Abstract
Understanding factors influencing sustained use of long-lasting insecticide-treated nets (LLIN) in areas of declining malaria transmission is critical to sustaining control and may facilitate elimination. From 2008 to 2013, 655 households in Choma District, Zambia, were randomly selected and residents were administered a questionnaire and malaria rapid diagnostic test. Mosquitoes were collected concurrently by light trap. In a multilevel model, children and adolescents of 5–17 years of age were 55% less likely to sleep under LLIN than adults (odds ratio [OR] = 0.45; 95% confidence interval [CI] = 0.35, 0.58). LLIN use was 80% higher during the rainy season (OR = 1.8; CI = 1.5, 2.2) and residents of households with three or more nets were over twice as likely to use a LLIN (OR = 2.1; CI = 1.4, 3.1). For every increase in 0.5 km from the nearest health center, the odds of LLIN use decreased 9% (OR = 0.9; CI = 0.88, 0.98). In a second multilevel model, the odds of LLIN use were more than twice high if more than five mosquitoes (anopheline and culicine) were captured in the house compared with households with no mosquitoes captured (OR = 2.1; CI = 1.1, 3.9). LLIN use can be sustained in low-transmission settings with continued education and distributions, and may be partially driven by the presence of nuisance mosquitoes.
Introduction
Several interventions are widely used for malaria control and prevention, and one of the most cost-effective and widely available methods is long-lasting insecticide-treated nets (LLINs). Substantial increases in funding for malaria control and the procurement and distribution of LLINs have been associated with declines in malaria burden.1 LLINs were associated with decreases in malaria incidence, mortality, and associated morbidity such as anemia in various settings.2 Unfortunately, these gains may be followed by resurgence of malaria in regions with high transmission potential if control efforts are not sustained.3–9 As transmission and perceived risk decline, LLIN access and use need to be maintained.
LLIN access is defined as one LLIN per two persons in a single household. The Zambian 2011–2015 Strategic Plan calls for universal net coverage, defined as “ensuring all sleeping spaces in targeted households are covered” by a bed net.10 LLINs in Zambia are distributed to all ages and have been free since 2005.11 A total of 2,738,835 LLINs were distributed in 2012. Challenges to achieving universal coverage are getting sufficient funding and ensuring that nets are successfully delivered. The Zambian 2012 Malaria Indicator Survey identified a gap between overall LLIN ownership and use; 68% of households surveyed reporting owning at least one net, but only 49% of household members reported sleeping under a net the night before the survey.10,12
To address this gap between LLIN ownership and use, the World Health Organization recommends behavior change interventions including information, education, communication campaigns, and post-distribution “hang-up campaigns” to ensure continued, proper use of LLINs.13 The Zambian 2011–2015 Malaria Operational Plan stated that it aims to strengthen behavior change communication for malaria prevention, particularly, appropriate and consistent LLIN use.10 As transmission declines, maintaining high coverage of vector control measures such as LLINs and promotion of their continued use are critical, even as perceived risk declines.10,14 A recent study conducted in Tanzania identified sustained use of LLINs after a significant decrease in transmission, but the authors argued that as perceived risk declines use may not be sustained.15 Ensuring continued use entails a better understanding of LLIN user preferences, use patterns, alternative uses for LLINs, and factors influencing LLIN use.16–18
Little data exist on sustained use of LLINs in regions of declining malaria transmission. LLIN use was evaluated over a 6-year period in a region of declining malaria transmission in southern Zambia. Trends in use over time and between seasons were assessed, and demographic factors associated with sustained high use of LLINs were identified. In addition, the association between total number of mosquitoes (culicine and anopheline) captured in the household and LLIN use was quantified. Identifying factors and trends associated with sustained use of LLINs is critical to sustaining progress in malaria control and may facilitate elimination.
Methods
Study site and population.
The study was conducted in the catchment area of Macha Hospital in Choma District, Southern Province, Zambia, between February 2008 and December 2013. The single rainy season lasts from November through April, followed by a cool dry season from April to August and a hot dry season through November. Malaria transmission peaks during the rainy season.19 The primary malaria vector is Anopheles arabiensis. The hospital catchment area is populated by villagers living in small, scattered homesteads. The prevalence of malaria declined in this area over the past decade from 13.7% in 2006 to 5.7% in 2010.20 Artemesinin combination therapies (ACTs) were introduced as first-line antimalarial therapy in Zambia in 200221 and into the study area in 2004. In Zambia, LLINs are distributed through antenatal care (ANC) clinics and additional mass distribution campaigns.12 LLINs were widely distributed in the study area in 200722 and more than 11,000 LLINs were distributed from nine health posts in the catchment area of Macha Hospital in June 2012, according to the Office of the Macha Hospital Environmental Technician.
The development of the sampling frame and enumeration of households were reported in detail elsewhere.22 In brief, satellite images were used to construct a sampling frame from which households were selected by simple random sampling for enrollment. A total of 6,210 structures were enumerated. Households were enrolled into prospective longitudinal and cross-sectional surveys depending on the month in which they were selected. Cross-sectional households were selected and enrolled every other month, and were visited once. Cross-sectional surveys were done every other month between 2008 and 2013. Longitudinal households were selected and visited every other month, beginning in 2008. If a household declined to participate or moved, a new longitudinal house was selected from a backup replacement list of randomly selected households. Households could enter or exit the longitudinal cohort and the number of follow-up visits ranged from 2 to 35. After comparing LLIN use at longitudinal follow-up visits with LLIN use at cross-sectional and the first longitudinal household visit, the analyses were restricted to households enrolled in the longitudinal cohort that reported owning at least one LLIN.
The study was approved by the Institutional Review Board of the Tropical Diseases Research Center, Ndola, Zambia, and the Institutional Review Board of the Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD. Informed consent was translated into Chitonga, and obtained from adult participants and the parents or guardians of children.
Survey data.
A trained field team conducted household visits. Coordinates of households were recorded using global positioning system devices and Euclidean distance to the nearest health facility was calculated using ArcGIS v9.2 (ESRI, Redlands, CA). During each study visit, a questionnaire was administered to consenting participants older than 16 years of age and the guardians of participants younger than 16 years of age. Data collected included demographic information, history of recent malaria and antimalarial treatment, reported health-seeking behavior, knowledge of malaria transmission and prevention, and ownership and use of LLIN the night before the visit. A blood sample was collected by finger prick for a malaria rapid diagnostic test (RDT) (ICT Diagnostics, Cape Town, South Africa). The RDT-positive participants were offered treatment with artemether–lumefantrine (Coartem®, Novartis, Basel, Switzerland). In a subset of households visited during 2012 and 2013, overnight mosquito collections were conducted using Centers for Disease Control and Prevention (CDC) light traps and both anopheline and culicine mosquitoes were enumerated.
Statistical analysis.
The proportion of individuals reporting sleeping under LLIN the night before the survey visit was compared between first visits (cross-sectional households and first visit to longitudinal households) and follow-up visits (longitudinal households only). LLIN ownership and use over time were analyzed by month and season for both longitudinal and cross-sectional visits. Among longitudinal participants who reported owning a LLIN, demographic and household-level variables were compared between those who reported sleeping under a LLIN and those who reported not sleeping under a LLIN using χ2 test for proportions and t-test for differences in means. A multilevel longitudinal model with random intercepts was constructed to assess factors associated with LLIN use adjusting for individuals clustered within households and repeated measures over time for individuals. The outcome of interest was individual self-reported LLIN use the night before. Variables associated with LLIN use in univariate models were included in the multilevel longitudinal model using a P value cutoff of 0.1. Study time was modeled as a quadratic function of time and season was included using an indicator variable (rainy season versus dry season). Model fit was assessed using the receiver operating characteristic (ROC) area C-statistic. A LLIN distribution occurred in Southern Province in June 2012, so a binary variable was constructed to examine LLIN ownership and use before and after June 2012. Qualitative questions regarding LLIN ownership and use were tabulated.
A generalized estimating equation (GEE) logistic regression model was used to investigate the association between the total number of mosquitoes captured by CDC light trap and LLIN use among a subset of households in which both anopheline and culicine mosquitoes were enumerated.
Results
Temporal trends in LLIN use.
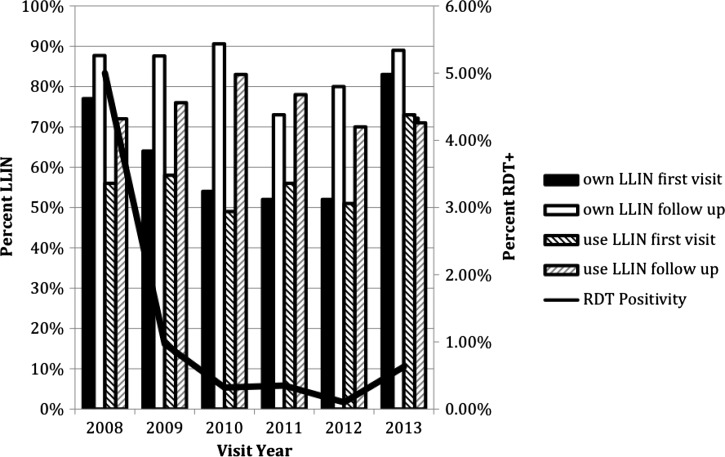
A total of 585 cross-sectional and 70 longitudinal households were enrolled between February 2008 and December 2013. The parasite prevalence as measured by RDT among the cross-sectional visits and first visit to longitudinal households was 8.4% in 2008 but declined to 2.1% in 2013. The proportion of participants in the cross-sectional and first longitudinal surveys who reported owning a LLIN was 77% in 2008, 64% in 2009, 54% in 2010, 52% in 2011, 52% in 2012, and 83% in 2013 (Figure 1 ). Of the participants who reported owning a LLIN, the proportion who reported using a LLIN the night before was 56% in 2008, 58% in 2009, 49% in 2010, 56% in 2011, 51% in 2012, and 73% in 2013 (Figure 1).
Figure 1.
Reported long-lasting insecticide-treated nets (LLIN) use at cross-sectional and the first visit to longitudinal households, compared with reported use during follow-up visits to longitudinal households: 2008–2013.
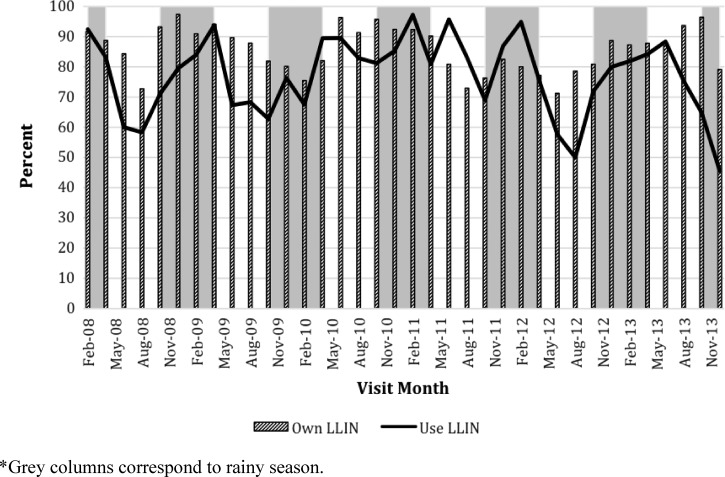
The longitudinal surveys included 399 individuals residing in 66 households in which at least one LLIN was reportedly owned, and followed for an average of six visits (minimum two and maximum 36 visits) for 3,689 observations. LLIN ownership was consistently higher during follow-up visits of the longitudinal households (58% use at cross-sectional and first visits as compared with 77% during follow-up) (unadjusted P value = 0.01). The proportion of participants reporting LLIN use was higher at follow-up visits compared with initial visits for each year, except for 2013 (Figure 1). LLIN ownership and use followed a seasonal trend, with a slightly higher use during the rainy season from November to April (74%) compared with the dry season (65.7%; P = 0.001) (Figure 2 ). Both LLIN ownership and use were significantly higher after the LLIN distribution in June 2012 for first visits (58% ownership and 52% use pre-distribution compared with 75% ownership and 70% use post distribution) and longitudinal follow-up visits (80% ownership and 78% use pre-distribution compared with 86% ownership and 75% use post distribution).
Figure 2.
Reported long-lasting insecticide-treated nets (LLIN) ownership and use among longitudinal households per month from 2008 to 2013.
Factors associated with LLIN use.
Individual- and household-level characteristics were compared in univariate analyses between those who reported using a LLIN and those who did not among participants in the longitudinal surveys. Among residents of households that owned a LLIN, 74.7% of individuals over the age of 18 years used their LLIN, 66.2% between 5 and 17 years of age used their LLIN, and 72.7% of individuals younger than 4 years used their LLIN. In the rainy season, LLIN owners were more likely to report using their LLIN (74.3% versus 55.1%). Households that owned more than three LLINs reported higher use (90% versus 74%). Similarly, households with appropriate access (defined as one LLIN per two people per household) reported higher LLIN use (71.4% versus 61.1%; P = 0.0001). LLIN users were more likely to reside closer to a health facility (median distance of 3.5 km versus 6.9 km) (Table 1).
Table 1.
Individual- and household-level factors associated with reported LLIN use among households followed longitudinally that owned at least one LLIN at the baseline visit, 2008–2013
| Individual-level variables | ||
|---|---|---|
| Use LLIN | Unadjusted P value | |
| Number of individuals | 275 | – |
| Age category in years | 0.25 | |
| 0–4 | 72.7% | – |
| 5–17 | 66.2% | – |
| ≥ 18 | 74.7% | – |
| Male | 72.9% | 0.4 |
| Female | 69.0% | – |
| RDT positive | 55.6% | 0.66 |
| Rainy season | 74.3% | 0.001 |
| Dry season | 55.1% | – |
| Learned about malaria from community health worker | 70.3% | 0.82 |
| Household-level variables | ||
| Any LLIN users in household | Unadjusted P value | |
| Number of households | 48 | – |
| Number of children < 5 years in the household | 0.02 | |
| 0 children < 5 years | 84.8% | – |
| 1 or more children < 5 years | 69.8% | – |
| Household education | – | 0.03 |
| Primary | 79.6% | – |
| Secondary or higher | 70.2% | – |
| More than 3 ITNs in the house | 90.0% | 0.003 |
| Appropriate coverage (1 net per 2 people) | 71.0% | 0.0001 |
| Distance to clinic per 500 m (median [minimum, maximum]) | 7.1 (0.17, 26.1) | 0.01 |
ITN = insecticide-treated net; LLIN = long-lasting insecticide-treated net; RDT = rapid diagnostic test.
In a multilevel longitudinal model that adjusted for individual- and household-level clustering, several individual- and household-level factors were associated with LLIN use among residents of longitudinal households that owned at least one LLIN. Compared with adults older than 18 years, children younger than 5 years of age were 35% less likely to sleep under LLIN (P < 0.0001) and children and adolescents 5 to 17 years of age were 55% less likely to sleep under LLIN (P = 0.001) (Table 2). Participants who reported learning about malaria from a community health worker (CHW) were 50% more likely to use their LLIN compared with those who learned from a different source such as from radio or at school (P = 0.001) (Table 2). Residents of households with more than three LLINs were twice as likely as to use a LLIN than residents of households with fewer than three LLINs (P = 0.001). Individuals had 80% higher odds of using a LLIN during the rainy season than the dry season (P < 0.0001). LLIN use increased slightly over time (about 9% per 6 months) despite the low parasite prevalence (Table 2). Although LLIN use was significantly higher after the LLIN distribution in 2012, this association was not significant after controlling for distance to the nearest health facility. Since the household-level random intercept variance estimate was larger than the individual-level random intercept variance estimate, greater differences between households were observed than the variation between individuals within a household. The model was considered a strong fit for the data (ROC Area C-statistic of 0.82).
Table 2.
Multilevel model of individual- and household-level factors associated with reported LLIN use, 2008–2013
| Factors | OR* (95% CI) |
|---|---|
| Number of observations | 3,689 |
| Fixed effects | |
| Household level | |
| Distance to nearest facility (per 0.5 km) | 0.93 (0.88, 0.98) |
| Own > 3 LLINs | 2.1 (1.4, 3.1) |
| Post 2012 LLIN distribution | 1.7 (0.94, 3.2) |
| Rainy season | 1.8 (1.5, 2.2) |
| Number of children < 5 years in the household | 1.0 (0.89, 1.2) |
| Individual level | |
| Female | 0.98 (0.78, 1.2) |
| Age category | |
| ≥ 18 years | REF |
| 0–4 years | 0.66 (0.48, 0.90) |
| 5–17 years | 0.45 (0.35, 0.58) |
| Learned about malaria from a CHW | 1.5 (1.2, 2.0) |
| Random effects | Variance (SE) |
| Household random effect | 1.2 (0.17) |
| Individual random effect | 0.35 (0.11) |
CHW = community health worker; CI = confidence interval; LLIN = long-lasting insecticide-treated net; OR = odds ratio; SE = standard error; REF = reference.
Mosquitoes and LLIN use.
The association between LLIN use and the total number of mosquitoes (anophelines and culicines) captured in the household was measured in all cross-sectional and longitudinal households visited during 2012 and 2013 (238 households). A total of 2,647 mosquitoes were captured; 665 anophelines and 1,982 culicines. Controlling for season and whether the visit was a follow-up or baseline visit, the odds of using a LLIN increased with increasing number of total mosquitoes captured by CDC light trap in the household. As compared with households with no mosquitoes, the odds of LLIN use increased 19% where one to four mosquitoes were captured (OR = 1.2, CI = 0.8, 1.9), and more than doubled in households with five or more mosquitoes (OR = 2.1 [1.1, 3.9]) (Table 3). This association approached significance when restricted to anopheline mosquitoes only; households with any anophelines caught were 64% more likely to use their LLIN(s) (OR = 1.64, CI = 0.99, 2.72).
Table 3.
GEE model of the association between total number of mosquitoes and LLIN use in a subset of surveyed households between 2012 and 2013
| Fixed effects | OR | 95% CI | P value |
|---|---|---|---|
| Number of observations | 703 | – | – |
| 0 mosquitoes | REF | – | – |
| 1–4 mosquitoes | 1.19 | (0.75, 1.89) | 0.46 |
| ≥ 5 mosquitoes | 2.11 | (1.14, 3.93) | 0.02 |
| Rainy season | 0.83 | (0.56, 1.24) | 0.36 |
| Follow-up visit vs. baseline visit | 2.76 | (1.57, 4.85) | 0.001 |
| Random effects | Estimate (SE) | – | – |
| Household random effect | 3.73 (0.39) | – | – |
CI = confidence interval; LLIN = long-lasting insecticide-treated net; OR = odds ratio.
Qualitative survey of LLIN use.
Almost half of participants who did not own a LLIN reported that they were too expensive (42%) despite the history of free LLIN distribution in the community (Table 4). Of the 1,654 participants who owned a LLIN, 250 (16%) did not use a LLIN because there were no mosquitoes, 76 (4%) reported that LLINs did not protect against mosquitoes, 139 (7%) reported that they were unable to hang the net over their sleeping space and 100 (5%) reported that their net was too old to use (Table 4). Other answers included that it was not the rainy season (1%).
Table 4.
Qualitative responses for reasons not owning or using a LLIN at baseline visit (includes among cross-sectional households) in Macha, 2008–2013
| What is the reason you do not own a LLIN in your house? | |
|---|---|
| N | 2,647 |
| It is too expensive | 1,073 (42.1%) |
| No mosquitoes around | 58 (2.3%) |
| Bed nets not available | 52 (2%) |
| Change my sleeping space too often | 30 (1.2%) |
| Not enough nets for everyone in the house in the house | 28 (1.1%) |
| Do not know where to buy one | 27 (1.1%) |
| It does not protect against mosquitoes/insects | 23 (0.9%) |
| It is too hot under the net | 10 (0.39%) |
| What is the reason you do not use a LLIN? | |
| N | 1,654 |
| No mosquitoes around | 250 (16.2%) |
| Cannot hang it over my sleeping space | 139 (6.5%) |
| The net I have is too old | 100 (4.6%) |
| Does not protect against mosquitoes/insects | 76 (3.5%) |
| Chang my sleeping space too often | 53 (2.5%) |
| There is not enough space under the net/I feel closed in | 26 (1.2%) |
| It is not the rainy/malaria season | 24 (1.1%) |
| The net is itchy | 17 (0.8%) |
| It is too hot under the net | 13 (1%) |
LLIN = long-lasting insecticide-treated net.
Discussion
LLIN use in the study area was high over the 6-year study period, during which the prevalence of malaria declined from 8.4% to 2.1%. Although malaria indicator surveys measure malaria prevalence and LLIN ownership and use with cross-sectional surveys, this study identified individual- and household-level factors influencing LLIN use over time and included data on the number of mosquitoes captured by CDC light traps within study households. Maintaining high LLIN use is critical to sustain malaria control and reduce the risk of resurgence. However, this will not be possible unless high ownership is maintained through frequent LLIN distributions and effective promotion of LLIN use.23
Quantitative and qualitative studies suggest a range of factors associated with sleeping under a LLIN; however, these studies were mainly conducted in areas where the malaria burden was high.24–30 In low-transmission regions in Uganda, Swaziland, and Zambia, reasons for not using LLIN included the low density of mosquitoes and the infrequency of malaria.31–33 In contrast, a recent qualitative study in Zanzibar, where malaria prevalence decreased from 50% to less than 2% in 15 years, found that caretakers strongly believed in the protection afforded by LLINs despite the reduction of malaria risk.5,14,34 Although malaria was no longer considered a common disease, caretakers associated high mosquito density with increased risk of malaria.14 Other factors associated with bed net use in Zanzibar were instructions from health-care workers or hearing about malaria in the media.14 Attrition in LLIN ownership and use also is associated with the aging and degradation of nets. Nets will be discarded or used for other activities more commonly in areas where perceived risk is low.8,17 These findings suggest that if malaria transmission is reduced, personal protective measures such as LLINs may no longer be used. That LLIN ownership and use were higher in longitudinal households suggest that repeated messaging may be useful to ensure sustained use.
Several individual- and household-level variables were associated with sustained LLIN use in this region of declining malaria transmission in southern Zambia. Households enrolled in the longitudinal cohort reported slightly higher LLIN use over time, perhaps because frequent visits from the field team increased awareness about malaria and the benefits of LLINs. Factors related to LLIN coverage and ownership were significantly associated with higher use, for example, owning three or more nets or living closer to a health facility. Living closer to a health facility was associated with higher LLIN ownership and use, suggesting distributions that originate from the health facility may not achieve universal access.35–37 Although LLIN use increased despite declining malaria transmission, our comparison of households visited once and households with repeated study visits suggest that without continued LLIN distributions and educational campaigns, LLIN use will likely decrease over time.
Children and adolescents of 5–17 years old were the least likely to sleep under LLIN. In Kenya, children aged 5–14 years reported significantly lower LLIN use.38 School-based LLIN distributions and educational campaigns may help target this high-risk age group, especially if coupled with educational campaigns. Traditionally, LLINs are targeted to children younger than 5 years and pregnant women, often excluding older children. A recent study in Zanzibar concluded that future behavior change communications should expand current messages of the potential benefits of net use in addition to protection against malaria, including a “good night's sleep” and protection from other insects.15
In response to qualitative questions, participants reported that deterrents to LLIN use were that they were too expensive, it was too hot or there were few mosquitoes during the dry season. Recent studies in Kenya and Tanzania also identified strong seasonality to LLIN use.25,30,38 This seasonality in use may be related to temperature (LLINs are often described as uncomfortable to sleep under when it is hot) and mosquito density in households (LLIN use is higher when mosquitoes are present). Previous studies in Zanzibar, Kenya, and Ghana found the presence of culicine mosquitoes to be associated with LLIN use.14,30,39 Promoting the protection that LLINs can offer against nuisance mosquitoes in public health messages may increase LLIN use.
There were some limitations to these analyses, including the fact that LLIN use was self-reported. Although ownership and use were high, LLINs may not have adequately provided protection because the nets were improperly hung, in poor physical condition (e.g., holes) or no longer had effective concentrations of insecticide. A recent study in the same area identified significant degradation of LLINs.40 Because few cases of malaria were identified, the effectiveness of LLINs could not be measured.
These findings support free universal LLIN distribution, ongoing education and “hang up campaigns” as successful strategies to increase LLIN use.25 If LLINs are delivered solely through health facilities, ownership and use declined as distance to the nearest health facility increases,35 as supported by these findings. Thus, additional distribution mechanisms are necessary, with frequently repeated messages regarding LLIN use and their benefits to ensuring high levels of LLIN use are sustained. As transmission declines, ensuring continued LLIN use is necessary to sustain malaria control, and may be helpful to pursue malaria elimination.
ACKNOWLEDGMENTS
We acknowledge the effort and support of the Macha Research Trust staff and field team. We are grateful to the communities of Choma District for their participation.
Footnotes
Financial support: This work was supported by the Johns Hopkins Malaria Research Institute, the Bloomberg Family Foundation and the Division of Microbiology and Infectious Diseases, National Institutes of Allergy and Infectious Diseases, National Institutes of Health as part of the International Centers of Excellence for Malaria Research (U19 AI089680).
Authors' addresses: Jessie Pinchoff, Department of International Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, E-mail: jpinchoff@gmail.com. Harry Hamapumbu, Limonty Simubali, Jennifer C. Stevenson, and Philip E. Thuma, Macha Research Trust, Macha, Southern Province, Zambia, E-mails: harry.hamapumbu@macharesearch.org, limonty.simubali@macharesearch.org, jennyc.stevenson@macharesearch.org, and phil.thuma@macharesearch.org, Tamaki Kobayashi, Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, E-mail: tkobayas@jhsph.edu. Douglas E. Norris, Johns Hopkins Malaria Research Institute, Department of Molecular Microbiology and Immunology, Johns Hopkins University Bloomberg School of Public Health, , Baltimore, MD, E-mail: douglas.norris@jhu.edu. Elizabeth Colantuoni, Department of Biostatistics, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, E-mail: ejohnso2@jhmi.edu. William J. Moss, Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, E-mail: wmoss1@jhu.edu.
References
- 1.O'Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet. 2010;10:545–555. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- 2.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria (review) Cochrane Libr. 2004:CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Jaenisch T, Sullivan DJ, Dutta A, Deb S, Ramsan M, Othman MK, Gaczkowski R, Tielsch J, Sazawal S. Malaria incidence and prevalence on Pemba Island before the onset of the successful control intervention on the Zanzibar Archipelago. Malar J. 2010;9:32. doi: 10.1186/1475-2875-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najera JA, Gonzalez-Silva M, Alonso PL. Some lessons for the future from the Global Malaria Eradication Programme (1955–1969) PLoS Med. 2011;8:e1000412. doi: 10.1371/journal.pmed.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauch J, Gu J, Maellem M, Ali A, Gosling R, Baltzell K. Perception of malaria risk in a setting of reduced malaria transmission: a qualitative study in Zanzibar. Malar J. 2013;12:1–17. doi: 10.1186/1475-2875-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi T, Chishimba S, Shields T, Hamapumbu H, Mharakurwa S, Thuma P, Glass G, Moss WJ. Temporal and spatial patterns of serologic responses to Plasmodium falciparum antigens in a region of declining malaria transmission in southern Zambia. Malar J. 2012;11:438. doi: 10.1186/1475-2875-11-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee PW, Liu CT, do Rosario VE, de Sousa B, Rampao H, Shaio MF. Potential threat of malaria epidemics in a low transmission area, as exemplified by Sao Tome and Principe. Malar J. 2010;9:264. doi: 10.1186/1475-2875-9-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou G, Afrane YA, Vardo-Zalik AM, Atieli H, Zhong D, Wamae P, Himeidan YE, Minakawa N, Githeko AK, Yan G. Changing patterns of malaria epidemiology between 2002 and 2010 in western Kenya: the fall and rise of malaria. PLoS One. 2011;6:e20318. doi: 10.1371/journal.pone.0020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moonen B, Cohen JM, Snow RW, Slutsker L, Drakeley C, Smith DL, Abeyasinghe RR, Rodriguez MH, Maharaj R, Tanner M, Targett G. Operational strategies to achieve and maintain malaria elimination. Lancet. 2010;376:1592–1603. doi: 10.1016/S0140-6736(10)61269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NMCP . National Malaria Control Programme Strategic Plan for FY 2011–2015: “Consolidating Malaria Gains for Impact.”. Lusaka, Zambia: National Malaria Control Programme, Ministry of Health; 2012. [Google Scholar]
- 11.WHO . World Malaria Report 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 12.MOH . Zambia National Malaria Indicator Survey 2012. Lusaka, Zambia: Ministry of Health, Government of the Republic of Zambia; 2012. [Google Scholar]
- 13.WHO . World Malaria Report 2012. WHO Global Malaria Programme. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 14.Beer N, Ali AS, Eskilsson H, Jansson A, Abdul-Kadir FM, Rotllant-Estelrich G, Abass AK, Wabwire-Mangen F, Bjorkman A, Kallander K. A qualitative study on caretakers' perceived need of bed-nets after reduced malaria transmission in Zanzibar, Tanzania. BMC Public Health. 2012;12:606. doi: 10.1186/1471-2458-12-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenker HM, Loll D, Rweyamamu D, Ali AS. A good night's sleep and the habit of net use: perceptions of risk and reasons for net use in Bukoba and Zanzibar. Malar J. 2013;12:203. doi: 10.1186/1475-2875-12-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisele TP, Thwing J, Keating J. Claims about the misuse of insecticide-treated mosquito nets: are these evidence-based? PLoS Med. 2011;8:e1001019. doi: 10.1371/journal.pmed.1001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batisso E, Habte T, Tesfaye G, Getachew D, Tekalegne A, Kilian A, Mpeka B, Lynch C. A stitch in time: a cross-sectional survey looking at long lasting insecticide-treated bed net ownership, utilization, and attrition in SNNPR, Ethiopia. Malar J. 2012;11:1–15. doi: 10.1186/1475-2875-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minakawa N, Dida G, Sonye GO, Futami K, Kaneko S. Unforeseen misuses of bed nets in fishing villages along Lake Victoria. Malar J. 2008;7:165. doi: 10.1186/1475-2875-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kent RJ, Thuma PE, Mharakurwa S, Norris DE. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am J Trop Med Hyg. 2007;76:267–274. [PMC free article] [PubMed] [Google Scholar]
- 20.Mharakurwa S, Thuma PE, Norris DE, Mulenga M, Chalwe V, Chipeta J, Munyati S, Mutamu S, Mason PR, Southern Africa ICEMR Team Malaria epidemiology and control in southern Africa. Acta Trop. 2011;121:202–206. doi: 10.1016/j.actatropica.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steketee RW, Sipilanyambi N, Chimumbwa J, Banda JJ, Mohamed A, Miller J, Basu S, Miti SK, Campbell CC. National malaria control and scaling up for impact: the Zambian experience through 2006. Am J Trop Med Hyg. 2008;79:45–52. [PubMed] [Google Scholar]
- 22.Moss WJ, Hamapumbu H, Kobayashi T, Shields T, Kamanga A, Clennon J, Mharakurwa S, Thuma PE, Glass G. Use of remote sensing to identify spatial risk factors for malaria in a region of declining transmission: a cross-sectional and longitudinal community. Malar J. 2011;10:163. doi: 10.1186/1475-2875-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beer N, Ali AS, Shakely D, Elfving K, Al-Mafazy AW, Msellem M, Petzold M, Bjorkman A, Kallander K. High effective coverage of vector control interventions in children after achieving low malaria transmission in Zanzibar, Tanzania. Malar J. 2013;12:38. doi: 10.1186/1475-2875-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loha E, Tefera K, Lindtjorn B. Freely distributed bed-net use among chano mille residents, south Ethiopia: a longitudinal study. Malar J. 2013;12:23. doi: 10.1186/1475-2875-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beer N, Ali AS, de Savigny D, Al-Mafazy AA, Ramsan M, Abass AK, Omari RS, Bjorkman A, Kallander K. System effectiveness of a targeted free mass distribution of long lasting insecticidal nets in Zanzibar, Tanzania. Malar J. 2010;9:1–9. doi: 10.1186/1475-2875-9-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulford J, Hetzel MW, Bryant M, Siba PM, Mueller I. Reported reasons for not using a mosquito net when one is available: a review of the published literature. Malar J. 2011;10:1–10. doi: 10.1186/1475-2875-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Basteiro AL, Schwabe C, Aragon C, Baltazar G, Rehman AM, Matias A, Nseng G, Kleinschmidt I. Determinants of bed net use in children under five and household bed net ownership on Bioko Island, equatorial Guinea. Malar J. 2011;10:179. doi: 10.1186/1475-2875-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moiroux N, Boussari O, Djenontin A, Damien G, Cottrell G, Henry MC, Guis H, Corbel V. Dry season determinants of malaria disease and net use in Benin, west Africa. PLoS One. 2012;7:e30558. doi: 10.1371/journal.pone.0030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winch PJ, Makemba AM, Makame VR, Mfaume MS, Lynch MC, Premji Z, Minjas JN, Shiff CJ. Social and cultural factors affecting rates of regular retreatment of mosquito nets with insecticide in Bagamoyo District, Tanzania. Trop Med Int Health. 1997;2:760–770. doi: 10.1046/j.1365-3156.1997.d01-376.x. [DOI] [PubMed] [Google Scholar]
- 30.Alaii JA, Hawley WA, Kolczak MS, ter Kuile FO, Gimnig JE, Vulule JM, Odhacha A, Oloo AJ, Nahlen BL, Phillips-Howard PA. Factors affecting use of permethrin-treated bed nets during a randomized controlled trial in western Kenya. Am J Trop Med Hyg. 2003;68:137–141. [PubMed] [Google Scholar]
- 31.Sutcliffe CG, Kobayashi T, Hamapumbu H, Shields T, Kamanga A, Mharakurwa S, Thuma PE, Glass G, Moss WJ. Changing individual-level risk factors for malaria with declining transmission in southern Zambia: a cross-sectional study. Malar J. 2011;10:324. doi: 10.1186/1475-2875-10-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hlongwana KW, Mabaso MLH, Kunene S, Govender D, Maharaj R. Community knowledge, attitudes and practices (kap) on malaria in Swaziland: a country earmarked for malaria elimination. Malar J. 2009;8:29. doi: 10.1186/1475-2875-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ndyomugyenyi R, Magnussen P, Clarke S. Malaria treatment-seeking behaviour and drug prescription practices in an area of low transmission in Uganda: implications for prevention and control. Trans R Soc Trop Med Hyg. 2007;101:209–215. doi: 10.1016/j.trstmh.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Haji KA, Khatib BO, Smith S, Ali AS, Devine GJ, Coetzee M, Majambere S. Challenges for malaria elimination in Zanzibar: pyrethroid resistance in malaria vectors and poor performance of long-lasting insecticide nets. BMC Public Health. 2012;6:82. doi: 10.1186/1756-3305-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson PS, Mathanga DP, Campbell CH, Jr, Wilson ML. Distance to health services influences insecticide-treated net possession and use among six to 59 month-old children in Malawi. Malar J. 2012;11:18. doi: 10.1186/1475-2875-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsen DA, Keating J, Miller J, Bennett A, Changufu C, Katebe C, Eisele TP. Barriers to insecticide-treated mosquito net possession 2 years after a mass free distribution campaign in Luangwa District, Zambia. PLoS One. 2010;5:e13129. doi: 10.1371/journal.pone.0013129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Meara WP, Smith N, Ekal E, Cole D, Ndege S. Spatial distribution of bednet coverage under routine distribution through the public health sector in a rural district in Kenya. PLoS One. 2011;6:e25949. doi: 10.1371/journal.pone.0025949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atieli HE, Zhou G, Afrane Y, Lee MC, Mwanzo I, Githeko AK, Yan G. Insectice treated net (ITN) ownership, usage, and malaria transmission in the highlands of western Kenya. Parasit Vectors. 2011;4:1–10. doi: 10.1186/1756-3305-4-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adongo PB, Kirkwood B, Kendall C. How local community knowledge about malaria affects insecticide-treated net use in northern Ghana. Trop Med Int Health. 2005;10:366–378. doi: 10.1111/j.1365-3156.2005.01361.x. [DOI] [PubMed] [Google Scholar]
- 40.Norris DE, Norris LC. Efficacy of long-lasting insecticidal nets in use in Macha, Zambia, against the local Anopheles arabiensis population. Malar J. 2011;10:254. doi: 10.1186/1475-2875-10-254. [DOI] [PMC free article] [PubMed] [Google Scholar]