Abstract
Little is known about the type and longevity of the humoral response to cryptosporidial infections in developing countries. We evaluated serum antibody response to Cryptosporidium gp15 in 150 sets of maternal, preweaning and postinfection/end-of-follow-up sera from children followed up to 2 years of age to determine the influence of maternal and preweaning serological status on childhood cryptosporidiosis. Fifty two percent (N = 78) of mothers and 20% (N = 30) of children were seropositive preweaning. However, most positive preweaning samples from children were collected early in life indicating transplacental transfer and subsequent rapid waning of antibodies. Although 62% (N = 94) of children had a parasitologically confirmed cryptosporidial infection (detected by stool polymerase chain reaction) during the follow-up, only 54% (N = 51) of children were seropositive postinfection. Given there were striking differences in seropositivity depending on when the sample was collected, even though Cryptosporidium was detected in the stool of the majority of the children, this study indicates that antibodies wane rapidly. During follow-up, the acquisition or severity of cryptosporidial infections was not influenced by maternal (P = 0.331 and 0.720, respectively) as well as the preweaning serological status of the child (P = 0.076 and 0.196, respectively).
Introduction
Cryptosporidium is an important cause of gastroenteritis worldwide. In endemic regions, cryptosporidiosis is widely distributed within and across populations, ranging from self-limiting and/or asymptomatic infections in healthy people to life-threatening infections in immunocompromised individuals. Transmission of Cryptosporidium is predominantly through the fecal-oral route by the ingestion of oocysts, but can also occur by person-to-person contact and zoonotic infection.1,2 Individuals across all ages are affected, but in developing countries, the disease is seen predominantly in children where hygiene may be low and safe drinking water is scarce.3 The excretion of environmentally resistant oocysts into water sources results in contaminated water being a risk factor for cryptosporidiosis in industrialized countries.4–6 However, we have shown that provision of safe drinking water did not alter acquisition of infection or disease in young children in an urban slum in India,6 possibly indicating multiple modes of transmission in a contaminated setting.
Earlier studies on Cryptosporidium infections were based on screening by microscopic examination of stool samples.7 With the advent of molecular tools for detection of Cryptosporidium by polymerase chain reaction (PCR) at the small-subunit rRNA and at multiple other loci, the epidemiology, environmental sources, routes of transmission, genetic diversity, and parasite species–host dynamics have been more intensively studied.8–11
Serological assays based on the detection of Cryptosporidium-specific immunoglobulin G (IgG) identify more infections than conventional techniques such as microscopy or antigen detection.12–14 Cryptosporidial infection results in IgM-, IgG-, and IgA-specific serum antibody responses to the 17- (also called gp15)15 and 27-kDa (also called cp23)16 antigens of various Cryptosporidium subtypes and species.17–20 The antibody response after cryptosporidial infection appears to develop rapidly, peaking within 3–9 weeks and wanes to baseline levels by 5–6 months.17,21,22 Cell-mediated immunity is known to be important for protection from and resolution of cryptosporidial infections, but the role of antibody responses are not well understood.23,24 The humoral and interferon-γ-mediated cellular response induced by the gp15 (17 kDa) antigen of Cryptosporidium have been postulated to be protective,25 and therefore measuring antigen-specific cryptosporidial antibodies may be important in estimation of the protection conferred against disease by natural infection and reinfection in children. In addition, the role of maternal antibodies in susceptibility to infection during early childhood remains undefined.
This study was undertaken to determine the influence of the serological status of the mother on early childhood acquisition of cryptosporidiosis, the time to primary infection, and whether cryptosporidial antibodies in children could be used to predict risk of future infection or disease.
Materials and Methods
Study subjects and samples.
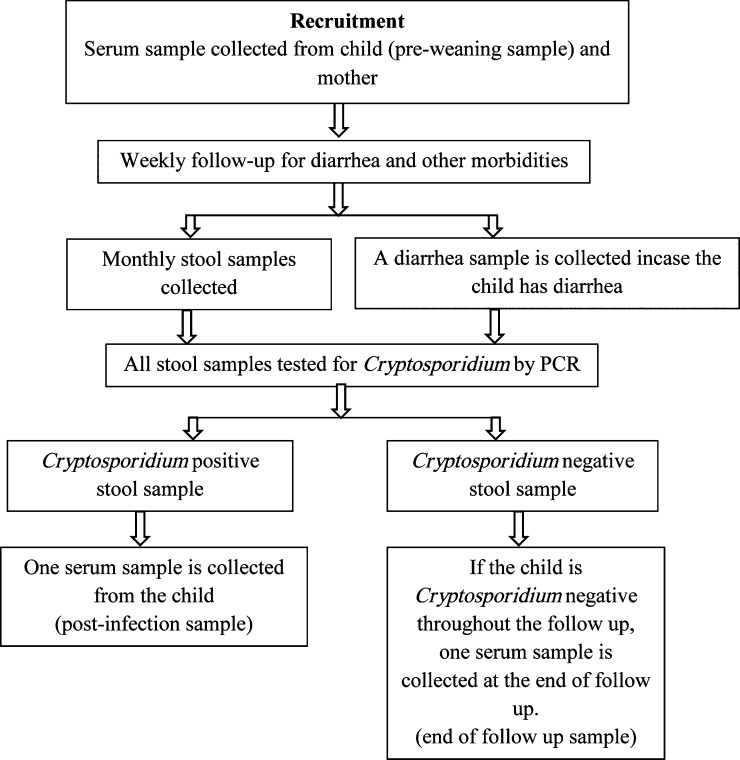
A total of 176 exclusively breast-fed children (defined as infants who received no food other than breast milk, either solid or liquid [including water], with the exception of oral rehydration solution or drops/syrups of vitamins, minerals, or medicines26) were recruited in a study investigating the protective efficacy of bottled water on childhood cryptosporidiosis in a semi-urban slum in Vellore, southern India.6,27 Based on the area of residence, families of the children received bottled (N = 90, protected) or municipal (N = 86, unprotected) drinking water, and the children were followed up until they attained 2 years of age; 160 (90.9%) of the 176 children completed the follow-up. Additional details of child recruitment and follow-up have been described previously.27 Surveillance stool samples were collected every month and diarrheal stool samples collected every time a child had an episode of diarrhea (defined as three or more loose, watery stools in a 24-hour period28). An infection was defined as symptomatic if a stool sample collected within ±7 days of a diarrheal episode was positive for Cryptosporidium spp. and asymptomatic if there was no diarrheal episode within a week before or after the detection of Cryptosporidium spp. in the stool sample.6 A blood sample was collected from mothers and exclusively breast-fed children at recruitment. In the event of a cryptosporidial infection, a blood sample was collected from the study subject as early as possible (not later than 6 months) after the first parasitologically confirmed infection (identified by stool PCR). At the end of 2 years of follow-up, a blood sample was collected from all children negative for cryptosporidiosis by fecal examination to ascertain missed cryptosporidial infections by serology (Figure 1 ). The study was approved by the Institutional Review Boards of the Christian Medical College, Vellore, India, and Tufts University Health Sciences Campus, Boston, MA, and written informed consent was obtained from parents or legal guardians of all children before enrollment.
Figure 1.
Follow-up and sample collection for 176 children recruited into a study on protection from cryptosporidial infection by bottled drinking water.
Screening for Cryptosporidium spp.
All fecal samples were screened for Cryptosporidium spp. by 18S rRNA PCR on DNA extracted using a QIAamp Stool DNA Minikit (Qiagen Inc., Valencia, CA).8,9 In brief, this is a two-step nested-PCR followed by restriction fragment length polymorphism (RFLP) to identify Cryptosporidium species and genotypes. The PCR primers and cycling conditions and restriction enzymes have been described previously.8,9
Enzyme-linked immunosorbent assay for anti gp15 IgG antibodies in serum.
Quantitation of serum IgG levels to the immunodominant gp15 antigen by enzyme-linked immunosorbent assay (ELISA) was carried out using recombinant (r)gp15 protein expressed in the pET46 vector (Novagen, EMD Biosciences, Inc., Merck KGaA, Darmstadt, Germany) as described previously6 and coated on a 96-well microtiter plate (Costar; Corning Inc., Corning, NY) overnight at 4°C in carbonate/bicarbonate buffer (pH 9.6) at a concentration of 0.5 μg of (r)gp15 protein/50 μL/well. Phosphate buffered saline (PBS) containing 0.05% Tween 20 was used to wash off excess antigen. To prevent nonspecific binding, the plates were blocked with 200 μL PBS containing 0.25% bovine serum albumin (BSA) (Sigma Aldrich, St. Louis, MO) for 2 hours at 37°C. Subsequently, 50 μL/well of serum samples diluted at 1:100 and 1:200 in PBS with 0.25% BSA along with a standard of pooled human IgG (Iviglob EX [5 g/100 mL]; VHB Life Science Ltd., Maharashtra, India) diluted serially from 1:50 to 1:3,200 was added to the wells. Wells at the periphery of the plate were used as blanks. Negative control sera (negative by ELISA and western blot analysis using Cryptosporidium parvum oocyst lysate as antigen) were run on each plate and incubated for 1 hour at 37°C. Plates were washed five times and 50 μL/well of alkaline phosphatase–conjugated goat antihuman IgG (γ-chain specific) (Sigma Aldrich) was added and incubated for 1 hour at 37°C. The plates were washed five times and 50 μL/well of substrate solution (100 mM Tris-HCl, pH 9.5, 100 mM NaCl, 5 mM MgCl2) containing 4-nitrophenyl phosphate disodium salt hexahydrate (1 mg/mL) (Sigma Aldrich) was added and incubated at room temperature for 15 minutes. The reaction was stopped with 50 μL/well of 0.1 M EDTA. Absorbance was measured at 405 nm using a microplate reader (ELX800; Biotek Instruments, Winooski, VT).17
The quantity of anti (r)gp15 IgG was determined by comparison of the optical density (OD) from sample wells to a standard curve generated by serial dilutions of pooled human IgG as described above. All samples and standards were run in duplicates, and the average OD of the blank wells in the periphery of the plate was subtracted. Each point on the standard curve was considered valid if the mean OD value was within a predetermined range and each plate was considered valid if at least 5 points of the standard curve were available and the mean negative control OD was < 0.1. The OD of all standards and samples was considered valid if the difference between the 2 replicates was < 0.1, and a coefficient of variation of < 15%. GraphPad Prism, Version 4.0, La Jolla, CA, was used to calculate unknown values for the samples from the linear part of the sigmoidal dose–response curve. The value obtained was multiplied by the dilution factor of 1 and 2 for 1:100 and 1:200 dilutions, respectively, and the results were expressed as arbitrary units (AU). The mean AU of 1:100 and 1:200 dilutions for each sample was calculated and considered valid if they had a coefficient of variation of < 15%.
Any sample with a detectable antibody level was considered seropositive. The proportions of seropositive maternal, pre- and postinfection samples were calculated along with geometric mean concentrations (GMCs) and 95% confidence intervals (95% CIs). Children were considered to have seroconverted if they were seronegative preweaning, but became seropositive postinfection or at the end of follow-up.
Statistical analysis.
Data were entered in duplicate using Epi-Info 2002 (CDC, Atlanta, GA). The two entry datasets were compared to detect missing values or discrepancies between them, and identical values were saved to a master database to be used for all subsequent analyses. The data analysis was performed in STATA 10.1 for Windows software (StataCorp, College Station, TX). Continuous variables were compared using two-tailed t test if normally distributed or using Mann–Whitney U test if the distribution was skewed. Categorical variables were compared using χ2 test if the expected cell count was ≥ 5 or using Fisher's exact test if the expected cell count was < 5.
Results
Of the 160 children who completed the follow-up until 2 years of age, a complete set of maternal, preweaning and postinfection (N = 94), or end- of-follow-up (N = 56) serum samples as per protocol were available for 150 (94%). Children for whom the complete set of serum samples were available were more likely to belong to a joint or an extended family (P = 0.044), had a larger family size (P = 0.017), and were exclusively breast-fed for a longer duration of 4.8 (3.6–5.7) months compared with children with an incomplete set of serum samples (3.5 (1.9–5.3) months, P = 0.029). Other sociodemographic characteristics such as gender, socioeconomic status, presence of toilet, and animals at home and household hygiene were comparable between children with and without a complete set of serum samples (Table 1). There was no difference in the proportion of children with complete sets of serum samples between the bottled (73/90, 81%) and municipal (77/86, 90%) water groups (P = 0.115).
Table 1.
Comparison of baseline characteristics between children who had complete sets of serum samples (N = 150) and those who did not (N = 26)
| Children with complete set of serum samples | Children without complete set of serum samples | P value | |
|---|---|---|---|
| Male child | 83 (55%) | 12 (46%) | 0.386‡ |
| Median (IQR) birth weight (in kg)* | 2.9 (2.7–3.1) | 2.9 (2.5–3.3) | 0.806§ |
| Median (IQR) family size | 6 (4–7) | 5 (4–6) | 0.017§ |
| Nuclear family | 66 (44%) | 17 (65%) | 0.044‡ |
| Crowded living conditions (≥ 5 per room) | 58 (39%) | 6 (23%) | 0.127‡ |
| Presence of older sibling(s) | 104 (69%) | 15 (58%) | 0.242‡ |
| Median (IQR) age of the mother (in years) | 24 (21–26) | 25 (23–26) | 0.146§ |
| Median (IQR) years of completed maternal education | 7 (3–10) | 8 (6–10) | 0.162§ |
| Living in a “kutcha” house† | 28 (19%) | 6 (23%) | 0.599‡ |
| Median (IQR) duration of exclusive breast-feeding (in months) | 4.8 (3.6–5.7) | 3.5 (1.9–5.3) | 0.029§ |
| Low socioeconomic status | 99 (66%) | 18 (69%) | 0.747‡ |
| Firewood as the primary cooking mode | 78 (52%) | 12 (46%) | 0.582‡ |
| Presence of toilet in the house | 92 (62%) | 10 (63%) | 0.953‡ |
| Presence of animal(s) in the house | 44 (29%) | 6 (23%) | 0.514‡ |
| Good household hygiene | 41 (27%) | 6 (23%) | 0.651‡ |
IQR = interquartile range
Data on birth weight and presence of toilet missing for 9 and 11 children, respectively.
“Kutcha” house: a house with wall and roof of mud/tin/asbestos/thatch.
Tests of significance: ‡χ2 test; §Mann–Whitney U test.
Comparison of maternal and preweaning antibody levels.
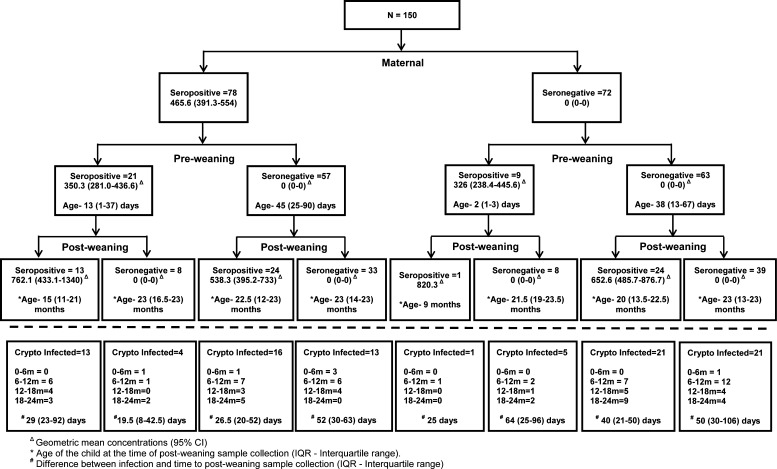
Of the 150 maternal samples, 78 (52%) were seropositive with a GMC of 465.6 (95% CI = 391.3–554) AU and 72 (48%) were seronegative. The proportion of seropositive mothers was comparable between the bottled (58%) and municipal (47%) water groups (P = 0.187), as were the GMCs (524.1 versus 405.2 AU, P = 0.092) (Table 2).
Table 2.
Proportion of seropositive mothers and children at the start and end of follow-up and geometric mean levels of anti-gp15 IgG antibodies
| Sample tested | All children (N = 150) | Bottled water (N = 73) | Municipal water (N = 77) | |||
|---|---|---|---|---|---|---|
| Number seropositive (%) | GMC (95% CI) AU | Number seropositive (%) | GMC (95% CI) AU | Number seropositive (%) | GMC (95% CI) AU | |
| Maternal | 78 (52) | 465.6 | 42 (58) | 524.1 | 36 (47) | 405.2 |
| (391.3–554) | (401.3–684.6) | (326.4–503) | ||||
| Child preweaning | 30 (20) | 342.7 | 19 (26) | 315.9 | 11 (14) | 394.6 |
| (289–406.4) | (259.4–384.6) | (278.5–559.1) | ||||
| Child postinfection/end of follow-up | 62 (41) | 627.8 | 31 (42) | 609.3 | 31 (40) | 646.8 |
| (517.6–761.4) | (451.0–823.2) | (499.1–838.1) | ||||
AU = arbitrary units; CI = confidence interval; GMC = geometric mean concentration.
Among the 150 children, 30 (20%) preweaning samples were seropositive with a GMC of 342.7 (95% CI = 289.0–406.4) AU and 120 (80%) were seronegative. There was no significant difference between proportion of children seropositive preweaning in the bottled (26%) and municipal (14%) water groups (P = 0.072). The preweaning GMCs were also comparable between children in the bottled and municipal water groups (315.9 versus 394.6 AU, P = 0.254) (Table 2).
The median (interquartile range [IQR]) age of children at the time of collection of the preweaning sera was 34 (13–65) days. It was 34 (10–67) and 33 (13–63) days among children in the bottled and municipal water groups, respectively (P = 0.891). However, children who were seropositive had their samples collected at an earlier age (median [IQR] = 4 [1–20] days) than those who were seronegative (42 [18–70] days, P < 0.001). This trend was similar among children in the bottled (4 [1–37] versus 46 [18–79] days, P = 0.002) as well as the municipal (3 [1–13] versus 40 [18–66] days, P < 0.001) water group.
When the mother–child paired sera were considered, 21 (26.9%) children of the 78 seropositive mothers were seropositive with a GMC of 350.3 (95% CI = 281.0–436.6) AU, whereas only 9 (12.5%) children of the 72 seronegative mothers were seropositive with a GMC of 326 (95% CI = 238.4–445.6) AU, indicating a significant association between the presence of antibodies in mothers and their children preweaning (P = 0.027). A dose–response relationship between maternal antibody levels and the likelihood of a child being seropositive preweaning, was also noticed (Table 3).
Table 3.
Proportion of preweaning and postinfection/end-of-follow-up child seropositivity based on low, middle, and high GMC on antibodies to cryptosporidial gp15 in mothers
| Maternal serological status (n) | Child preweaning seropositive (%) | P value | Child postinfection/end-of-follow-up seropositive (%) | P value |
|---|---|---|---|---|
| Seronegative (72) | 9 (12.5) | – | 25 (34.7) | – |
| Seropositive (78) | 21 (26.9) | 0.002 | 37 (47.4) | 0.114 |
| Based on GMC tertiles of seropositive mothers | ||||
| Lower (26) | 3 (11.5) | 0.006 | 10 (38.5) | 0.123 |
| Middle (26) | 7 (26.9) | 16 (61.5) | ||
| Upper (26) | 11 (42.3) | 11 (42.3) | ||
GMC = geometric mean concentration.
Serological response to cryptosporidial infections.
Sixty two (41%) of the 150 children with complete sample sets were seropositive in their postinfection/end-of-follow-up samples. There was no difference between proportion of seropositive children, postinfection, in the bottled (42%) and the municipal (40%) water groups (P = 0.784). The postinfection/end-of-follow-up GMCs were also comparable between children in the bottled and the municipal water groups (609.3 versus 646.8 AU, P = 0.667) (Table 2).
During their 2-year follow-up period, 94 (62%) of 150 children had a parasitologically confirmed cryptosporidial infection; of them 51 (54%) also had detectable antibodies in their postinfection serum samples with a GMC of 666.2 (95% CI = 538.1–824.6). In addition, 11 (20%) of the 56 children who did not have a parasitologically confirmed cryptosporidial infection had detectable antibody levels in their end-of-follow-up serum sample with a GMC of 476.8 ((95% CI = 290.8–781.7). The postinfection/end-of-follow-up GMCs were comparable between children with and without a parasitologically confirmed cryptosporidial infection (P = 0.159).
The median (IQR) duration between a parasitologically confirmed cryptosporidial infection and the collection of postinfection serum sample was 38 (23–64) days. Children who were seropositive had their postinfection serum samples collected earlier (29 [21–50] days post detection) than seronegative children (50 [24–96] days post detection, P = 0.041).
Maternal and preweaning serological status and cryptosporidial infections.
There was no difference between seropositive and seronegative mothers in the proportion of children who were stool positive for Cryptosporidium spp. (46/78, 59% children of seropositive mothers and 48/72, 67% children of seronegative mothers, P = 0.331). There was no difference in the postinfection/end-of-follow-up seropositivity between children of seropositive and seronegative mothers (47% versus 35%, P = 0.114).
Among the 120 children who were seronegative preweaning, 71 (59%) developed a parasitologically confirmed cryptosporidial infection during the follow-up, compared with 23/30 (77%) seropositive children (P = 0.076). The postinfection/end-of-follow-up seropositivity status was comparable in children with or without a positive preweaning sample (47% versus 40%, P = 0.507). Interestingly, however, all 11 children with parasitologically negative but serologically positive cryptosporidial infection were seronegative preweaning, indicating seroconversion following an infection missed by the study sampling.
Severity of infection and serological status in mothers and children.
Of the 94 children with cryptosporidiosis, 67 (71%) had an asymptomatic first infection, whereas 27 (29%) had diarrhea with the first infection. There was no difference in seropositivity between children who had asymptomatic (39/67, 58%) or symptomatic (12/27, 44%) infections (P = 0.258). Seropositive children with asymptomatic infection had higher gp15 IgG levels (752.6, 582.7–972.0) postinfection than those with symptomatic infections (448.1, 327.9–612.2), but this difference was not statistically significant (P = 0.060).
When the severity of infections were analyzed by maternal serological status, children of seronegative and seropositive mothers were equally likely to have a symptomatic infection (48% versus 52%, P = 0.720). However, children with a negative preweaning serum sample were almost twice as likely to have cryptosporidial diarrhea as children with a positive sample, although this difference was not statistically significant (32% versus 17%, P = 0.196).
Discussion
Antibodies to gp15 were evaluated within a quasi-experimental study investigating the protective efficacy of bottled and municipal water against cryptosporidial infections in children < 2 years of age.6,27 There are very few studies that have examined sera for antibodies against cryptosporidial proteins at multiple time points in children in whom longitudinal follow-up, with or without stool sampling for Cryptosporidium, has been undertaken. This study recruited exclusively breast-fed children and demonstrated a high correlation between seropositivity of mothers and their children in samples collected before weaning, indicating efficient transplacental transfer of antibodies as had been described in Brazilian infants,29 but differing from data in Bedouin infants where it was estimated that about one-third of the cryptosporidial antibodies were transplacental.30 However, the antigens used in the three studies were different, with cp23 antigen in Brazil, calf oocyst lysate in Bedouin infants, and gp15 in this study (Table 4). The gp15 and cp23 are sporozoite-derived antigens that are considered specific and immunodominant.14,17,29 It would be interesting to estimate and compare the two immunodominant antigens (gp15 and cp23) to better understand the serological response to cryptosporidial antigens, transplacentally and after an infection.
Table 4.
Selected serological studies with longitudinal follow-up or paired (pre- and postinfection) sampling
| Study site(s) | Year | Type of study | Age group(s) | Serological method | Antigen | Key outcomes | Reference |
|---|---|---|---|---|---|---|---|
| United States* | 1989 | Pre/post | 24–58 years | ELISA | Oocysts lysate | 32% had initial detectable levels of Cryptosporidium IgG | 31 |
| Seroconversion patterns for 6 weeks (5%), 1 year (14%), and 2 years (13.6%) | |||||||
| 1 year group: seropositivity increased from 27% to 39% after 1 year, 41% had detectable levels at different sampling times | |||||||
| 2 year group: seropositivity increased from 36% to 73% after 1 year, 82% had detectable levels at different sampling times | |||||||
| Manila, Philippines | 1990 | Longitudinal | 1–24 months | ELISA | Oocysts lysate | No increase in antibody levels after 1–6 weeks follow-up | 32 |
| Melbourne, Australia, and Goroka, Papua New Guinea | 1994 | Longitudinal | 1–84 months | ELISA | Oocysts lysate | Antibodies peak 3–6 weeks after infection and fell to baseline levels by 6 months | 21 |
| Seropositivity rose from 15% (< 6 months) to 64% (> 2 years) in Papua New Guinea | |||||||
| Seropositivity rose from 3% (< 6 months) to 11% (> 2 years) in Melbourne | |||||||
| Texas, United States | 1997 | Pre/post (longitudinal sampling) | 20–45 years | Immunoblot and ELISA | 15/17 and 27 kDa | Fewer oocysts were excreted by volunteers with preexisting IgG antibodies to 27-kDa antigen compared with volunteers without the antibody | 33 |
| IgG reactivity to the 17-kDa antigen was higher in asymptomatic than symptomatic infected volunteers | |||||||
| IgG reactivity to the three antigens peaked by day 32 postinoculation | |||||||
| Oregon, United States | 1998 | Pre/post | 18–60 years | Western blot | 15/17 and 27 kDa | Mean antibody level after 2 years remained at 91% of the initial value for the 15/17-kDa antigen | 34 |
| Mean antibody level after 2 years declined to 54% of the initial value for the 27-kDa antigen | |||||||
| Negev, Israel | 2001 | Longitudinal | 0–2 years | ELISA | Oocysts lysate | Infants had one-third the level of antibodies found in mothers | 30 |
| Level of IgG antibodies dropped significantly by 6 months of age | |||||||
| Seroconversion rate of 42% to Cryptosporidium around 6–23 months of age | |||||||
| Seroprevalence 13% (< 5 years), 38% (5–13 years), and 58% (14–21 years) | |||||||
| Cryptosporidium antigen detected in 11% (6 months) and 48% (23 months) | |||||||
| Texas, United States | 2004 | Pre/post | 18–45 years | ELISA | TRAP-C1 | Uninfected individuals showed higher reactivity at baseline compared with infected individuals | 35 |
| Increase in antibody response was seen in days 30 and 45 compared with days 0 and 5 | |||||||
| Dhaka, Bangladesh | 2004 | Case–control (pre/post sampling) | ≤ 5 years | ELISA | Oocysts lysate | 64% seropositivity in cases and 57% in controls | 36 |
| Significant increase in IgG levels in cases compared with controls in follow-up | |||||||
| Lima, Peru | 2006 | Pre/post | 1 month–10 years | ELISA | 17 and 27 kDa | Peak antibody detection was at 15.3 and 26.7 months of age | 18 |
| Antibody levels were higher during the second serological response | |||||||
| Antibody response increases with age and infection experience | |||||||
| Dhaka, Bangladesh | 2011 | Case–control (pre/post sampling) | 15 days–60 months | ELISA | gp15 | Increase in follow-up IgG levels significantly greater in cases than controls | 19 |
| Significant increase in IgG levels response to Cryptosporidium parvum and Cryptosporidium hominis gp15 | |||||||
| Vellore, India | 2011 | Longitudinal | Birth–3 years | ELISA | gp15 | Increase in serum IgG levels after first episode of cryptosporidial diarrhea | 17 |
| Peak response between 8 and 11 weeks (∼9 weeks) postexposure | |||||||
| Serological response to infection did not depend on baseline values | |||||||
| Dhaka, Bangladesh | 2012 | Case–control (pre/post sampling) | 15 days–60 months | ELISA | Cp23 | Increase in follow-up IgG, IgM, IgA levels significantly greater in cases than controls | 20 |
| Cases with acute diarrhea had significantly greater serum IgA and IgM responses than those with persistent diarrhea | |||||||
| Vellore, India | 2012 | Longitudinal | 0–2 years | ELISA | gp15 | Seropositivity: maternal (89.2%), child at 3.5 months (31.8%), and child at 2 years (94.6%) | 37 |
| No difference in serum IgG levels in mothers and children between cases and controls | |||||||
| 76.7% remained seropositive or seroconverted at 9 months | |||||||
| Seroconversion at 9 months irrespective of the serological status of the mother | |||||||
| Leogane, Haiti | 2014 | Longitudinal | 3 weeks–11.5 years | Multiplex bead assay | 17 and 27 kDa | 97.9% had one serologically positive episode throughout the 10-year period | 38 |
| 28.9% had secondary Cryptosporidium IgG response | |||||||
| IgG responses to Cryptosporidium tend to increase with age |
ELISA = enzyme-linked immunosorbent assay; IgG = immunoglobulin G; “TRAP-C1 = thrombospondin-related adhesive protein of Cryptosporidium-1.
Samples obtained from U.S. Peace Corps volunteers before and after service posting in western Africa.
Previous studies have shown that most maternally acquired antibodies fall to low levels by the 6th month of life, rendering 60–80% of children seronegative.29,30 Also, antibody responses to cryptosporidial gp15 antigen have been predicted to peak at approximately 3–9 weeks after an episode of cryptosporidial diarrhea and wanes to baseline levels by 5–6 months.17,21,22 In this study, however, only one-fourth of children of seropositive mothers had detectable antibodies in their preweaning samples, although they were collected within the first 4 months of birth. Moreover, only 54% children with a parasitologically confirmed cryptosporidial infection had detectable antibodies postinfection, despite samples being collected within 9 weeks of infection (Figure 2 ). Striking difference in seropositivity was noticed depending on when the preweaning or the postinfection sample was collected. Children in whom preweaning/postinfection samples were collected earlier were more likely to be seropositive. Taken together, these observations suggest that cryptosporidial antibodies possibly wane much faster than what was previously believed. A half-life of 12 weeks has been estimated for antibodies to the 27- and 17-kDa (gp15) cryptosporidial antigens in adults12; but there is no information on the half-life of the anti-gp15 IgG antibodies in children in developing countries and whether it differs from that in adults.
Figure 2.
Flowchart showing the serological status of mothers and children (pre- and postweaning). Infections identified by stool polymerase chain reaction (PCR) or serology are shown below the dashed line for each category.
After the loss of maternal or preweaning antibodies, about half of the study children who were exposed to cryptosporidial antigen did not develop or sustain an immune response detectable in the postinfection or end-of-follow-up sample. The lack of detection of antibodies might have been either because no immune response was mounted by the host, with Cryptosporidium detection by PCR not indicating replicating parasites, but presence in the gut possibly as part of an extremely contaminated environment, or because the immune response was short lived and the timing of sample collection was too late to detect antibodies. The presence of cryptosporidial antibodies might be expected to prevent binding of Cryptosporidium to the intestinal epithelium and therefore replication in the gut.15,23,33,39
At the end of 2 years, 41% of children were seropositive in this study, which was similar to the study in a Brazilian peri-urban area where 41.2% seropositivity was seen in children aged between 0 and 4 years.40 Several other studies have reported seropositivity to Cryptosporidium ranging from 46% to 89%,29,30,36,40–44 and this may depend on several factors involving the level of exposure affected by sanitation and hygiene, drinking water, food, socioeconomic status, environmental conditions and animal exposure, inter-study and population differences and, more importantly, variations in the ELISA procedures (cryptosporidial antigens [crude oocysts versus sporozoite antigens], serum dilutions, and methods used in the calculation of antibody titers) (Table 4). Previous studies in the same study area used (r)gp15 that was expressed in the pET 32/Xa/LIC vector (Novagen, EMD Biosciences, Inc., Merck KGaA, Darmstadt, Germany), which encodes a fusion tag including thioredoxin, S-tag, and His tag.17,37 To control for the possibility of nonspecific antibody responses to the fusion tags, a recombinant control protein containing only the fusion was also used, and the OD from the control protein was subtracted from that of the (r)gp15 protein and a semiquantitative assay with antibody levels calculated based on OD values was used. By contrast, this study used (r)gp15 expressed in the pET46 vector (Novagen, EMD Biosciences, Inc., Merck KGaA, Darmstadt, Germany), which encodes only a 6-His tag. Since human sera did not react with the His tag alone, a control protein was not used. Compared with the previous study, using this modified assay there were marked differences with 94.6% of children seropositive by 2 years of age in the earlier study compared with 41% in this study. Maternal seropositivity was also low at 52% in this study, compared with 89% in the previous study.37 It is probable that nonspecific antibody responses were reduced significantly by using the 6-His tag–encoded(r)gp15 antigen, therefore resulting in a more specific quantitative assay for measuring gp15 antibody levels.
In this study, the proportion of cryptosporidial infections was slightly higher in children of seronegative mothers compared with children of seropositive mothers (67% versus 59%). Also, children who were seronegative preweaning were slightly more susceptible to symptomatic infections than seropositive children (32% versus 17%). Although not a marked effect, these observations might indicate partial protection by preexisting antibodies.39 Higher antibody levels in asymptomatic compared with symptomatic children could be due to the booster effect of secondary exposures to the cryptosporidial antigen.33,38
In conclusion, our study demonstrated serological evidence of frequent exposure to Cryptosporidium spp. in early life, which may persist into adulthood, as demonstrated by the acquisition and increase in antibodies in a majority of children and the finding of antibodies to gp15 in mothers. However, the maternal antibody status did not influence acquisition or severity of infection or antibody levels in children. Antibodies also appeared to wane rapidly in children, but because there was limited follow-up of children after the second blood sample, the level of protection from infection or disease in children with and without antibodies cannot be determined. The modified assay used in this study demonstrated a more specific antibody response toward the cryptosporidial gp15 antigen. Prospective future studies with longitudinal sampling methods that combine parasitological and serological data would enable us to better understand the correlates of protection against cryptosporidial infections.
ACKNOWLEDGMENTS
We thank the parents and children from the semi-urban slums of Vellore for their participation and support. We also thank the field workers for their monitoring of the cohort and the support staff of the Wellcome Trust Research Laboratory, Division of Gastrointestinal Sciences, Christian Medical College, Vellore, India, for their help.
Footnotes
Financial support: This work was supported by National Institute of Allergy and Infectious Diseases (grant nos. R01 A1075452 to Gagandeep Kang). Robin P. Lazarus and Rajiv Sarkar were supported by a Fogarty International Center training grant (no. D43 TW007392 to Gagandeep Kang).
Authors' addresses: Robin P. Lazarus, Sitara S. R. Ajjampur, Rajiv Sarkar, Jayanthy C. Geetha, Ashok D. Prabakaran, Vasanth Velusamy, and Gagandeep Kang, Division of Gastrointestinal Sciences, Christian Medical College, Tamil Nadu, India, E-mails: robin.l@cmcvellore.ac.in, sitararao@cmcvellore.ac.in, rsarkar@cmcvellore.ac.in, jayanthyraghav@gmail.com, ashokdani@gmail.com, veluvasanth.cmc@gmail.com, and gkang@cmcvellore.ac.in. Elena N. Naumova, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, E-mail: elena.naumova@tufts.edu. Honorine D. Ward, Division of Geographic Medicine and Infectious Diseases, Department of Medicine, Tufts-New England Medical Center, Boston, MA, E-mail: hward@tuftsmedicalcenter.org.
References
- 1.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fayer R, Morgan U, Upton SJ. Epidemiology of Cryptosporidium: transmission, detection and identification. Int J Parasitol. 2000;30:1305–1322. doi: 10.1016/s0020-7519(00)00135-1. [DOI] [PubMed] [Google Scholar]
- 3.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 4.Smith HV, Rose JB. Waterborne cryptosporidiosis: current status. Parasitol Today. 1998;14:14–22. doi: 10.1016/s0169-4758(97)01150-2. [DOI] [PubMed] [Google Scholar]
- 5.LeChevallier MW, Norton WD, Lee RG. Occurrence of Giardia and Cryptosporidium spp. in surface water supplies. Appl Environ Microbiol. 1991;57:2610–2616. doi: 10.1128/aem.57.9.2610-2616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkar R, Ajjampur SS, Prabakaran AD, Geetha JC, Sowmyanarayanan TV, Kane A, Duara J, Muliyil J, Balraj V, Naumova EN, Ward H, Kang G. Cryptosporidiosis among children in an endemic semiurban community in southern India: does a protected drinking water source decrease infection? Clin Infect Dis. 2013;57:398–406. doi: 10.1093/cid/cit288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber R, Bryan RT, Bishop HS, Wahlquist SP, Sullivan JJ, Juranek DD. Threshold of detection of Cryptosporidium oocysts in human stool specimens: evidence for low sensitivity of current diagnostic methods. J Clin Microbiol. 1991;29:1323–1327. doi: 10.1128/jcm.29.7.1323-1327.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajjampur SS, Gladstone BP, Selvapandian D, Muliyil JP, Ward H, Kang G. Molecular and spatial epidemiology of cryptosporidiosis in children in a semiurban community in south India. J Clin Microbiol. 2007;45:915–920. doi: 10.1128/JCM.01590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao L, Escalante L, Yang C, Sulaiman I, Escalante AA, Montali RJ, Fayer R, Lal AA. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl Environ Microbiol. 1999;65:1578–1583. doi: 10.1128/aem.65.4.1578-1583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awad-el-Kariem FM, Robinson HA, Dyson DA, Evans D, Wright S, Fox MT, McDonald V. Differentiation between human and animal strains of Cryptosporidium parvum using isoenzyme typing. Parasitology. 1995;110:129–132. doi: 10.1017/s0031182000063885. [DOI] [PubMed] [Google Scholar]
- 11.Nagamani K, Pavuluri PR, Gyaneshwari M, Prasanthi K, Rao MI, Saxena NK. Molecular characterisation of Cryptosporidium: an emerging parasite. Indian J Med Microbiol. 2007;25:133–136. doi: 10.4103/0255-0857.32719. [DOI] [PubMed] [Google Scholar]
- 12.Priest JW, Li A, Khan M, Arrowood MJ, Lammie PJ, Ong CS, Roberts JM, Isaac-Renton J. Enzyme immunoassay detection of antigen-specific immunoglobulin g antibodies in longitudinal serum samples from patients with cryptosporidiosis. Clin Diagn Lab Immunol. 2001;8:415–423. doi: 10.1128/CDLI.8.2.415-423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Priest JW, Bern C, Roberts JM, Kwon JP, Lescano AG, Checkley W, Cabrera L, Moss DM, Arrowood MJ, Sterling CR, Gilman RH, Lammie PJ. Changes in serum immunoglobulin G levels as a marker for Cryptosporidium sp. infection in Peruvian children. J Clin Microbiol. 2005;43:5298–5300. doi: 10.1128/JCM.43.10.5298-5300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Priest JW, Kwon JP, Moss DM, Roberts JM, Arrowood MJ, Dworkin MS, Juranek DD, Lammie PJ. Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigens. J Clin Microbiol. 1999;37:1385–1392. doi: 10.1128/jcm.37.5.1385-1392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cevallos AM, Zhang X, Waldor MK, Jaison S, Zhou X, Tzipori S, Neutra MR, Ward HD. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect Immun. 2000;68:4108–4116. doi: 10.1128/iai.68.7.4108-4116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perryman LE, Jasmer DP, Riggs MW, Bohnet SG, McGuire TC, Arrowood MJ. A cloned gene of Cryptosporidium parvum encodes neutralization-sensitive epitopes. Mol Biochem Parasitol. 1996;80:137–147. doi: 10.1016/0166-6851(96)02681-3. [DOI] [PubMed] [Google Scholar]
- 17.Ajjampur SS, Sarkar R, Allison G, Banda K, Kane A, Muliyil J, Naumova E, Ward H, Kang G. Serum IgG response to Cryptosporidium immunodominant antigen gp15 and polymorphic antigen gp40 in children with cryptosporidiosis in south India. Clin Vaccine Immunol. 2011;18:633–639. doi: 10.1128/CVI.00464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Priest JW, Bern C, Xiao L, Roberts JM, Kwon JP, Lescano AG, Checkley W, Cabrera L, Moss DM, Arrowood MJ, Sterling CR, Gilman RH, Lammie PJ. Longitudinal analysis of Cryptosporidium species-specific immunoglobulin G antibody responses in Peruvian children. Clin Vaccine Immunol. 2006;13:123–131. doi: 10.1128/CVI.13.1.123-131.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allison GM, Rogers KA, Borad A, Ahmed S, Karim MM, Kane AV, Hibberd PL, Naumova EN, Calderwood SB, Ryan ET, Khan WA, Ward HD. Antibody responses to the immunodominant Cryptosporidium gp15 antigen and gp15 polymorphisms in a case-control study of cryptosporidiosis in children in Bangladesh. Am J Trop Med Hyg. 2011;85:97–104. doi: 10.4269/ajtmh.2011.11-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borad AJ, Allison GM, Wang D, Ahmed S, Karim MM, Kane AV, Moy J, Hibberd PL, Ajjampur SS, Kang G, Calderwood SB, Ryan ET, Naumova E, Khan WA, Ward HD. Systemic antibody responses to the immunodominant p23 antigen and p23 polymorphisms in children with cryptosporidiosis in Bangladesh. Am J Trop Med Hyg. 2012;86:214–222. doi: 10.4269/ajtmh.2012.11-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groves VJ, Lehmann D, Gilbert GL. Seroepidemiology of cryptosporidiosis in children in Papua New Guinea and Australia. Epidemiol Infect. 1994;113:491–499. doi: 10.1017/s0950268800068503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar R, Ajjampur SS, Ward HD, Kang G, Naumova EN. Analysis of human immune responses in quasi-experimental settings: tutorial in biostatistics. BMC Med Res Methodol. 2012;12:1. doi: 10.1186/1471-2288-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borad A, Ward H. Human immune responses in cryptosporidiosis. Future Microbiol. 2010;5:507–519. doi: 10.2217/fmb.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theodos CM. Innate and cell-mediated immune responses to Cryptosporidium parvum. Adv Parasitol. 1998;40:87–119. doi: 10.1016/s0065-308x(08)60118-9. [DOI] [PubMed] [Google Scholar]
- 25.Preidis GA, Wang HC, Lewis DE, Castellanos-Gonzalez A, Rogers KA, Graviss EA, Ward HD, White AC., Jr Seropositive human subjects produce interferon gamma after stimulation with recombinant Cryptosporidium hominis gp15. Am J Trop Med Hyg. 2007;77:583–585. [PubMed] [Google Scholar]
- 26.WHO e-Library of Evidence for Nutrition Actions (eLENA) 2015. http://www.who.int/elena/titles/exclusive_breastfeeding/en/ Available at. Accessed April 30, 2015.
- 27.Sarkar R, Sivarathinaswamy P, Thangaraj B, Sindhu KN, Ajjampur SS, Muliyil J, Balraj V, Naumova EN, Ward H, Kang G. Burden of childhood diseases and malnutrition in a semi-urban slum in southern India. BMC Public Health. 2013;13:87. doi: 10.1186/1471-2458-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris SS, Cousens SN, Lanata CF, Kirkwood BR. Diarrhoea—defining the episode. Int J Epidemiol. 1994;23:617–623. doi: 10.1093/ije/23.3.617. [DOI] [PubMed] [Google Scholar]
- 29.Cox MJEK, Massad E, Azevedo RS. Age-specific seroprevalence to an immunodominant Cryptosporidium sporozoite antigen in a Brazilian population. Epidemiol Infect. 2005;133:951–956. doi: 10.1017/S0950268805004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robin GFD, Orr N, Sela T, Slepon R, Ambar R, Dagan R, Le Blancq S, Deckelbaum RJ, Cohen D. Cryptosporidium infection in Bedouin infants assessed by prospective evaluation of anticryptosporidial antibodies and stool examination. Am J Epidemiol. 2001;153:194–201. doi: 10.1093/aje/153.2.194. [DOI] [PubMed] [Google Scholar]
- 31.Ungar BL, Mulligan M, Nutman TB. Serologic evidence of Cryptosporidium infection in US volunteers before and during Peace Corps service in Africa. Arch Intern Med. 1989;149:894–897. [PubMed] [Google Scholar]
- 32.Laxer MA, Alcantara AK, Javato-Laxer M, Menorca DM, Fernando MT, Ranoa CP. Immune response to cryptosporidiosis in Philippine children. Am J Trop Med Hyg. 1990;42:131–139. doi: 10.4269/ajtmh.1990.42.131. [DOI] [PubMed] [Google Scholar]
- 33.Moss DM, Chappell CL, Okhuysen PC, DuPont HL, Arrowood MJ, Hightower AW, Lammie PJ. The antibody response to 27-, 17-, and 15-kDa Cryptosporidium antigens following experimental infection in humans. J Infect Dis. 1998;178:827–833. doi: 10.1086/515377. [DOI] [PubMed] [Google Scholar]
- 34.Frost FJ, Calderon RL, Muller TB, Curry M, Rodman JS, Moss DM, de la Cruz AA. A two-year follow-up survey of antibody to Cryptosporidium in Jackson County, Oregon following an outbreak of waterborne disease. Epidemiol Infect. 1998;121:213–217. doi: 10.1017/s095026889800898x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okhuysen PC, Chappell CL, Crabb JH, Sterling CR, DuPont HL. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J Infect Dis. 1999;180:1275–1281. doi: 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- 36.Khan WA, Rogers KA, Karim MM, Ahmed S, Hibberd PL, Calderwood SB, Ryan ET, Ward HD. Cryptosporidiosis among Bangladeshi children with diarrhea: a prospective, matched, case-control study of clinical features, epidemiology and systemic antibody responses. Am J Trop Med Hyg. 2004;71:412–419. [PubMed] [Google Scholar]
- 37.Sarkar RAS, Muliyil J, Ward H, Naumova EN, Kang G. Serum IgG responses and seroconversion patterns to Cryptosporidium gp15 among children in a birth cohort in south India. Clin Vaccine Immunol. 2012;19:849–854. doi: 10.1128/CVI.00051-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moss DM, Priest JW, Hamlin K, Derado G, Herbein J, Petri WA, Jr, Lammie PJ. Longitudinal evaluation of enteric protozoa in Haitian children by stool exam and multiplex serologic assay. Am J Trop Med Hyg. 2014;90:653–660. doi: 10.4269/ajtmh.13-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chappell CL, Okhuysen PC, Sterling CR, Wang C, Jakubowski W, Dupont HL. Infectivity of Cryptosporidium parvum in healthy adults with pre-existing anti-C. parvum serum immunoglobulin G. Am J Trop Med Hyg. 1999;60:157–164. doi: 10.4269/ajtmh.1999.60.157. [DOI] [PubMed] [Google Scholar]
- 40.Teixeira MC, Barreto ML, Melo C, Silva LR, Moraes LR, Alcantara-Neves NM. A serological study of Cryptosporidium transmission in a periurban area of a Brazilian northeastern city. Trop Med Int Health. 2007;12:1096–1104. doi: 10.1111/j.1365-3156.2007.01892.x. [DOI] [PubMed] [Google Scholar]
- 41.McDonald AC, Mac Kenzie WR, Addiss DG, Gradus MS, Linke G, Zembrowski E, Hurd MR, Arrowood MJ, Lammie PJ, Priest JW. Cryptosporidium parvum-specific antibody responses among children residing in Milwaukee during the 1993 waterborne outbreak. J Infect Dis. 2001;183:1373–1379. doi: 10.1086/319862. [DOI] [PubMed] [Google Scholar]
- 42.Leach CT, Koo FC, Kuhls TL, Hilsenbeck SG, Jenson HB. Prevalence of Cryptosporidium parvum infection in children along the Texas-Mexico border and associated risk factors. Am J Trop Med Hyg. 2000;62:656–661. doi: 10.4269/ajtmh.2000.62.656. [DOI] [PubMed] [Google Scholar]
- 43.Frost FJ, Kunde TR, Muller TB, Craun GF, Katz LM, Hibbard AJ, Calderon RL. Serological responses to Cryptosporidium antigens among users of surface- vs. ground-water sources. Epidemiol Infect. 2003;131:1131–1138. doi: 10.1017/s0950268803001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zu SXLJ, Barrett LJ, Fayer R, Shu SY, McAuliffe JF, Roche JK, Guerrant RL. Seroepidemiologic study of Cryptosporidium infection in children from rural communities of Anhui, China and Fortaleza, Brazil. Am J Trop Med Hyg. 1994;51:1–10. doi: 10.4269/ajtmh.1994.51.1. [DOI] [PubMed] [Google Scholar]