Abstract
Individual differences in subjective responses to alcohol consumption represent genetically-mediated biobehavioral mechanisms of alcoholism risk (i.e., endophenotype). The objective of this review is three-fold: (1) to provide a critical review the literature on subjective response to alcohol and to discuss the rationale for its conceptualization as an endophenotype for alcoholism; (2) to examine the literature on the neurobiological substrates and associated genetic factors subserving individual differences in subjective response to alcohol; and (3) to discuss the treatment implications of this approach and to propose a framework for conceptualizing, and systematically integrating, endophenotypes into alcoholism treatment.
Keywords: alcohol, subjective responses, endophenotypes, genetics, pharmacogenetics, behavioral marker, craving, alcoholism
Introduction
Alcohol is a commonly used addictive substance with multiple behavioral and neurobiological effects. As a psychoactive compound, it can elicit a spectrum of behavioral effects, which include gregariousness, anxiolysis, aggression, loss of executive functions and cognitive deficits. A host of pharmacokinetic factors (i.e., absorption, distribution in the tissues, and rate of metabolism - primarily in the liver) contribute to the intensity and duration of ethanol’s effects, whereas an array of pharmacodynamic factors, determine the behavioral and subjective effects of ethanol on the brain. The spectrum of subjective responses to alcohol is attributed to the ability of ethanol to inhibit or to activate multiple neural pathways. Specifically, how a person responds to alcohol will ultimately depend on how the neural pathways are organized in that individual and the extent to which certain pathways are inhibited or activated. It is well known that there is substantial variability in the subjective response to alcohol and that differences in the subjective experiences of ethanol’s effects appear to play a significant role in the predisposition to alcohol abuse1 and dependence (e.g., Schuckit & Smith, 1996; Schuckit et al., 1996).
In light of the significance of subjective response to alcohol as a putative biobehavioral marker of alcoholism risk2, the objective of this manuscript is three-fold. First is to provide a critical review the empirical literature on subjective response to alcohol and to discuss the rationale for its conceptualization as an endophenotype for alcoholism. Second is to examine the literature on the neurobiological substrates and associated genetic factors subserving individual differences in subjective response to alcohol. This will be done by systematically considering two important pharmacological processes relevant to alcohol’s biobehavioral effects, namely pharmacokinetics and pharmacodynamics. Third is to discuss the treatment implications of considering subjective responses to alcohol as endophenotypes and to propose a framework for conceptualizing and systematically integrating endophenotypes into treatment approaches for alcoholism. Finally, limitations and future directions will be discussed.
1. Subjective Responses to Alcohol as Endophenotypes
1.1. Subjective Responses to Alcohol
Individuals vary widely in their subjective experience of the pharmacological and neurobehavioral effects of alcohol upon its consumption. Pharmacological effects focus on the cellular and physiological effects of alcohol while subjective experiences focus on an individual’s self-reported perceptions of the effects of the substance. While some individuals may be more or less sensitive to the positively reinforcing and stimulant effects of alcohol, others report higher sensitivity to the aversive sedative effects. Recent research, primarily from alcohol administration studies, has documented the substantial variability in individuals’ subjective responses to alcohol and has shown that differences in these subjective experiences may play a significant role in the predisposition to alcohol use and misuse (e.g., Schuckit & Smith, 1996). Importantly, recent studies have shown that subjective response to alcohol represents a heritable phenotype (Heath & Martin, 1991; Viken et al., 2003).
So what constructs encompass subjective responses to alcohol? Schuckit and colleagues produced the early seminal work on the assessment of self-reported subjective response to alcohol by measuring self-reported subjective intoxication during alcohol administration sessions (i.e., alcohol challenge) (e.g., Schuckit, 1980). In the context of Schuckit’s work, the primary measure of subjective responses to alcohol is the Subjective High Assessment Scale (SHAS). The SHAS consists of various positive and negative mood-related adjectives, in addition to a single item ad-hoc scale of “feeling high.” Principal components analysis of the SHAS suggested that the “maximum terrible feelings” construct loaded into a first factor and accounted for 46% of the total variance (Schuckit, 1985), thereby suggesting that the SHAS may be most sensitive to the unpleasant effects of alcohol. Perhaps the most compelling evidence that subjective responses to alcohol predict alcohol use and misuse comes from a longitudinal study of sons of alcohol dependent probands and controls, suggesting that individuals who demonstrated low response to alcohol in the laboratory (measured by the SHAS) were significantly more likely to develop alcoholism at 8-year follow-up (Schuckit & Smith, 1996).
More recent work has suggested that alcohol’s pharmacological effects may be biphasic in nature (Earleywine, 1994a; Earleywine, 1994b; Earleywine & Martin, 1993; Erblich et al., 2003; Martin et al., 1993). Specifically, it has been documented that when blood alcohol levels are rising (i.e., the ascending limb of intoxication), alcohol produces robust stimulatory and other pleasurable subjective effects (Earleywine & Martin, 1993; Erblich et al., 2003). Conversely, when blood alcohol levels are declining (i.e., the descending limb of intoxication), alcohol’s effects are largely sedative and unpleasant (Earleywine & Martin, 1993; Erblich et al., 2003). This conceptualization of the effects of alcohol argues for the construct of subjective responses to be further parsed out into stimulant and sedative effects. Indeed, the Biphasic Alcohol Effects Scale (BAES; Earleywine & Martin, 1993) has been developed to directly assess the stimulant and sedative aspects of intoxication in alcohol administration studies. When subjective responses to alcohol are divided into stimulant and sedative effects, studies have shown that greater alcohol-induced stimulation and reinforcement is associated with increased alcohol consumption (Lewis & June, 1990; Wise & Bozarth, 1987), whereas greater subjective experiences of the sedative and unpleasant effects of alcohol are associated with decreased alcohol use (Leigh, 1987; O’Malley & Maisto, 1984).
In addition to the measures described above, various measures of mood states have been used in alcohol administration studies in order to capture the “mood-altering” effects of alcohol. Some of the most widely used measures of mood include the Profile of Mood States (POMS; McNair et al., 1971) and the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988). Although the concurrent use of these multiple measures provides more comprehensive assessment of individual differences in the subjective experience of alcohol consumption, they also raise issues regarding the core construct(s) of subjective responses to alcohol and how to best define it (them). Moreover, the multiple assessments of subjective intoxication further complicate the integration of findings in the alcohol administration literature.
In very global terms, the subjective effects of alcohol can be conceptualized in two broad domains, namely reinforcing (positively and negative) and punishing (aversive). However, these effects are not necessarily orthogonal to one another and may be concomitantly experienced to varying degrees within a single drinking episode. In order to empirically address the interrelationships among assessments of subjective intoxication used in alcohol administration studies, Table 1 presents previously unpublished data on the correlations among the SHAS, BAES, POMS and urge to drink (measured by the Alcohol Urge Questionnaire; AUQ, Bohn et al., 1995; MacKillop, 2006). These assessments were administered in the context of an intravenous alcohol administration study (n = 49; 23 women) of hazardous college drinkers. Pearson product-moment correlations are presented for the assessment point in which the target Breath Alcohol Concentration (BrAC) was 0.06 (for details see Ray et al., 2006).
Table 1.
Correlations among Measures of Subjective Responses to Alcohol during the Ascending Limb of the Breath Alcohol Concentration (BrAC = 0.06).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. SHAS | |||||||
| 2. BAES Stimulation | 0.38** | ||||||
| 3. BAES Sedation | 0.71*** | 0.08 | |||||
| 4. POMS Vigor | 0.03 | 0.72*** | −0.14 | ||||
| 5. POMS Tension | 0.14 | −0.02 | 0.12 | −0.10 | |||
| 6. POMS Neg Mood | −0.04 | −.027 | 0.21 | −0.29* | 0.48*** | ||
| 7. POMS Pos Mood | 0.29* | 0.61*** | 0.13 | 0.63*** | −0.32* | −0.43** | |
| 8. AUQ Urge to drink | 0.43** | 0.59*** | 0.15 | 0.41** | −0.06 | −0.20 | 0.49*** |
Note: Previously unpublished data from Ray, McGeary, Marshall, and Hutchison (2006); n = 49. SHAS: Subjective High Assessment Scale; BAES: Biphasic Alcohol Effects Scale (Subscale: Stimulation and Sedation); POMS: Profile of Mood States, Short Version (Subscales: Vigor, Tension, Negative and Positive Mood); AUQ: Alcohol Urge Questionnaire.
p < .05;
p < .01;
p < .001
Results of these correlational analyses indicate that the SHAS is most strongly correlated with the sedative effects of alcohol (BAES, sedation), although it is also moderately correlated with alcohol-induced stimulation (BAES, stimulation), which is in turn consistent with the notion that the SHAS contains both positive and negative adjectives related to alcohol’s subjective effects. Conversely, the BAES stimulation subscale was most strongly associated with vigor and positive mood, whereas its association with the sedation subscale was small and non-significant. Interestingly, the BAES stimulation subscale was the strongest predictor of urge to drink alcohol, accounting for approximately 36% of the variance in self-reported urge. Similar relationships among these assessments of the subjective effects of alcohol were recently reported in an independent sample of hazardous drinkers (Ray et al., 2007) and in previous studies where indexes of vigor and physiological reactivity were inversely related to SHAS scores (Conrod, Peterson, & Pihl, 2001). Taken together, these findings highlight the need for further research on the optimal conceptualization and assessment of the construct of subjective responses to alcohol. Such research is critical in order to advance the field by increasing consistency in the findings of alcohol administration studies, whether they focus on biobehavioral factors or underlying genetic risk for alcohol use disorders.
An important effort towards resolving discrepancies in the alcohol administration literature comes from the work of Newlin and Thompson (1990). In the context of their review of alcohol challenge studies of sons of alcohol dependent parents and controls, they proposed the influential differentiator model for understanding psychobiological responses to alcohol as a function of family history. This model can be applied more broadly, to conceptualizing individual differences in responses to alcohol. Newlin and Thompson’s (1990) differentiator model proposes that responses to alcohol may be accentuated during the rising blood alcohol curve (BAC) (i.e., acute sensitization) and attenuated during the falling BAC (i.e., acute tolerance). The authors propose that sons of alcohol dependent individuals may be both more sensitive to the rewarding effects of alcohol during the rising limb of the BAC and less sensitive to the unpleasant effects of alcohol when BAC is dropping. Importantly, acute tolerance and acute sensitization occur within session and represent a useful way to capture the “snap shot” of alcohol’s effects obtained in a single administration session. This model has influenced efforts to parse out the phenotype of subjective response to alcohol into rewarding (primarily during the rising limb of BAC) and unpleasant (most salient during the descending limb of BAC).
This approach is somewhat consistent with the psychomotor stimulant theory of addictions which posits that the stimulatory and rewarding effects of addictive substances, including alcohol, share a common underlying biological mechanism and that individuals who experience greater alcohol-induced reward are thought to be more likely to develop alcohol problems (Wise & Bozarth, 1987). However, just as alcoholism represents a heterogeneous disorder (discussed in more detail below), family history of alcoholism constitutes a heterogeneous, and rather crude, measure of genetic risk. Newlin and Thompson (1990) note the role of pharmacokinetics and neurobiological and genetic differences, primarily via family history of alcoholism, underlying variation in response to alcohol. Recent developments in the understanding of both the genetic underpinnings of pharmacokinetic and pharmacodynamic processes underlying subjective responses to alcohol will be reviewed herein.
In summary, subjective responses to alcohol represent an important phenotype for alcohol use and misuse. Research evidence suggests that the way in which individuals’ experience the pharmacological effects of alcohol influences their subsequent use of alcohol (e.g., Lewis & June, 1990; Wise & Bozarth, 1987) and the risk of developing an alcohol use disorder (Schuckit & Smith, 1996). Moreover, subjective responses to alcohol are heritable (Heath & Martin, 1991; Viken et al., 2003) and informative regarding the neuropharmacological effects of alcohol and their biological and genetic bases. The differentiator model of response to alcohol (Newlin & Thompson, 1990) has been influential in distinguishing two related and biologically-mediated processes that emerge during a single alcohol administration session, namely acute tolerance and acute sensitization. In the next sections we will first introduce the concept of endophenotypes in psychiatric research and then discuss the conceptualization of subjective responses to alcohol as an endophenotype for alcoholism.
1.2. Endophenotypes
Behavioral and clinical scientists are largely interested in the behavioral manifestation of a given disorder, which can be thought of as a phenotype. The behavioral phenotypes used in many of the association studies, mostly consisting of diagnostic phenotypes such as alcohol abuse or dependence, are influenced by many different genetic as well as environmental factors. Because there are so many different factors that influence whether an individual receives a diagnosis of alcohol abuse or dependence, it has become increasingly important to identify more specific and narrowly defined behavioral phenotypes (i.e., intermediate phenotypes or “endophenotypes”) that are related to the larger disorder. Endophenotypes are thought to facilitate research in the neurobiology of psychiatric disorders by being more homogenous and narrowly defined than the larger diagnostic phenotype (Gottesman & Gould, 2003). Recent research in behavioral genetics has focused on identifying specific genes underlying individual differences in the vulnerability for the development of an alcohol use disorder. In light of such efforts, the identification of more narrow behavioral phenotypes, or endophenotypes, for alcoholism has received increased attention (e.g., Hines et al., 2005), as is the case for most psychiatric disorders (Burmeister, 1999; Gottesman & Gould, 2003). A good endophenotype should be narrowly defined, readily identifiable, and related to the disorder of interest (Hutchison et al., 2002). When used correctly, endophenotypes for psychiatric disorders are expected to increase the power to detect specific genes underlying the risk for a given disorder.
In the abovementioned critical review of alcohol administration studies, Newlin and Thompson (1990) described psychobiological markers as characteristics, other than disease symptoms, that identify individuals who are most likely to develop a specific disorder. Importantly, psychobiological makers should be distinguished from the manifestation of prolonged drinking and there may be multiple psychobiological makers for alcoholism given that this disorder is likely to have a multidimensional etiology. The description of psychobiological markers by Newlin and Thompson (1990) and their putative utility in advancing alcoholism research closely resembles the concept of endophenotypes, as described in the psychiatric literature (Burmeister, 1999; Gottesman and Gould, 2003). A recent review of endophenotypes for alcoholism (Hines, Ray, Hutchison, and Tabakoff, 2005), suggested a number of candidate endophenotypes, such as behavioral and physiological traits (e.g., subjective responses to alcohol, alcohol metabolism, alcohol craving, and electrophysiological measures) and biochemical traits (e.g., monoamine oxidase and β-endorphins). The next section will review the conceptual framework and empirical data suggesting that subjective responses to alcohol constitute useful endophenotypes for alcoholism.
1.3 Subjective Responses to Alcohol as Endophenotypes
It is important to systematically evaluate phenotypes in order to determine whether or not they constitute an adequate, and potentially useful, endophenotype for the disorder of interest. Tsuang, Faraone, and Lyons (1993) have put forth specific criteria for evaluating endophenotypes. In this section, subjective responses to alcohol will be reviewed in light of their a-priori criteria: (1) specificity; (2) state-independence; (3) heritability; (4) familial association; (5) cosegregation; and (6) biological and clinical plausibility.
Specificity refers to the expectation that the endophenotype is more strongly associated with the disease of interest than to other psychiatric disorders. Given that subjective responses to alcohol are directly dependent upon alcohol consumption and its associated pharmacological effects, the argument can also be made that this phenotype is highly specific to the disorder of interest. However, the argument can be made that similar mechanisms of pharmacological response may be in play for substances other than alcohol, which in turn would argue for it being a broader phenotype for substance use disorders. Patterns of cross-tolerance between alcohol and benzodiazepines, for example, have been widely documented in the animal and human literature (e.g., Toki et al., 1996). Moreover, common neurotransmitter systems and pathways are involved in the reinforcing effects of multiple substances, as is the case for the dopaminergic and opioidergic systems, for example (Bond et al., 1998; Herz, 1997; Wise & Bozarth, 1987). In summary, responses to alcohol may be an alcohol-specific phenotype, although future research is needed to determine its specificity in relation to other substances of abuse and their common reward pathways, which in turn could help explain the high comorbidity between alcohol use disorders and other substance use disorders.
State-independence refers to the phenotype being stable and not simply a reflection of the disease process. As discussed above, the work of Schuckit and colleagues found that subjective responses to alcohol measured in the laboratory before the development of alcohol-related problems predict the development of alcohol use disorders at 8-year follow-up (Schuckit & Smith, 1996). That is true of even in the case of individuals who were relatively alcohol-naïve at the time of the alcohol challenge. Significantly less is known about the state-independence of alcohol’s reinforcing effects and its association to alcohol use disorders. Heritability is an important criterion for evaluating endophenotypes and represents the degree to which the phenotype is influenced by genetic variance. Ideal endophenotypes are heritable and one would wish for a highly heritable endophenotype very much in the same way we expect our behavioral measures to be reliable, so as to reduce “noise.” Subjective responses to alcohol, measured in an experimental twin study, had a heritability estimate of 60% (Viken et al., 2003). This study used a 22-item measure called Sensation Scale (Maisto et al., 1980), which included items such as drowsy, light headed, and dizzy. Similar estimates, ranging between 0.4 and 0.6, were obtained in an Australian twin study in which subjective response to alcohol was measured across levels of BAC by a single item, namely “how drunk to you feel now” (Heath & Martin, 1991). A more recent laboratory study of the offspring of fathers who completed an alcohol challenge 20 years earlier revealed a significant positive parent-offspring association for subjective feelings of intoxication and body sway, among family history positive individuals (Schuckit et al., 2005). Although not providing direct evidence of heritability, this study is consistent with prior reports of the genetic influences on these endophenotypes and provides support for its reliability. A recent experimental twin study has also shown that subjective responses to nicotine in the laboratory are substantially heritable (Ray et al., 2007b), yet further work establishing the heritability of certain facets of subjective responses to alcohol are certainly warranted.
Familial association refers to the expectation that the endophenotype will be more prevalent among relatives of affected probands, as compared to controls. To that end, there is substantial evidence that subjective responses to alcohol are influenced by family history of alcoholism (e.g., Conrod et al., 1997; Pollock, 1992; Schuckit & Gold, 1998) and a recent study has shown that the number of alcohol dependent relatives was significantly associated with subjective response to alcohol in the laboratory (Schuckit et al., 2005). However, as reviewed by Newlin and Thompson (1990), the broader literature on alcohol challenge studies with sons of alcohol dependent parents and controls is mixed with regard to several behavioral and biological markers.
Cosegregation, in turn, is the expectation that the endophenotype will be more prevalent among the affected relatives compared to the unaffected relatives of ill probands. To date, there have been no animal or human studies of cosegregation patterns for alcohol endophenotypes. Lastly, biological and clinical plausibility refers to the assumption that the endophenotype will bear a conceptual relationship to the disorder of interest. Subjective responses to alcohol are conceptually related to the clinical construct of alcoholism in that individuals who are more sensitive to the rewarding and positive effects of alcohol are thought to crave alcohol more (e.g., consistent with the results in Table 1) and to drink more (Lewis & June, 1990; Wise & Bozarth, 1987), whereas sensitivity to the unpleasant effects of alcohol may deter alcohol use and serve as a protective factor, as discussed below. From a biological standpoint, there is evidence that subjective responses to alcohol are informative regarding neurobiological and genetic factors underlying alcoholism. This evidence will be reviewed in the next sections focusing on pharmacodynamic and pharmacokinetic factors subserving the endophenotype of response to alcohol.
In summary, subjective responses to alcohol appears to meet several of the criteria for evaluating an endophenotype put forth by Tsuang, Faraone, and Lyons (1993). However, the most consistent evidence comes from studies using the SHAS, which, as discussed above, seems to capture both positive and negative aspects of intoxication, arguably loading most strongly on the negative facets. Therefore, additional work capturing the reinforcing and stimulant effects of alcohol and carefully examining its utility as an endophenotype for alcoholism is warranted.
2. Pharmacodynamics
Alcohol intoxication is a complex pharmacological process involving multiple neurotransmitter systems and producing a host of physiological and behavioral effects (Grobin et al., 1998; Herz, 1997). As reviewed above, research, primarily from alcohol administration studies, has provided valuable insight into the subjective effects of alcohol ingestion indicating that alcohol’s effects are biphasic in nature (Earleywine, 1994a; Earleywine, 1994b; Earleywine & Martin, 1993; Erblich et al., 2003; Martin et al., 1993). It has been documented that during the ascending limb of intoxication alcohol produces robust stimulatory and other pleasurable subjective effects, whereas during the descending limb alcohol’s effects are predominantly sedative and unpleasant (Earleywine & Martin, 1993; Erblich et al., 2003).
Despite the body of research suggesting that the pharmacological effects of alcohol vary by limb of BAC, human laboratory studies have largely failed to consider limb of intoxication when examining the subjective responses to alcohol. Based on the known pharmacokinetics of alcohol and methods of calculating circulating alcohol (Brick, 2006) the limb of intoxication can often be broadly inferred in most alcohol administration studies via the time elapsed between alcohol intake and assessment, but there is nonetheless substantial ambiguity in the literature. Considering limb of intoxication may also be critically important in evaluating the subjective effects of alcohol and medications, such as naltrexone, that are thought to alter the subjective responses to alcohol. This is especially important given that the effects of alcohol clearly vary by limb of the BAC, with well-documented limb-dependent alcohol effects on expectancies (Dunn & Earleywine, 2001), memory (Soderlund et al. 2005), cognitive performance (Pihl et al., 2003), and the unpleasant subjective effects of alcohol (Evans & Levin, 2004). Specifically, light drinkers3 are more likely than heavy drinkers to activate negative and sedating alcohol expectancies associated with the descending limb (Dunn & Earleywine, 2001) and report greater dislike of the alcohol during that limb (Evans & Levin, 2004). Impairments in executive cognitive functioning were found to be greater during the descending limb of intoxication (Pihl et al., 2003) and word recognition was found to be impaired only on the ascending limb (Soderlund et al., 2005). Moreover, limb of BAC was proposed as an important feature of the differentiator model (Newlin and Thompson, 1990) of subjective response to alcohol. Together, these findings argue for research that takes into account limb of the BAC when examining the subjective effects of alcohol as well as medications thought to work by altering alcohol’s subjective effects (e.g., reducing its rewarding effects; increasing its unpleasant effects).
2.1 Neurobiology and Genetics
There are multiple neurotransmitter systems underlying the subjective effects of alcohol. Given the complexity of the neurobiological and behavioral effects of alcohol we have recently argued for the importance of considering the subjective effects of alcohol a moving target subserved by multiple neurotransmitter systems (Ray et al., in press). For example, the opiodergic system is thought to mediate some of the rewarding pharmacological effects of alcohol, such as feelings of euphoria and analgesia (Bond et al., 1998; Herz, 1997; Kreek, 1996), and these effects, in turn, are thought to be most prominent during the ascending limb of the BAC.
Given the recognition that the opioidergic system may underlie some of the reinforcing effects of alcohol, there has been recent scientific interest in the genes encoding for endogenous opioid receptors, with a particular focus on the mu-opioid receptor gene (OPRM1). One of the most widely studied polymorphisms of the OPRM1 gene is the +118A/G SNP located in the +118 position in exon 1, which codes for the AsnAsp40 substitution (rs1799971). Molecular studies of this polymorphism initially suggested that the A to G substitution affects receptor activity for endogenous ligand β-endorphin leading to a gain in function, such that the Asp40 variant (i.e., G allele) was though to bind β-endorphin three times stronger than the Asn40 (i.e., A) allele (Bond et al., 1998). However, a more recent study of the functional significance of this SNP suggested that the Asp allele has deleterious effects on both mRNA and protein yield, leading to a loss of function, rather than a gain (Zhang et al., 2005).
Several studies have tested the relationship between the A118G SNP of the OPRM1 gene and substance use disorders, particularly alcoholism and opioid dependence and the results are largely inconsistent. While some investigations have found support for the association between the A118G SNP and alcohol or opioid dependence (Kranzler et al., 1998; Schinka et al., 2002; Tan et al., 2003; Town et al., 1999), several studies have failed to find an association (Arias et al., 2005; Bergen et al., 1997; Crowley et al., 2003; Franke et al., 2001; Gelernter et al., 1999; Loh et al., 2004). However, recent laboratory-based research used an endophenotype-driven approach to studying the effects of the A118G SNP of the OPRM1 gene. Instead of measuring alcohol dependence per se, this study tested the association between this SNP and measures of subjective response to alcohol (Ray & Hutchison, 2004). Results revealed that individuals with at least one copy of the G allele showed greater subjective response to the effects of alcohol measured by subjective intoxication, sedation and stimulation, and changes in mood states, as compared to participants who were homozygous for the A allele. These findings have been recently replicated in an independent sample (Ray & Hutchison, 2007) and this candidate gene has been of interest as a potential mediator of the effects of naltrexone for the treatment of alcoholism (Anton et al., 2008; Oslin et al., 2003). Similar findings were reported in animal models with male rhesus macaques. Here the equivalent OPRM1 polymorphism was associated with increased alcohol response, consumption, and preference (Barr et al., 2007). More broadly, these results provide an example of how endophenotypes may be used to advance our understanding of the pathophysiology of alcoholism. Specifically, subjective responses to alcohol are informative regarding the underlying neuropharmacological effects of alcohol – in suggesting that endogenous opioids may be involved in the rewarding subjective effects of alcohol – which in turn may be used to elucidate candidate genes subserving those effects.
Although the neurotransmitter systems underlying the sedative subjective effects of alcohol have not been clearly characterized, there is some evidence that these effects are mediated by glutamatergic antagonism (Krystal et al., 1998). Likewise, the sedative and anxiolytic effects of alcohol, more prominent during the descending limb, are thought to be mediated by γ-Aminobutric Acid (GABA) neurotransmission (Buck, 1996; Grobin et al., 1998). As discussed above, understanding the substrates underlying subjective responses to alcohol has the potential to improve gene identification by focusing on a more narrowly defined and biologically-relevant phenotype. To that end, a number of genetic association studies have examined candidate genes for level of response to alcohol, as defined by Schuckit and colleagues (e.g., Schuckit & Smith, 1996; Schuckit et al., 1999). Results to date have not been entirely consistent, yet there is some support for the role of variation in the GABAAα6 and serotonin transporter in the subjective response to alcohol in the laboratory (Corbin, Fromme, and Bergenson, 2006; Fromme et al., 2004; Schuckit et al., 1999). In a recent review of the literature on genes contributing to low level of response to alcohol, Schuckit, Smith, & Kamijin (2004) highlighted several candidate gene findings with possible pharmacodynamic effects on level of response to alcohol and alcoholism risk. These findings included genes relating to alterations in the GABAergic system, with a particular focus on GABAA and its subunits, the serotonin transporter gene, opioid and cannabinoid systems, and genes involved in second messenger systems (e.g., G proteins and protein kinases) (Schuckit, Smith, & Kamijin, 2004). These authors concluded that there is evidence to support the benefits of conducting genetic studies, both linkage and association, on subjective responses to alcohol as an endophenotype for alcoholism. As suggested by Schuckit and colleagues (2004), the ultimate goal of this line of research is to identify genes that underlie alcohol-related phenotypes, such as level of response to alcohol, and evaluate genetic factors that interact with environmental factors to determine the risk for alcoholism, which in turn can be translated into improved treatment approaches.
3. Pharmacokinetics
In addition to the pharmacodynamic effects reviewed above, another source of genetic influence on the subjective effects of alcohol comes from genetically-mediated differences in the metabolism of alcohol as it travels through the body, or in other words, alcohol’s pharmacokinetics. When alcohol is consumed, its metabolic breakdown is a three-step hepatic process in which the alcohol is first oxidized into acetaldehyde by the enzyme alcohol dehydrogenase (ADH) and is then further metabolized into acetate, and other byproducts, by the enzyme aldehyde dehydrogenase (ALDH). The genes responsible for these enzymes exert important influences on the subjective effects of alcohol because they determine the relative levels of these metabolites over the course of alcohol metabolism. Indeed, genes underlying the pharmacokinetics of alcohol are among the best characterized in terms of their influence on subjective responses to alcohol and alcoholism risk.
3.1 Neurobiology and Genetics
Genetic influences are first evident at the first step in the metabolism of alcohol. As noted above, alcohol is initially broken down in acetaldehyde by ADH. Of the multiple forms of the ADH, the class I isozymes (ADH1A, ADH1B, and ADH1C) play the predominant role in metabolizing alcohol (Edenberg, 2007; Lee et al., 2006). The genes responsible for these enzymes are closely linked on chromosome 4 and the ADH1B and ADH1C genes (coding for the respective isoenzymes) have been determined to have functional polymorphisms. In the case of ADH1B, three polymorphisms of the gene have been identified, of which two (ADH1B-2, ALDH1B-3) are associated with faster enzymatic activity compared to the ADH1B-1 allele, with both resulting in a 70- to 80-fold greater turnover rate. In the case of ADH1C, two variants have been characterized, ADH1C-1 and ADH1C-2, and there is evidence that ADH1C-1 is associated with approximately half the alcohol turnover compared to ADH1C-2. In addition, some Native American groups have been found to carry a third variant of the ADH1C gene (Osier et al., 2002), but that has not been extensively studied. Most importantly, variation in these genes substantially affects the speed at which an individual metabolizes alcohol into acetaldehyde. For example, the speed of oxidation is estimated to be eight times faster for a man who is homozygous for the ADH1B-2 and ADH1C-1 alleles, as compared to a man who is homozygous for the ADH1B-1 and ADH1C-2 alleles (Lee et al., 2006).
The behavioral and subjective consequences of possession of genetic variants that affect ADH enzymatic activity to increase the presence of acetaldehyde are acutely aversive in nature, including flushing, headache, tachycardia, and nausea. As a result, possession of the ADH1B-2 allele has been demonstrated to reduce the risk of AUDs in populations where the frequency of this allele is high, such as East Asians (Luczak et al. 2006; Thomasson et al. 1991; Whitfield 2002). In addition, even in populations where the allele frequency is lower, a protective effect has been demonstrated for individuals of European and African ancestry (Whitfield, 2002) and individuals of Jewish descent (Luczak et al., 2002; Hasin et al., 2002). Similarly, Edenberg et al. (2006) found that possession of the ADH1B-3 allele reduces the risk of alcoholism in African-Americans, although not in Europeans. Wall et al. (2003) found the ADH1B-3 allele had a protective effect among Southwest American Indians. With regard to ADH1C, there is evidence that the faster metabolism of alcohol (i.e., greater accumulation of acetaldehyde), mediated by the possession of the ADH1C-1 allele, has protective effects (Chen et al. 1999; Choi et al. 2005; Osier et al. 1999). However, these effects are complicated by the fact that the allele is commonly inherited with ADH1B-2 (i.e., these markers are in linkage disequilibrium; LD), making it unclear whether this is an independent effect. In addition, the dynamic and multidimensional nature of these behavioral phenotypes suggest that non-linear models may be required to capture their complexity (Buscema, 1998).
In addition to the relatively clear effects of genetic effects at the first stage of metabolism, even more robust findings are evident in terms of genetic influences at the second stage of alcohol metabolism, the oxidation of acetaldehyde into acetate. Two ALDH enzymes are responsible for metabolizing acetaldehyde and are encoded by the eponymous genes, ALDH2 and ALDH1A1, on chromosomes 12 and 9, respectively. ALDH2 has two variants, ALDH2-1 and ALDH2-2, and the latter results in an inactive form of the enzyme that cannot metabolize acetaldehyde into acetate. Further, this variant is dominant, so possession of even one ALDH2-2 copy results in almost no hepatic ALDH2 activity (Crabb et al, 1989). Consequently, ALDH2-2 carriers experience a build-up of acetaldehyde following alcohol consumption, again causing a syndrome of unpleasant effects, including flushing, headache, tachycardia, and nausea. From a functional standpoint, as with the case for polymorphisms affecting acetaldehyde levels via ADH genes, the aversive reaction to alcohol resulting from the ALDH2-2 allele has been robustly demonstrated to result in a substantial protective effect against alcoholism in a number of studies (e.g., Chen et al., 1999, Luczak et al., 2006; Thomasson et al., 1991). In behavioral terms, these individuals experience unpleasant effects of alcohol upon consumption, which makes them less likely to consume alcohol and sharply decreases their risk for developing an AUD. Importantly, the narrowly-defined phenotype of unpleasant subjective responses to alcohol (i.e., “flushing” response) has helped scientists further understand the neurobiology of alcohol and its effects, as well as genetic factors underlying this posited protective behavioral phenotype.
In terms of the specific magnitude, a recent large-scale meta-analysis found that ALDH2-2 genotype reduced the risk of alcoholism by 25%-90%. Indeed, in over 4,000 individuals, only 3 cases of alcoholism were identified for ALDH2-2 homozygotes (Luczak et al., 2006). Of note, as with ADH genes, this protective effect generally pertains to individuals of East Asian ancestry for whom the ALDH2-2 allele is relatively common. Conversely, this polymorphism is rare in individuals of European or African ancestry (Oota et al., 2004). Interestingly, despite its strong putative protective effect, there is evidence the effects of ALDH2 status interact with environmental influences also. Higuchi et al. (1994) report that, from 1979 to 1992, the percentage of Japanese individuals with alcohol dependence who possessed an ALDH2-2 allele increased from 2.5% to 13%, suggesting that the protective effect of the polymorphism was reduced by the greater social acceptability of alcohol use in Japanese culture.
Based on these potent effects via variation in the ALDH2 gene, there is also considerable interest in the ALDH1A1 gene. In contrast to the ALDH2 gene, which is responsible for mitochrondrial ALDH activity, ALDH1A1 is responsible for cytosolic ALDH activity (Edenberg, 2007). Variants of the ALDH1A1 gene occur at relatively low frequencies and findings to date have been mixed. For example, Ehlers et al. (2004) found that Southwest California Indians possessing the less functional variant (i.e., resulting in greater acetaldehyde buildup) experienced a protective effect against alcoholism, but Hansell et al. (2005) did not find a protective effect in an Australian community sample. At this point, although variation in ALDH1A1 is promising candidate for exerting similar effects on aversive subjective effects of alcohol and in turn affecting AUD risk, the data are far from definitive.
Taken together, the preceding genetic variants affect the pharmacokinetics of alcohol and further underscore the importance that genetic influences on the subjective effects of alcohol have on risk for AUD. Allelic variation that either results in faster initial accumulation of acetaldehyde (ADH-related genes) or slower breakdown of acetaldehyde (ALDH-related genes) largely determine whether an individual will experience an acutely aversive reaction to alcohol and, in turn, significantly affects the risk for the development of AUD. As such, acetaldehyde accumulation syndrome, including its behavioral effects, is a prototypical intermediate phenotype, intervening between genetic variation and disease liability. Although substantially more remains to be understood about the genes responsible for alcohol’s metabolism, these findings are highly illustrative of an intermediate phenotype approach, and may also provide a model system for potential interventions.
4. Treatment Applications
There are multiple ways by which alcoholism endophenotypes may be used to improve treatment processes and outcomes. The first approach consists of using endophenotypes as biobehavioral risk markers that can inform secondary prevention efforts through improved identification of individuals at risk. The second, and perhaps most developed approach, consists of using endophenotypes as treatment targets of pharmacological or psychosocial interventions. To that end, it has been postulated that medications that affect the subjective effects of alcohol may hold particular promise for the treatment of alcoholism. For example, two of the four currently FDA-approved pharmacotherapies for alcoholism are thought to work by altering subjective responses to alcohol, namely naltrexone and disulfiram (Antabuse®). Specifically, several studies have shown that naltrexone, an opioid antagonist, alters subjective responses to alcohol by: dampening feelings of alcohol-induced stimulation (Drobes et al., 2004; Ray & Hutchison, 2007; Swift et al., 1994) and alcohol “high” (Volpicelli et al., 1995), decreasing ratings of liking and enjoyment of the alcohol intoxication (McCaul et al., 2001; Ray & Hutchison, 2007), and increasing self-reported fatigue, tension, and confusion (King et al., 1997; Ray et al., in press). Naltrexone’s effects are thought to be mediated through the blockade of opioid receptors, which in turn are associated with the reinforcing effects of alcohol upon consumption (Bond et al., 1998; Herz, 1997).
Conversely, disulfiram is a compound that exerts its effects via the same pharmacokinetic pathway associated with the ALDH2-2 allele (reviewed above). In the context of alcohol’s metabolism, disulfiram is an aldehyde dehydrogenase blocker that results in an increase in acetaldehyde after ingested alcohol is oxidized via ADH. This results in a dramatic change in subjective response to alcohol through a set of aversive symptoms caused by acetaldehyde buildup (e.g., nausea, flushing, and tachycardia). These acutely aversive consequences of alcohol consumption are thought to serve as a deterrent to alcohol consumption. Unfortunately, a major limitation of disulfiram’s use in practice is compliance (Suh et al., 2006); which is also problematic for other addiction pharmacotherapies (e.g., Kranzler et al., 2008). In summary, the biological and behavioral mechanisms of action of both naltrexone and disulfiram exemplify the potential utility of subjective response to alcohol as an endophenotype and its applications to treatment approaches for alcoholism.
An important extension of using endophenotypes, such as subjective responses to alcohol, as pharmacological treatment targets is that information regarding underlying neurobiology and genetics can then be integrated in efforts towards optimizing treatment. Pharmacogenetics represents one important approach towards optimizing treatments for alcoholism on the basis of genetic variance. Specifically, pharmacogenetics is a field of research that seeks to understand individual differences in the metabolism and efficacy of drugs by identifying genetic factors that account for variability in pharmacotherapy effects, both in terms of pharmacodynamics and treatment efficacy (Evans & Johnson, 2001). The field of pharmacogenetics has grown rapidly and has greatly benefited from advancements in molecular genetic tools for identifying gene polymorphisms, developments in bioinformatics and functional genomics, and new findings from the human genome project (Evans & Johnson, 2001). The foremost goal of this line of research is to optimize drug therapy by identifying genetic factors that predict who is more likely to respond to certain pharmacotherapies and who will not respond, therefore matching patients to medications on the basis of genetic factors.
A few studies to date have investigated genetic polymorphisms in the context of pharmacotherapies for alcohol dependence. One of such studies has found that a polymorphism of the µ-opioid receptor gene (OPRM1) was associated with clinical response to naltrexone among alcohol dependent patients (Oslin et al., 2003). The relationship was such that individuals with at least one copy of the variant allele (the A118G SNP of the OPRM1 gene) showed lower relapse rates and longer time to return to heavy drinking when treated with naltrexone (Oslin et al., 2003). These findings have been recently replicated in the COMBINE Study (Anton et al., 2008), such that carriers of the G allele receiving Medication Management (MM) showed a significant decrease in heavy drinking days, as compared to homozygotes for the A allele. In addition, 87% of carriers of the G allele had a good clinical outcome to naltrexone + MM, as compared to 55% of homozygotes for the A allele; and the groups did not differ in their response to MM plus placebo (Anton et al., 2008). And overall, naltrexone was superior to placebo in the COMBINE Study when delivered in combination with MM (Anton et al., 2006). However, one recent study did not find support for the moderating role of OPRM1 genotype on clinical response to naltrexone in a sample of male veterans (Gelernter et al., 2007). Moreover, a placebo-controlled laboratory study of naltrexone suggested that individuals who were carriers of the G allele of the OPRM1 gene showed significantly greater naltrexone-induced blunting of alcohol “high,” as compared to individuals who were homozygote for the A allele (Ray & Hutchison, 2007). These findings suggest that the differential clinical response to naltrexone, discussed above, may be due to differential blunting of the subjective experience of alcohol reward as a function of genotype, which in turn suggests a biobehavioral mechanism for this important pharmacogenetic relationship. Future studies are certainly needed to probe for this relationship before individualized treatment approaches may be implemented, including consideration of issues such as the differential allele frequencies of this genotype among various ethnic groups (Arias, Feinn, & Kranzler, 2006).
Limitations notwithstanding, the recent literature on the pharmacogenetics of naltrexone provides an example of how endophenotype-driven approaches may be useful tools for advancing the knowledge of alcoholism etiology, neurobiology, and ultimately, treatment efficacy and mechanisms. Translational approaches such as these have the potential to inform clinical practice by identifying individuals who are more likely to benefit from a given pharmacotherapy on the basis of genetic factors. Importantly, a similar framework may be use for optimizing psychosocial interventions that can target certain alcoholism endophenotypes (e.g., craving). In an interesting application of behavioral genetics to optimizing treatment, Bauer and colleagues (2007) reported that variation within the GABRA2 gene thought to increase the risk for alcoholism (Edenberg et al., 2004), predicted response to the psychosocial interventions tested in Project MATCH. Specifically, the low-risk allele was associated with more robust differences in drinking outcomes in the trial, enhancing the superiority of Twelve-Step Facilitation (TSF) over Cognitive Behavioral Therapy (CBT) and Motivational Enhancement Therapy (MET) (Bauer et al., 2007). In short, these results indicate that the assessment of genetic liability may also be important to studies of the efficacy of psychosocial interventions. More broadly, these results allude to the importance of integrating biological and psychosocial variables to more fully capture the clinical phenomenon of alcoholism.
4.1 An Endophenotype-Pharmacogenetic Model
In light of the literature on endophenotypes for alcoholism and their potential to advance etiological and treatment approaches to this disorder, we have developed a conceptual model that ties together alcohol endophenotypes to genetic and pharmacological treatments of alcoholism. To that end, we propose that focusing on alcohol endophenotypes, such as subjective responses to alcohol, and genetic variants subserving those endophenotypes represent important steps towards parsing out the effects of medications on drinking outcomes. More specifically, we posit that alcohol endophenotypes and genetic factors can be used to improve our understanding of pharmacotherapies for alcoholism in several ways (see Figure 1). First, endophenotypes for alcoholism, such as alcohol craving and subjective responses to alcohol, have been shown to predict drinking behavior and the risk for developing alcohol use disorders (e.g, Hines, Ray, Hutchison, and Tabakoff, 2006; Hutchison et al., 2002; Ray, Hutchison, and Bryan, 2006; Schuckit & Smith, 1996; Tidey et al., 2008). Second, medications found to operate at the level of endophenotypes, such as craving and subjective responses to alcohol (e.g., Monti & MacKillop, 2007; Monti et al., 2001; Ray & Hutchison, 2007), may ultimately be effective in reducing drinking. In a recent example of this approach, a laboratory study found that aripiprazole increased the sedative effects of alcohol and decreased its euphoric and stimulant effects, those effects are thought to capture the mechanisms of action of Aripiprazole for alcoholism (Kranzler et al., in press). Third, genetic variants appear to underlie the expression of alcohol endophenotypes such as craving (e.g., Hutchison et al., 2002) and subjective responses to alcohol (e.g., Fromme et al., 2004; Ray and Hutchison, 2004; 2007; Schuckit et al. 2004). Fourth, genetic variants associated with alcohol endophenotypes may, in turn, be used to predict responses to pharmacotherapies thought to affect those endophenotypes (Ray & Hutchison, 2004; 2007).
Figure 1.
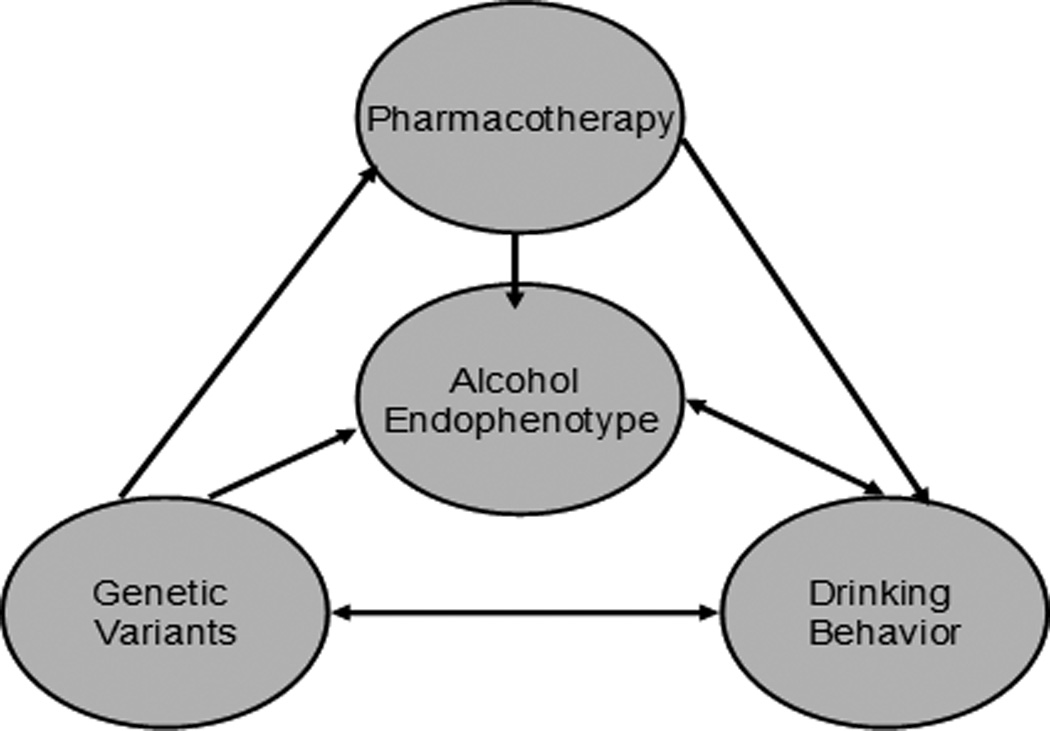
An endophenotype-pharmacogenetic model of alcoholism treatment
In sum, we argue that a theory-driven endophenotype pharmacogenetic approach can be used to enhance the pharmacological treatment of alcoholism4. Pharmacotherapies, in turn, may be useful in the context of multiple psychosocial treatment platforms and orientations, including abstinence-based and harm-reduction models. This model is interdisciplinary by nature, as it integrates aspects of behavioral genetics, pharmacology, clinical and experimental psychology. Focusing on theory-driven alcohol endophenotypes and genetic and neurobiological factors underlying these phenotypes may help us elucidate the mechanisms of action of pharmacotherapies, as well as moderators of response. Moreover, this approach has the potential to enhance the translation of basic science to treatment, as it more directly connects endophenotypes and genetic variants to pharmacotherapy outcomes for alcohol dependence. Ultimately, the endophenotype-pharmacogenetic model described herein offers a potentially useful framework for better understanding how endophenotypes and genetic factors concomitantly influence responses to pharmacotherapies for alcoholism. Similar approaches may be useful in optimizing psychosocial interventions by targeting more specific and narrowly defined components of the risk for alcoholism (i.e., prevention efforts) or the clinical syndrome itself (i.e., treatment efforts).
5. Future Directions
The literature reviewed herein indicates a number of possibilities for future research with the ultimate goal of translating basic findings into improved treatments for alcoholism. Specifically, as suggested by Insel and Quirion (2005), increased knowledge about the pathophysiology of mental disorders should lead to treatments that are more specific, effective, and accessible. Recent efforts to identify responders to naltrexone as a treatment for alcoholism (e.g., Anton et al., 2008; Ray & Hutchison, 2007) represent initial attempts to develop treatments that are more specific, and as a result, also more efficacious. These efforts are also consistent with the personalized treatment approaches pursued in various fields of medicine. Further characterizing the role of environmental factors (i.e., “E”) and their singular and interactive effects on endophenotypes and disease phenotypes represents a necessary step to more fully understand and treat disorders of complex genetics. Translating some of the promising endophenotypes for alcoholism, such as subjective response to alcohol, into constructs that are measurable outside of the laboratory may help scientists capture the full picture. For example, using ecological momentary assessment (EMA) technology is a promising alternative to capturing subjective response to alcohol, as well as other important environmental and psychological variables, outside of the laboratory setting (e.g., Miranda et al., 2008; Tidey et al., 2008). Likewise, the combination of laboratory, functional imaging (i.e., fMRI), and genetics may be especially helpful in “connecting the dots” by understanding how functional polymorphisms affect brain structure and function, and ultimately behavior (e.g., McClernon et al., 2007; Filbey, Ray et al., in press). Finally, the field would benefit from the development of novel intermediate phenotypes, and the refinement of existing phenotypes, to further identify intervening processes/mechanisms by which specific genes exert their influences.
From a pharmacotherapy standpoint, several future directions should be considered. For example, further understanding the pharmacokinetics of medications, including genetically-determined differences in drug metabolism, may be helpful in elucidating medication-induced effects on the endophenotypes of interest, such as craving and subjective responses to alcohol. Considering medication pharmacokinetics also raises possibilities for differential pharmacotherapy delivery, such as depo versus oral formulations, and daily versus as needed (i.e., PRN) medication administration. In summary, the study of subjective responses to alcohol has evolved significantly over the past three decades. From the initial characterization of differential response to alcohol by sons of alcohol dependent parents, as compared to controls, to the more recent line of work using subjective response to alcohol as an endophenotype in genetic association studies and pharmacological trials. Nevertheless, considerable work has yet to be done before these advances can be translated into clinical practice. Specifically, further refining the phenotype of subjective response to alcohol and considering both genetic and environmental determinants represent two important steps in the direction of increasing consistency in the literature leading to clinical applications. The endophenotype-driven pharmacogenetic model offers a useful framework for systematically integrating endophenotypes in etiological and treatment models of alcoholism with the ultimate goal of translating these findings into improved prevention and intervention approaches.
Acknowledgements
LAR was supported by a grant (T32 AA007459) from the National Institute on Alcohol Abuse and Alcoholism.
Glossary
- Behavioral marker
a trait or phenotype that is required for the expression of a disorder of interest and that can inform clinical and/or genetic studies
- Cravin
an inherently subjective experience described as a state of desire or wanting
- Endophenotypes
internal phenotypes, not obvious to the untrained eye, which can fill the gap between gene and the behavior or disorder of interest
- Pharmacodynamics
the study of biochemical and physiological effects of drugs, such as for the example, drug-receptor interactions
- Pharmacokinetics
a field of pharmacology that is concerned with drug absorption, distribution, metabolism and excretion; or in other words, how the body processes drug substances
- Pharmacogenetics
the field of research that seeks to understand individual differences in the metabolism and efficacy of drugs by examining genetic variability
- Phenotype
any observable trait or characteristic of an individual that is thought to be the result of genes, environment, and their interaction. Research in psychiatric genetics often focuses on diagnostic phenotypes
- Subjective responses to alcohol
this construct refers to an individual’s subjective experience of alcohol’s effects, such as feelings of intoxication or “high,” sedation, stimulation, mood alterations, and overall enjoyment of the intoxication effects
Footnotes
The journal’s style utilizes the category substance abuse as a diagnostic category. Substances are used or misused; living organisms are and can be abused. Editor’s note.
Risk is hereby defined as the predisposition to developing alcoholism under certain environmental conditions, such as alcohol exposure.
Drinking level was defined using a composite drinking score averaging the number of standard drinks consumed per occasion and the average number of drinking occasions per month.
Treatment can be briefly and usefully defined as a planned, goal directed, temporally structured change process, of necessary quality, appropriateness and conditions (endogenous and exogenous; micro to macro levels)), which is bounded (culture, place, time, etc.) and can be categorized into professional-based, tradition-based, mutual-help based (AA, NA, etc.) and self-help ("natural recovery") models. There are no unique models or techniques used with substance users, of whatever types and heterogeneities, which are not also used with non-substance users. In the West, with the relatively new ideology of "harm reduction" and the even newer Quality of Life (QOL) treatment-driven model there are now a new set of goals in addition to those derived from/associated with the older tradition of abstinence driven models. Editor’s note.
References
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Archives of General Psychiatry. 2008;65(2):135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donavan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence, the COMBINE study: A randomized controlled trial. Journal of the American Medical Association. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Arias A, Feinn R, Kranzler HR. Association of an Asn40Asp (A118G) polymorphism in the mu-opioid receptor gene with substance dependence: a meta-analysis. Drug and Alcohol Dependence. 2006;83:262–268. doi: 10.1016/j.drugalcdep.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt M, Lindell SG, Chen SA, Goldman D, Suomi SJ, Higley JD, Heilig M. Association of a functional polymorphism in the mu-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Archives of General Psychiatry. 2007;64(3):369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Covault J, Harel O, Das S, Gelernter J, Anton R, Kranzler HR. Variation in GABRA2 predicts drinking behavior in project MATCH subjects. Alcoholism: Clinical and Experimental Research. 2007;31(11):1780–1787. doi: 10.1111/j.1530-0277.2007.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Kokoszka J, Peterson R, Long JC, Virkkunen M, Linnoila M, Goldman D. µ opioid receptor gene variants: Lack of association with alcohol dependence. Molecular Psychiatry. 1997;2:490–494. doi: 10.1038/sj.mp.4000331. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human µ-opioid receptor gene alters β-endorphin binding and activity: Possible implications for opiate addiction. Neurobiology. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brick J. Standardization of alcohol calculations in research. Alcoholism: Clinical and Experimental Research. 2006;30:1276–1287. doi: 10.1111/j.1530-0277.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- Buck KJ. Molecular genetic analysis of the role of GABAergic systems in the behavioral and cellular actions of alcohol. Behavior Genetics. 1996;26:313–323. doi: 10.1007/BF02359387. [DOI] [PubMed] [Google Scholar]
- Burmeister M. Basic concepts in the study of diseases with complex genetics. Biological Psychiatry. 1999;45(5):522–532. doi: 10.1016/s0006-3223(98)00316-3. [DOI] [PubMed] [Google Scholar]
- Busceman M. A brief overview and introduction to artificial neural networks. Substance use & Misuse. 1998;37(8–10):1093–1148. doi: 10.1081/ja-120004171. [DOI] [PubMed] [Google Scholar]
- Chen C-C, Lu R-B, Chen Y-C, et al. Interaction between the functional polymorphisms of the alcohol-metabolism genes in protection against alcoholism. American Journal of Human Genetics. 1999;65:795–807. doi: 10.1086/302540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IG, Son HG, Yang BH, et al. Scanning of genetic effects of alcohol metabolism gene (ADH1B and ADH1C) polymorphisms on the risk of alcoholism. Human Mutation. 2005;26(3):224–234. doi: 10.1002/humu.20209. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology. 2001;157:20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- Conrod P, Peterson J, Pihl R, Mankowski S. Biphasic effects of alcohol on heart rate are influenced by alcoholic family history and rate of alcohol ingestion. Alcoholism: Clinical and Experimental Research. 1997;21:140–149. [PubMed] [Google Scholar]
- Corbin WR, Fromme K, Bergeson SE. Preliminary data on the association among the serotonin transporter polymorphism, subjective alcohol experiences, and drinking behavior. Journal of Studies on Alcohol. 2006;67(1):5–13. doi: 10.15288/jsa.2006.67.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb DW, Edenberg HJ, Bosron WF, Li T-K. Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity: The inactive ALDH2(2) allele is dominant. Journal of Clinical Investigation. 1989;83:314–316. doi: 10.1172/JCI113875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JJ, Oslin DW, Patkar AA, DeMaria PA, Jr, O’Brien CP, Berrettini WH, Grice DE. A genetic association study of the mu opioid receptor and severe opioid dependence. Psychiatric Genetics. 2003;13:169–173. doi: 10.1097/00041444-200309000-00006. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Vornin K. Effects of naltrexone and namefene on subjective response to alcohol among non-treatment seeking alcoholics and social drinkers. Alcoholism: Clinical and Experimental Research. 2004;28:1362–1370. doi: 10.1097/01.alc.0000139704.88862.01. [DOI] [PubMed] [Google Scholar]
- Dunn ME, Earleywine M. Activation of alcohol expectations in memory in relation to limb of the blood alcohol curve. Psychology of Addictive Behaviors. 2001;15:18–24. doi: 10.1037/0893-164x.15.1.18. [DOI] [PubMed] [Google Scholar]
- Earleywine M, Erblich J. Distraction does not impair memory during intoxication: support for the attention-allocation model. Journal of Studies of Alcohol. 1995;56:444–448. doi: 10.15288/jsa.1995.56.444. [DOI] [PubMed] [Google Scholar]
- Earleywine M. Confirming the factor structure of the anticipated biphasic alcohol effects scale. Alcoholism: Clinical and Experimental Research. 1994a;18:861–866. doi: 10.1111/j.1530-0277.1994.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Earleywine M. Anticipated biphasic effects of alcohol vary with risk for alcoholism: a preliminary report. Alcoholism: Clinical and Experimental Research. 1994b;18:711–714. doi: 10.1111/j.1530-0277.1994.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Earleywine M, Martin C. Anticipated stimulant and sedative effects of alcohol vary with dosage and limb of the blood alcohol curve. Alcoholism: Clinical and Experimental Research. 1993;17:135–139. doi: 10.1111/j.1530-0277.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30(1):5–13. [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Xuei X, Chen HJ, et al. Association of alcohol dehydrogenase genes with alcohol dependence: A comprehensive analysis. Human Molecular Genetics. 2006;15(9):1539–1549. doi: 10.1093/hmg/ddl073. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li T-K, Nurnberger JI, Jr, O’Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. American Journal of Human Genetics. 2004;74(4):705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Spence JP, Wall TL, et al. Association of ALDH1 promoter polymorphisms with alcohol-related phenotypes in southwest California Indians. Alcoholism: Clinical and Experimental Research. 2004;28(10):1481–1486. doi: 10.1097/01.alc.0000141821.06062.20. [DOI] [PubMed] [Google Scholar]
- Erblich J, Earleywine M. Behavioral undercontrol and subjective stimulant and sedative effects of alcohol intoxication: independent predictors of drinking habits? Alcoholism: Clinical and Experimental Research. 2003;27:44–50. doi: 10.1097/01.ALC.0000047300.46347.CE. [DOI] [PubMed] [Google Scholar]
- Erblich J, Earleywine M, Erblich B, Bovbjerg DH. Biphasic stimulant and sedative effects of ethanol: are children of alcoholics really different? Addictive Behaviors. 2003;28:1129–1139. doi: 10.1016/s0306-4603(02)00221-6. [DOI] [PubMed] [Google Scholar]
- Evans WE, Johnson JA. Pharmacogenomics: The inherited basis for interindividual differences in drug response. Annual Review of Genomics and Human Genetics. 2001;2:9–39. doi: 10.1146/annurev.genom.2.1.9. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR. Differential response to alcohol in light and moderate female social drinkers. Behavioural Pharmacology. 2004;15:167–181. [PubMed] [Google Scholar]
- Franke P, Wang T, Nöthen MM, Knapp M, Neidt H, Albrecht S, Jahnes E, Propping P, Maier W. Nonreplication of association between µ-opioid-receptor gene (OPRM1) A118G polymorphism and substance dependence. American Journal of Medical Genetics. 2001;105:114–119. [PubMed] [Google Scholar]
- Fromme K, de Witt H, Hutchison KE, Ray L, Corbin WR, Cook TA, Wall TL. Biological and behavioral markers of alcohol sensitivity. Alcoholism Clinical and Experimental Research. 2004;28(2):247–256. doi: 10.1097/01.alc.0000113420.28472.25. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Gueorguieva R, Kranzler HR, Zhang H, Cramer J, Rosenheck R, Krystal JHVA Cooperative Study #425 Study Group. Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA Cooperative Study. Alcoholism Clinical and Experimental Research. 2007;31(4):555–563. doi: 10.1111/j.1530-0277.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells J. Genetics of two µ opioid receptor gene (OPRM1) exon I polymorphisms: Population studies, and allele frequencies in alcohol- and drug-dependent subjects. Molecular Psychiatry. 1999;4:476–483. doi: 10.1038/sj.mp.4000556. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Hansell NK, Pang D, Heath AC, et al. Erythrocyte aldehyde dehydrogenase activity: Lack of association with alcohol use and dependence or alcohol reactions in Australian twins. Alcohol and Alcoholism. 2005;40(5):343–348. doi: 10.1093/alcalc/agh168. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG. Intoxication after an acute dose of alcohol: an assessment of its association with alcohol consumption patterns by using twin data. Alcoholism: Clinical and Experimental Research. 1991;15:122–128. doi: 10.1111/j.1530-0277.1991.tb00529.x. [DOI] [PubMed] [Google Scholar]
- Heath AC, Phil D. Genetic influences on alcoholism risk: A review of adoption and twin studies. Alcohol Health and Research World. 1995;19(3):166–171. [PMC free article] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology. 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Matsushita S, Imazeki H, et al. Aldehyde dehydrogenase genotypes in Japanese alcoholics. Lancet. 1994;343:741–742. doi: 10.1016/s0140-6736(94)91629-2. [DOI] [PubMed] [Google Scholar]
- Hines L, Ray LA, Hutchison KE, Tabakoff B. Alcoholism: The dissection for endophenotypes. Dialogues in Clinical Neuroscience. 2005;7:153–163. doi: 10.31887/DCNS.2005.7.2/lhines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, McGeary J, Smolen A, Bryan A, Swift RM. The DRD4 VNTR polymorphism moderates craving after alcohol consumption. Health Psychology. 2002;21(2):139–146. [PubMed] [Google Scholar]
- Insel TR, Quirion R. Psychiatry as a clinical neuroscience discipline. Journal of the American Medical Association. 2005;294(17):2221–2224. doi: 10.1001/jama.294.17.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O’Brien CP. Effects of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology. 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Pierucci-Lagha A, Chan G, Douglas K, Arias AJ, Oncken C. Effects of aripiprazole on subjective and physiological responses to alcohol. Alcoholism: Clinical and Experimental Research. doi: 10.1111/j.1530-0277.2007.00608.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Stephenson JJ, Montejano L, Wang S, Gastfriend DR. Persistence with oral naltrexone for alcohol treatment: implications for health-care utilization. Addiction. 2008;103(11):1801–1808. doi: 10.1111/j.1360-0443.2008.02345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Gelernter J, O’Malley S, Hernandez-Avila CA, Kaufman D. Association of alcohol or other drug dependence with alleles of the µ opioid receptor gene (OPRM1) Alcoholism: Clinical and Experimental Research. 1998;22:1359–1362. [PubMed] [Google Scholar]
- Kreek MJ. Opioid receptors: Some perspectives from early studies of their role in normal physiology, stress responsivity, and in specific addictive diseases. Neurochemical Research. 1996;21:1469–1488. doi: 10.1007/BF02532387. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Webb E, Cooney NL, Karper LP, Namanworth S, Stetson P, Trevisan LA, Charney DS. Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Archives of General Psychiatry. 1998;55:354–360. doi: 10.1001/archpsyc.55.4.354. [DOI] [PubMed] [Google Scholar]
- Lee SL, Chau GY, Yao CT, et al. Functional assessment of human alcohol dehydrogenase family in ethanol metabolism: Significance of first-pass metabolism. Alcoholism: Clinical and Experimental Research. 2006;30(7):1132–1142. doi: 10.1111/j.1530-0277.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- Leigh BC. Beliefs about the effects of alcohol on self and others. Journal of Studies of Alcohol. 1987;48:467–475. doi: 10.15288/jsa.1987.48.467. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, June HL. Neurobehavioral studies of ethanol reward and activation. Alcohol. 1990;7:213–219. doi: 10.1016/0741-8329(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Loh EW, Fann CS, Chang YT, Chang CJ, Cheng AT. Endogenous opioid receptor genes and alcohol dependence among Taiwanese men. Alcoholism: Clinical and Experimental Research. 2004;28:15–19. doi: 10.1097/01.ALC.0000106303.41755.B8. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Glatt SJ, Wall TJ. Meta-analyses of ADLH2 and ADH1B with alcohol dependence in Asians. Psychological Bulletin. 2006;132(4):607–621. doi: 10.1037/0033-2909.132.4.607. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Shea SH, Carr LG, et al. Binge drinking in Jewish and non-Jewish white college students. Alcoholism: Clinical and Experimental Research. 2002;26(12):1773–1778. doi: 10.1097/01.ALC.0000042150.71818.A0. 2002. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Connors GJ, Tucker JA, McCollam JB. Validation of the Sensation Scale, a measure of subjective physiological responses to alcohol. Behaviour Research and Therapy. 1980;18:37–43. doi: 10.1016/0005-7967(80)90067-4. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism: Clinical and Experimental Research. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- MacKillop J. Factor structure of the alcohol urge questionnaire under neutral conditions and during a cue-elicited urge state. Alcoholism: Clinical and Experimental Research. 2006;30:1315–1321. doi: 10.1111/j.1530-0277.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Stauffer R, Lee SM, Rohde CA. Naltrexone dampens ethanol-induced cardiovascular and hypothalamic-pituitary-adrenal axis activation. Neuropsychopharmacology. 2001;25:537–547. doi: 10.1016/S0893-133X(01)00241-X. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Thode CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology. 2000;22:480–492. doi: 10.1016/S0893-133X(99)00147-5. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hutchison KE, Rose JE, Kozink RV. DRD4 VNTR polymorphism is associated with transient fMRI-BOLD responses to smoking cues. Psychopharmacology. 2007;194(4):433–441. doi: 10.1007/s00213-007-0860-6. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego: Educational & Industrial Testing Service; 1971. [Google Scholar]
- Miranda R, Jr, MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, Swift R, Ray L, McGeary J. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcohol Clin Exp Res. 2008;32(3):489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Monti PM, MacKillop J. Advances in the treatment of craving for alcohol and tobacco. In: Miller PM, Kavanagh DJ, editors. Translation of Addictions Sciences into Practice: Update and Future Directions. Amsterdam, The Netherlands: Elsevier Press; 2008. [Google Scholar]
- Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, Brown RA, Gordon A, Abrams DB, Niaura RS, Asher MK. Naltrexone and cue exposure with coping and communication skills training for alcoholics: Treatment process and 1-year outcomes. Alcoholism: Clinical and Experimental Research. 2001;25:1634–1647. [PubMed] [Google Scholar]
- Newlin DB, Thompson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychological Bulleting. 1990;108(3):383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- O’Malley S, Maisto S. Factors affecting the perception of intoxication: dose, tolerance, and setting. Addictive Behaviors. 1984;9:111–120. doi: 10.1016/0306-4603(84)90049-2. [DOI] [PubMed] [Google Scholar]
- Oota H, Pakstis AJ, Bonne-Tamir B. The evolution and population genetics of the ADLH2 locus: Random genetic drift, selection, and low levels of recombination. Annals of Human Genetics. 2004;68(Pt. 2):93–109. doi: 10.1046/j.1529-8817.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- Osier MV, Pakstis AJ, Kidd JR. Linkage disequilibrium at the ADH2 and ADH3 loci and risk of alcoholism. American Journal of Human Genetics. 1999;64(4):1147–1157. doi: 10.1086/302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osier MV, Pakstis AJ, Goldman D, et al. A proline-threonine substitution in codon 351 of ADH1C is common in Native Americans. Alcoholism: Clinical and Experimental Research. 2002;26(12):1759–1763. doi: 10.1097/01.ALC.0000042013.13899.75. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Paylan SS, Gentes-Hawn A, Hoaken PNS. Alcohol affects executive cognitive functioning differentially on the ascending versus descending limb of the blood alcohol concentration curve. Alcoholism: Clinical and Experimental Research. 2003;27:773–779. doi: 10.1097/01.ALC.0000065434.92204.A1. [DOI] [PubMed] [Google Scholar]
- Pollock VE. Meta-analysis of subjective sensitivity to alcohol in sons of alcoholics. American Journal of Psychiatry. 1992;149:1534–1538. doi: 10.1176/ajp.149.11.1534. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, MacKillop J, Miranda R, Jr, Audette A, Swift R, Monti PM. Effects of naltrexone during the descending limb of the blood alcohol curve. American Journal on Addictions. doi: 10.1080/10550490802138400. in press. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: A double-blind placebo-controlled study. Archives of General Psychiatry. 2007;64(9):1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Ray LA, Meskew-Stacer S, Hutchison KE. The relationship between prospective self-rating of alcohol sensitivity and craving and experimental results from two alcohol challenge studies. Journal on Studies of Alcohol and Drugs. 2007;68:379–384. doi: 10.15288/jsad.2007.68.379. [DOI] [PubMed] [Google Scholar]
- Ray LA, Audette A, Dicristoforo S, Odell K, Kaiser A, Hutchison KE. Behavioral, laboratory, and genetic correlates of low level of response to alcohol; Poster presented at the annual meeting of the Research Society on Alcoholism; Chicago, IL. 2007a. [Google Scholar]
- Ray LA, Rhee SH, Stallings MS, Knopik V, Hutchison KE. Examining the heritability of a laboratory-based smoking endophenotype: Initial Results from an experimental twin study. Twin Research and Human Genetics. 2007b;10(4):546–553. doi: 10.1375/twin.10.4.546. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, Bryan A. Psychosocial predictors of treatment outcome, dropout, and change processes in a pharmacological clinical trial for alcohol dependence. Addictive Disorders and Their Treatment. 2006;5(4):179–190. [Google Scholar]
- Ray LA, McGeary J, Marshall E, Hutchison KE. Risk factors for alcohol misuse: Integrating heart rate response to alcohol, alcohol sensitivity, and personality factors. Addictive Behaviors. 2006;31:1959–1973. doi: 10.1016/j.addbeh.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene and sensitivity to the effects of alcohol in humans. Alcoholism: Clinical and Experimental Research. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Schinka JA, Town T, Abdullah L, Crawford FC, Ordorica PI, Francis E, Hughes P, Graves AB, Mortimer JA, Mullan M. A functional polymporphism within the µ-opioid receptor gene and risk for abuse of alcohol and other substances. Molecular Psychiatry. 2002;7:224–228. doi: 10.1038/sj.mp.4000951. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. Journal of Studies on Alcohol. 1980;41:242–249. doi: 10.15288/jsa.1980.41.242. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Archives of General Psychiatry. 1984;41:879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Ethanol-induced changes in body sway in men at high alcoholism risk. Archives of General Psychiatry. 1985;42:375–379. doi: 10.1001/archpsyc.1985.01790270065007. [DOI] [PubMed] [Google Scholar]
- Scuckit MA, Gould EO. A simultaneous evaluation of multiple markers of ethanol/placebo challenges in sons of alcoholics and controls. Archives of General Psychiatry. 1988;211:11–216. doi: 10.1001/archpsyc.1988.01800270019002. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Archives of General Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger JI. Alcohol challenges in young men from alcohol pedigrees and control families: A report from the COGA project. Journal of Studies on Alcohol. 1996;57:368–377. doi: 10.15288/jsa.1996.57.368. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Mazzanti C, Smith TL, Ahmed U, Radel M, Iwata N, Goldman D. Selective genotyping for the role of 5-HT2A, 5-HT2C, and GABA alpha 6 receptors and the serotonin transporter in the level of response to alcohol: a pilot study. Biological Psychiatry. 1999;45(5):647–651. doi: 10.1016/s0006-3223(98)00248-0. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J. The search for genes contributing to the low level of response to alcohol: patterns of findings across studies. Alcoholism: Clinical and Experimental Research. 2004;28(10):1449–1458. doi: 10.1097/01.alc.0000141637.01925.f6. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Danko GP. A cross-generational comparison of alcohol challenges at about age 20 in 40 father-offspring pairs. Alcoholism: Clinical and Experimental Research. 2005;29(11):1921–1927. doi: 10.1097/01.alc.0000187154.94681.65. [DOI] [PubMed] [Google Scholar]
- Soderlund H, Parker ES, Schwartz BL, Tulving E. Memory encoding and retrieval on the ascending and descending limbs of the blood alcohol concentration curve. Psychopharmacology. 2005;182:305–317. doi: 10.1007/s00213-005-0096-2. [DOI] [PubMed] [Google Scholar]
- Suh JJ, Pettinati HM, Kampman KM, O’Brien CP. The status of disulfiram: a half of a century later. Journal of Clinical Psychopharmacology. 2006;26(3):290–302. doi: 10.1097/01.jcp.0000222512.25649.08. [DOI] [PubMed] [Google Scholar]
- Swift RM, Whelihan W, Kusnetsov O, Buongiorno G, Hsuing H. Naltrexone-induced alterations in human ethanol intoxication. American Journal of Psychiatry. 1994;151:1463–1467. doi: 10.1176/ajp.151.10.1463. [DOI] [PubMed] [Google Scholar]
- Tan E, Tan C, Karupathivan U, Yap EP. Mu opioid receptor gene polymorphisms and heroin dependence in Asian populations. Neuroreport. 2003;14:569–572. doi: 10.1097/00001756-200303240-00008. [DOI] [PubMed] [Google Scholar]
- Thomasson HR, Edenberg HJ, Crabb DW, et al. Alcohol and aldehyde dehydrogenase genotypes and alcoholism in Chinese men. American Journal of Human Genetics. 1991;48(4):677–681. [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranda R, Jr, McGeary JE, MacKillop J, Swift RM, Abrams DB, Shiffman S, Paty JA. Moderators of naltrexone’s effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcoholism: Clinical and Experimental Research. 2008;32(1):58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki S, Saito T, Nabeshima A, Hatta S, Watanabe M, Takahata N. Changes in GABAA receptor function and cross-tolerance to ethanol in diazepam-dependent rats. Alcoholism: Clinical and Experimental Research. 1996;20(1 Suppl):40A–44A. doi: 10.1111/j.1530-0277.1996.tb01726.x. [DOI] [PubMed] [Google Scholar]
- Town T, Abdullah L, Crawford F, Schinka J, Ordorica PI, Francis E, Hughes P, Duara R, Mullan M. Association of a functional µ-opioid receptor allele (+118A) with alcohol dependency. American Journal of Medical Genetics. 1999;88:458–461. [PubMed] [Google Scholar]
- Tsuang MT, Faraone SV, Lyons MJ. Identification of the phenotype in psychiatric genetics. European Archives of Psychiatry and Clinical Neuroscience. 1993;243:131–142. doi: 10.1007/BF02190719. [DOI] [PubMed] [Google Scholar]
- Viken RJ, Rose RJ, Morzorati SL, Christian JC, Li T-K. Subjective intoxication in response to alcohol challenge: Heritability and covariation with personality, breath alcohol level, and drinking history. Alcoholism: Clinical and Experimental Research. 2003;27(5):795–803. doi: 10.1097/01.ALC.0000067974.41160.95. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Watson NT, King AC, Sherman CE, O’Brien CP. Effects of naltrexone on alcohol “high” in alcoholics. American Journal of Psychiatry. 1995;152:613–615. doi: 10.1176/ajp.152.4.613. [DOI] [PubMed] [Google Scholar]
- Wall TL, Carr LG, Ehlers CL. Protective association of genetic variation in alcohol dehydrogenase with alcohol dependence in Native American Mission Indians. American Journal of Psychiatry. 2003;160(1):41–46. doi: 10.1176/appi.ajp.160.1.41. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whitfield JB. Alcohol dehydrogenase and alcohol dependence: Variation in genotype-associated risk between populations. American Journal of Human Genetics. 2002;71(5):1247–1250. doi: 10.1086/344287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Review. 1987;94:469–492. [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by A118G variant. Journal of Biological Chemistry. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]


