Abstract
To improve the knowledge base of Borrelia in north Africa, we tested 257 blood samples collected from febrile patients in Oran, Algeria, between January and December 2012 for Borrelia species using flagellin gene polymerase chain reaction sequencing. A sequence indicative of a new Borrelia sp. named Candidatus Borrelia algerica was detected in one blood sample. Further multispacer sequence typing indicated this Borrelia sp. had 97% similarity with Borrelia crocidurae, Borrelia duttonii, and Borrelia recurrentis. In silico comparison of Candidatus B. algerica spacer sequences with those of Borrelia hispanica and Borrelia garinii revealed 94% and 89% similarity, respectively. Candidatus B. algerica is a new relapsing fever Borrelia sp. detected in Oran. Further studies may help predict its epidemiological importance.
Relapsing fever borreliae are arthropod-borne pathogens causing mild to deadly septicemia and miscarriage.1 In Africa, cultured representatives include tick-borne Borrelia crocidurae, Borrelia duttonii, and Borrelia hispanica transmitted by Ornithodoros soft ticks and louse-borne Borrelia recurrentis.1 Borreliae are fastidious bacteria responsible for various febrile presentations, most commonly malaria-like symptoms.1,2 Borreliae have been documented in patients with tick-borne relapsing fever, however little is known regarding Borrelia in north Africa.2 Borrelia crocidurae has been detected with a 2.5% prevalence in Ornithodoros sonrai ticks,3 while Lyme group Borrelia garinii was recently detected in Ixodes ricinus ticks, collected from El Ghora, Algeria.4 In addition, at least 10 different relapsing fever–causing borreliae have been documented in Africa, including five different borreliae in humans and five different borreliae in nonhuman hosts.2 The former includes pathogens classified as B. hispanica, B. crocidurae, B. duttonii, and B. recurrentis.2 Although relapsing fever–causing Borrelia may form one genetic species, they differ in their vector, host range, and disease spectra protein profile by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.5–7 Accordingly, molecular tools can be used to discriminate these different Borrelia.8,9 Here, using such molecular tools, we detected sequences indicative of a new Borrelia sp. named Candidatus Borrelia algerica in a blood sample from a patient with prolonged fever in Oran, Algeria.
We studied 257 blood samples collected from febrile patients in Oran between January and December 2012. Interviews, sampling (3–4 mL blood in ethylenediaminetetraacetic acid [EDTA] tubes) and a medical examination were performed on each individual with a fever (an axillary temperature > 37.5°C) and a questionnaire was completed by each patient. We have previously reported the presence of Coxiella burnetii,10 Rickettsia felis, and Plasmodium spp. in this patient series.11 A 200 μL sample of whole blood was used for DNA extraction performed using a QIAamp DNA Micro Kit according to the manufacturer protocols (Qiagen, Hilden, Germany). The samples were handled appropriately to avoid cross-contamination. The quality of the DNA handling and extraction was verified by real-time polymerase chain reaction (RT-PCR) for the housekeeping gene encoding beta-actin12 (Table 1). Borrelia was then detected in the samples using a 16S rRNA gene sequence-based system, as previously described.8,9 Two sets of negative controls (DNA of blood from a nonfebrile patient and sterile water) and a positive control (B. recurrentis DNA) were also analyzed in each run. All positive and negative controls demonstrated the expected results in all tests and Borrelia spp. were detected in four (1.6%) patients.
Table 1.
Primers and probes used in this study
| Microorganism detected | Targeted sequences | Primers (5′-3′) | Probes | Reference | |
|---|---|---|---|---|---|
| Forward | Reverse | ||||
| Borrelia spp. | 16S | AGCCTTTAAAGCTTCGCTTGTAG | GCCTCCCGTAGGAGTCTGG | 6-FAM CCGGCCTGAGAGGGTGAACGG-TAMRA | 11 |
| MST2 | TTTTTGCTAAAATTAACCCTTTTCA | CTCATTTTAATTTCCTTACCCCTA | 11 | ||
| MST3 | GCAGGTGGCTGTTAACCACT | ATGTGGGGAATGCACTCTTT | 11 | ||
| MST5 | CCTGAGTCGATATGGGCACT | CAACCTGACATATCTTACTCAATTCAT | 11 | ||
| MST6 | GGGTTCGAATCCCATTTTCT | CTCTGGGACGCCTCTTAATG | 11 | ||
| MST7 | TTCGCCACTGAATGTATTGC | TGCCAATGTTCTTGTTGGTC | 11 | ||
| flaB | TAATACGTCAGCCATAAATGC | GCTCTTTGATCAGTTATCATTC | 11 | ||
| Borrelia crocidurae | glpQ | CCTTGGATACCCCAAATCATC | GGCAATGCATCAATTCTAAAC | 6-FAM-ATGGACAAATGACAGGTCTTAC-NFQ | 11 |
| Borrelia duttonii/Borrelia recurrentis | rec N | GATGATGTAATTTCTAATGAAGGATG | TCTTTGACCAAAATTCCCCTAA | 6-VIC-GCAAGTGATGAGTTTAGACGTTGTTTA-TAMRA | 11 |
| Borrelia hispanica | rec C | AAATTGCAACCAAGCATACAAA | TCGTCCAAATTTGATAGAGGTG | 6-VIC-AGCTTAAAAAATAATATTGTCAAAGG-NFQ | 11 |
| beta-actin | CATGCCATCCTGCGTCTGGA | CCGTGGCCATCTCTTGCTCG | 6-FAM-CGGGAAATCGTGCGTGACATTAAG-TAMRA | 11 | |
MST = multispacer sequence typing.
To confirm our results, multispacer sequence typing was performed on the four positive samples, as previously described8 (Table 1). Only one blood sample resulted in positive amplification and sequencing of the two spacers (GenBank LN626643 and LN626644). Concatenation of the spacer sequences indicated that this Borrelia sp. had 97% similarity with B. crocidurae, B. duttonii, and B. recurrentis. Moreover, the in silico comparison of these spacer sequences with those of B. hispanica (AYOU00000000.1) and B. garinii (AYAJ01000003.1) revealed 94% and 89% similarity, respectively, indicating a new Borrelia species, that we named Candidatus B. algerica. Candidatus B. algerica DNA was then tested by a second RT-PCR assay targeting the glpQ gene for B. crocidurae, the recN gene for B. duttonii/B. recurrentis and the recC gene for B. hispanica, as previously described.9 The results of all assays were negative, providing further evidence of a new species. Finally, Candidatus B. algerica DNA was tested by flaB gene PCR sequencing8,9 and the sequences (LN626647) were compared with those available in the GenBank, EMBL, and DJB databases using the gapped BLASTN 2.0.5 program in the National Center for Biotechnology Information server. Candidatus B. algerica showed 99.6% sequence similarity with B. duttonii (CP000976.1) and 99.3% similarity with B. crocidurae (GU357619.1) (Figure 1 ).
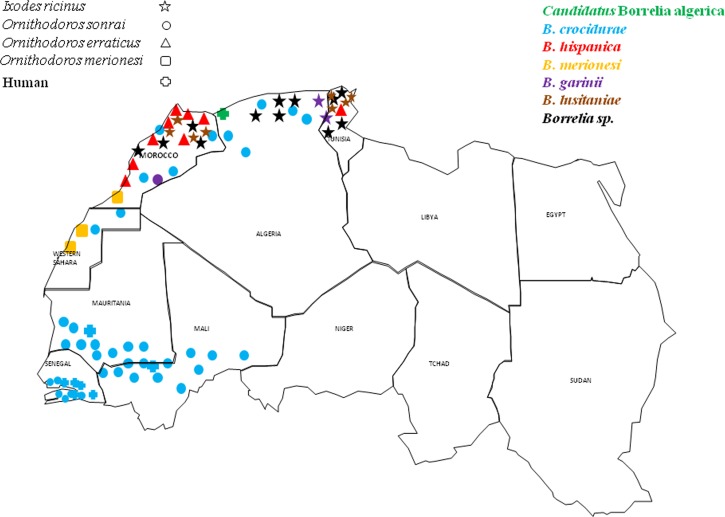
Figure 1.
Geographical distribution of relapsing fever-causing borreliae in northwestern Africa.
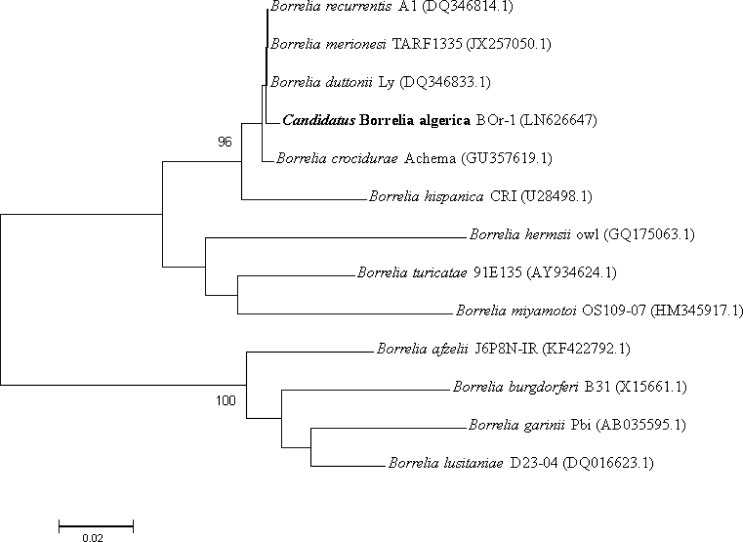
Borrelia lusitaniae is a species within the complex Borrelia burgdorferi sensu lato and is by far the predominant Borrelia species detected in I. ricinus ticks in Tunisia and Morocco.13,14 Borrelia miyamotoi also belongs to the relapsing fever borreliae group and may cause relapsing fever and Lyme disease-like symptoms throughout the Holarctic region of the world, because of its widespread prevalence in the tick vector I. ricinus.15,16 A phylogenetic tree based on the 735-bp flaB gene was constructed using the MEGA software (www.megasoftware.net) and revealed that Candidatus B. algerica clustered with relapsing fever borreliae, differing from B. recurrentis and B. duttonii (Figure 2 ).
Figure 2.
Phylogenetic tree of Candidatus Borrelia algerica. Bootstrap values > 95% are indicated at the nodes (the GenBank accession numbers are indicated in brackets).
We believe that our results are accurate, as all molecular assays have previously been evaluated and are routinely used in our reference center. Furthermore, all negative controls were negative in each molecular assay. Lyme disease has been previously suspected in 21 Algerian patients17; however, these cases were diagnosed serologically by B. burgdorferi enzyme-linked immunosorbent assay, without confirmation by western blotting.17 Antigenic cross-reactions between Lyme-disease-group and relapsing-fever-group borreliae may suggest that these infections could have been caused by other Borrelia spp. of the relapsing fever group.
In conclusion, we have determined that Candidatus B. algerica is a new relapsing fever Borrelia sp. detected in Oran. Clinicians and microbiologists need to be aware of these data to further predict its epidemiological importance. Further surveys of arthropod populations should be conducted in north Africa to isolate and examine the geographic distribution of Candidatus B. algerica.
Footnotes
Authors' addresses: Aurélien Fotso Fotso and Emmanouil Angelakis, URMITE, Faculté de Médecine, Marseille, France, E-mails: aurelien74000618@yahoo.fr and angelotasmanos@msn.com. Nadjet Mouffok, Service des Maladies Infectieuses, Centre Hospitalo-Universitaire d'Oran, Oran, Algeria, E-mail: nadjmouf_31@yahoo.fr. Michel Drancourt, Méditerranée Infection, Aix Marseille Université, Marseille, France, and Unité de Recherche sur les Maladies Infectieuses et Tropicale Emergentes, Marseille, France, E-mail: michel.drancourt@univ-amu.fr. Didier Raoult, URMITE UMR 6236, IRD 198, Aix Marseille Université, Marseille, France, E-mail: didier.raoult@gmail.com.
References
- 1.Cutler SJ, Abdissa A, Trape JF. New concepts for the old challenge of African relapsing fever borreliosis. Clin Microbiol Infect. 2009;15:400–406. doi: 10.1111/j.1469-0691.2009.02819.x. [DOI] [PubMed] [Google Scholar]
- 2.Elbir H, Raoult D, Drancourt M. Relapsing fever borreliae in Africa. Am J Trop Med Hyg. 2013;89:288–292. doi: 10.4269/ajtmh.12-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trape JF, Diatta G, Arnathau C, Bitam I, Sarih M, Belghyti D, Bouattour A, Elguero E, Vial L, Mane Y, Balde C, Prugnolle F, Chauvancy G, Mahe G, Granjon L, Duplantier JM, Durand P, Renaud F. The epidemiology and geographic distribution of relapsing fever borreliosis in west and north Africa, with a review of the Ornithodoros erraticus complex (Acari: Ixodida) PLoS One. 2013;8:e78473. doi: 10.1371/journal.pone.0078473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benredjem W, Leulmi H, Bitam I, Raoult D, Parola P. Borrelia garinii and Rickettsia monacensis in Ixodes ricinus ticks, Algeria. Emerg Infect Dis. 2014;20:1776–1777. doi: 10.3201/eid2010.140265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elbir H, Abi-Rached L, Pontarotti P, Yoosuf N, Drancourt M. African relapsing fever borreliae genomospecies revealed by comparative genomics. Front Public Health. 2014;2:43. doi: 10.3389/fpubh.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderaro A, Gorrini C, Piccolo G, Montecchini S, Buttrini M, Rossi S, Piergianni M, Arcangeletti MC, De CF, Chezzi C, Medici MC. Identification of Borrelia species after creation of an in-house MALDI-TOF MS database. PLoS One. 2014;9:e88895. doi: 10.1371/journal.pone.0088895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fotso Fotso A, Mediannikov O, Diatta G, Almeras L, Flaudrops C, Parola P, Drancourt M. MALDI-TOF mass spectrometry detection of pathogens in vectors: the Borrelia crocidurae/Ornithodoros sonrai paradigm. PLoS Negl Trop Dis. 2014;8:e2984. doi: 10.1371/journal.pntd.0002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbir H, Gimenez G, Sokhna C, Bilcha KD, Ali J, Barker SC, Cutler SJ, Raoult D, Drancourt M. Multispacer sequence typing relapsing fever borreliae in Africa. PLoS Negl Trop Dis. 2012;6:e1652. doi: 10.1371/journal.pntd.0001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elbir H, Henry M, Diatta G, Mediannikov O, Sokhna C, Tall A, Socolovschi C, Cutler SJ, Bilcha KD, Ali J, Campelo D, Barker SC, Raoult D, Drancourt M. Multiplex real-time PCR diagnostic of relapsing fevers in Africa. PLoS Negl Trop Dis. 2013;7:e2042. doi: 10.1371/journal.pntd.0002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angelakis E, Mediannikov O, Socolovschi C, Mouffok N, Bassene H, Tall A, Niangaly H, Doumbo O, Znazen A, Sarih M, Sokhna C, Raoult D. Coxiella burnetii-positive PCR in febrile patients in rural and urban Africa. Int J Infect Dis. 2014;28C:107–110. doi: 10.1016/j.ijid.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Mediannikov O, Socolovschi C, Edouard S, Fenollar F, Mouffok N, Bassene H, Diatta G, Tall A, Niangaly H, Doumbo O, Lekana-Douki JB, Znazen A, Sarih M, Ratmanov P, Richet H, Ndiath MO, Sokhna C, Parola P, Raoult D. Common epidemiology of Rickettsia felis infection and malaria, Africa. Emerg Infect Dis. 2013;19:1775–1783. doi: 10.3201/eid1911.130361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safont M, Angelakis E, Richet H, Lepidi H, Fournier PE, Drancourt M, Raoult D. Bacterial lymphadenitis at a major referral hospital in France from 2008 to 2012. J Clin Microbiol. 2014;52:1161–1167. doi: 10.1128/JCM.03491-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younsi H, Sarih M, Jouda F, Godfroid E, Gern L, Bouattour A, Baranton G, Postic D. Characterization of Borrelia lusitaniae isolates collected in Tunisia and Morocco. J Clin Microbiol. 2005;43:1587–1593. doi: 10.1128/JCM.43.4.1587-1593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarih M, Jouda F, Gern L, Postic D. First isolation of Borrelia burgdorferi sensu lato from Ixodes ricinus ticks in Morocco. Vector Borne Zoonotic Dis. 2003;3:133–139. doi: 10.1089/153036603768395834. [DOI] [PubMed] [Google Scholar]
- 15.Hansford KM, Fonville M, Jahfari S, Sprong H, Medlock JM. Borrelia miyamotoi in host-seeking Ixodes ricinus ticks in England. Epidemiol Infect. 2015;143:1079–1087. doi: 10.1017/S0950268814001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alem A, Hadji N. Clinico-serologic study of Lyme disease in Algeria (1996–1999) Arch Inst Pasteur Alger. 1999;63:49–58. [Google Scholar]