Abstract
Malaria disproportionately affects young children. Clinical trials in African children showed that dihydroartemisinin–piperaquine (DP) is an effective antimalarial and has a longer posttreatment prophylactic (PTP) effect against reinfections than other artemisinin-based combination therapies, including artemether–lumefantrine (AL). Using a previously developed Markov model and individual patient data from a multicenter African drug efficacy trial, we assessed the economic value of the PTP effect of DP versus AL in pediatric malaria patients from health-care provider's perspective in low-to-moderate and moderate-to-high transmission settings under different drug co-payment scenarios. In low-to-moderate transmission settings, first-line treatment with DP was highly cost-effective with an incremental cost-effectiveness ratio of US$5 (95% confidence interval [CI] = −76 to 196) per disability-adjusted life year (DALY) averted. In moderate-to-high transmission settings, DP first-line treatment led to a mean cost saving of US$1.09 (95% CI = −0.88 to 3.85) and averted 0.05 (95% CI = −0.08 to 0.22) DALYs per child per year. Our results suggested that DP might be superior to AL for first-line treatment of uncomplicated childhood malaria across a range of transmission settings in Africa.
Introduction
The burden of Plasmodium falciparum malaria remains high, disproportionally affecting children under 5 years of age with little or no immunity in sub-Saharan Africa.1 Prompt and effective oral antimalarial treatment can help achieve parasitological cure and prevent complications including death in malaria patients, and may reduce onward malaria transmission. Artemisinin-based combination therapies (ACTs) are the preferred choice for first-line treatment of uncomplicated P. falciparum malaria to reduce morbidity and mortality, and slow the emergence and spread of antimalarial drug resistance. The WHO-recommended list includes five ACTs: artemether–lumefantrine (AL), artesunate–amodiaquine (AS–AQ), artesunate–mefloquine (AS–MQ), artesunate–sulfadoxine–pyrimethamine (AS–SP), and dihydroartemisinin–piperaquine (DP).2 Of these ACTs, AL is the most widely used and heavily subsidized co-formulated drug in malaria-endemic countries.3 DP is the latest addition to the WHO-recommended ACTs and is being increasingly adopted by African countries as a first- or second-line antimalarial treatment following its regulatory approval by the European Medical Agency in 2011.4
Large-scale multicenter trials showed that DP is as efficacious and safe as AL in the initial treatment of uncomplicated P. falciparum malaria in African children in a wide range of transmission settings.5,6 Potential advantages of DP over AL include a simplified three-dose (versus six-dose) treatment regimen and a reduction in early posttreatment reinfection rates, confirmed by the reductions in overall treatment failure rates observed in several clinical trials.5–10 This clinically relevant benefit of DP over AL is likely to be due to the longer elimination half-life of piperaquine (2–3 weeks) compared with lumefantrine (3–6 days).11 A recent Cochrane review supported the growing evidence in African settings that DP has a longer posttreatment prophylactic (PTP) effect than AL, which may last up to 63 days after treatment.12
In a previous analysis, we demonstrated that DP was superior to AL from both the clinical and economic perspectives for first-line treatment of uncomplicated P. falciparum malaria in children in moderate-to-high transmission settings based on the pooled data on reinfection rates after treatment with DP and AL from a large drug efficacy trial in 12 sites distributed over seven African countries.13 During the preparation of this article, Okell and others14 published a model-based cost-effectiveness analysis, incorporating pharmacokinetic–pharmacodynamic factors and transmission-reducing effects of DP and AL, which confirmed our findings in such settings. In this follow-up study, we extended our previous analysis to assess how the economic value of the PTP effect varied with malaria transmission intensity from the health-care provider perspective, using individual patient data on reinfection rates after treatment with DP and AL from another large-scale pediatric trial conducted in five African countries (Burkina Faso, Kenya, Mozambique, Uganda, and Zambia).5
Methods
Decision analytical model.
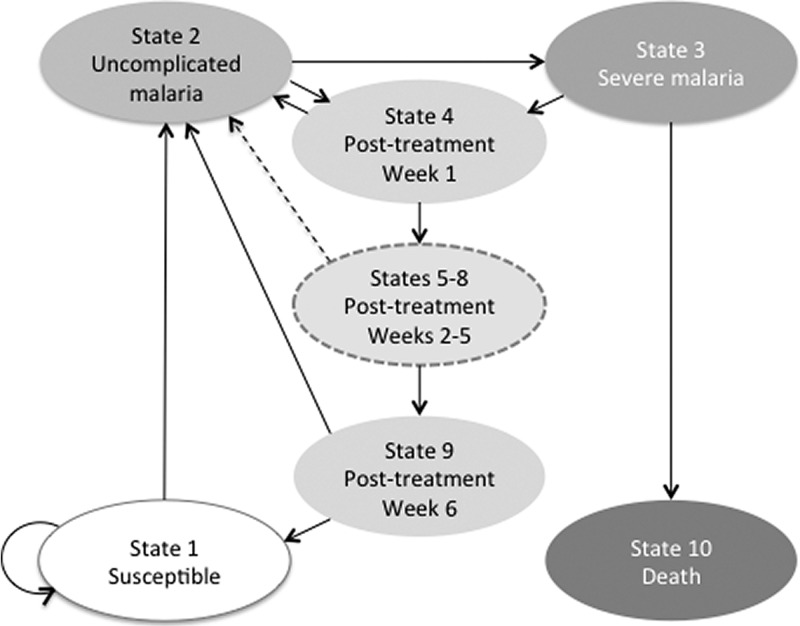
A previously developed Markov decision analytical model (TreeAge Pro 2014; TreeAge Software Inc., Williamstown, MA), which simulated the progression of malarial disease and the risk of recurrent malaria in children in weekly cycles over a period of 1 year, was used to perform this cost-effectiveness analysis (Figure 1 ).13 The Markov model had 10 mutually exclusive health states (State 1: susceptible; State 2: uncomplicated malaria; State 3:severe malaria; States 4–9: posttreatment from week 1 to week 6, respectively; and State 10: dead). The posttreatment states were subdivided into temporary states, known as tunnel states, which allowed the model to incorporate the time-dependent nature of the PTP effect of DP and AL after treatment. All model assumptions are summarized in Box 1.
Figure 1.
Markov model.
Box 1. Model assumptions.

The individual patient data on reinfection rates from a multicenter clinical trial of ACTs conducted in African children aged 6–59 months in a wide range of malaria transmission settings were used to calculate the transition probabilities for the Markov model.5 We assumed that higher rates of early treatment failure corresponded to a higher risk of reinfection after treatment and hence a higher transmission setting. The clinical and economic benefit of the PTP effect of DP is expected to increase in settings with higher risk of early reinfection after treatment. To assess the impact of reinfection risk, we used the Kaplan–Meier estimator to calculate the weekly hazard rates for recurrent malaria in settings with low-to-moderate treatment failure (day 42 treatment failure < 35%) and moderate-to-high treatment failure (day 42 treatment failure > 35%). The low-to-moderate failure scenario combined individual patient data from the four trial sites in Uganda, Kenya, Mozambique, and Zambia, whereas the moderate-to-high failure scenario used individual patient data from a single trial site in Burkina Faso (Supplemental Table 1). All Kaplan–Meier survival curves are provided in Supplemental Figure 1.
The follow-up period in the trial was 42 days. We, therefore, assumed conservatively that any difference in the weekly hazard rates for recurrent malaria between the two treatment groups would vanish 6 weeks after treatment, and that all treated children would be susceptible to reinfection based on the level of malaria endemicity in each transmission setting. Similar to the previous analysis, we estimated the hazard rate for uncomplicated malarial disease in susceptible children by taking the average of the estimated weekly hazard rates of recurrent malaria in both treatment arms at week 6 in each transmission setting. The estimated baseline and weekly reinfection hazard rates are listed in Supplemental Table 2.
Published estimates were used for the probability of developing severe malaria after failing first-line oral treatment,15 the case fatality rate for severe malaria after inpatient treatment, and the proportion of severe malaria survivors with persisting neurological sequelae in African settings16 (Table 1).
Table 1.
Markov model input variables
| Input variable | Distribution | Distribution parameters |
|---|---|---|
| Proportion of treated uncomplicated episodes progressing to severe malaria15 | Beta | 0.02 (α = 3; β = 156) |
| Proportion of severe malaria survivors having persisting NS16 | Beta | 0.00995 (α = 27; β = 2,686) |
| Case fatality rate for severe malaria after inpatient care16 | Beta | 0.109 (α = 297; β = 2,416) |
| Disability weight for treated uncomplicated and severe malaria18 | Point estimate | 0.211 |
| Disability weight for permanent NS18 | Point estimate | 0.436 |
| Uncomplicated malaria treatment | ||
| DP cost per course of treatment20 | Uniform | Min-max: 0.66–0.93 |
| AL cost per course of treatment20 | Uniform | Min-max: 0.43–0.83 |
| Severe malaria treatment (inpatient care) | ||
| Cost of drugs per child21 | Uniform | Min-max: 3.65–4.90 |
| Cost of diagnostic investigations21 | Uniform | Min-max: 6.98–31.31 |
| Cost of hospital bed day21 | Point estimate | 11.52 |
| Length of hospital stay (days) when patient recovers fully24 | Triangle | Mode 4.5 (min-max: 3–7) |
| Length of hospital stay (days) when patient recovers with NS23 | Triangle | Mode 10 (min-max: 8–12) |
| Length of hospital stay (days) when patient dies (assumed) | Triangle | Mode 2 (min-max: 1.6–2.4) |
AL = artemether–lumefantrine; DP = dihydroartemisinin piperaquine; Max = maximum; Min = minimum; NS = neurological sequelae.
All costs are in U.S. dollars for the year 2013.
We estimated the mean difference in costs (incremental costs) and the mean difference in health outcomes (incremental health outcomes) of the two treatment strategies per patient over a 1-year period. Incremental cost-effectiveness ratios (ICERs), which are the ratio of the incremental costs to the incremental health outcomes, were calculated in U.S. dollars (US$) for the year 2013. All input parameters, their distributions, and data sources are listed in Table 1.
Estimating health outcomes.
The model began with all children in the susceptible state. During each weekly cycle, susceptible children were subjected to a baseline hazard rate for uncomplicated malarial disease and flowed through the subsequent health states. Those not contracting malaria remained in the susceptible state. We assumed that all children with uncomplicated malaria received prompt treatment with DP or AL, and would either recover and enter the posttreatment states, or develop severe malaria. Depending on the choice of antimalarial drug, children in the posttreatment states were subjected to a weekly hazard rate for recurrent malaria during the first 6 weeks after the treatment (Supplemental Table 1). Children who remained free of recurrent malaria for 6 weeks after the treatment entered the susceptible state. We assumed that all children in the “severe malaria” state would receive inpatient care and a course of oral antimalarial treatment, and would either recover fully or with permanent neurologic sequelae and enter the posttreatment state for a period of 6 weeks, or die (dead).
We measured incremental health outcomes in terms of episodes of uncomplicated and severe malaria averted and disability-adjusted life years (DALYs) averted. DALYs are a composite health metric that combines years of life lost because of premature mortality with years of life lived with disability. An average life expectancy of 57.25 years for children aged 1–4 years is used based on the life tables for African men and women for the WHO subregion with high child and adult mortality.17 The disability weight for uncomplicated and severe episodes of malaria was both set at 0.211, and the disability weight for permanent treated neurological sequelae was at 0.436.18 DALYs were discounted at 3% as per recommendation of the WHO.19 Age weighting was not used; we valued a year of healthy life equally at all ages.
Estimating costs.
We considered the costs of oral antimalarial treatment of uncomplicated malaria episodes and the costs of inpatient treatment of severe malaria episodes over 1 year (Table 1). We excluded the costs of illness accruing to patients because of the assumed health-care provider perspective. In the baseline analysis, the price per course of treatment was US$0.66 and US$0.93 for children receiving three times 20/160 mg and three times 40/320 mg DP tablets, respectively, and US$0.43 and US$0.83 for children receiving six times 20/120 mg and six times 40/240 mg non-dispersible AL, respectively.20 We also considered a number of co-payment scenarios for DP and AL, which were set at 70%, 85%, and 97%, representing previously negotiated co-payment percentages for ACTs by the Affordable Medicines Facility-malaria for a number of African countries.20
We used the published estimates of inpatient care costs at primary level hospitals from a Kenyan costing study21 and adjusted the costs reported in 2005–2013 for inflation using the consumer price index.22 Drug and diagnostic investigation costs ranged between US$3.65–4.90 and US$6.98–31.31 per child, respectively. Costs per hospital stay per patient were calculated per day at a rate of US$11.52 per bed.21 The average length of hospital stay for a severe malaria patient varies based on the patient's health outcome. The average length of hospital stay was 4.5 days if the patient had a full recovery and 10 days if the patient recovered with neurologic sequelae.23,24 Most deaths in severe malaria patients occur within 24–48 hours of hospital admission. We, therefore, assumed an average hospital stay of 2 days if the patient died. Because the time horizon of the cost-effectiveness analysis was 1 year, costs warranted no discounting.
Sensitivity analysis.
We conducted probabilistic sensitivity analysis to assess the uncertainty in key model parameters and the robustness of the results to key model assumptions. Parameter ranges for disease input variables and their associated distributions were obtained from the published literature. Uncertainty in cost input parameters was captured using a simple uniform distribution between higher and lower values reported, since no information on the distribution was available. Monte Carlo simulation technique (10,000 iterations) was performed by selecting a value for each input variable from its distribution (Table 1) for each iteration. In addition, we conducted univariate sensitivity analyses to assess the relative contribution of key model parameters to uncertainty (Supplemental Figures 2 and 3).
Results
Table 2 shows the incremental outcomes of the cost-effectiveness analysis for first-line treatment with DP versus AL in low-to-moderate and moderate-to-high transmission settings. In the low-to-moderate treatment failure scenario, first-line treatment with DP compared with AL averted an estimated 0.22 (95% confidence interval [CI] = 0.17–0.27) episodes of uncomplicated malaria and 0.004 (95% CI = −0.003 to 0.014) episodes of severe malaria per child over 1 year. First-line treatment with DP resulted in a mean estimated health benefit of 0.01 (95% CI = −0.05 to 0.09) DALYs averted at additional cost of US$0.07 (95% CI = −1.07 to 1.12) per child over 1 year. The mean ICER of DP first-line treatment was thereby US$5 (95% CI = −76 to 196) per DALY averted. The health benefits of first-line treatment with DP increased in the moderate-to-high treatment failure scenario. In this scenario, first-line treatment with DP was estimated to avert, on average, 0.86 (95% CI = 0.47–1.31) episodes of uncomplicated malaria and 0.015 (95% CI = −0.001 to 0.047) episodes of severe malaria per child over a 1-year period. The estimated mean health benefit of first-line treatment with DP was 0.05 (95% CI = −0.08 to 0.22) DALYs averted per child and the estimated mean cost saving was US$1.09 (95% CI = −0.88 to 3.85) per child over a 1-year period. Figure 2 illustrates estimated incremental costs and DALYs averted per child per year across different malaria transmission settings. The results of the probabilistic sensitivity analysis showed that first-line treatment with DP was the economically dominant strategy (i.e., first-line treatment with DP is less costly and have better health outcomes than first-line treatment with AL) in approximately 48% (95% CI = 47–49%) and 72% (95% CI = 71–73%) of all iterations in the low-to-moderate and the moderate-to-high transmission scenarios, respectively (Supplemental Figures 4 and 5). The results of the univariate sensitivity analyses demonstrated that ICERs (US$ per DALY averted) are most sensitive to the drug costs of AL and DP in both transmission settings (Supplemental Figures 2 and 3).
Table 2.
Results of cost-effectiveness analysis
| Transmission setting | Low to moderate | Moderate to high |
|---|---|---|
| Mean (95% CI) | Mean (95% CI) | |
| Uncomplicated malaria episodes: DP treatment | 3.1 (2.7–3.5) | 3.9 (3.2–4.6) |
| Uncomplicated malaria episodes: AL treatment | 3.3 (2.9–3.8) | 4.8 (4.0–5.6) |
| Uncomplicated malaria episodes averted | 0.22 (0.17–0.27) | 0.86 (0.47–1.31) |
| Severe malaria episodes averted | 0.004 (−0.003 to 0.014) | 0.015 (−0.001 to 0.047) |
| Incremental costs | 0.07 (−1.07 to 1.12) | −1.09 (−3.85 to 0.88) |
| DALYs averted | 0.01 (−0.05 to 0.09) | 0.05 (−0.08 to 0.22) |
| ICER (US$ per DALY averted) | 5 (−76 to 196) | Dominant strategy |
AL = artemether–lumefantrine; CI = confidence interval; DALYs = disability-adjusted life years; DP = dihydroartemisinin–piperaquine; ICER = incremental cost-effectiveness ratio.
All values are calculated per child over 1 year and all costs are in U.S. dollars for the year 2013.
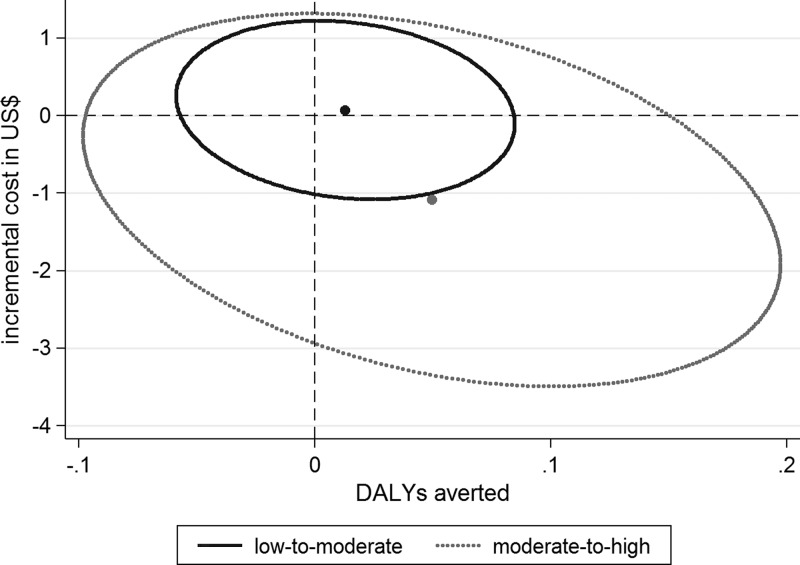
Figure 2.
Incremental costs and disability-adjusted life years (DALYs) averted across different malaria transmission settings (all costs are in U.S. dollars for the year 2013). The figure illustrates the mean number (dots) and 95% CI (ellipse) of DALYs averted and cost savings in U.S. dollars per child over a 1-year period.
Table 3 shows the effect of different co-payment percentages for DP and AL on the estimated mean cost savings per child per year across different transmission settings. In the low-to-moderate transmission setting, although first-line treatment with DP cost, on average, an additional US$0.07 (95% CI = −1.07 to 1.12) per child per year under no co-payment scenario, at 70%, 85%, and 97% co-payment, DP was a cost-saving treatment strategy with an estimated mean cost saving of US$0.19 (95% CI = −0.44 to 1.04), US$0.25 (95% CI = −0.33 to 1.10), and US$0.29 (95% CI = −0.28 to 1.13), respectively. In the moderate-to-high transmission setting scenario, the estimated mean cost saving with DP first-line treatment was US$1.09 (95% CI = −0.88 to 3.85) per child per year for no drug co-payment scenario, and the mean cost saving was US$1.14 (95% CI = −0.24–3.58), US$1.17 (95% CI = −0.15–3.54), and US$1.20 (95% CI = −0.11–3.60) per child per year at 70%, 85%, and 97% co-payment, respectively.
Table 3.
Cost savings under different drug co-payment scenarios across different malaria transmission setting
| Transmission setting | Low to moderate | Moderate to high |
|---|---|---|
| Mean cost saving per child per year (95% CI) | ||
| No co-payment | −0.07 (−1.12 to 1.07) | 1.09 (−0.88 to 3.85) |
| 70% co-payment | 0.19 (−0.44 to 1.04) | 1.14 (−0.24 to 3.58) |
| 85% co-payment | 0.25 (−0.33 to 1.10) | 1.17 (−0.15 to 3.54) |
| 97% co-payment | 0.29 (−0.28 to 1.13) | 1.20 (−0.11 to 3.60) |
CI = confidence interval.
All costs are in U.S. dollars for the year 2013. A negative value represents an additional cost per child per year.
Discussion
This analysis assessed the economic value of the PTP effect of DP versus AL for first-line treatment of uncomplicated P. falciparum malaria in African children in different malaria transmission settings and extended the results of our earlier analysis in moderate-to-high transmission settings to low-to-moderate transmission settings.13 We used a set of primary datasets from a large multicenter drug trial with a follow-up period of 42 days,5 whereas our previous analysis was based on a different multicenter trial with an extended follow-up period of 63 days.6 Our current analysis estimated that DP first-line treatment would result in, on average, an additional cost of US$0.07 per child per year in low-to-moderate transmission settings and a cost saving of US$1.09 per child per year in moderate-to-high transmission settings. Across these two transmission settings, the incremental health benefit was 0.01 and 0.05 DALYs averted per child per year for DP versus AL for first-line treatment of uncomplicated childhood malaria, respectively. These values are in line with our previous findings (a cost saving of US$0.96 and 0.03 DALYs averted per child per year in moderate-to-high transmission setting),13 representing lower and higher estimates for the estimated incremental costs and health benefits of first-line treatment with DP across transmission settings. With an ICER of US$5 per DALY averted, DP first-line treatment of uncomplicated P. falciparum malaria proved to be a highly cost-effective strategy in low-to-moderate transmission settings and an economically dominant strategy in moderate-to-high transmission settings. These results suggested that from an economic perspective DP might be superior to AL for first-line treatment of uncomplicated malaria in African children across a range of transmission settings.
Policy makers need to select optimal treatment strategies that suit the local malaria epidemiology and thus weigh the potential advantages of first-line treatment with DP, such as the PTP benefit after treatment, against the potential disadvantages, such as the costs and the resources required to make a change in first-line treatment policy for uncomplicated malaria. It is important to note that a higher rate of early treatment failure reflects a higher risk of reinfection after treatment, which might be due to a number of factors including local transmission rates that were highest at the clinical trial site representing our moderate-to-high reinfection scenario (Nanoro, Burkina Faso; EIR 100-160 reported for the year 2003).5 Besides transmission intensity, reinfection rates are likely to be influenced by local circumstances and personal behavior, such as availability and use of insecticide-treated bed nets and availability and accessibility of prompt malaria treatment for all age groups. Thus, local data on posttreatment failure rates should be considered before the implementation of DP first-line therapy for children with uncomplicated malaria.
Besides local treatment failure rates, drug prices are an important factor to be considered, which may vary substantially. Indeed, widely differing drug co-payment amounts have been previously negotiated for a number of African countries by the Global Fund.20 Our model incorporated treatment costs arising from different sources, including the cost of antimalarials and diagnostic procedures and the cost of inpatient care for severe malaria. Increasing drug co-payment percentages make first-line treatment with DP more cost saving because it is more expensive than AL per course of treatment. The impact of drug co-payment also varies between transmission settings; the higher the transmission intensity the greater the additional health benefits associated with the PTP effect of treatments. The effect of increasing drug co-payment percentages on cost savings is small in the moderate-to-high transmission setting (Table 3) because the difference in the drug costs per course of treatment is almost exactly offset by the difference in the number of malaria episodes estimated in the two treatment arms. It is important to note that we applied equal co-payment percentages to DP and AL, representing the currently negotiated and established figures for ACTs. If different co-payment percentages applied to DP and AL then their costs per course of treatment would gain considerable importance in the analysis of incremental costs.
Besides the parameters considered in our analysis, a number of additional factors may affect the overall benefit of first-line treatments for malaria, including semi-immunity in older children and adults, transmission-reducing effects of antimalarials,8 dosing modality and regimens, and resistance to artemisinin derivatives and their partner drugs. In the case of DP and AL, Mori and others25 reported a superior cost-effectiveness of DP treatment under the assumption of increased compliance owing to a simplified treatment regimen with DP. Although both treatments are 3-day oral regimens, DP is administered once daily whereas AL is given twice daily and ideally with a fatty meal to increase drug absorption. Therefore, DP can potentially improve patient compliance and treatment effectiveness and hence case management of pediatric patients.26 However, this potential benefit could not be evaluated in a clinical trial where drug intake was closely monitored and ensured5 and was not considered in our analysis, potentially underestimating the effectiveness of first-line treatment with DP over AL in usual care settings. Very recently, Okell and others14 published a detailed cost-effectiveness analysis of DP versus AL for first-line treatment of entire populations at risk in African countries, taking variations in transmission intensity and access to treatment. In line with the results of this analysis and our previous analysis, the authors concluded that the economic benefits of DP increased with increasing malaria transmission intensity, and longer acting ACTs should be targeted in areas with higher transmission. In areas with low transmission, the authors prioritized cheaper ACTs with gametocytocidal effects. Our analysis, which is limited to a pediatric population, bolsters our previous findings by showing that DP remains the preferred treatment option from an economic perspective for young African children under the age of 5 years for the treatment of uncomplicated malaria across all transmission settings.
This analysis has the same main limitation with our previous analysis that the longitudinal follow-up of patients is limited and the data on the long-term PTP effect of DP compared with AL is lacking. In this analysis, we assumed that the prophylactic effect of both DP and AL would vanish 6 weeks after treatment. Our primary dataset was limited to a follow-up period of 6 weeks. Starting from week 7 posttreatment, we assumed that equal reinfection hazard rates applied to both treatment arms. This might have led to an underestimation of the benefit of DP over AL because the prophylactic effect of DP may last up to 63 days after treatment. The only available study on the long-term prophylactic effect of DP versus AL treatment was conducted in a limited number of patients in an area with high malaria endemicity in Uganda, and reported a decreasing prophylactic benefit from DP treatment over time, suggesting that slowly eliminated antimalarial drugs could do little against the overwhelming risk of reinfection with malaria in such settings.27 We also did not consider the possibility that new infections were suppressed but not eliminated within the first 6 weeks after treatment. Although there is currently no evidence for this, if it were true this would have resulted in an overestimation of the real benefit of DP. It is important that the potential benefit of first-line treatment with DP and other slowly eliminated drugs be assessed and validated by clinical trials with longer-term follow-up periods.
The results of the currently available cost-effectiveness analyses suggest that DP can be the appropriate treatment option in patients of all ages living in areas of high malaria transmission and with high risk of early reinfection posttreatment. In settings with lower endemicity and reinfection risk, the use of DP might be restricted to patients with a higher risk of malaria morbidity, such as young pediatric malaria patients, which might lead to additional benefits within the context of drug resistance through the use of multiple first-line treatment strategies.28 A dispersible formulation of DP suitable for use in children between the ages of 6 months and 5 years is currently under development, and a targeted deployment of this drug can play an effective role in reducing the disease burden in children living in malaria-endemic areas.
Supplementary Material
ACKNOWLEDGMENTS
Johannes Pfeil is the recipient of a HRCMM (Heidelberg Research Center for Molecular Medicine) Career Development Fellowship. Quique Bassat has a fellowship from the program Miguel Servet of the ISCIII (Plan Nacional de I+D+I 2008-2011, grant no. CP11/00269). Ambrose Talisuna is supported by the MRC/DfID/Wellcome Trust Joint Global Health Trial scheme (grant no. MR/K007351/1).
Footnotes
Authors' addresses: Johannes Pfeil, Parasitology Unit, Department for Infectious Diseases, University Hospital Heidelberg, Heidelberg, Germany, and General Pediatrics Unit, Center for Childhood and Adolescent Medicine, University Hospital Heidelberg, Heidelberg, Germany, E-mail: johannes.pfeil@med.uni-heidelberg.de. Steffen Borrmann, Institute for Tropical Medicine, University of Tübingen, Tübingen, Germany, E-mail: sborrmann@me.com. Quique Bassat, ISGlobal, Barcelona Centre for International Health Research (CRESIB), Hospital Clínic—Universitat de Barcelona, Barcelona, Spain, E-mail: quique.bassat@isglobal.org. Modest Mulenga, Tropical Diseases Research Centre, Ndola, Zambia, E-mail: mulengam@tdrc.org.zm. Ambrose Talisuna, Department of Public Health Research, University of Oxford-KEMRI-Wellcome Trust Research Programme, Nairobi, Kenya, E-mail: atalisuna@kemri-wellcome.org. Yesim Tozan, College of Global Public Health, New York University, New York, NY, E-mail: tozan@nyu.edu.
References
- 1.World Health Organization World Malaria Report 2014. :2014. ISBN 9789241564830. [Google Scholar]
- 2.World Health Organization . Guidelines for the Treatment of Malaria. 2010. 2nd edition. [Google Scholar]
- 3.World Health Organization . Global Supply of Artemether-Lumefantrine before, during, and after the Memorandum of Understanding between WHO and Novartis. 2011. http://www.who.int/malaria/areas/treatment/MoU_termination_report_may2011.pdf Available at. [Google Scholar]
- 4.Ubben D, Poll EM. MMV in partnership: the Eurartesim® experience. Malar J. 2013;12:211. doi: 10.1186/1475-2875-12-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassat Q, Mulenga M, Tinto H, Piola P, Borrmann S, Menendez C, Nambozi M, Valea I, Nabasumba C, Sasi P, Bacchieri A, Corsi M, Ubben D, Talisuna A. Dihydroartemisinin-piperaquine and artemether-lumefantrine for treating uncomplicated malaria in African children: a randomised, non-inferiority trial. PLoS One. 2009;4:e7871. doi: 10.1371/journal.pone.0007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Four Artemisinin-Based Combinations Study Group A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med. 2011;8:e10011. doi: 10.1371/journal.pmed.1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamya MR, Yeka A, Bukirwa H, Lugemwa M, Rwakimari JB, Staedke SG, Talisuna AO, Greenhouse B, Nosten F, Rosenthal PJ, Wabwire-Mangen F, Dorsey G. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: a randomized trial. PLoS Clin Trials. 2007;2:e20. doi: 10.1371/journal.pctr.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawa P, Shekalaghe SA, Drakeley CJ, Sutherland CJ, Mweresa CK, Baidjoe AY, Manjurano A, Kavishe RA, Beshir KB, Yussuf RU, Omar SA, Hermsen CC, Okell L, Schallig HD, Sauerwein RW, Hallett RL, Bousema T. Malaria transmission after artemether-lumefantrine and dihydroartemisinin-piperaquine: a randomized trial. J Infect Dis. 2013;207:1637–1645. doi: 10.1093/infdis/jit077. [DOI] [PubMed] [Google Scholar]
- 9.Yeka A, Dorsey G, Kamya MR, Talisuna A, Lugemwa M, Rwakimari JB, Staedke SG, Rosenthal PJ, Wabwire-Mangen F, Bukirwa H. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treating uncomplicated malaria: a randomized trial to guide policy in Uganda. PLoS One. 2008;3:e2390. doi: 10.1371/journal.pone.0002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeka A, Tibenderana J, Achan J, D'Alessandro U, Talisuna AO. Efficacy of quinine, artemether-lumefantrine and dihydroartemisinin-piperaquine as rescue treatment for uncomplicated malaria in Ugandan children. PLoS One. 2013;8:e53772. doi: 10.1371/journal.pone.0053772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price RN, Douglas NM. Artemisinin combination therapy for malaria: beyond good efficacy. Clin Infect Dis. 2009;49:1638–1640. doi: 10.1086/647947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zani B, Gathu M, Donegan S, Olliaro PL, Sinclair D. Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst Rev. 2014;1:CD010927. doi: 10.1002/14651858.CD010927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfeil J, Borrmann S, Tozan Y. Dihydroartemisinin-piperaquine vs. artemether-lumefantrine for first-line treatment of uncomplicated malaria in African children: a cost-effectiveness analysis. PLoS One. 2014;9:e95681. doi: 10.1371/journal.pone.0095681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okell LC, Cairns M, Griffin JT, Ferguson NM, Tarning J, Jagoe G, Hugo P, Baker M, D'Alessandro U, Bousema T, Ubben D, Ghani AC. Contrasting benefits of different artemisinin combination therapies as first-line malaria treatments using model-based cost-effectiveness analysis. Nat Commun. 2014;5:5606. doi: 10.1038/ncomms6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubell Y, Dondorp A, Guérin PJ, Drake T, Meek S, Ashley E, Day NPJ, White NJ, White LJ. Artemisinin resistance—modelling the potential human and economic costs. Malar J. 2014;13:452. doi: 10.1186/1475-2875-13-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubell Y, Riewpaiboon A, Dondorp AM, von Seidlein L, Mokuolu OA, Nansumba M, Gesase S, Kent A, Mtove G, Olaosebikan R, Ngum WP, Fanello CI, Hendriksen I, Day NP, White NJ, Yeung S. Cost-effectiveness of parenteral artesunate for treating children with severe malaria in sub-Saharan Africa. Bull World Health Organ. 2011;89:504–512. doi: 10.2471/BLT.11.085878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez ADAO, Guillot M, Inoue M, Ferguson BD, Salomon JA. Life Tables for 191 Countries for 2000: Data, Methods, Results. Geneva, Switzerland: World Health Organization; 2001. (GPE discussion paper no. 40) Health Systems Performance Assessment Peer Review. Technical documentation. IV Outcomes: Population Health. [Google Scholar]
- 18.Murray C, Lopez A. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. 1st edition. Cambridge, MA: Harvard School of Public Health (ISBN-13: 978-0674354487); 1996. [Google Scholar]
- 19.Edejer TT, Baltussen R, Adam T, Hutubessy R, Acharya A, Evands DB, Murray CJL. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. Geneva, Switzerland: World Health Organization; 2003. p. 68. (ISBN 9241546018) [Google Scholar]
- 20.The Global Fund ACT Prices under the Affordable Medicines Facility-Malaria, Fact Sheet. 2013. http://www.theglobalfund.org/Documents/amfm/AMFm_ACTPrice_Factsheet_en/ Available at.
- 21.Ayieko P, Akumu AO, Griffiths UK, English M. The economic burden of inpatient paediatric care in Kenya: household and provider costs for treatment of pneumonia, malaria and meningitis. Cost Eff Resour Alloc. 2009;7:3. doi: 10.1186/1478-7547-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bureau of Labor Statistics, Consumer Price Index, CPI Inflation Calculator http://www.bls.gov/cpi/ Available at. Accessed January 29, 2014.
- 23.Goodman C, Mills A. Economic Analysis of Malaria Control in Sub-Saharan Africa. Geneva, Switzerland: Global Forum for Health Research; 2000. [Google Scholar]
- 24.Shillcutt S, Morel C, Goodman C, Coleman P, Bell D, Whitty CJ, Mills A. Cost-effectiveness of malaria diagnostic methods in sub-Saharan Africa in an era of combination therapy. Bull World Health Organ. 2008;86:101–110. doi: 10.2471/BLT.07.042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori AT, Ngalesoni F, Norheim OF, Robberstad B. Cost-effectiveness of dihydroartemisinin-piperaquine compared with artemether-lumefantrine for treating uncomplicated malaria in children at a district hospital in Tanzania. Malar J. 2014;13:363. doi: 10.1186/1475-2875-13-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ewing VL, Terlouw A, Kapinda A, Pace C, Richards E, Tolhurst R, Lalloo D. Perceptions and utilization of the anti-malarials artemether-lumefantrine and dihydroartemisinin-piperaquine in young children in the Chikhwawa District of Malawi: a mixed methods study. Malar J. 2015;14:13. doi: 10.1186/s12936-014-0528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arinaitwe E, Sandison TG, Wanzira H, Kakuru A, Homsy J, Kalamya J, Kamya MR, Vora N, Greenhouse B, Rosenthal PJ, Tappero J, Dorsey G. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for falciparum malaria: a longitudinal, randomized trial in young Ugandan children. Clin Infect Dis. 2009;49:1629–1637. doi: 10.1086/647946. [DOI] [PubMed] [Google Scholar]
- 28.Boni MF, Smith DL, Laxminarayan R. Benefits of using multiple first-line therapies against malaria. Proc Natl Acad Sci USA. 2008;105:14216–14221. doi: 10.1073/pnas.0804628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.