Abstract
Acute respiratory tract infections (ARTIs) are a common reason for unnecessary antibiotic prescriptions worldwide. Our objective was to determine if providing access to rapid influenza test results could reduce antibiotic prescriptions for ARTIs in a resource-limited setting. We conducted a prospective, pre-post study from March 2013 to October 2014. Outpatients presenting to a hospital in Sri Lanka were surveyed for influenza-like illness–onset of fever ≥ 38.0°C and cough in prior 7 days. Enrolled patients were administered a structured questionnaire, physical examination, and nasal/nasopharyngeal sampling for rapid influenza A/B testing. Influenza test results were released only during phase 2 (January–October 2014). We enrolled 571 patients with ILI–316 in phase 1 and 241 in phase 2. The proportion positive for influenza was 46.5% in phase 1 and 28.6% in phase 2, P < 0.001. Between phases, antibiotic prescriptions decreased from 81.3% to 69.3% (P = 0.001) among all patients and from 83.7% to 62.3% (P = 0.001) among influenza-positive patients. On multivariable analysis, a positive influenza result during phase 2 was associated with lower odds of antibiotic prescriptions (OR = 0.50, 95% CI = 0.26–0.95). This prospective study suggests that providing access to rapid influenza testing may reduce unnecessary antibiotic prescriptions in resource-limited settings.
Introduction
Acute respiratory tract infections (ARTIs) result in millions of ambulatory care visits worldwide each year.1,2 Although the majority of ARTIs are caused by viruses such as rhinovirus, adenovirus, influenza, and respiratory syncytial virus, ARTIs are one of the most common reasons for being prescribed an antibiotic in the outpatient setting.3–5 In the United States, over half of outpatient visits for ARTIs result in an antibiotic prescription, with up to 55% of these prescriptions being unnecessary at an excess cost of $726 million annually.6,7 Antibiotic overprescription, in addition to contributing to increased health-care costs, is associated with adverse drug reactions and the development of antimicrobial resistance.6,8
In resource-limited settings, antibiotic overprescription for ARTIs appears even more common. In rural Thailand, 82% of patients presenting to outpatient departments with influenza-like illness (ILI) received an antibiotic prescription.9 In southern Sri Lanka, we previously showed that 81% of outpatients with ILI received a prescription for an antibiotic when influenza test results were not available to clinicians, although almost 50% of these patients had confirmed influenza.10 The reasons for antibiotic overprescription are likely many, and include a lack of access to rapid and specific diagnostic tests that distinguish viral versus bacterial infections, providers' lack of knowledge regarding appropriate prescribing guidelines, and patients' expectations for an antibiotic prescription.1 In resource-limited settings, additional factors such as high patient-to-provider ratios and inability to ensure necessary follow-up may contribute to excess antibiotic prescriptions.9
Recently, rapid, antigen-based tests for influenza have been developed that have high sensitivity and specificity.11 Studies from higher-income countries indicate that providing access to rapid influenza test results can result in a decreased proportion of antibiotic prescriptions for ARTIs.12–16 Prospective studies from low- or middle-income country (LMIC) settings are lacking. We performed a prospective, pre-post study of outpatients with ILI presenting to a tertiary care hospital in southern Sri Lanka to assess the impact of providing access to rapid influenza testing on antibiotic prescribing patterns.
Methods
Study design and setting.
This was a one-group, pre-post study performed in the Outpatient Department (OPD) of Teaching Hospital Karapitiya (THK), the largest (1,500 bed) tertiary care hospital in southern Sri Lanka. The OPD of this public hospital serves > 1,000 patients daily between 8 am and 8 pm and is staffed by approximately 10 physicians. Phase 1 (pretest) of the study was conducted from March to November 2013, and phase 2 (posttest) was conducted from January to October 2014. No enrollment occurred in December 2013 (holiday period). Study procedures were similar during the two phases, except that the individual rapid influenza test result was available to patients and clinicians before any clinical decision making in phase 2, but was not available during phase 1. In addition, patients in phase 1 were consented and enrolled after seeing clinical providers to prevent unnecessary delays in obtaining clinical care. The participation of clinicians in either phase was not tracked, and hence there may have been turnover in care providers between the two phases.
Study enrollment.
All children and adults presenting to the OPD were screened for the presence of ILI by a study physician. Consecutive patients ≥ 1 year of age were enrolled if they met the definition of ILI as defined by the World Health Organization: tympanic temperature ≥ 38°C/100.4°F and acute onset of cough in the past 7 days without alternative diagnosis.17 Screening was performed from 8 am to 3 pm Monday–Friday and 8 am–12 pm on Saturday. Consent was obtained from patients ≥ 18 years of age and the guardians of patients 1–17 years, and assent was obtained from patients 12–17 years. Enrolled patients were administered a standardized questionnaire in Sinhala, the local language, and a structured physical examination was conducted. Collection of a nasopharyngeal sample was attempted in all patients; patients unable to tolerate nasopharyngeal sample collection had a nasal sample collected instead. Patients received standard clinical assessment and treatment including a physical examination, additional diagnostic testing, and prescriptions from their routine care providers in the OPD. Details regarding patients' clinical diagnoses and management were recorded. Study personnel were not involved in clinical decision making or treatment at any point, and routine clinical personnel were not involved in any study procedures. Patients enrolled during the second phase of the study were administered a structured questionnaire by telephone within 1–4 weeks of their OPD visit to gather further information regarding the course of their illness and additional treatments received. Ethical approval for this study was obtained from the Ruhuna University Ethical Review Committee, Duke University Institutional Review Board, and Johns Hopkins Medicine Institutional Review Board.
Rapid influenza testing.
The nasal/nasopharyngeal sample was used immediately for rapid influenza testing using the Veritor Flu A + B system (Becton, Dickinson and Company, Franklin Lakes, NJ). This rapid chromatographic immunoassay detects influenza A and B viral nucleoprotein antigens from nasal and nasopharyngeal swabs using a single processed sample. During phase 1, the result of the rapid influenza test was used solely for surveillance purposes and only released to clinicians in aggregate. Following approval of the test for clinical use in this study by the Sri Lanka Ministry of Health, individual results were released to patients and clinicians during phase 2 (starting in January 2014). Before the second phase of the study, an informational session was conducted with physicians in the OPD regarding the test and its performance characteristics. To measure the isolated effect of providing results from the rapid diagnostic test, we did not provide education regarding the clinical interpretation of the influenza result; instead, we referred clinicians to the comprehensive guidelines on influenza management provided by the Sri Lanka Ministry of Health. The Veritor system's performance characteristics were previously documented by Hassan and others, who showed that in pediatric patients the sensitivity and specificity of the test when compared with polymerase chain reaction (PCR) were 90.2% and 99.1%, respectively, for influenza A, and 87.5% and 100%, respectively, for influenza B.11
Statistical analysis.
For the sample size calculation, it was estimated that 85% of influenza-positive patients would receive antibiotic prescriptions at baseline, based on prior data from outpatients with confirmed influenza in Thailand.9 Prior studies document that a decrease as large as 25–30% in antibiotic prescriptions is possible when access to rapid influenza testing is available.12,16,18 To detect a 25% decrease in antibiotic prescriptions associated with the release of rapid test results in influenza-positive patients, with α= 0.05 and β= 0.20, at least 57 patients who were influenza positive by rapid test were required in each phase. Phase 1 of the study was continued longer than required given the delay in obtaining approval for release of rapid influenza test results to clinicians; data from continued testing in this phase were used for ILI surveillance purposes. Sociodemographic and clinical characteristics of patients between phases 1 and 2 were compared using the Fisher exact test for categorical variables and the Kruskall–Wallis test for continuous variables. Bivariable and multivariable logistic regressions were carried out for each phase separately to assess the association (odds ratios [ORs] with 95% confidence intervals [CIs]) between receipt of antibiotic prescriptions and patients' sociodemographic and clinical characteristics during each phase. Variables were tested for collinearity before inclusion in the multivariable models. For multivariable regression, sociodemographic characteristics, exposures, or clinical symptoms that were significant at less than 0.05 on bivariable analysis for both adults and children were included. In addition, age and rapid influenza test positivity were included in the models, since these variables were thought a priori to have an important impact on antibiotic prescriptions. STATA, version 11 (STATACorp, College Station, TX), was used for all statistical analyses.
Results
Study cohort.
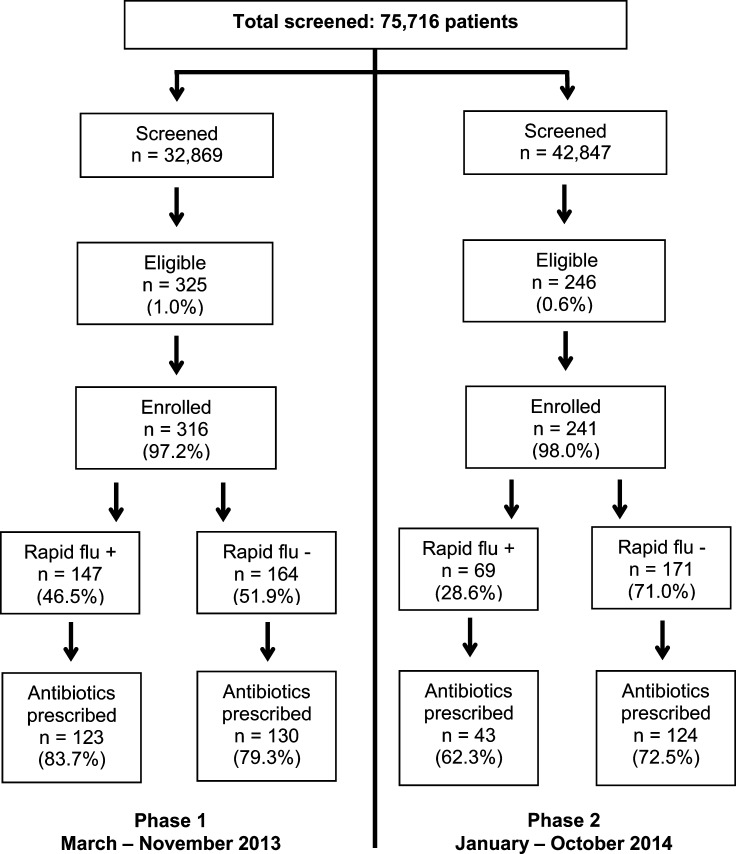
During the study period, 75,716 patients were screened for ILI: 32,869 in phase 1 and 42,847 in phase 2 (Figure 1 ). Of screened patients, 571 (0.7%) were eligible for enrollment, with more patients eligible during phase 1 than phase 2 (1.0% versus 0.6%, P < 0.001). A total of 557 patients were enrolled: 316 in phase 1 and 241 in phase 2. Among those eligible, there was no difference in the proportion enrolled between the two phases (97.2% versus 96.8%, P = 0.81).
Figure 1.
Flow chart of outpatients in southern Sri Lanka who were screened for influenza-like illness (ILI), enrolled, positive for influenza by rapid test, and prescribed an antibiotic, March 2013–October 2014. For 6 (1.1%) enrolled patients, a respiratory specimen was either not obtained or returned an invalid result.
Sociodemographic characteristics.
Table 1 lists the sociodemographic characteristics and prior exposures of patients enrolled in the two phases of the study. Patients in phase 1 tended to be older (14.2 years versus 11.3 years, P = 0.009), although the proportion of adults was not significantly different between the two phases (42.7% overall). Patients in phase 1 lived closer to the hospital (5 km versus 10 km, P = 0.02). The educational level of adults in the two phases was similar, with 33.2% overall having a 12th grade education or higher (P = 0.16). Patients in phase 1 were less likely to report a prior medical visit for the same illness (24.8% versus 33.3%, P = 0.04). Other sociodemographic and exposure characteristics between the two phases were similar, including 44.2% of patients reporting contact with a sick person with similar illness in the past month.
Table 1.
Sociodemographic characteristics, exposures, and clinical symptoms among patients with influenza-like illness (ILI) in southern Sri Lanka, March 2013–October 2014
| Characteristic | First phase, N = 316 | Second phase, N = 241 | P value |
|---|---|---|---|
| Age (years) | 14.2 (6.6–39.6) | 11.3 (5.3–29.5) | 0.009 |
| Male | 175 (55.4%) | 120 (49.8%) | 0.20 |
| Distance to THK (km) | 5 (3–15) | 10 (4–20) | 0.02 |
| Sick contact in past month | 137 (43.5%) | 109 (46.2%) | 0.55 |
| Travel in past month | 54 (17.1%) | 33 (13.8%) | 0.29 |
| Prior medical visit for same illness | 78 (24.8%) | 79 (33.3%) | 0.04 |
| Prior antibiotic use for same illness—yes/unsure | 70 (22.2%) | 44 (18.3%) | 0.29 |
| Number of days fever | 2 (1–3) | 2 (2–4) | < 0.001 |
| Number of days cough | 2 (1–3) | 3 (2–4) | < 0.001 |
| Rhinitis/congestion | 188 (59.5%) | 222 (92.1%) | < 0.001 |
| Sore throat | 159 (50.3%) | 95 (39.4%) | 0.01 |
| Shortness of breath | 84 (26.6%) | 4 (1.7%) | < 0.001 |
| Pain with breathing | 54 (17.1%) | 5 (2.1%) | < 0.001 |
| Anorexia | 244 (77.2%) | 109 (45.2%) | < 0.001 |
| Vomiting | 65 (20.6%) | 35 (14.5%) | 0.08 |
| Abdominal pain | 22 (7.0%) | 16 (6.6%) | 1.00 |
| Headache | 251 (79.4%) | 161 (66.8%) | 0.001 |
| Fatigue | 259 (82.0%) | 130 (53.9%) | < 0.001 |
| Arthralgias | 226 (71.5%) | 99 (41.1%) | < 0.001 |
| Myalgias | 232 (73.4%) | 97 (40.3%) | < 0.001 |
THK = Teaching Hospital Karapitiya.
Bold indicates P values significant at less than 0.05. Characteristics between the two phases of the study are compared using Fisher's exact test for categorical variables and the Kruskall–Wallis test for continuous variables.
Clinical characteristics.
There were significant differences in patients' clinical characteristics between the two phases (Table 1). Patients in phase 1 presented sooner after onset of fever or cough (P < 0.001). Patients in phase 1 were more likely to report sore throat (50.3% versus 39.4%), shortness of breath (26.6% versus 1.7%), pain with breathing (17.1% versus 2.1%), headache (79.4% versus 66.8%), fatigue (82.0% versus 53.9%), arthralgias (71.5% versus 41.1%), and myalgias (73.4% versus 40.3%). However, patients in phase 1 were less likely to experience rhinitis/congestion (59.5% versus 92.1%).
Rapid influenza positivity.
The proportion of patients who tested positive for influenza was greater in phase 1: 147 (46.5%) versus 69 (28.6%), P < 0.001. Influenza A accounted for the majority of cases in both phases (64.0% versus 94.2%, P < 0.001). Patients in phase 1 were older (16.3 years versus 12.9 years, P = 0.04), although the proportion of adults was similar in the two phases. No other sociodemographic features were significantly different between influenza-positive patients in the two phases. Of clinical characteristics, many of the differences observed between patients in phases 1 and 2 remained when considering only influenza-positive patients (data not shown). Influenza-positive patients in phase 1 had a shorter duration of fever or cough (P < 0.001), and were more likely to experience shortness of breath (29.9% versus 2.9%), pain with breathing (26.5% versus 4.4%), anorexia (83.0% versus 58.0%), fatigue (87.8% versus 75.4%), arthralgias (81.6% versus 60.9%), and myalgias (81.6% versus 62.3%) than influenza-positive patients in phase 2. However, influenza-positive patients in phase 1 were less likely to experience rhinitis/congestion (57.1% versus 100%). All differences in clinical symptoms remained when comparing the 94 influenza A-positive patients in phase 1 with the 65 influenza A-positive patients in phase 2 (data not shown).
Clinical management and antibiotic prescriptions.
Overall, 20.5% of patients reported previously using an antibiotic or possible antibiotic for the same illness, with no significant difference between the two phases. However, at the OPD visit, patients in phase 1 were more likely to be prescribed antibiotics (81.3% versus 69.3%, P = 0.001). Penicillins (289, 68.2%), first generation cephalosporins (89, 21.0%), erythromycin (21, 5.0%), and fluoroquinolones (9, 2.1%) were the most commonly prescribed antibiotics overall. There were more diagnostic tests such as complete blood counts ordered in phase 1, but this was not statistically significant (20.6% versus 15.4%, P = 0.12).
Similar differences in clinical management existed between the two phases when comparing only patients who were influenza positive. Influenza-positive patients were more likely to be prescribed antibiotics in phase 1 than in phase 2 (83.7% versus 62.3%, P = 0.001). A similar decrease in antibiotic use was seen between phases in influenza-negative patients, but this was not statistically significant (79.3% versus 72.5%, P = 0.16). The ordering of additional diagnostic tests was similar in the two phases in influenza-positive patients (21.1% versus 17.4%, P = 0.59). No patients were admitted for treatment with antiviral medications for influenza (the OPD does not allow outpatient prescription of antivirals such as oseltamivir).
Features associated with antibiotic prescriptions.
Sociodemographic characteristics, exposures, and clinical features associated with the receipt of an antibiotic prescription were assessed (Table 2). This was done separately for each phase, since the receipt of the rapid influenza test result in the second phase may have influenced prescribing patterns. In phase 1, adults who missed more days of work (P = 0.02) and patients with longer duration of fever (P = 0.05) were more likely to be prescribed an antibiotic. In addition, the diagnosis of upper respiratory tract infection was more likely to be associated with an antibiotic prescription (P = 0.002), while the diagnosis of unspecified viral fever was less likely to be associated with an antibiotic prescription (P < 0.001). Patients who had an additional diagnostic test ordered were also more likely to be prescribed an antibiotic (P = 0.03). In the unadjusted analysis, rapid influenza test positivity was not significantly associated with antibiotic prescriptions (48.6% among patients prescribed antibiotics versus 41.4% in patients not prescribed antibiotics, P = 0.38).
Table 2.
Sociodemographic characteristics, exposures, and clinical features associated with antibiotic prescriptions among patients with influenza-like illness (ILI), southern Sri Lanka, March 2013–October 2014
| Characteristic | Phase 1 | Phase 2 | ||||
|---|---|---|---|---|---|---|
| Antibiotic prescribed, N = 257 | No antibiotic prescribed, N = 59 | P value | Antibiotic prescribed, N = 167 | No antibiotic prescribed, N = 74 | P value | |
| Age (years) | 13.1 (6.4–37.1) | 24.4 (7.7–47.8) | 0.14 | 12.9 (5.7–35.8) | 8.3 (5.2–24.3) | 0.08 |
| Adults ≥ 18 years | 112 (43.6%) | 33 (55.9%) | 0.11 | 71 (42.5%) | 22 (29.7%) | 0.06 |
| Male | 147 (57.2%) | 28 (47.5%) | 0.19 | 84 (50.3%) | 36 (48.7%) | 0.89 |
| Distance to THK (km) | 5 (3–15) | 5.5 (3–15) | 0.91 | 10 (4–20) | 8 (4–15) | 0.53 |
| Sick contact in past month | 109 (42.6%) | 28 (47.5%) | 0.56 | 75 (46.3%) | 34 (46.0%) | 1.00 |
| Travel in past month | 47 (18.3%) | 7 (11.9%) | 0.34 | 22 (13.3%) | 11 (14.9%) | 0.84 |
| Prior medical visit for same illness | 63 (24.6%) | 15 (25.9%) | 0.87 | 63 (38.2%) | 16 (22.2%) | 0.02 |
| Prior antibiotic use for same illness—yes/unsure | 57 (22.2%) | 13 (22.0%) | 1.00 | 38 (22.8%) | 6 (8.1%) | 0.006 |
| Days missed | ||||||
| Work (adults) | 1 (0–2) | 0 (0–1) | 0.02 | 0 (0–2) | 0 (0–1) | 0.68 |
| School (children) | 1 (0–2) | 1 (0–1) | 0.54 | 1 (0–2) | 1 (0–2) | 0.63 |
| Fever days | 2 (1–3) | 2 (1–2) | 0.05 | 3 (2–4) | 2 (2–3) | 0.02 |
| Cough days | 2 (1–3) | 2 (1–3) | 0.28 | 3 (2–4) | 2 (2–3) | 0.008 |
| Rhinitis/congestion | 155 (60.3%) | 33 (55.9%) | 0.56 | 157 (94.0%) | 65 (87.8%) | 0.12 |
| Sore throat | 134 (52.1%) | 25 (42.4%) | 0.20 | 68 (40.7%) | 27 (36.5%) | 0.57 |
| Shortness of breath | 70 (27.2%) | 14 (23.7%) | 0.63 | 4 (2.4%) | 0 (0%) | 0.32 |
| Pleuritic chest pain | 44 (17.1%) | 10 (17.0%) | 1.00 | 3 (1.8%) | 2 (2.7%) | 0.64 |
| Anorexia | 199 (77.4%) | 45 (76.3%) | 0.86 | 81 (48.5%) | 28 (37.8%) | 0.16 |
| Vomiting | 56 (21.8%) | 9 (15.3%) | 0.29 | 24 (14.4%) | 11 (14.9%) | 1.00 |
| Abdominal pain | 19 (7.4%) | 3 (5.1%) | 0.78 | 9 (5.4%) | 7 (9.5%) | 0.27 |
| Headache | 204 (79.4%) | 47 (79.7%) | 1.00 | 119 (71.3%) | 42 (56.8%) | 0.04 |
| Fatigue | 211 (82.1%) | 48 (81.4%) | 0.85 | 95 (56.9%) | 35 (47.3%) | 0.21 |
| Arthralgias | 183 (71.2%) | 43 (72.9%) | 0.87 | 73 (43.7%) | 26 (35.1%) | 0.26 |
| Myalgias | 187 (72.8%) | 45 (76.3%) | 0.63 | 71 (42.5%) | 26 (35.1%) | 0.32 |
| Clinical diagnosis | ||||||
| Upper respiratory illness | 63 (24.5%) | 4 (6.8%) | 0.002 | 112 (67.1%) | 39 (52.7%) | 0.04 |
| Lower respiratory illness | 41 (16.0%) | 5 (8.5%) | 0.16 | 17 (10.2%) | 3 (4.1%) | 0.13 |
| Unspecified viral illness | 102 (39.7%) | 43 (72.9%) | < 0.001 | 11 (6.6%) | 12 (16.2%) | 0.03 |
| Influenza | 0 | 0 | – | 26 (15.6%) | 20 (27.0%) | 0.05 |
| Positive rapid influenza test | 123 (48.6%) | 24 (41.4%) | 0.38 | 43 (25.8%) | 26 (35.6%) | 0.13 |
| Additional diagnostic test ordered | 59 (23.0%) | 6 (10.2%) | 0.03 | 30 (18.0%) | 7 (9.5%) | 0.12 |
THK = Teaching Hospital Karapitiya.
Bold indicates P values significant at less than 0.05. Characteristics between patients who received antibiotic prescriptions and those who did not receive antibiotic prescriptions are compared using Fisher's exact test for categorical variables and the Kruskall–Wallis test for continuous variables. The two phases of the study are analyzed separately, since the result from the rapid influenza test was only available to clinicians in the second phase and may have influenced antibiotic prescribing patterns
In phase 2, having a prior medical visit for the same illness was associated with an antibiotic prescription (P = 0.02). In addition, a longer duration of fever (P = 0.02), longer duration of cough (P = 0.008), and headache (P = 0.04) were associated with antibiotic prescriptions. As in phase 1, the diagnosis of upper respiratory tract infection was more likely to be associated with an antibiotic prescription (P = 0.04) and the diagnosis of unspecified viral fever was less likely to be associated with an antibiotic prescription (P = 0.03). In phase 2, patients who received antibiotic prescriptions were less likely to be rapid influenza test positive (25.8% versus 35.6%), but this was not statistically significant (P = 0.13). Among the 69 patients who were rapid influenza positive in phase 2, the only sociodemographic or clinical variable associated with antibiotic prescriptions was a history of prior possible antibiotic use for the same illness (27.9% versus 3.9%, P = 0.02, data not shown).
In phase 1, the final variables included in the multivariable model were age, days of fever, and influenza rapid test positivity (Table 3). In phase 2, the final variables included in the multivariable model were age, days of fever, days of cough, headache, prior medical visit for the same illness, possible prior antibiotic use for the same illness, and rapid influenza test positivity. Having a longer duration of fever (OR = 1.29, 95% CI = 1.00–1.66) was associated with receipt of an antibiotic prescription in phase 1. In phase 2, older age (OR = 1.02, 95% CI = 1.00–1.04) was positively associated with antibiotic prescriptions and rapid influenza test positivity (OR = 0.50, 95% CI = 0.26–0.95) was negatively associated with antibiotic prescriptions.
Table 3.
Sociodemographic characteristics, exposures, and clinical symptoms associated with antibiotic prescriptions among patients with influenza-like illness (ILI), southern Sri Lanka, March 2013–October 2014
| Characteristic | Phase 1 | Phase 2 | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (years) | 0.99 (0.97–1.00) | 0.07 | 1.02 (1.00–1.04) | 0.05 |
| Days of fever | 1.29 (1.00–1.66) | 0.05 | 1.04 (0.79–1.37) | 0.77 |
| Days of cough | – | – | 1.14 (0.89–1.45) | 0.30 |
| Headache | – | – | 1.70 (0.90–3.22) | 0.10 |
| Prior visit for same illness | – | – | 1.26 (0.53–3.04) | 0.60 |
| Prior antibiotic for illness | – | – | 2.71 (0.84–8.73) | 0.10 |
| Rapid test positive | 1.38 (0.77–2.47) | 0.28 | 0.50 (0.26–0.95) | 0.04 |
CI = confidence interval; OR = odds ratio.
Multivariable logistic regression was used to analyze the two phases separately since the result from the rapid influenza test was only available to clinicians in the second phase and may have influenced antibiotic prescribing patterns.
Follow-up encounter.
Telephone follow-up interviews were conducted in the second phase in 209 (86.7%) of patients within 1 month of the visit. Median time to follow-up was 16 days (interquartile range = 12–22). On follow-up, 199 (95.2%) patients were well, 5 (2.4%) had the same illness, and 2 (1.0%) had a new febrile illness. A total of 28 (13.4%) patients reported a new fever in a household member in the week following the OPD visit. Among 146 followed up patients who were prescribed antibiotics at the OPD visit, 139 (95.2%) took the medication but 48 (32.9%) were unaware that the medication was an antibiotic. Twenty-six (12.4%) patients reported another visit to a provider after the initial visit. Among patients on whom follow-up was conducted, 7 (3.4%) reported receiving a new antibiotic since the OPD visit and 23 (10.8%) were unsure.
Discussion
Our study is the first prospective study from a resource-limited setting to assess the impact of rapid influenza testing on antibiotic prescriptions in patients with ILI. In this one-group, pre-post study of outpatients with ILI in southern Sri Lanka, 81% of patients were prescribed an antibiotic when results from rapid influenza testing were not available. Knowledge of results from rapid influenza testing was associated with a 10% decrease in antibiotic prescriptions when comparing all patients between the two phases, and with a 20% decrease in antibiotic prescriptions when comparing influenza-positive patients between the two phases. In the second phase, a positive rapid influenza test result was associated with 50% lower odds of receiving an antibiotic prescription. Our results suggest that providing access to rapid influenza testing may reduce unnecessary antibiotic prescriptions for ILI in resource-limited settings.
The high frequency of antibiotic prescriptions for outpatients with ILI is consistent with results from other resource-limited regions. In Turkey, 100% of children presenting to an emergency room with ILI were prescribed an antibiotic.18 In China, antibiotics were prescribed for 78% of colds and 94% of acute bronchitis in outpatients attending primary health-care clinics.19 Data from Sri Lanka are limited, but Lucas and others20 showed that 47% of patients with acute respiratory infections, admitted to the largest public pediatric hospital in the country, may have been treated unnecessarily with antibiotics.
The high baseline proportion of antibiotic prescriptions in this study is likely due to a multitude of factors. Diagnostic tests, which are important in providing clinicians with feedback regarding their decision making and in improving the specificity of their treatment, are limited in this setting. The short clinician–patient interaction time in this busy OPD, where more than 1,000 patients are typically seen daily and individual patient visits tend to last less than 10 minutes, may leave little time for optimal clinical decision making. The lack of medical records and care continuity in the OPD prevents the implementation of techniques such as repeat visits for delayed antibiotic prescriptions, which have been shown to reduce antibiotic prescriptions to less than 40%.21 Patients' expectations and providers' perceptions of those expectations may also play a role. One study from Hong Kong showed that patient satisfaction and the belief that patients who wanted antibiotics would obtain them anyway were the most common reasons given by family physicians who self-identified themselves as overprescribing antibiotics for ARTIs.22
The drop in antibiotic prescriptions associated with and potentially caused by providing rapid influenza test results is encouraging, and is consistent with results from studies in the developed world. A retrospective observational study of children with acute respiratory symptoms presenting to a U.S. emergency department (ED) revealed that children who tested positive using a rapid influenza test were significantly less likely to receive antibiotics than children who tested negative (20% versus 53%).14 In a randomized trial, Bonner and others12 showed that ED physicians' knowledge of the rapid influenza test result was associated with lower antibiotic use in influenza-positive children (7.3% versus 24.5%), fewer diagnostic tests ordered, and shorter length of stay in the ED. Only two studies to date have studied the impact of rapid influenza tests in LMIC settings, where antibiotic use is generally more prevalent and less regulated. A retrospective analysis of outpatients presenting with ILI to five OPDs in Thailand showed that patients who tested positive for influenza were less likely to be prescribed antibiotics than patients who tested negative (73% versus 87%).9 A cross-sectional study from Turkey showed that children with ILI who tested positive for influenza were less likely to be prescribed antibiotics than children who tested negative (100% versus 68%).18 Our study is the first prospective study from a LMIC to study the impact of rapid influenza testing on antibiotic use, and indicates that providing access to rapid influenza tests results may be associated with 50% lower odds of receiving an antibiotic prescription among influenza-positive patients.
Although the results of our study are promising, solely providing access to rapid influenza testing is likely not sufficient to make a sustained impact. Sixty-two percent of patients who tested positive for influenza in the second phase still received a prescription for an antibiotic; multiple reasons may be responsible for this continued high prevalence of antibiotic prescriptions. Fear of missing a bacterial pneumonia or bacterial superinfection may have played a role in antibiotic overprescription. In addition, we only conducted a one-time informational session with physicians in the OPD regarding the performance characteristics of the rapid influenza test before the release of results in the second phase. New staff may have joined the OPD after the informational session and may have been unaware of the implications of the test result. To measure the isolated effect of providing results from the rapid diagnostic test, we did not provide education regarding the clinical interpretation of the influenza test result; we referred clinicians to the comprehensive guidelines provided by the Sri Lanka Ministry of Health. Including an educational component, in addition to providing access to the rapid influenza test results, may have resulted in a more significant decrease in antibiotic prescriptions. Patients' expectations for a prescription in this public hospital setting, where medications are provided free of charge, may also have contributed to the continued high proportion of antibiotic prescriptions. Studies from other settings indicate that a multifaceted approach that includes both provider and patient education is required to solve a problem as complex as overprescription of antibiotics.2,23 Further studies to determine clinicians' reasons for providing an antibiotic prescription in the face of a positive influenza test result need to be conducted in this setting. In addition, the cost-effectiveness of using advanced diagnostics such as rapid influenza tests, which still cost approximately $20 (well in excess of average daily wages in Sri Lanka) must be examined against individual patient and public health benefits such as fewer adverse drug reactions and decreased antimicrobial resistance.24
There are several limitations to our study. To improve logistical flow and reduce delay in care, eligible patients in phase 1 were enrolled after seeing the OPD clinician whereas eligible patients in phase 2 were enrolled before seeing the OPD clinician. Hence, patients admitted to the hospital from the OPD would have been excluded in phase 1. However, the severity of illness in general in the OPD is low; patients with severe illness who are likely to require hospitalization are triaged to a separate Emergency Treatment Unit. Our study was designed as a quasi-experimental, pre-post study and not as a randomized controlled trial, because the local ethics board did not feel comfortable with a randomized design in the OPD setting. Hence, underlying differences between patients in phases 1 and 2, even when only comparing influenza-positive patients, may have contributed to decreased antibiotic prescriptions. However, identical enrollment procedures were used in both phases, and analysis of patients in only the second phase indicated that a positive rapid influenza test result was associated with 50% lower odds of receiving an antibiotic prescription, suggesting that the rapid influenza test was important in this decrease.
In conclusion, our prospective, pre-post study suggests that providing access to rapid influenza testing may reduce antibiotic overprescription in an outpatient, LMIC setting among patients with ARTIs. A multifaceted approach that includes provider reinforcement, patient education, and improved access to rapid and affordable diagnostic tests is likely necessary to make a meaningful and sustained impact on the global problem of antibiotic overuse and antimicrobial resistance.
ACKNOWLEDGMENTS
We would like to thank the study team consisting of research assistants, phlebotomists, and laboratory technicians who were involved in this work, as well as the OPD physicians and patients who participated in this study. We also acknowledge Becton, Dickinson, and Company for providing the Veritor rapid influenza test kits.
Footnotes
Financial support: This work was supported by the NIH Research Training Grant #R25 TW009337 funded by the Fogarty International Center and the National Institute of Mental Health.
Disclosure: Woods has served in an advisory capacity to Becton, Dickinson, and Company.
Authors' addresses: L. Gayani Tillekeratne, Global Health Institute, Duke University, Durham, NC, E-mail: gayani.tillekeratne@dm.duke.edu. Champica K. Bodinayake, Ajith Nagahawatte, and Vasantha Devasiri, Departments of Medicine, Microbiology, and Pediatrics, Ruhuna University, Galle, Sri Lanka, E-mails: bodinayake@yahoo.co.uk, ajithnagahawatte@yahoo.co.uk, and vdevasiri@gmail.com. Dhammika Vidanagama and Wasantha Kodikara Arachchi, Teaching Hospital Karapitiya, Galle, Sri Lanka, E-mails: dhammikasv@yahoo.com and kody@sltnet.lk. Ruvini Kurukulasooriya, Duke-Ruhuna Collaborative Research Center, Ruhuna University, Galle, Sri Lanka, E-mail: ruhunasearch@gmail.com. Aruna Dharshan De Silva, Genetech, Research Institute, Colombo, Sri Lanka, E-mail: dslv90@yahoo.com. Truls Østbye, Department of Community and Family Medicine, Duke University, Durham, NC, and Global Health Institute, Duke University, Durham, NC, E-mail: truls.ostbye@dm.duke.edu. Megan E. Reller, Department of Pathology, Johns Hopkins University, Baltimore, MD, E-mail: mreller1@jhmi.edu. Christopher W. Woods, Department of Medicine, Duke University, Durham, NC, and Global Health Institute, Duke University, Durham, NC, E-mail: chris.woods@duke.edu.
References
- 1.Zaas AK, Garner BH, Tsalik EL, Burke T, Woods CW, Ginsburg GS. The current epidemiology and clinical decisions surrounding acute respiratory infections. Trends Mol Med. 2014;20:579–588. doi: 10.1016/j.molmed.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758–766. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peltola V, Ruuskanen O. Editorial commentary: respiratory viral infections in developing countries: common, severe, and unrecognized. Clin Infect Dis. 2008;46:58–60. doi: 10.1086/524020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA. 1995;273:214–219. [PubMed] [Google Scholar]
- 5.Donnelly JP, Baddley JW, Wang HE. Antibiotic utilization for acute respiratory tract infections in U.S. emergency departments. Antimicrob Agents Chemother. 2014;58:1451–1457. doi: 10.1128/AAC.02039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzales R, Malone DC, Maselli JH, Sande MA. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33:757–762. doi: 10.1086/322627. [DOI] [PubMed] [Google Scholar]
- 7.Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278:901–904. [PubMed] [Google Scholar]
- 8.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J., Jr The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 9.Bhavnani D, Phatinawin L, Chantra S, Olsen SJ, Simmerman JM. The influence of rapid influenza diagnostic testing on antibiotic prescribing patterns in rural Thailand. Int J Infect Dis. 2007;11:355–359. doi: 10.1016/j.ijid.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Tillekeratne L, Bodinayake C, Nagahawatte A, Vidanagama D, Devasiri V, Kodikara Arachchi W, Kurukulasooriya R, De Silva A, Ostbye T, Reller M, Woods C. An under-recognized influenza epidemic identified by rapid influenza testing, southern Sri Lanka, 2013. Am J Trop Med Hyg. 2015;92:1023–1029. doi: 10.4269/ajtmh.14-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan F, Nguyen A, Formanek A, Bell JJ, Selvarangan R. Comparison of the BD Veritor System for Flu A+B with the Alere BinaxNOW influenza A&B card for detection of influenza A and B viruses in respiratory specimens from pediatric patients. J Clin Microbiol. 2014;52:906–910. doi: 10.1128/JCM.02484-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonner AB, Monroe KW, Talley LI, Klasner AE, Kimberlin DW. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003;112:363–367. doi: 10.1542/peds.112.2.363. [DOI] [PubMed] [Google Scholar]
- 13.Esposito S, Marchisio P, Morelli P, Crovari P, Principi N. Effect of a rapid influenza diagnosis. Arch Dis Child. 2003;88:525–526. doi: 10.1136/adc.88.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noyola DE, Demmler GJ. Effect of rapid diagnosis on management of influenza A infections. Pediatr Infect Dis J. 2000;19:303–307. doi: 10.1097/00006454-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Falsey AR, Murata Y, Walsh EE. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med. 2007;167:354–360. doi: 10.1001/archinte.167.4.ioi60207. [DOI] [PubMed] [Google Scholar]
- 16.Sharma V, Dowd MD, Slaughter AJ, Simon SD. Effect of rapid diagnosis of influenza virus type a on the emergency department management of febrile infants and toddlers. Arch Pediatr Adolesc Med. 2002;156:41–43. doi: 10.1001/archpedi.156.1.41. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . WHO Interim Global Epidemiological Surveillance Standards for Influenza. Geneva, Switzerland: World Health Organization; 2012. pp. 1–61. [Google Scholar]
- 18.Ozkaya E, Cambaz N, Coskun Y, Mete F, Geyik M, Samanci N. The effect of rapid diagnostic testing for influenza on the reduction of antibiotic use in paediatric emergency department. Acta Paediatr. 2009;98:1589–1592. doi: 10.1111/j.1651-2227.2009.01384.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Wang P, Wang X, Zheng Y, Xiao Y. Use and prescription of antibiotics in primary health care settings in China. JAMA Intern Med. 2014;174:1914–1920. doi: 10.1001/jamainternmed.2014.5214. [DOI] [PubMed] [Google Scholar]
- 20.Lucas M, Liyanage U, Lokukankanamage L. A study of antibiotic usage in acute respiratory infections in children. Sri Lankan Journal of Child Health. 2001;30:5–7. [Google Scholar]
- 21.Little P, Moore M, Kelly J, Williamson I, Leydon G, McDermott L, Mullee M, Stuart B. Delayed antibiotic prescribing strategies for respiratory tract infections in primary care: pragmatic, factorial, randomised controlled trial. BMJ. 2014;348:g1606. doi: 10.1136/bmj.g1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam TP, Lam KF. What are the non-biomedical reasons which make family doctors over-prescribe antibiotics for upper respiratory tract infection in a mixed private/public Asian setting? J Clin Pharm Ther. 2003;28:197–201. doi: 10.1046/j.1365-2710.2003.00485.x. [DOI] [PubMed] [Google Scholar]
- 23.Holloway K. Promoting the rational use of antibiotics. Regional adviser, essential drugs and other medicines, World Health Organization, Regional Office for South-East Asia. Regional Health. 2011;15:122–130. [Google Scholar]
- 24.Montalto NJ. An office-based approach to influenza: clinical diagnosis and laboratory testing. Am Fam Physician. 2003;67:111–118. [PubMed] [Google Scholar]