building or pisciculture. However, aquaculture is becoming increasingly widespread among settlers in some areas of the Amazon35,62 and its practice may increase over time, changing malaria epidemiology.
We believe more studies are necessary to improve our understanding of the forest fringe and how the deforestation process affects A. darlingi binomics. The importance of size, number, shape, and density of forest remnants on mosquito survival and surface water availability needs better evaluation. The effect of diverging species of secondary growth on mosquito binomics is of paramount importance, deserving prompt investigation, specially the time necessary for mosquito recolonization, after deforestation occurs. We believe that the importance of ecotone biology research may have been previously underscored and hope that this study may spur a new field of investigation. Also, in multi-intervention, environmentally directed programs, quantitative modeling studies of tradeoffs among different malaria mitigation efforts, in terms of their coverage and overall effects, may provide important information for control strategies.
Supplementary Material
ACKNOWLEDGMENTS
We thank the personnel of FUNASA in Roraima, especially Ducinéia de Aguiar. To Ulisses Confalonieri for providing kind assistance with all necessary infrastructure during field work, as well as support during planning and execution.
Footnotes
Financial support: This work was supported by the Inter-American Institute for Global Change Research.
Authors' addresses: Fábio S. M. Barros, Department of Zoology, Centro de Ciências Biológicas, Universidade Federal de Pernambuco, Pernambuco, Brazil, E-mail: fsaito1@yahoo.com.br. Nildimar A. Honório, Departamento de Entomologia, Laboratório de Transmissores de Hematozoários, Instituto Oswaldo Cruz-Fiocruz, Rio de Janeiro, Brazil, E-mail: honorio@ioc.fiocruz.br.
References
- 1.Castro MC, Monte-Mor RL, Sawyer DO, Singer BH. Malaria risk on the Amazon frontier. Proc Natl Acad Sci USA. 2006;103:2452–2457. doi: 10.1073/pnas.0510576103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawyer DR. Frontier malaria in the Amazon region of Brazil: Types of Malaria Situations and Some Implications for Control. Brasília, Brazil: Pan American Health Organization/WHO/Special Programme for Research Training in Tropical Diseases; 1988. [Google Scholar]
- 3.Singer BH, de Castro MC. Agricultural colonization and malaria on the Amazon frontier. Ann N Y Acad Sci. 2001;954:184–222. doi: 10.1111/j.1749-6632.2001.tb02753.x. [DOI] [PubMed] [Google Scholar]
- 4.Vasconcelos CH, Novo EM, Donalisio MR. Use of remote sensing to study the influence of environmental changes on malaria distribution in the Brazilian Amazon. Cad Saude Publica. 2006;22:517–526. doi: 10.1590/s0102-311x2006000300006. [DOI] [PubMed] [Google Scholar]
- 5.Olson SH, Gangnon R, Silveira GA, Patz JA. Deforestation and malaria in Mancio Lima County, Brazil. Emerg Infect Dis. 2010;16:1108–1115. doi: 10.3201/eid1607.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira-Ferreira J, Lourenco-de-Oliveira R, Teva A, Deane LM, Daniel-Ribeiro CT. Natural malaria infections in anophelines in Rondonia State, Brazilian Amazon. Am J Trop Med Hyg. 1990;43:6–10. [PubMed] [Google Scholar]
- 7.Tadei WP, Thatcher BD. Malaria vectors in the Brazilian Amazon: anopheles of the subgenus Nyssorhynchus. Rev Inst Med Trop Sao Paulo. 2000;42:87–94. doi: 10.1590/s0036-46652000000200005. [DOI] [PubMed] [Google Scholar]
- 8.Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, Pinedo-Cancino V, Patz JA. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. [PubMed] [Google Scholar]
- 9.Yasuoka J, Levins R. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am J Trop Med Hyg. 2007;76:450–460. [PubMed] [Google Scholar]
- 10.Vittor AY, Pan W, Gilman RH, Tielsch J, Glass G, Shields T, Sanchez-Lozano W, Pinedo VV, Salas-Cobos E, Flores S, Patz JA. Linking deforestation to malaria in the Amazon: characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am J Trop Med Hyg. 2009;81:5–12. [PMC free article] [PubMed] [Google Scholar]
- 11.Silva-Nunes M, Codeco CT, Malafronte RS, da Silva NS, Juncansen C, Muniz PT, Ferreira MU. Malaria on the Amazonian frontier: transmission dynamics, risk factors, spatial distribution, and prospects for control. Am J Trop Med Hyg. 2008;79:624–635. [PubMed] [Google Scholar]
- 12.Silva NS, da Silva-Nunes M, Malafronte RS, Menezes MJ, D'Arcadia RR, Komatsu NT, Scopel KK, Braga EM, Cavasini CE, Cordeiro JA, Ferreira MU. Epidemiology and control of frontier malaria in Brazil: lessons from community-based studies in rural Amazonia. Trans R Soc Trop Med Hyg. 2010;104:343–350. doi: 10.1016/j.trstmh.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Barros FSM, Arruda ME, Gurgel HC, Honorio NA. Spatial clustering and longitudinal variation of Anopheles darlingi (Diptera: Culicidae) larvae in a river of the Amazon: the importance of the forest fringe and of obstructions to flow in frontier malaria. Bull Entomol Res. 2011;101:643–658. doi: 10.1017/S0007485311000265. [DOI] [PubMed] [Google Scholar]
- 14.Furley P. The Forest Frontier. London, United Kingdom: Routledge; 1994. [Google Scholar]
- 15.Barros FSM, de Aguiar DB, Rosa-Freitas MG, Luitgards-Moura JF, Gurgel Hda C, Honorio NA, de Arruda ME, Tsouris P, Vasconcelos SD. Distribution summaries of malaria vectors in the northern Brazilian Amazon. J Vector Ecol. 2007;32:161–167. doi: 10.3376/1081-1710(2007)32[161:dsomvi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Rosa-Freitas MG, Tsouris P, Peterson AT, Honorio NA, Barros FSM, de Aguiar DB, Gurgel Hda C, de Arruda ME, Vasconcelos SD, Luitgards-Moura JF. An ecoregional classification for the state of Roraima, Brazil: the importance of landscape in malaria biology. Mem Inst Oswaldo Cruz. 2007;102:349–357. doi: 10.1590/s0074-02762007005000052. [DOI] [PubMed] [Google Scholar]
- 17.Barros FSM, Arruda ME, Vasconcelos SD, Luitgards-Moura JF, Confalonieri U, Rosa-Freitas MG, Tsouris P, Lima-Camara TN, Honorio NA. Parity and age composition for Anopheles darlingi root (Diptera: Culicidae) and Anopheles albitarsis Lynch-Arribalzaga (Diptera: Culicidae) of the northern Amazon Basin, Brazil. J Vector Ecol. 2007;32:54–68. doi: 10.3376/1081-1710(2007)32[54:paacfa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Barros FSM, Honorio NA, Arruda ME. Mosquito anthropophily: implications on malaria transmission in the northern Brazilian Amazon. Neotrop Entomol. 2010;39:1039–1043. doi: 10.1590/s1519-566x2010000600029. [DOI] [PubMed] [Google Scholar]
- 19.Barros FSM, Honorio NA, Arruda ME. Survivorship of Anopheles darlingi (Diptera: Culicidae) in relation with malaria incidence in the Brazilian Amazon. PLoS One. 2011;6:e22388. doi: 10.1371/journal.pone.0022388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Service M . Mosquito Ecology: Field Sampling Methods. London, United Kingdom: Chapman and Hall; 1993. [Google Scholar]
- 21.Achee NL, Grieco JP, Andre RG, Roberts DR, Rejmankova E. Experimental evaluation of overhanging bamboo in Anopheles darlingi larval habitat selection in Belize, central America. J Vector Ecol. 2006;31:145–151. doi: 10.3376/1081-1710(2006)31[145:eeoobi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Barros FSM, Honorio NA, Arruda ME. Temporal and spatial distribution of malaria within an agricultural settlement of the Brazilian Amazon. J Vector Ecol. 2011;36:159–169. doi: 10.1111/j.1948-7134.2011.00153.x. [DOI] [PubMed] [Google Scholar]
- 23.Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference. Stat Med. 1995;14:799–819. doi: 10.1002/sim.4780140809. [DOI] [PubMed] [Google Scholar]
- 24.Komp WHW. The anopheline mosquitoes of the Caribbean region. Bull Nat Inst Health. 1942;179:1–195. [Google Scholar]
- 25.Manguin S, Roberts DR, Andre RG, Rejmankova E, Hakre S. Characterization of Anopheles darlingi (Diptera: Culicidae) larval habitats in Belize, central America. J Med Entomol. 1996;33:205–211. doi: 10.1093/jmedent/33.2.205. [DOI] [PubMed] [Google Scholar]
- 26.Rozendaal JA. Observations on the distribution of anophelines in Suriname with particular reference to the malaria vector Anopheles darlingi. Mem Inst Oswaldo Cruz. 1990;85:221–234. doi: 10.1590/s0074-02761990000200014. [DOI] [PubMed] [Google Scholar]
- 27.Stage HH, Geijskes DC. Observations on mosquito and malaria control in the Caribbean area; Suriname. Mosq News. 1946;6:177–180. [PubMed] [Google Scholar]
- 28.Nagm L, Luitgards-Moura J, Neucamp C, Barros F, Honório N, Tsouris P, Rosa-Freitas M. Affinity and diversity indices for Anopheline immature forms. Rev Inst Med trop S Paulo. 2007;49:309–316. doi: 10.1590/s0036-46652007000500007. [DOI] [PubMed] [Google Scholar]
- 29.Barros FSM, Vasconcelos SD, Arruda ME, Confalonieri UE, Luitgards-Moura JF, Honorio NA. Tetrahymenidae infection in mosquito populations in a malaria-endemic region of the Amazon. J Invertebr Pathol. 2006;91:199–201. doi: 10.1016/j.jip.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Deane LM, Causey OR, Deane MP. Notas sobre a distribuição e a biologia dos anofelinos das regiões nordestina e amazônica do Brasil. Revista do Serviço Especial de Saúde Pública. 1948;1:827–966. [Google Scholar]
- 31.Charlwood JD, Wilkes TJ. Studies on the age-composition of samples of Anopheles darlingi Root (Diptera: Culicidae) in Brazil. Bull Entomol Res. 1979;69:337–342. [Google Scholar]
- 32.Moutinho PR, Gil LH, Cruz RB, Ribolla PE. Population dynamics, structure and behavior of Anopheles darlingi in a rural settlement in the Amazon rainforest of Acre, Brazil. Malar J. 2011;10:174. doi: 10.1186/1475-2875-10-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vittor AYG R, Tielsch J, Glass G, Shields T, Patz JA. Baltimore, MD: John Hopkins Bloomberg School of Public Health; 2002. Deforestation and the presence of Anopheles darlingi larvae in the Peruvian Amazon. PhD thesis. [Google Scholar]
- 34.Hiwat H, Bretas G. Ecology of Anopheles darlingi root with respect to vector importance: a review. Parasit Vectors. 2011;4:177. doi: 10.1186/1756-3305-4-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tadei WP, Thatcher BD, Santos JM, Scarpassa VM, Rodrigues IB, Rafael MS. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am J Trop Med Hyg. 1998;59:325–335. doi: 10.4269/ajtmh.1998.59.325. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues IB, Tadei WP, Santos RC, Santos SO, Bagio JB. Malaria control: efficacy of Bacillus sphaericus 2362 formulates against Anopheles species in artifitial pisciculture tanks and breeding sites in pottery areas. Rev de Pat Trop. 2008;37:161–177. [Google Scholar]
- 37.Saraiva MGG, Amorim RDS, Moura MAS, Martinez-Espinosa FE, Barbosa MGV. Expansão urbana e distribuição espacial da malária no município de Manaus, Estado do Amazonas. Rev Soc Bras Med Trop. 2009;42:515–522. doi: 10.1590/s0037-86822009000500008. [DOI] [PubMed] [Google Scholar]
- 38.Barros FSM, Honorio NA. Man biting rate seasonal variation of malaria vectors in Roraima, Brazil. Mem Inst Oswaldo Cruz. 2007;102:299–302. doi: 10.1590/s0074-02762007005000024. [DOI] [PubMed] [Google Scholar]
- 39.Saul A, Belizario VY, Bustos MD, Espino F, Lansang MA, Salazar NP, Torres E. Stability of malaria in a community in Bataan, the Philippines: prospects for control. Acta Trop. 1997;63:267–273. doi: 10.1016/s0001-706x(96)00626-2. [DOI] [PubMed] [Google Scholar]
- 40.Gaudart J, Poudiougou B, Dicko A, Ranque S, Toure O, Sagara I, Diallo M, Diawara S, Ouattara A, Diakite M, Doumbo OK. Space-time clustering of childhood malaria at the household level: a dynamic cohort in a Mali village. BMC Public Health. 2006;6:286. doi: 10.1186/1471-2458-6-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bousema T, Drakeley C, Gesase S, Hashim R, Magesa S, Mosha F, Otieno S, Carneiro I, Cox J, Msuya E, Kleinschmidt I, Maxwell C, Greenwood B, Riley E, Sauerwein R, Chandramohan D, Gosling R. Identification of hot spots of malaria transmission for targeted malaria control. J Infect Dis. 2010;201:1764–1774. doi: 10.1086/652456. [DOI] [PubMed] [Google Scholar]
- 42.Midega JT, Smith DL, Olotu A, Mwangangi JM, Nzovu JG, Wambua J, Nyangweso G, Mbogo CM, Christophides GK, Marsh K, Bejon P. Wind direction and proximity to larval sites determines malaria risk in Kilifi District in Kenya. Nat Commun. 2012;3:674. doi: 10.1038/ncomms1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bautista CT, Chan AS, Ryan JR, Calampa C, Roper MH, Hightower AW, Magill AJ. Epidemiology and spatial analysis of malaria in the northern Peruvian Amazon. Am J Trop Med Hyg. 2006;75:1216–1222. [PubMed] [Google Scholar]
- 44.Sawyer DR, Sawyer DO. Malaria on the Amazon Frontier: Economic and Social Aspects of Transmission and Control. Belo Horizonte, Brazil: CEDEPLAR; 1987. [PubMed] [Google Scholar]
- 45.Assis MC. São José dos Campos. Brazil: INPE; 2011. Abordagens Espaciais para Caracterização dos Condicionantes Socioambientais Associados ao Risco de Malária em Novas Fronteiras na Amazônia: O Caso de Lábrea, Amazonas. [Google Scholar]
- 46.Maheu-Giroux M, Casapia M, Soto-Calle VE, Ford LB, Buckeridge DL, Coomes OT, Gyorkos TW. Risk of malaria transmission from fish ponds in the Peruvian Amazon. Acta Trop. 2010;115:112–118. doi: 10.1016/j.actatropica.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Sawyer DR, Sawyer DO. Advancing the Health in Developing Countries: The Role of Social Research. Westport, CT: Auburn House; 1992. [Google Scholar]
- 48.Barata RC. Malaria in Brazil: trends in the last ten years. Cad Saude Publica. 1995;11:128–136. doi: 10.1590/s0102-311x1995000100019. [DOI] [PubMed] [Google Scholar]
- 49.Keiser J, Singer BH, Utzinger J. Reducing the burden of malaria in different eco-epidemiological settings with environmental management: a systematic review. Lancet Infect Dis. 2005;5:695–708. doi: 10.1016/S1473-3099(05)70268-1. [DOI] [PubMed] [Google Scholar]
- 50.Sawyer DO, Silveira CM. Malaria in the Machadinho Settlement Project. Belo Horizonte, Brazil: CEDEPLAR; 1985. [Google Scholar]
- 51.Valle D, Clark JS, Zhao K. Enhanced understanding of infectious diseases by fusing multiple datasets: a case study on malaria in the western Brazilian Amazon region. PLoS One. 2011;6:e27462. doi: 10.1371/journal.pone.0027462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valle D, Clark J. Conservation efforts may increase malaria burden in the Brazilian Amazon. PLoS One. 2013;8:e57519. doi: 10.1371/journal.pone.0057519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kakitani I, Forattini OP. Parity and ovarian development of Anopheles albitarsis l.s.in irrigated agroecosystem field. Rev Saude Publica. 2000;34:33–38. doi: 10.1590/s0034-89102000000100007. [DOI] [PubMed] [Google Scholar]
- 54.Singer B, de Castro MC. Enhancement and suppression of malaria in the Amazon. Am J Trop Med Hyg. 2006;74:1–2. [PubMed] [Google Scholar]
- 55.Sawyer DO, Monte-Mór RL. Malaria Prevalence and Environmental Factors in Early Stages of Settlement Project. Brasília, Brazil: Pan American Health Organization; 1992. [Google Scholar]
- 56.Hahn MB, Gangnon RE, Barcellos C, Asner GP, Patz JA. Influence of deforestation, logging, and fire on malaria in the Brazilian Amazon. PLoS One. 2014;9:e85725. doi: 10.1371/journal.pone.0085725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miguel RB, Peiter PC, de Albuquerque H, Coura JR, Moza PG, Costa Ade P, Brasil P, Suarez-Mutis MC. Malaria in the state of Rio de Janeiro, Brazil, an Atlantic Forest area: an assessment using the health surveillance service. Mem Inst Oswaldo Cruz. 2014;109:634–640. doi: 10.1590/0074-0276130558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pina-Costa A, Brasil P, Di Santi SM, de Araujo MP, Suarez-Mutis MC, Santelli AC, Oliveira-Ferreira J, Lourenco-de-Oliveira R, Daniel-Ribeiro CT. Malaria in Brazil: what happens outside the Amazonian endemic region. Mem Inst Oswaldo Cruz. 2014;109:618–633. doi: 10.1590/0074-0276140228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gómez LT., Sr . Economic Evaluation of Pisciculture in Loreto. Case Studies: Fish-Farms on the Iquitos-Nauta road. Lima, Peru: Instituto de investigaciones de la Amazonía Peruana; 2009. [Google Scholar]
- 60.Simpson JM. Aquaculture Production, Contributing to the Transmission of Malaria in the Peruvian Amazon. New York, NY: Department of Ecology, Evolution and Environmental Biology, Columbia University; 2006. [Google Scholar]
- 61.Reis IC, Honório NA, Barros FSM, Barcellos C, Kitron U, Camara DCP, Pereira GR, Keppeler EC, Silva-Nunes M, Codeço CT. Epidemic and endemic malaria transmission related to fish farming ponds in the Amazon frontier. PLoS One. 2015;10:e0137521. doi: 10.1371/journal.pone.0137521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costa KM, de Almeida WA, Magalhaes IB, Montoya R, Moura MS, de Lacerda MV. Malaria in Cruzeiro do Sul (western Brazilian Amazon): analysis of the historical series from 1998 to 2008. Rev Panam Salud Publica. 2010;28:353–360. doi: 10.1590/s1020-49892010001100005. [DOI] [PubMed] [Google Scholar]
- 63.Woolhouse ME, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JL, Ndhlovu PD, Quinnell RJ, Watts CH, Chandiwana SK, Anderson RM. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci USA. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith DL, McKenzie FE, Snow RW, Hay SI. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol. 2007;5:e42. doi: 10.1371/journal.pbio.0050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gu W, Utzinger J, Novak RJ. Habitat-based larval interventions: a new perspective for malaria control. Am J Trop Med Hyg. 2008;78:2–6. [PubMed] [Google Scholar]
- 66.Gu W, Regens JL, Beier JC, Novak RJ. Source reduction of mosquito larval habitats has unexpected consequences on malaria transmission. Proc Natl Acad Sci USA. 2006;103:17560–17563. doi: 10.1073/pnas.0608452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Menach A, McKenzie FE, Flahault A, Smith DL. The unexpected importance of mosquito oviposition behaviour for malaria: non-productive larval habitats can be sources for malaria transmission. Malar J. 2005;4:23. doi: 10.1186/1475-2875-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.