Abstract
Aims/hypothesis
Maternal metabolic alterations are essential to achieve healthy pregnancy outcomes, but increasing maternal parity may be associated with long-term metabolic dysfunction risk. As existing data are limited by study design, our aim was to employ robust metabolic measures to determine whether or not physiological pregnancy alterations in maternal metabolic function persist at 1 year postpartum.
Methods
We evaluated 21 healthy women, of whom 11 had an interval pregnancy (IP) and assessment at preconception, during pregnancy and 1 year postpartum, and 10 had no IP and assessment at baseline and a 1 year interval. Assessment measures included body composition, insulin sensitivity and response, and basal metabolic rate. For each measure, IP vs no IP and time intervals within each group were compared using nonparametric analyses, reporting median (IQR).
Results
IP and no IP women were similar at enrolment, and no IP women had similar metabolic profiles at enrolment and the 1 year interval. IP women exhibited expected metabolic changes during pregnancy compared with preconception. In IP women, preconception and postpartum measures, including fat mass (20.7 [13.7–37.4] kg vs 18.4 [13.8–41.3] kg; p = 0.2), total insulin response (AUC 11,459 [9,230–13,696] pmol/ml × min vs 11,522 [5,882–17,404] pmol/ml × min; p = 0.9), insulin sensitivity (0.12 [0.06–0.13] mg [kg fat-free mass (FFM)]−1min−1 vs 0.11 [0.10–0.15] mg [kg FFM]−1min−1; p=0.1) and basal metabolic rate (0.092 [0.092–0.105] kJ min−1 FFM vs 0.096 [0.088–0.096] kJ min−1 FFM; p=0.5), were similar.
Conclusions/interpretation
Our findings suggest pregnancy might not irreversibly alter maternal metabolic profile, measured at preconception through to 1 year postpartum. This result might be explained by a return to pre-pregnancy weight.
Keywords: Basal metabolic rate, Insulin response, Insulin sensitivity, Maternal metabolism, Preconception, Pregnancy
Introduction
Pregnancy is characterised by a 50% decrease in insulin sensitivity from early pregnancy through to the third trimester [1]. This change is an essential metabolic adaptation that facilitates provision of maternal nutrients to the growing fetus [2–4]. Postpartum return to preconception insulin resistance and sensitivity would be an equally important physiological and metabolic adaptation. Without it, one can presume pregnancy itself, or increasing parity, has a long-lasting adverse impact on maternal metabolism.
Pregnancy has been viewed as a window to future health, with diagnoses such as gestational diabetes and hypertension, and pre-eclampsia acting as potential early markers of later metabolic and cardiovascular dysfunction [5]. Beyond these diagnoses, pregnancy itself has been implicated in future metabolic alterations. Large-scale epidemiological studies have reported associations with increasing parity and risk of metabolic syndrome [6–8] and type 2 diabetes mellitus [9, 10]. Causal relationships, however, cannot be established in these retrospective or secondary analyses, and they have not compared pre- with post-pregnancy metabolic profiles. On the contrary, small, robust human studies using the euglycaemic–hyperinsulinaemic clamp suggest a postpartum trend towards preconception metabolic profile. In a cross-sectional study, insulin sensitivity 3 days postpartum approached that of non-pregnant women with normal glucose tolerance [11]. Insulin sensitivity in normoglycaemic obese women was higher at 16 weeks postpartum compared with the third trimester [12] and in lean women showed a significant (74%) improvement when 1 year postpartum measures were compared with late pregnancy measures [2]. None of these studies, however, included pre-pregnancy metabolic measures, so whether or not pre- and post-pregnancy metabolic profiles differ remains uncertain.
Understanding the relationship between changes in maternal metabolism before and after pregnancy is essential, as clues to future health and long-term chronic disease risk may be evident during pregnancy and the postpartum period [5]. If specific abnormalities in the maternal metabolic profile persist postpartum, these may provide clues as to where efforts should be directed during pregnancy to potentially ameliorate these long-term metabolic stresses. We hypothesised that the pregnancy-associated changes in insulin sensitivity, beta cell function and basal metabolic rate, each related to long-term metabolic health of the individual, persist at 1 year postpartum. To test this hypothesis and overcome limitations of published work, we performed a long-term longitudinal, observational study of metabolic profile measures in women at preconception, in late pregnancy and approximately 1 year after delivery.
Methods
Study participants
We conducted a prospective, longitudinal, observational study in the Clinical Research Unit (CRU) of the Clinical and Translational Science Collaborative at MetroHealth Medical Center, Case Western Reserve University (Cleveland, OH, USA). The study protocol was approved by the Institutional Review Board at MetroHealth Medical Center. Healthy, non-pregnant women were recruited via local advertisements and mass media. Women were enrolled as a convenience sample as they presented in response to recruitment efforts and met inclusion criteria. Each participant provided written informed consent before study enrolment.
Inclusion criteria for any individual consisted of women aged 20–45 years with no history of glucose intolerance, hyperlipidaemia or hypertension. The women were non-smokers and not using hormonal contraception at enrolment or at any point throughout the study procedures. All participants were in the follicular phase of a normal menstrual cycle and not lactating for study procedures. Confirmation that inclusion criteria were met was ascertained at recruitment via patient-reported history and at an initial study visit via physical exam and laboratory testing. No enrolled women were excluded at this stage. Women were not matched to study group, but over 10 years, women who planned a pregnancy and conceived comprised the interval pregnancy (IP) group. Women who were evaluated but did not become pregnant for various reasons became our comparison, no IP, group.
For women in the IP group, anthropometric and metabolic measurements were assessed at enrolment (preconception), 34–36 weeks of pregnancy (pregnancy), and approximately 1 year postpartum following a term delivery (postpartum). Postpartum study procedures were conducted after cessation of lactation and after monthly menses had resumed.
Women in the no IP group provided an internal control of the methodologies and possible metabolic change over time. Anthropometric and metabolic measurements, which were identical to study group women, were assessed at enrolment (baseline) and approximately 1 year later (1 year interval).
Study procedures
All enrolled women had a single consultation with a nutritionist 2 weeks before their first CRU study visit. Study procedures were explained, and participants were instructed to follow a diet low in simple sugars and saturated fat and high in complex carbohydrates, and to maintain a 3 day dietary intake log. Activity was assessed by the self-administered Minnesota Leisure Time Physical Activity Questionnaire [13]. Subsequently, participants attended three separate study visits in the CRU to complete all anthropometric and metabolic measurements specified for each study time point.
Anthropometric measurements
Height without shoes was measured with a stadiometer to the nearest 1.0 mm and weight with a tared hospital gown was measured with a calibrated scale (Toledo; Toledo, OH, USA) to the nearest 0.01 kg. BMI was calculated and represented as kg/m2.
Body composition analysis
Body composition was estimated using hydrodensitometry with adjustment for residual lung volume using a nitrogen washout technique, as previously described [14]. Calculations of percentage body fat were specific to non-pregnant [15, 16], late gestation [17] and postpartum [16] time points. Total fat mass and fat-free mass, reported to the nearest 0.01 kg, were measured using the same technique.
IVGTT
The IVGTT was performed with a 0.5 g/kg glucose load as previously described and specific to an ideal body weight <120% and >120% of ideal body weight [3]. The AUCs for the first phase (0–5 min; pmol/ml×min), second phase (>5–60 min; pmol/ml×min) and total insulin responses (0–60 min; pmol/ml×min) were measured using the trapezoidal rule [3].
Insulin sensitivity
Insulin sensitivity was estimated using the hyperinsulinaemic–euglycaemic clamp, performed after an 8 h fast, as described by DeFronzo et al [18]. The clamp was based on a 40 mU/m2 insulin infusion for 2 h. Steady state was measured during the last 40 min of the glucose infusion. The variable glucose infusion rate maintained plasma glucose level constant at 5 mmol/l (90 mg/dl). We report insulin sensitivity as the glucose infusion rate divided by the mean insulin concentration achieved during the clamp, defined for these purposes as the insulin sensitivity index (ISI; mg [kg fat-free mass (FFM)]−1min−1). Basal endogenous (hepatic) glucose production was measured using 6-62H2 glucose, as previously described by Steele [19], in seven women in the IP group (mg [kg FFM]−1min−1).
Indirect calorimetry
Resting metabolic rate was measured using indirect calorimetry as previously described [3], continuously for 1 h before the clamp. The route of nutrient use, oxidation or storage (glycogen and lipogenesis) was calculated, adjusting for urine urea nitrogen excretion. We measured resting metabolic rate (kJ/min), carbohydrate (mg/min) and fat oxidation per min (mg/min), and adjusted initial robust measures for FFM (kJ min−1 FFM, mg min−1 FFM).
OGTT
During pregnancy, after a 10 h overnight fast, women in the IP group were administered a 100 g 3 h OGTT. Gestational diabetes was diagnosed using criteria established by the National Diabetes Data Group [20]. These criteria were standard during the time our study was conducted, and 27% (3/11) were diagnosed with gestational diabetes mellitus. These three women remained in the IP group for all analyses.
Statistical analysis
We compared enrolment demographic, medical history, laboratory data and study specific metabolic measurements between no IP (baseline) and IP (preconception) groups using Fisher’s Exact for categorical and Wilcoxon rank-sum tests for continuous variables. Continuous metabolic measures for no IP group women were compared between baseline and the 1 year interval using the Wilcoxon signed-rank test. Metabolic measures for IP group women were compared between preconception and pregnancy, and preconception and postpartum time points separately. The Wilcoxon signed-rank test was used for these paired comparisons. Parametric analyses were also performed, with results consistent with nonparametric analyses. We report total n (%) and medians with interquartile range (IQR) for categorical and nonparametric continuous variables, respectively, and p<0.05 was considered significant.
Results
At total of 21 women were enrolled and all completed the study procedures. Eleven women comprised the IP group and 10 comprised the no IP group. At enrolment, the groups had similar baseline characteristics and enrolment weight and BMI (Table 1). In addition, all women were normoglycaemic and had normal hepatic, thyroid and renal function, and all had similar activity levels as measured by the Minnesota Leisure Time Physical Activity Questionnaire (data not shown). The time from enrolment to the 1 year interval in the no IP group was 13.4±1.4 months, and from preconception to postpartum in the IP group was 31.9±15.1 months.
Table 1.
Enrolment/preconception characteristics of IP vs no IP groups
| Characteristic | IP group (n=11) | No IP group (n=10) |
|---|---|---|
| Median maternal age, years (IQR) | 29 (27–36) | 35 (30–40) |
| Race | ||
| White, n (%) | 10 (91) | 9 (90) |
| Non-white, n (%) | 1 (9) | 1 (10) |
| Parous | ||
| Yes, n (%) | 2 (18) | 3 (30) |
| Education | ||
| High school, n (%) | 2 (18) | 2 (20) |
| College/graduate school, n (%) | 9 (82) | 8 (80) |
| Diabetes in first-degree relative | ||
| Yes, n (%) | 3 (27) | 4 (40) |
| Median weight, kg (IQR) | 59.7 (53.5–85.6) | 65.5 (56.3–86.9) |
| Median height, cm (IQR) | 165.4 (163.2–168.8) | 164.3 (161.5–167.9) |
| Median BMI, kg/m2 (IQR) | 23.8 (19.2–31.4) | 24.2 (20.6–34.8) |
p value is non-significant (p≥0.05) for all IP vs No IP comparisons
No IP group
The 10 women in the no IP group provided an internal control to demonstrate reproducibility of study measures and to evaluate any expected change over time in the absence of pregnancy. Baseline no IP group and preconception IP group women had similar body composition, insulin response, ISI and metabolic rate (Table 2). Among the no IP group, no study measures changed significantly over the 1-year study interval (Table 3).
Table 2.
Enrolment/preconception metabolic measures of IP vs no IP groups
| Characteristic | IP group (n=11) | No IP group (n=10) |
|---|---|---|
| Body composition | ||
| Weight, kg | 59.7 (53.5–85.6) | 65.5 (56.3–86.9) |
| FFM, kg | 42.5 (39.0–49.2) | 45.4 (41.8–48.5) |
| Fat mass, kg | 20.7 (13.7–37.4) | 19.1 (13.1–39.2) |
| Body fat, % | 30.0 (24.1–43.8) | 29.6 (24.2–46.0) |
| Insulin response, pmol/ml×min | ||
| 1st phase | 1,472 (1,229–2,188) | 1,278 (875–2,278) |
| 2nd phase | 9,181 (7,799–11,911) | 10,793 (4,257–15,939) |
| Total response | 11,459 (9,230–13,696) | 11,945 (5,237–23,384) |
| Insulin sensitivity, mg (kg FFM)−1min−1 | 0.12 (0.06–0.13) | 0.12 (0.06–0.14) |
| Indirect calorimetry | ||
| Basal metabolic rate, kJ min−1 FFM | 0.092 (0.092–0.105) | 0.090 (0.080–0.096) |
| Carbohydrate oxidation, mg min−1 FFM | 2.67 (1.69–3.53) | 2.07 (1.72–2.76) |
| Fat oxidation, mg min−1 FFM | 0.89 (0.67–1.03) | 0.75 (0.60–0.95) |
p value is non-significant (p≥0.05) for all IP vs no IP comparisons
All values are median (IQR)
Table 3.
Enrolment vs 1 year interval metabolic measures in the no IP group
| Characteristic | Enrolment | 1-year interval |
|---|---|---|
| Body composition | ||
| Weight, kg | 65.5 (56.3–86.9) | 65.1 (57.9–91.6) |
| FFM, kg | 45.4 (41.8–48.5) | 45.3 (41.2–48.4) |
| Fat mass, kg | 19.1 (13.1–39.2) | 20.5 (15.3–40.4) |
| Body fat, % | 29.6 (24.2–46.0) | 33.1 (26.1–44.2) |
| Insulin response, pmol/ml×min | ||
| 1st phase | 1,278 (875–2,278) | 1,132 (667–2,354) |
| 2nd phase | 10,793 (4,257–15,939) | 9,862 (4,361–19,245) |
| Total response | 11,945 (5,237–23,384) | 11,598 (5,834–20,689) |
| Insulin sensitivity, mg (kg FFM)−1min−1 | 0.12 (0.06–0.14) | 0.13 (0.08–0.15) |
| Indirect calorimetry | ||
| Basal metabolic rate, kJ min−1 FFM | 0.090 (0.080–0.096) | 0.088 (0.088–0.092) |
| Carbohydrate oxidation, mg min−1 FFM | 2.07 (1.72–2.76) | 2.28 (1.61–2.63) |
| Fat oxidation, mg min−1 FFM | 0.75 (0.60–0.95) | 0.90 (0.69–1.11) |
p value is non-significant (p≥0.05) at enrolment and the 1 year interval
All values are median (IQR)
IP group
The metabolic changes from preconception through to late pregnancy were as anticipated and as previously reported (Table 4) [1, 4]. Maternal fat mass and percentage body fat, however, were not significantly different in pregnancy compared with preconception. All preconception compared with postpartum measures are shown in Table 4 and are summarised below.
Table 4.
IP group metabolic measures preconception vs pregnancy and preconception vs postpartum
| Characteristic | Preconception | Pregnancy | Preconception vs pregnancy |
Postpartum | Preconception vs postpartum |
|---|---|---|---|---|---|
| p | p | ||||
| Body composition | |||||
| Weight, kg | 59.7 (53.5–85.6) | 77.2 (62.9–94.3) | 0.003 | 61.7 (54.9–89.1) | 0.100 |
| FFM, kg | 42.5 (39.0–49.2) | 54.7 (46.7–57.5) | 0.003 | 44.3 (38.8–49.6) | 0.400 |
| Fat mass, kg | 20.7 (13.7–37.4) | 24.2 (14.9–38.7) | 0.100 | 18.4 (13.8–41.3) | 0.200 |
| Body fat, % | 30.0 (24.1–43.8) | 30.6 (24.8–40.2) | 0.400 | 29.3 (25.2–46.2) | 0.400 |
| Insulin response, pmol/ml×min | |||||
| 1st phase | 1,472 (1,229–2,188) | 3,563 (1,577–4,598) | 0.003 | 1,500 (972–2,834) | 0.300 |
| 2nd phase | 9,181 (7,799–11,911) | 21,002 (18,397–36,350) | 0.003 | 8,265 (4,910–14,744) | 0.700 |
| Total response | 11,459 (9,230–13,696) | 24,273 (19,981–40,218) | 0.003 | 11,522 (5,882–17,404) | 0.900 |
| Insulin sensitivity, mg (kg FFM)−1min−1 | 0.12 (0.06–0.13) | 0.06 (0.04–0.08) | 0.003 | 0.11 (0.10–0.15) | 0.100 |
| BEGP, mg (kg FFM)−1min−1 [n=7] | 2.86 (2.79–3.00) | 3.37 (2.73–3.90) | 0.100 | 3.03 (2.80–3.42) | 0.200 |
| Indirect calorimetry | |||||
| Basal metabolic rate, kJ min−1 FFM | 0.092 (0.092–0.105) | 0.100 (0.096–0.105) | 0.070 | 0.096 (0.088–0.096) | 0.500 |
| Carbohydrate oxidation, mg min−1 FFM | 2.67 (1.69–3.53) | 2.80 (2.26–3.69) | 0.500 | 2.18 (1.65–3.31) | 0.500 |
| Fat oxidation, mg min−1 FFM | 0.89 (0.67–1.03) | 1.09 (0.62–1.38) | 0.300 | 1.03 (0.65–1.31) | 0.500 |
| Indirect calorimetry, robust | |||||
| Basal metabolic rate, kJ/min | 4.23 (3.68–4.72) | 5.06 (4.44–6.32) | 0.003 | 4.06 (3.43–5.06) | 0.300 |
| Carbohydrate oxidation, mg/min | 117.3 (83.8–161.0) | 146.0 (114.0–179.0) | 0.080 | 97.7 (75.9–145.1) | 0.300 |
| Fat oxidation, mg/min | 38.3 (27.9–48.6) | 6.3 (33.1–73.5) | 0.080 | 43.1 (31.2–63.9) | 0.500 |
All values are median (IQR)
Body composition measures included weight, FFM, fat mass and percentage body f a t estimated using hydrodensitometry with correction for residual lung volume. Preconception and postpartum measures were similar for weight, FFM, fat mass and percentage body fat.
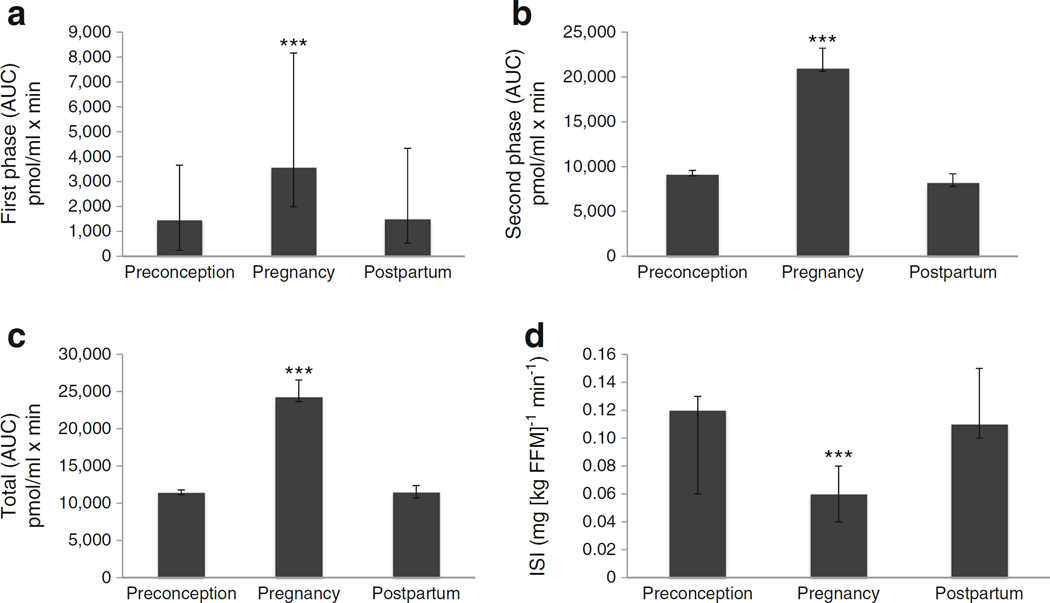
Insulin response, measured using the IVGTT, differed across preconception, pregnancy and postpartum time points among women in the IP group. Preconception and postpartum responses were similar for first phase, second phase and total insulin response (Table 4, Fig. 1). ISI, estimated using the hyperinsulinaemic–euglycaemic clamp, varied across study time points among the IP group. Preconception and postpartum ISI were similar (Table 4, Fig. 1). A subset of seven women in the IP group also had assessment of basal endogenous glucose production (BEGP). Preconception and postpartum BEGP was similar.
Fig. 1.
Measures of insulin response and sensitivity (ISI) among women in the IP group across preconception, pregnancy and postpartum study time points. (a) First phase insulin response, (b) second phase insulin response, (c) total insulin response and (d) ISI. ***p<0.001 vs preconception. Histograms, median. Error bars, IQR
Resting metabolic rate and carbohydrate and fat oxidation are reported, both adjusted for FFM and as robust unadjusted measures. Preconception and postpartum measures for all these variables were similar.
Metabolic measures that were significantly different preconception compared with pregnancy, and those that were similar preconception compared with postpartum, were also significantly different when pregnancy was compared with postpartum (data not shown).
Discussion
Our results demonstrate a return to preconception metabolic profile at 1 year postpartum. Specifically, we found expected pregnancy-related increases in maternal weight, FFM and insulin response, and decreased insulin sensitivity; each pregnancy-related change had returned to pre-pregnancy indices by 1 year postpartum. These measures were not significantly different at 1 year postpartum compared with preconception.
Our data extend the return to preconception metabolic status suggested by other studies that employed similar techniques for measuring insulin sensitivity and response. In an early, small study of four postpartum women, Ryan et al reported that insulin sensitivity at 3 days postpartum was similar to measurements in a non-pregnant control group of seven women [11]. Sivan et al reported changes from the second to third trimester and at approximately 16 weeks postpartum in a small cohort of obese women [12]. These authors reported significant postpartum improvement in insulin resistance compared with late third trimester, demonstrated by increased insulin-stimulated glucose disappearance and carbohydrate oxidation, and less insulin suppression of endogenous glucose production [12]. Such early postpartum improvements appear to continue, as shown by our 1 year postpartum data. Finally, Kirwan et al reported a 74% improvement in insulin sensitivity at 1 year postpartum compared with the late third trimester [2]. Our data demonstrate a similar improvement of 83% over the same time interval but also document that insulin sensitivity at 1 year postpartum mirrors preconception values. Overall, each of the earlier studies cast some doubt on the proposed link between parity and potentially irreversible metabolic changes but none could make any direct comparisons with a pre-pregnant condition. The current analysis does exactly that, and in contrast to our hypothesis, preconception and 1 year postpartum metabolic profile are similar.
Our data, however, contradict literature that has reported an association between parity and persistent alternations in insulin resistance, insulin sensitivity and basal metabolic rate. Large-scale cohort studies have followed or retrospectively assessed whether or not increased parity is linked to diabetes mellitus or metabolic syndrome. In the ARIC (Atherosclerosis Risk in Communities) study, adjusted logistic regression models showed the highest incidence rates of type 2 diabetes mellitus among women with five or more live births, although the authors acknowledged that much of this risk was also linked to sociodemographic factors and obesity [9]. A Chinese cohort similarly suggested a significant increase in type 2 diabetes mellitus risk with two, three or four or more live births, with a reported risk as much as 1.44 times that of nulliparous women [21]. A limitation common to both studies is the lack of important confounders, such as history of gestational diabetes, gestational weight gain and most importantly postpartum weight retention [9, 21]. Parity and metabolic syndrome, defined as prediabetes in some publications, have been linked with parity less consistently in other long-term cohort studies, which are also limited by study design and unable to establish causal relationships [6–8].
Strengths of the present study include its longitudinal design, internal control group and robust metabolic measures. Enrolling women at preconception enabled direct comparison of all metabolic measures from preconception through to late pregnancy and at 1 year postpartum. A similar cohort of women, whose metabolic profiles were measured at baseline and at a 1 year interval, in the absence of an IP, provided an important internal control of the effect of time and methodologies. As anticipated, all enrolled women (both IP and no IP) had overall similar metabolic profiles at the initial measure, and this did not change significantly over 1 year in women without an IP. Furthermore, specific measures of maternal body composition, insulin response and sensitivity, glucose production, and carbohydrate and fat oxidation extend beyond data available in most published work.
Some limitations must also be addressed. Other variables could impact a postpartum return of preconception metabolic profile but these were not specifically measured in this study. Activity and lactation, each of which has been linked to weight retention and loss [22–24], were measured to some degree. Activity was assessed with an objective questionnaire and was similar at baseline, and lactation was reported among nine of 11 postpartum women. However, activity was not measured postpartum, and lactation intensity and longevity was not quantified. Both activity and lactation might contribute to improved postpartum metabolic profile [22–24]. While these data are important, their absence does not alter the objective findings that a return to preconception insulin response and sensitivity accompanied a return to baseline body composition. While our sample size is small and we cannot rule out the possibility of type II error, replicating this longitudinal study with a larger sample would be impractical. Finally, these data in predominantly normal or overweight, white, educated women may not be generalisable to obese women and would need to be measured in that population. Nonetheless, these limitations do not take away from our findings but instead highlight essential data to be included in future research. Our robust metabolic assessments contribute important information on the metabolic changes from preconception through to the postpartum period and offer an alternative to the growing assumption that pregnancy itself alters maternal metabolism.
Our data did not support our hypothesis that pregnancy-associated changes in insulin sensitivity, or beta cell function, and basal metabolic rate would persist at 1 year postpartum. Instead, the current analyses suggest that the metabolic changes of pregnancy may not lead to permanent negative alterations in maternal metabolism. Others have shown excess gestational weight gain is associated with weight retention at 6 months postpartum [25]. Furthermore, increased weight is linked to decreased insulin resistance, as originally measured in a Pima Indian cohort by Swinburn et al [26]. Increasing parity, with weight gain and additional weight retention, may then indirectly be associated with metabolic syndrome and risk of glucose intolerance. For those women who are overweight or obese, a return to preconception weight is the minimal requirement. A 5–7% decrease, compared with preconception weight, may improve maternal metabolic condition for subsequent pregnancies [27]. Although our data reflect measurements in a predominantly normal and overweight group of women, we suggest that the return to preconception weight in our cohort may partially explain the parallel return to preconception metabolic profile. Until more definitive data become available, avoiding excessive gestational weight gain, defined by the 2009 Institute of Medicine criteria [28], remains advisable for all pregnant women.
Acknowledgments
We thank the nurses and staff of the General Clinical Research Center (now called the Clinical Research Unit) of the Clinical and Translational Science Collaborative at MetroHealth Medical Center, Case Western Reserve University (Cleveland, OH, USA), without whose assistance these studies would not have been possible.
Funding This work was supported by National Institutes of Health (NIH) grant no. HD-22965-19 and, in part, by the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- BEGP
Basal endogenous glucose production
- CRU
Clinical Research Unit
- FFM
Fat-free mass
- IP
Interval pregnancy
- IQR
Interquartile range
- ISI
Insulin sensitivity index
Footnotes
A portion of these data was presented in abstract format at the 70th Scientific Sessions of The American Diabetes Association (2010); abstract number 1950-P.
Contribution statement EKB wrote the manuscript, and researched and analysed the data. LP researched the data and contributed to the review of the manuscript review. SBA assisted with data analysis and provided critical review of manuscript drafts. SHM researched the data and provided critical review of manuscript drafts. PMC researched the data and provided critical review of all analysis and manuscript drafts. EKB is responsible for the integrity of the work as a whole and is the guarantor.
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 1991;165:1667–1672. doi: 10.1016/0002-9378(91)90012-g. [DOI] [PubMed] [Google Scholar]
- 2.Kirwan JP, Varastehpour A, Jing M, et al. Reversal of insulin resistance postpartum is linked to enhanced skeletal muscle insulin signaling. J Clin Endocrinol Metab. 2004;89:4678–4684. doi: 10.1210/jc.2004-0749. [DOI] [PubMed] [Google Scholar]
- 3.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–916. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 4.Catalano PM, Tyzbir ED, Wolfe RR, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol. 1993;264:E60–E67. doi: 10.1152/ajpendo.1993.264.1.E60. [DOI] [PubMed] [Google Scholar]
- 5.Saade GR. Pregnancy as a window to future health. Obstet Gynecol. 2009;114:958–960. doi: 10.1097/AOG.0b013e3181bf5588. [DOI] [PubMed] [Google Scholar]
- 6.Al-Farsi YM, Brooks DR, Werler MM, Cabral HJ, Al-Shafei MA, Wallenburg HC. Effect of high parity on the occurrence of prediabetes: a cohort study. Acta Obstet Gynecol Scand. 2010;89:1182–1186. doi: 10.3109/00016349.2010.501854. [DOI] [PubMed] [Google Scholar]
- 7.Gunderson EP, Jacobs DR, Jr, Chiang V, et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol. 2009;201:e1–e9. doi: 10.1016/j.ajog.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akter S, Jesmin S, Rahman MM, et al. Higher gravidity and parity are associated with increased prevalence of metabolic syndrome among rural Bangladeshi women. PLoS One. 2013;8:e68319. doi: 10.1371/journal.pone.0068319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholson WK, Asao K, Brancati F, Coresh J, Pankow JS, Powe NR. Parity and risk of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2006;29:2349–2354. doi: 10.2337/dc06-0825. [DOI] [PubMed] [Google Scholar]
- 10.Kritz-Silverstein D, Barrett-Connor E, Wingard DL. The effect of parity on the later development of non-insulin-dependent diabetes mellitus or impaired glucose tolerance. N Engl JMed. 1989;321:1214–1219. doi: 10.1056/NEJM198911023211802. [DOI] [PubMed] [Google Scholar]
- 11.Ryan EA, O'Sullivan MJ, Skyler JS. Insulin action during pregnancy. Studies with the euglycemic clamp technique. Diabetes. 1985;34:380–389. doi: 10.2337/diab.34.4.380. [DOI] [PubMed] [Google Scholar]
- 12.Sivan E, Chen X, Homko CJ, Reece EA, Boden G. Longitudinal study of carbohydrate metabolism in healthy obese pregnant women. Diabetes Care. 1997;20:1470–1475. doi: 10.2337/diacare.20.9.1470. [DOI] [PubMed] [Google Scholar]
- 13.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity–diabetes link. Diabetes. 1994;43:1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 15.Behnke AR. Anthropometric fractionation of body weight. J Appl Physiol. 1961;16:949–954. doi: 10.1152/jappl.1961.16.6.949. [DOI] [PubMed] [Google Scholar]
- 16.Brozek J. Body composition: the relative amounts of fat, tissue, and water vary with age, sex, exercise, and nutritional state. Science. 1961;134:920–930. doi: 10.1126/science.134.3483.920. [DOI] [PubMed] [Google Scholar]
- 17.Catalano PM, Wong WW, Drago NM, Amini SB. Estimating body composition in late gestation: a new hydration constant for body density and total body water. Am J Physiol. 1995;268:E153–E158. doi: 10.1152/ajpendo.1995.268.1.E153. [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 19.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N YAcad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 20.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 21.Tian Y, Shen L, Wu J, et al. Parity and the risk of diabetes mellitus among Chinese women: a cross-sectional evidence from the Tongji-Dongfeng cohort study. PLoS One. 2014;9:e104810. doi: 10.1371/journal.pone.0104810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkegaard H, Stovring H, Rasmussen KM, Abrams B, Sorensen TI, Nohr EA. How do pregnancy-related weight changes and breastfeeding relate to maternal weight and BMI-adjusted waist circumference 7 y after delivery? Results from a path analysis. Am J Clin Nutr. 2014;99:312–319. doi: 10.3945/ajcn.113.067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuebe AM, Kleinman K, Gillman MW, Rifas-Shiman SL, Gunderson EP, Rich-Edwards J. Duration of lactation and maternal metabolism at 3 years postpartum. J Womens Health (Larchmt) 2010;19:941–950. doi: 10.1089/jwh.2009.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunderson EP, Jacobs DR, Jr, Chiang V, et al. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: a 20-year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults) Diabetes. 2010;59:495–504. doi: 10.2337/db09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nohr EA, Vaeth M, Baker JL, Sorensen T, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr. 2008;87:1750–1759. doi: 10.1093/ajcn/87.6.1750. [DOI] [PubMed] [Google Scholar]
- 26.Swinburn BA, Nyomba BL, Saad MF, et al. Insulin resistance associated with lower rates of weight gain in Pima Indians. J Clin Invest. 1991;88:168–173. doi: 10.1172/JCI115274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wing RR, Bolin P, Brancati FL, et al. Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute of Medicine/National Research Council. Committee to Reexamine IOM Pregnancy Weight Guidelines, Food and Nutrition Board and Board on Children, Youth, and Families. Weight gain during pregnancy: reexamining the guidelines. National Academies Press; Washington, DC: 2009. [Google Scholar]