SUMMARY
Although microglia are implicated in nerve injury-induced neuropathic pain, how injured sensory neurons engage microglia is unclear. Here we demonstrate that peripheral nerve injury induces de novo expression of colony-stimulating factor 1 (CSF1) in injured sensory neurons. The CSF1 is transported to the spinal cord where it targets the microglial CSF1 receptor (CSF1R). Cre-mediated sensory neuron deletion of Csf1 completely prevented nerve injury-induced mechanical hypersensitivity and reduced microglia activation and proliferation. In contrast, intrathecal injection of CSF1 induces mechanical hypersensitivity and microglial proliferation. Nerve injury also upregulated CSF1 in motoneurons, where it is required for ventral horn microglial activation and proliferation. Downstream of CSF1R, we found that the microglial membrane adapter protein DAP12 is required for both nerve injury- and intrathecal CSF1-induced upregulation of pain-related microglial genes and the ensuing pain, but not for microglia proliferation. Thus, both CSF1 and DAP12 are potential targets for the pharmacotherapy of neuropathic pain.
INTRODUCTION
Neuropathic pain is a severe chronic pain condition characterized by ongoing mechanical hypersensitivity, where normally innocuous stimuli provoke intense pain1,2. As traditional pharmacotherapies are largely ineffective against neuropathic pain3, the search continues for mechanism(s) through which nerve damage triggers the pain. There is now considerable consensus that nerve damage alters pain transmission circuitry in the spinal cord dorsal horn2 and that microglia, the tissue-resident macrophages in the central nervous system (CNS)4,5, are important contributors to this process6–9. What underlies the activation of microglia, however, is still unclear. Interestingly, although activation of microglia is readily demonstrated after damage to the peripheral branch of the primary sensory neuron, microglia appear unresponsive to transection of the central branch, namely the dorsal root10 (Fig. 1a). Thus, injured sensory neurons in dorsal root ganglia (DRG) must release a signal that communicates with and activates spinal cord microglia1.
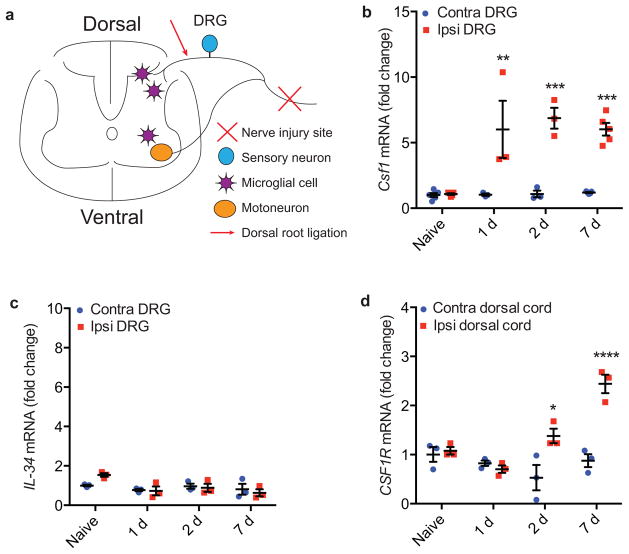
Figure 1. Csf1 and Csf1r are respectively induced in the DRG and dorsal spinal cord ipsilateral to the peripheral nerve injury.
(a) Schematic illustrating relevant neuroanatomy; (b) qRT-PCR illustrates Csf1 induction in the DRG ipsilateral to the peripheral nerve injury, compared to the contralateral side; (c) qRT-PCR shows that there is no induction of IL-34; (d) qRT-PCR illustrates Csf1r induction in the dorsal cord ipsilateral to the nerve injury compared to the contralateral side. N=3 mice/time point.
Although a host of studies have sought sensory neuron-derived factors, it is still unclear how injured neurons initiate microglia activation. For example, fractalkine (CX3CL1), a chemokine that is cleaved from the membrane of sensory neurons after peripheral nerve injury11, requires cathepsin S (CatS), a protease released by already activated microglia8. Thus, fractalkine may contribute to the maintenance of, but cannot be the initiating signal for microglia activation. Although the chemokines, CCL2 and CCL21 are reported to be induced in sensory neurons after nerve injury12,13, CCR2, the receptor for CCL2, is not expressed in microglia14, and deletion of CCL21 has no effect on nerve injury-induced microglia activation or proliferation13. Neuregulin-1 (NRG-1) has also been implicated, but NRG-1 is not induced in sensory neurons after nerve injury15. Another view holds that ATP released after nerve injury binds to the microglial P2X4 purinergic receptor to initiate microglia activation6,16. However, nerve injury-induced microglia activation is intact in P2X4 knockout mice17, and the source of ATP after nerve injury that binds the receptor has never been unequivocally identified6.
In addition to being activated, the microglia population expands after nerve injury18. Whether this expansion results from proliferation of local microglia or from infiltration of circulating monocytes is unclear. As both resident microglia and infiltrating monocytes express common markers, addressing the relative contribution of resident and infiltrating cells has been difficult. Using a model in which healthy bone marrow is transplanted into lethally irradiated recipients, Priller et al (2001) concluded that circulating monocytes infiltrate into the CNS and contribute to the expansion of the microglia population19. On the other hand, using chimeric mice generated by parabiosis, Ajami et al (2007) concluded that the microglia expansion in the facial nucleus after VIIth (facial) nerve injury or in the spinal cord in an ALS mouse model derives exclusively from self-renewal of resident microglia20. Regardless of the source of the proliferation, neither the identity nor the cellular origin of the factor(s) by which injured neurons trigger microglia proliferation in vivo is known.
To address these questions, here we performed RNA-Seq and recorded a significant induction of CSF1 (viz., macrophage colony stimulating factor, MCSF) in the injured DRG. The nerve injury-induced upregulation of CSF1 occurred not only in injured DRG sensory neurons, but also in ventral horn motoneurons. By Cre-mediated selective deletion of Csf1 from sensory neurons, we demonstrate that sensory neuron-derived CSF1 is required for the development of the neuropathic pain phenotype, as well as for microglia proliferation in the dorsal horn. Finally, we identified a critical downstream pathway in microglia, one that includes the membrane adaptor protein DAP12, in the generation of nerve injury and CSF1-induced neuropathic pain. However, nerve injury and CSF1-induced microglial proliferation are DAP12-independent.
RESULTS
De novo induction of CSF1 in injured sensory neurons
To identify the genes that are upregulated in DRG and dorsal horn after nerve injury and the signals through which injured sensory neurons interact with microglia to produce pain, we first performed an RNA-Seq analysis after nerve injury (Fig. 1a). Many studies have reported transcriptional changes after nerve injury, but few examined both DRG and spinal cord and most were performed using microarray21,22. We found a dramatic upregulation of colony-stimulating factor 1 (Csf1) in the ipsilateral DRG and of its receptor (Csf1r) in the ipsilateral dorsal cord after nerve injury (Supplementary Table 1). This finding is particularly important as CSF1 is an essential factor added to culture medium to expand microglia in vitro23, and CSF1R is required in vivo for microglia development24. In fact, Csf1r is among the earliest genes expressed in microglia progenitors in yolk sac during microglia development24,25. Importantly, the expression of IL-34, another CSF1R ligand26, did not change (Supplementary Table 1). qRT-PCR confirmed our finding that Csf1, but not Il-34, is induced in the DRG (Fig. 1b–c), and that Csf1r is induced in the dorsal spinal cord (Fig. 1d) after nerve injury.
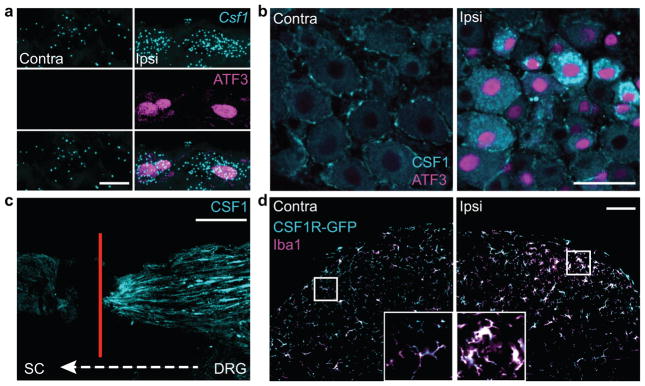
Subsequent in situ hybridization for Csf1 mRNA (Fig. 2a) and immunostaining for CSF1 protein (Fig. 2b) showed that CSF1 is induced in DRG neurons that co-expressed ATF3, a marker of cells with damaged peripheral axons27. In fact, one day after nerve injury, all CSF1-positive DRG neurons co-expressed ATF3 and ~80% of ATF3-positive neurons co-expressed CSF1 (Fig. 2b, Supplementary Fig. 1a–b). The CSF1 induction occurred in both small and large diameter DRG neurons (Fig. 2b), within 18 hours of the nerve injury and persisted for at least 3 weeks (Supplementary Fig. 1a–b). As we could not detect CSF1 in DRG neurons in the absence of injury (Fig. 2b), we conclude that nerve injury induces de novo CSF1 expression in the injured sensory neurons. We observed some CSF1 immunoreactivity in satellite cells of the DRG, but there was no change after nerve injury (Fig. 2b).
Figure 2. CSF1 is de novo induced in injured sensory neurons and transported to the spinal cord, where CSF1R is expressed exclusively in microglia.
(a) Co-expression of Csf1 mRNA (in situ hybridization) and ATF3 (immunostaining) in injured DRG neurons (1d post injury), compared to contralateral side. Scale bar: 10 μm; (b) Compared to the contralateral side, there is de novo CSF1 (immunostaining) in axotomized, ATF3 positive DRG neurons (1d post injury). Note that there is mild CSF1 immunoreactivity in satellite cells. Scale bar: 50 μm; (c) Concurrent L4 and L5 dorsal root ligation and peripheral nerve injury results in the accumulation of CSF1 protein (immunoreactivity) at the dorsal root ligature (4d post surgery). Red line denotes ligature site (see Fig. 1a). Scale bar: 200 μm; (d) Complete overlap of the microglial markers, Iba1 and GFP in the dorsal horn of a CSF1R-GFP reporter mouse. Both markers increase in the dorsal horn ipsilateral to the nerve injury (3d post injury) compared to the contralateral side. Inset: Control (left) and activated (right) microglia. Note the amoeboid morphology of activated microglia. Scale bar: 100 μm. Inset: maximum projection of Z-stack images.
Csf1 is transported to the spinal cord after nerve injury
To determine whether sensory neuron-derived de novo CSF1 is transported to the spinal cord, we ligated the L4 and L5 dorsal roots (between the DRG and spinal cord; Fig. 1a, arrow) after peripheral nerve injury and demonstrated accumulation of CSF1 at the ligatures (Fig. 2c). Co-expression in DRG neurons and at the ligature site of CSF1 and NPY (Supplementary Fig. 2a–b), a peptide that is upregulated in injured sensory neurons28, confirmed the intra-axonal transport of CSF1.
In spinal cord, CSF1R is expressed only in microglia
Next, using a CSF1R-GFP reporter mouse29, and by immunostaining for CSF1R, we found that CSF1R is expressed exclusively in spinal cord microglia and is indeed upregulated after nerve injury (Fig. 2d, Supplementary Fig. 2c–f). The fact that we did not observe a corresponding CSF1 increase in the dorsal horn suggests that CSF1 is rapidly released after its transport to the cord.
CSF1 is necessary and sufficient for microglia activation
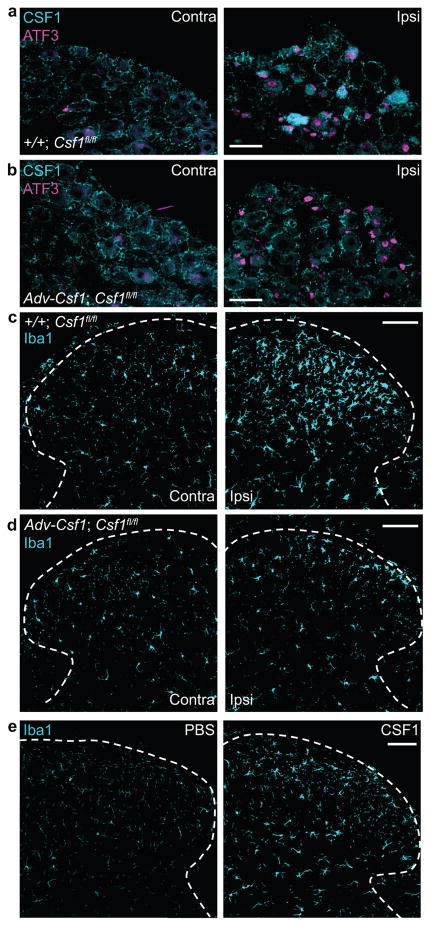
To investigate the functional significance of CSF1 upregulation in injured sensory neurons, we deleted Csf1 selectively from sensory neurons (Fig. 3a–b) by crossing a floxed Csf1 mouse30 with an Advillin-Cre mouse in which Cre-recombinase is expressed only in DRG sensory neurons31. Neither the morphology nor the density of spinal cord microglia contralateral to the nerve injury differed from that of wild type mice (Fig. 3c–d), indicating that microglial development is not compromised in these mice. However, nerve injury-induced microglia activation in the ipsilateral dorsal horn, demonstrated by increased Iba1 expression, was substantially reduced in these mice (Fig. 3c–d, Supplementary Fig. 3a), even though the ATF3 induction in the injured sensory neurons was preserved (Fig. 3b). To test whether CSF1, by itself, triggers microglial activation in vivo, we injected CSF1 intrathecally, once per day for three days and observed a profound activation of dorsal horn microglia, manifest as enhanced Iba1 expression (Fig. 3e, Supplementary Fig. 3b). Together, these results demonstrate that CSF1 induction in injured sensory neurons is necessary, and that CSF1 by itself, is sufficient, for nerve injury-induced microglia activation in the spinal cord dorsal horn.
Figure 3. Sensory neuron-derived CSF1 is necessary and CSF1, by itself, is sufficient for nerve injury-induced microglia activation in the dorsal horn.
(a) Injury-induced CSF1 and ATF3 in ipsilateral DRG neurons of a control mouse (+/+; Csf1 fl/fl) (3d post injury). Scale bar: 50 μm; (b) Despite complete loss of CSF1 induction in injured DRG neurons in Adv-Cre;Csf1 fl/fl mice (3d post injury), ATF3 expression persists. Note that the CSF1 immunoreactivity in satellite cells of the DRG is intact in the mutant mice. Scale bar: 50 μm; (c) Peripheral nerve injury-induced microglia activation (increased Iba1 expression) in the ipsilateral dorsal horn (3d post injury) in control animal (+/+; Csf1 fl/fl). Scale bar: 100 μm; (d) Csf1 deletion from sensory neurons (Adv-Cre; Csf1 fl/fl) reduces nerve injury-induced dorsal horn microglia activation. Note that the density and morphology of microglia in the spinal cord contralateral to the nerve injury is comparable between control (+/+; Csf1 fl/fl) and mutant (Adv-Cre; Csf1 fl/fl) mice. Scale bar: 100 μm. (e) Compared to PBS, intrathecal CSF1 activates microglia (increased Iba1 expression) in the dorsal horn. Scale bar: 100 μm. N=3 mice/condition.
CSF1 is necessary and sufficient for neuropathic pain
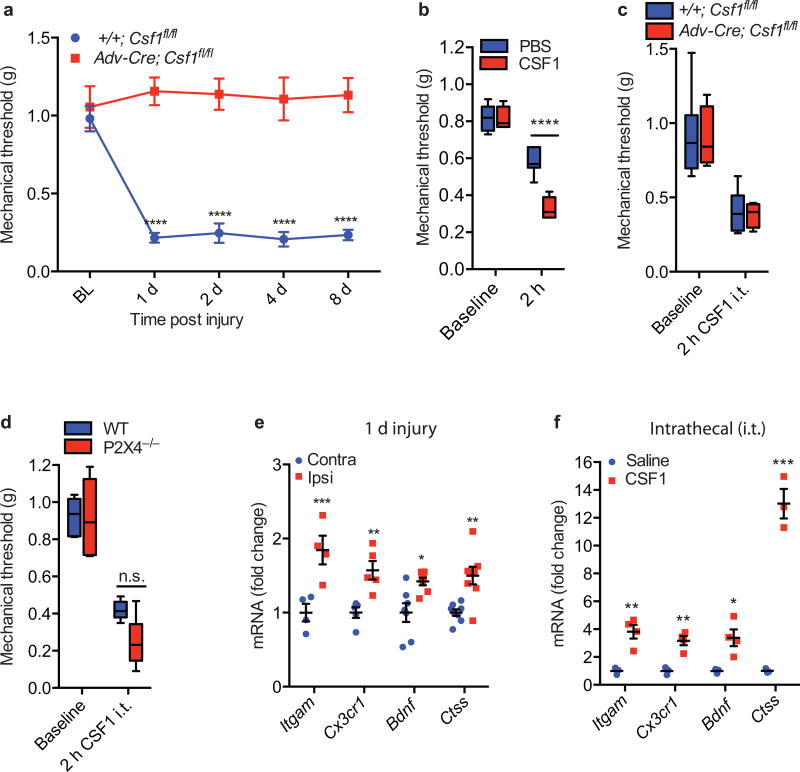
Next, we asked whether sensory neuron-derived CSF1 also contributes to the neuropathic pain produced by nerve injury. Fig. 4a and Supplementary Fig. 4a illustrate that Advillin-Cre-mediated Csf1 deletion from sensory neurons completely prevented nerve injury-induced mechanical hypersensitivity, the hallmark of neuropathic pain32. In these mutant mice, body weight (Supplementary Fig. 4b), motor activity (Supplementary Fig. 4c), response to acute noxious heat stimulation (Supplementary Fig. 4d–e), hindpaw formalin (inflammation)-induced nocifensive behaviors (Supplementary Fig. 4f) and numbers and neurochemical subpopulations of DRG neurons (Supplementary Fig. 4g–h) did not differ from wild type mice. Consistent with a sufficiency of the CSF1 contribution to the neuropathic pain phenotype, intrathecal injection of CSF1 provoked substantial mechanical hypersensitivity in both WT animals (Fig. 4b) and in the mice in which Csf1 was deleted from DRG neurons (Fig. 4c). Two hours after intrathecal CSF1 we also recorded morphological changes in dorsal horn microglia (Supplementary Fig 5a) and a small but substantial increase of Iba1 expression (Supplementary Fig 5b). Consistent with these findings, the microglia inhibitor, minocycline prevented the hypersensitivity produced by intrathecal CSF1 (Supplementary Fig 5c). Interestingly, although the P2X4 receptor is considered critical to the hypersensitivity following nerve injury6, intrathecal CSF1-induced mechanical hypersensitivity persists in P2X4 knockout mice (Fig. 4d).
Figure 4. Sensory neuron-derived CSF1 is necessary and CSF1, by itself, is sufficient for nerve injury-induced neuropathic pain (mechanical hypersensitivity).
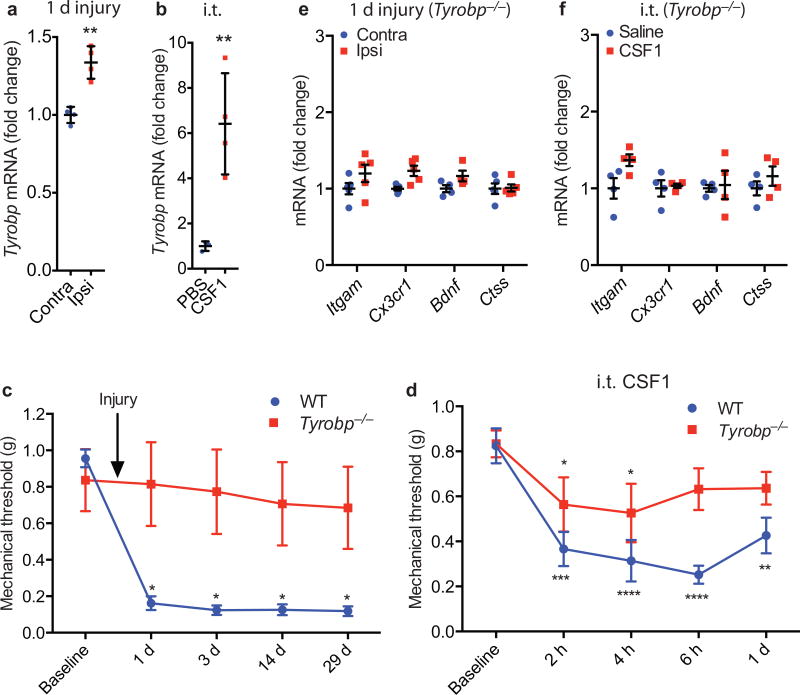
(a) Advillin-Cre mediated Csf1 deletion from sensory neurons prevents the development of nerve injury-induced mechanical hypersensitivity (n=5–6 mice/group); (b) The mechanical hypersensitivity produced by intrathecal CSF1 is significantly greater than that induced by the PBS vehicle (n=7 mice/group) and comparable to that produced by nerve injury; (c) Advillin-Cre mediated Csf1 deletion from sensory neurons had no effect on intrathecal CSF1-induced mechanical hypersensitivity (n=4–6 mice/group); (d) Intrathecal CSF1 induces comparable mechanical hypersensitivity in wild type and P2X4−/− mice (n=6 mice/group); (e) Neuropathic pain-related microglial genes are upregulated in the spinal cord 1 day post injury (n=4 mice/group); (f) Upregulation of the same set of microglial genes occurs in the dorsal horn after intrathecal injection of CSF1 (n=3–4 mice/group). Data are presented as mean ± SEM. n.s. represents “not significant”, with p=0.1332.
Finally, intrathecal CSF1 substantially upregulated several microglial genes (Fig. 4f), including Itgam (encoding CD11b), Cx3cr1, Bndf (brain-derived neurotrophic factor), and Ctss (encoding CatS). Many of these genes have been implicated in the development of neuropathic pain8,33. Interestingly, the same microglia genes are upregulated in the dorsal cord 1 day after nerve injury (Fig. 4e). We conclude that de novo induction of CSF1 in injured sensory neurons triggers the expression of neuropathic pain-relevant microglial genes in the dorsal spinal cord, as well as the ensuing neuropathic pain condition.
DAP12 mediates microglial gene upregulation and pain
We next addressed the signal transduction pathway downstream of the microglial CSF1R. Our RNA-Seq analysis of the dorsal spinal cord ipsilateral to the nerve injury revealed a substantial upregulation of Tyrobp, the gene that encodes DAP12 (Supplementary Table 1). We focused on DAP12 as it is central to adult microglial functionality5,34 and is induced in microglia in the XIIth nucleus after hypoglossal nerve injury35. qRT-PCR showed that the Tyrobp induction was substantial within 1 day of injury (Fig. 5a) and lasted for at least 7 days (Supplementary Fig. 6a). Intrathecal CSF1 also induced Tyrobp (Fig. 5b) and importantly, Tyrobp deletion36 completely prevented nerve injury and intrathecal CSF1-induced mechanical hypersensitivity (Fig. 5c–d), as well as the microglial gene upregulation (Fig. 5e–f), without affecting the de novo induction of CSF1 in sensory neurons (Supplementary Fig. 6b). The mild hypersensitivity induced by CSF1 in the Tyrobp−/− mice (Fig. 5d) is comparable to that produced by PBS vehicle in wild type mice (Fig. 4b). Motor activity and response to acute noxious heat stimulation in the Tyrobp−/− mice did not differ from those of wild type mice (Supplementary Fig. 6c–e). We conclude that DAP12 lies downstream of CSF1R and is necessary for the CSF1-CSFR1 triggered upregulation of pain-related microglial genes and of the consequent neuropathic pain condition. Interestingly, DAP12 is also required for hypoglossal nerve injury-induced expression of pro-inflammatory cytokines, including M1-phenotype markers35. Finally, in the rat, we found that DAP12 mechanisms also contribute to ongoing neuropathic pain. Autotomy (self-mutilation of a denervated limb) is presumed to arise from a persistent pain comparable to phantom limb pain. We found that basal levels of spinal cord DAP12 mRNA are substantially higher in a strain of rats with high autotomy (HA) scores37 than are DAP12 levels in rats that rarely develop this condition (low autotomy; LA). These DAP12 differences were present both before and after nerve injury (Supplementary Fig. 7).
Figure 5. DAP12 is required for nerve injury-induced microglia gene upregulation and neuropathic pain (mechanical hypersensitivity).
(a) Upregulation of Tyrobp mRNA (qRT-PCR) in the dorsal cord ipsilateral to nerve injury (1d); (b) Upregulation of Tyrobp mRNA (qRT-PCR) in the spinal cord after intrathecal CSF1; (c) Tyrobp−/− mice do not develop mechanical hypersensitivity after nerve injury (n=5–6 per group); (d) Intrathecal CSF1 does not provoke mechanical hypersensitivity in Tyrobp−/− mice (n=5 per group). The mild hypersensitivity observed in the Tyrobp−/− mice is comparable to that produced by PBS in wild type mice (See Fig. 4b); (e) Tyrobp−/− prevents nerve injury-induced upregulation of neuropathic pain-related microglial genes (1d post injury; n=4–5 mice/group). (f) Tyrobp−/− also prevents intrathecal CSF1-induced microglial gene induction (n=4–5 mice/group).
Nerve injury induces microglia self-renewal in spinal cord
In addition to establishing the neuropathic pain condition, peripheral nerve injury expands the spinal cord microglia population. Whether this expansion results from the infiltration of circulating monocytes or by self-renewal from local microglia remains controversial. Despite the comparable gene profile of microglia and monocytes, some genes (Csf1r and Cx3cr1) are expressed at higher levels in microglia; others (Trem1 and Trem3) are expressed exclusively in monocytes38. Our RNA-Seq analysis showed that although the microglia-enriched genes are upregulated, the monocyte specific genes remained undetectable after nerve injury (Supplementary Table 1). We confirmed these RNA-Seq findings by qRT-PCR (Supplementary Fig. 8) and conclude, in agreement with Ajami et al20, that monocytes do not significantly infiltrate the spinal cord after nerve injury. Rather, microglia expansion after nerve injury involves self-renewal of resident microglia.
CSF1 is necessary and sufficient for microglia self-renewal
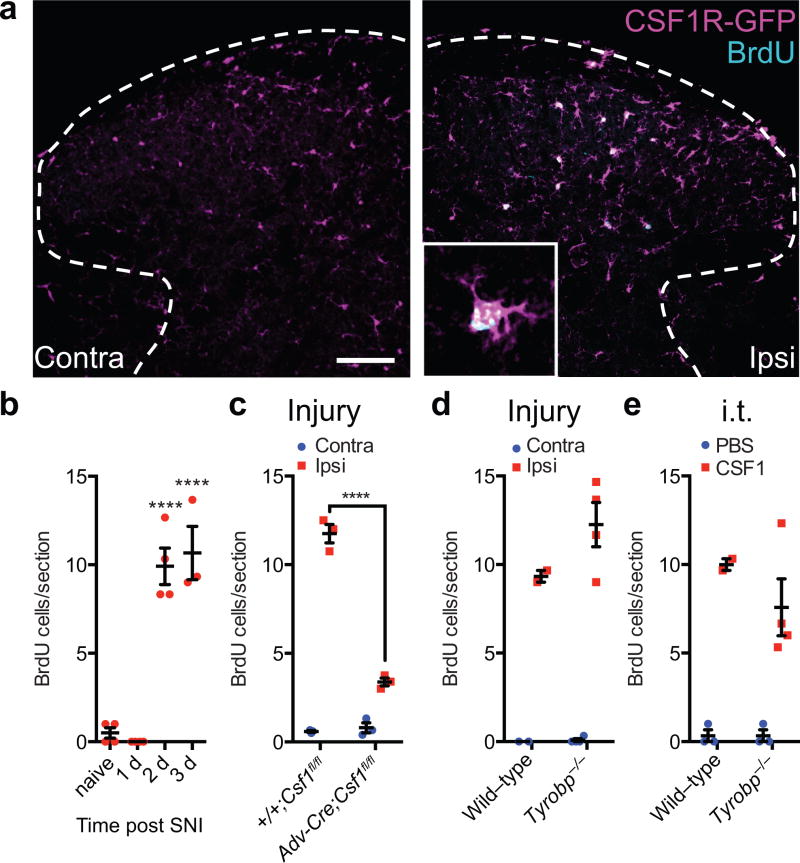
We next asked whether the de novo expression of CSF1 in injured sensory neurons is also required for nerve injury-induced microglia self-renewal in vivo. We first confirmed a previous report18 that nerve injury triggers dorsal horn microglia proliferation, demonstrated by incorporation of the thymidine analogue BrdU into CSF1R-expressing microglia (Fig. 6a). Three days following nerve injury, all dorsal horn BrdU+ cells expressed CSF1R (Fig. 6a), demonstrating that these proliferating cells originate from resident microglia, i.e., the proliferation reflects microglial self-renewal. Note that the microglia proliferation occurred after the CSF1 was induced. We detected no microglia proliferation in the dorsal horn at 1 day post injury (Fig. 6b), when CSF1 induction in sensory neurons is readily observed (Fig. 1b, 2a–b). Importantly, Advillin-Cre-mediated deletion of Csf1 from DRG neurons largely eliminated the nerve injury-induced dorsal horn microglia proliferation (Fig. 6c, Supplementary Fig. 9a). Finally, intrathecal injection of CSF1 also induced microglia proliferation in the dorsal horn (Fig. 6e, Supplementary Fig. 9c), comparable to that provoked by nerve injury (Fig. 6a–b). We conclude that sensory neuron-derived CSF1 is necessary, and CSF1 by itself, is sufficient for microglia proliferation/self-renewal in the dorsal horn.
Figure 6. Sensory neuron-derived CSF1 is necessary and sufficient for nerve injury-induced microglia proliferation in the dorsal horn.
(a) Double-labeling for BrdU (to label proliferating microglia) and GFP in the dorsal horn of a CSF1R-GFP mouse (SNI 3d). Note that all the BrdU+ cells express CSF1R. Inset: BrdU incorporation is limited to CSF1R-expressing microglia. Scale bar: 100 μm; Inset: maximum projection of Z-stack images. (b) Microglia proliferation (BrdU incorporation) begins 2d after injury (n=3–4 mice/group); (c) Advillin-Cre-mediated deletion of Csf1 from sensory neurons significantly decreases injury-induced dorsal horn microglia proliferation (3d post injury, n=3 mice/group); (d) Comparable nerve injury-induced microglia proliferation in wild type and Tyrobp−/− mice (3d post injury, n=3–4 mice/group); (e) Intrathecal CSF1 induces dorsal horn microglia proliferation in wild-type and this proliferation is preserved in Tyrobp−/− mice (n=3–4 mice/group, 3d post injury). Data are presented as mean ± SEM.
DAP12 is not required for microglia proliferation in vivo
As DAP12 is required for CSF1-induced proliferation of bone marrow-derived macrophages in vitro39, and as it lies downstream of spinal cord microglial CSF1R in regulating pain-related microglial gene expression in vivo (Fig. 5e–f), we expected that DAP12 also mediates microglia proliferation in vivo. To our surprise, however, Tyrobp deletion altered neither nerve injury nor intrathecal CSF1-induced dorsal horn microglial proliferation (Fig. 6d–e, Supplementary Fig. 9b,d). Thus, although nerve injury and CSF1-induced microglia gene induction and the consequent neuropathic pain condition are DAP12-dependent, nerve injury and CSF1-induced microglia proliferation/self-renewal involves a DAP12-independent pathway.
CSF1 is induced in injured motoneurons
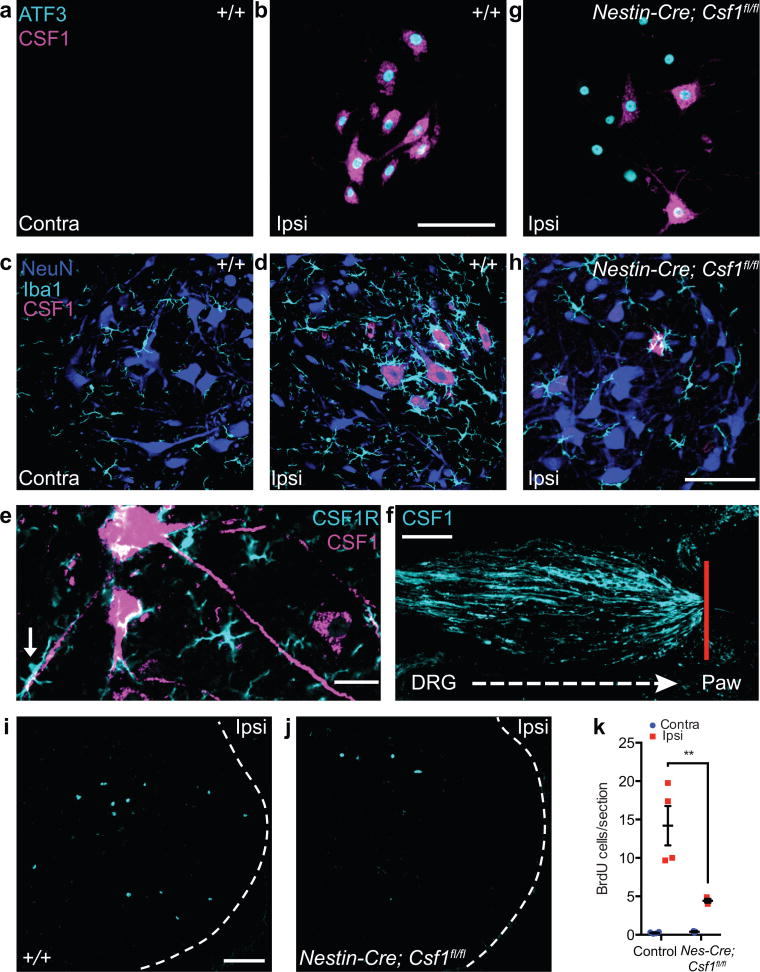
As the sciatic nerve contains sensory and motor axons, its transection damages both DRG sensory neurons and ventral horn motoneurons27 (Fig. 1a). As for sensory neurons, we observed dramatic CSF1 induction in axotomized (ATF3-expressing) motoneurons (Fig. 7a–b). The motoneuronal CSF1 induction occurred within 18 hours of the injury and persisted for at least 3 weeks (Supplementary Fig. 10). Virtually all ATF3-expressing motoneurons co-expressed CSF1, even 3 weeks after nerve injury (Fig. 7b, Supplementary Fig. 10). These results differ greatly from previous reports that found either no change in CSF140 or an induction of CSF1 in microglia, not neurons41, in the facial motor nucleus after VIIth nerve injury. Importantly, we found that the nerve injury-induced ventral horn microglia activation (enhanced Iba1 immunostaining) and microglial engulfment of motoneurons occurred only around motoneuron cell bodies and dendrities in which CSF1 expression increased (Fig. 7d, e). And just as sensory neuron-derived CSF1 is intraxonally transported (Fig. 2c, Supplementary Fig. 2b), so the induced motoneuronal CSF1 is also transported in axons that exit the spinal cord (Fig. 7e). Indeed, we observed accumulation of CSF1 at the peripheral nerve injury site (Fig. 7f). Thus, CSF1 is induced in both injured sensory and motoneurons and is axonally-transported to the dorsal horn and to the periphery, respectively.
Figure 7. CSF1 is upregulated in injured motoneurons and is required for nerve injury-induced microglia activation and proliferation in the spinal cord ventral horn.
(a) Neither CSF1 nor ATF3 was expressed in ventral horn motoneurons contralateral to nerve injury (3 d post injury). Scale bar represents 100 μm for (a), (b) and (g). (b) Coexpression of CSF1 and ATF3 (immunostaining) in axotomized ventral horn motoneurons (3 d post injury). (c) No microglia activation in contralateral ventral horn (8 d post injury). Scale bar represents 100 μm for (c), (d), and (h). (d) Activated ventral horn microglia (enhanced Iba1 expression) surrounded CSF1-expressing motoneurons (8 d post injury). (e) Motoneuron axons transported CSF1. Note the apposition of CSF1R-expressing microglia and a CSF1+ motoneuron dendrite (arrow). Scale bar represents 25 μm. (f) CSF1 accumulation at the peripheral nerve injury site (4 d post injury). Red line denotes ligature site. Scale bar represents 100 μm, (g) CSF1 upregulation was significantly reduced in Nestin–Cre; Csf1fl/fl mice, despite the persistent motoneuronal ATF3 expression. Given that ~30% of motoneurons continued to express CSF1 after injury, Nestin–Cre was likely not expressed in all motoneurons. (h) Csf1 deletion from the majority of CNS neurons (Nestin–Cre; Csf1fl/fl) reduced ventral horn microglia activation after injury. (i,j) Peripheral nerve injury (3 d) induced microglia proliferation in the ventral horn in wild–type (i), and this was greatly attenuated when Csf1 was deleted from CNS neurons (Nestin–Cre; Csf1fl/fl) (j). Scale bar represents 100 μm for (i) and (j). (k) Quantification of i and j (n = 3–4 mice per group). **P ≤ 0.01.
Ventral horn microglia proliferation is CSF1-dependent
To investigate the consequence of CSF1 induction in injured motoneurons, we crossed the floxed Csf1 mouse with a Nestin-Cre mouse, in which Cre-recombinase is expressed in most CNS neurons42. Nerve injury-induced ATF3 expression in axotomized motoneurons was not affected in these mice, but the CSF1 upregulation in motoneurons was substantially reduced (Fig. 7g). Only ~30% of ATF3+ motoneurons expressed CSF1 (Fig. 7g), compared to 100% of ATF3+ motoneurons in wild type mice (Fig. 7b). The residual expression of CSF1 in motoneurons presumably reflects incomplete Nestin-Cre-mediated recombination in motoneurons. Importantly, preventing CSF1 upregulation in motoneurons largely eliminated the nerve injury-induced microglia activation (Fig. 7h) and proliferation (Fig. 7i–k) in the ventral horn.
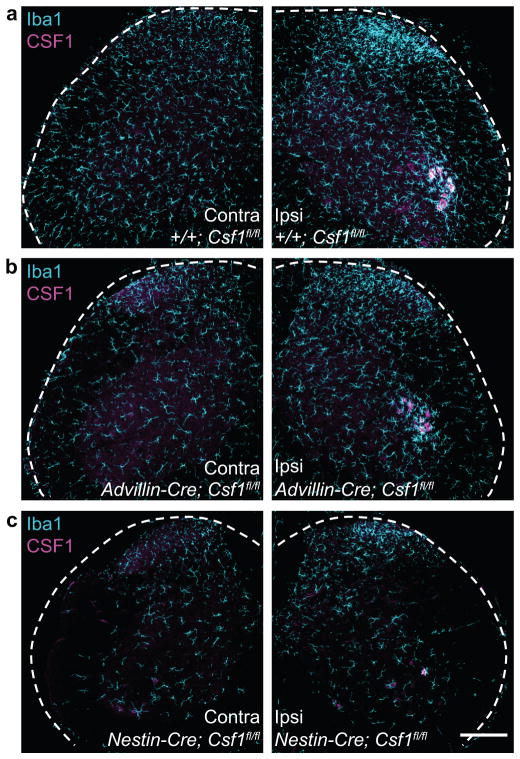
The topographic consequences of neuronal deletion of Csf1 was impressive. Deletion of Csf1 from sensory neurons (Adv-Cre, Fig 3b) altered neither motoneuronal CSF1 induction nor ventral horn microglial activation after nerve injury (Fig. 8a–b). Rather, the reduced nerve injury-induced microglia activation was limited to the dorsal horn, within the terminal field of the injured afferents (Fig. 8a–b). In contrast, deletion of Csf1 from CNS neurons (Nestin-Cre, Fig. 7g) markedly reduced nerve injury-induced microglia activation in the ventral horn (Fig. 8c). Note that baseline microglial density was also reduced in these mice (Fig. 8a,c). Despite this overall reduction, in these mice the nerve injury-induced CSF1 induction was preserved in sensory neurons (Supplementary Fig. 11), as was the dorsal horn microglial activation (Fig 8c).
Figure 8. Cre-mediated neuronal Csf1 deletion reveals topographic distribution of microglia activation after nerve injury.
(a) Peripheral nerve injury-induced microglia activation (increased Iba1 expression) in the ipsilateral dorsal and ventral horn, and upregulation of CSF1 in ventral horn motoneurons (3d post injury) in a control animal (+/+; Csf1 fl/fl); (b) Csf1 deletion from sensory neurons (Adv-Cre; Csf1 fl/fl) reduces injury-induced dorsal horn microglia activation. There is no effect on ventral horn microglia activation or on motoneuronal CSF1 induction; (c) Csf1 deletion from the majority of CNS neurons (Nestin-Cre; Csf1 fl/fl) reduces ventral horn microglia activation after injury (See also Fig. 7h). Note that the density of microglia in the spinal cord contralateral to the nerve injury is reduced in the mutant mice. Despite this overall reduction, microglia are still activated in the dorsal horn ipsilateral to the nerve injury in the mutant mice. Scale bar: 200 μm.
DISCUSSION
Although there is general agreement that microglia are important contributors to the neuropathic pain following peripheral nerve injury, how injured sensory neurons communicate with and activate microglia to produce this pain condition is not known. Here we demonstrate that injured sensory neurons de novo express CSF1 and transport it to the spinal cord, where it engages microglia via an interaction with microglial CSF1R. Via a DAP12-dependent microglial pathway, CSF1, in turn, upregulates microglial genes implicated in the neuropathic pain phenotype. Injured neuron-derived CSF1 also triggers a DAP12-independent microglia proliferation/self-renewal in the spinal cord.
Injured neuron-derived CSF1, microglia activation and pain
Although CSF1 is known for its in vitro colony-stimulating effect on cultured microglia23, its in vivo role is much less understood, largely because of limitations of the available in vivo animal models, notably the Csf1 point mutation op/op mouse43. As this mouse has a significant deficit in microglia development24, it is not ideal for the study of adult microglia functionality4. Moreover, because of the global Csf1 mutation in these mice, the contribution of CSF1 from a specific cell type cannot be assessed. Indeed, although it has been reported that nerve injury-induced microglial activation in the facial nucleus is attenuated in op/op mice44, another study concluded that the source of the CSF1 triggering the microglial response was microglia41, not neurons. Very recent studies used systemic administration of CSF1R inhibitors45. However, as the CSF1R has two ligands, CSF1 and IL-3426, the action of the inhibitor cannot be unequivocally attributed to CSF1 blockade. Also the source of relevant CSF1 cannot be determined with this approach.
We now demonstrate that CSF1 is dramatically and selectively induced in injured (ATF3-positive) sensory neurons after nerve injury and transported to the spinal cord where the CSF1R is also upregulated. Importantly, selective deletion of Csf1 from sensory neurons substantially reduced nerve injury-induced dorsal horn microglial activation and completely prevented the neuropathic pain behavioral phenotype. Furthermore, intrathecal injection of CSF1 produced both mechanical hypersensitivity and microglia activation. Taken together, these results provide the first evidence that upregulation of CSF1 in injured neurons is the critical contributor to nerve injury-induced microglia activation and neuropathic pain.
An important basis for our conclusion as to the essential contribution of sensory neuron-derived CSF1 came from our concurrent demonstration of the spinal cord upregulation of microglial CSF1R. In sharp contrast, although CCL21 is reportedly upregulated in the DRG after nerve injury13, none of the CCL21 receptors, namely CCR7 and CXCR346, is expressed in the dorsal cord, even after peripheral nerve injury (Supplementary Table 1). In fact, our RNA-Seq analysis could not even confirm the upregulation of CCL21 in the DRG (Supplementary Table 1). Finally, although it has been suggested that neurons express several membrane proteins (e.g. CD200) that inhibit microglia activity and that microglia activation results from downregulation of these inhibitory proteins47, we found no change in the levels of their corresponding genes in the DRG after nerve injury (Supplementary Table 1).
A microglial CSF1R-DAP12 pathway mediates neuropathic pain
There are many microglial genes implicated in neuropathic pain, but the microglial signaling pathways through which these genes are induced after nerve injury have yet to be fully defined. Here we demonstrate that the de novo expression of CSF1 in sensory neurons engages a DAP12-dependent pathway. Importantly, we found that this CSF1-CSF1R-DAP12 pathway lies upstream of microglial genes that are critical to neuropathic pain development6,48,49, including CatS, CX3CR1 (Fig. 4e–f, 5e–f), P2X4, Irf8, and Irf5 (Supplementary Fig. 12a–b). As DAP12 lies upstream of these genes, it follows that targeting DAP12 should be considered in the pharmacotherapy of neuropathic pain. Interestingly, although the CSF1-CSF1R-DAP12 pathway lies upstream of P2X4 gene induction, intrathecal CSF1 induced equivalent mechanical hypersensitivity in P2X4 mutant and WT mice (Fig. 4d). We conclude that the initial microglial signaling via CSF1R is P2X4-independent.
Nerve injury induces CSF1-dependent microglia self-renewal
Our finding that microglial, rather than monocyte specific genes, are upregulated in the spinal cord after nerve injury is consistent with a previous report of no significant monocyte infiltration after nerve injury20. Although it has been reported that Nestin-expressing microglia progenitor cells contribute to microglia repopulation after pharmacological depletion of microglia45, in our model we did not observe Nestin expression in any of the proliferating BrdU-positive cells (data not shown). In fact, all BrdU-positive cells expressed CSF1R, suggesting that expansion of the spinal cord microglia population after nerve injury results from self-renewal of resident microglia.
To our knowledge, however, little is known about the signal that triggers microglia self-renewal in vivo, after peripheral nerve injury or indeed in any neurological condition. Here we demonstrated that CSF1 induced in injured sensory and motoneurons is, in fact, the in vivo signal that transforms the microglia from a resident, homeostatic state into a highly proliferative one. As CSF1R signaling is required for microglia embryonic development25, our findings indicate that injury-induced adult microglia proliferation/self-renewal recapitulates the CSF1R-mediated pathway that is active in embryonic stem cells. However, although DAP12 is required for CSF1-induced proliferation of bone marrow derived macrophages in vitro39, we found that nerve injury and CSF1-induced microglia proliferation in vivo are DAP12-independent. Clearly, the signal transduction pathway that operates in adult microglia in vivo differs greatly from the pathway that in vitro studies identified from bone marrow-derived macrophages.
CSF1-induced microglial activation and proliferation
Microglia activation after nerve injury is typically concluded from enhanced expression of specific microglia markers, notably CD11b or Iba1, but self-renewal based proliferation of microglia is also a major manifestation of their activation. Here we distinguished two biomarkers of microglia activation (Supplementary Fig. 13). Within one day of nerve injury, before microglia proliferation (BrdU incorporation) occurred, we documented a DAP12-dependent induction of microglial genes, including many implicated in neuropathic pain. Among these genes are Ctss, which encodes CatS, the protease that is released by activated microglia to cleave fractalkine from neuronal membrane8, and BDNF, which is reported to be released from microglia, resulting in a shift of the anion gradient of pain transmission neurons33. This shift reduces GABAergic inhibitory control, which contributes to the ensuing hyperexcitability50. Only at two days after nerve injury did we detect the BrdU marker of microglial proliferation. Unlike microglial gene upregulation, however, neither nerve injury nor CSF1-induced microglia proliferation is DAP12-dependent. We conclude that the de novo expression of CSF1 after injury engages two distinct microglial processes, a DAP12-dependent pathway for microglia gene upregulation and the consequent neuropathic pain, and a DAP12-independent pathway for microglia proliferation/self-renewal. As the DAP12 pathway is presumably also engaged by the newly generated microglia, we suggest that interfering with this pathway will reduce both the resident and proliferating microglia contribution to the neuropathic pain phenotype.
METHODS
Animal lines
Animal experiments were approved by UCSF Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory animals. Wild type and CSF1R-EGFP mice were purchased from Jackson Laboratory. Tyrobp−/−36, Csf1 fl/fl30, Advillin-Cre31, Nestin-Cre42 and P2X4−/−51 mice and HA/LH rats37 were described previously. All experiments were performed in male animals.
Surgeries, injections and behavioral analysis
We performed either sciatic nerve ligation and transection (SNL, for DRG RNA-Seq and ventral horn microglia proliferation) or combined sciatic and femoral nerve transection (dorsal cord RNA-Seq) and the spared nerve injury (SNI) model of neuropathic pain32 for all other experiments. For SNI, we ligated and transected the sural and superficial peroneal branches of the sciatic nerve, leaving the tibial nerve intact. To analyze CSF1 transport from the DRG to the spinal cord, we ligated the ipsilateral L4 and L5 dorsal roots immediately after SNI. Intrathecal injections were made as previously described52. To study CSF1-induced microglia proliferation, we injected 10 μl of 3 ng/μl CSF1 (total of 30 ng) daily for 3 days. To study CSF1-induced microglial gene induction, we injected CSF1 twice within 24 h, with 17 h between the two injections. Spinal cord tissue was collected 24h after the first injection. Minocycline (40 mg/kg) was i.p. injected twice daily for three days and 1 hour before CSF1 intrathecal injection. All behavioral experiments were performed as previously reported in a blinded manner during the light cycle53,54.
RNA-Seq
Ipsilateral and contralateral DRGs and the dorsal quadrant of the spinal cords were collected 7d after nerve injury. RNA was purified with QIAgen RNeasy Mini Kit with DNase I digestion. RNA-Seq libraries were built with Epicentre ScriptSeq mRNA-Seq Library Preparation Kit and were sequenced by Illumina HiSeq 2000. Differential expression testing was performed using Cuffdiff 1.3.0 using default parameters. Resulting significant gene lists were filtered for genes with an absolute fold change greater than 2.
Immunohistochemistry
We immunostained tissue as previously described53, with the following antibodies: GFP (abcam), CSF1R (Millipore), CD11b (Abcam), ATF3 (Santa Cruz), CSF1 (R&D), NPY (gift from Clark J. Allen), Iba1 (Wako), NeuN (Millipore), BrdU (Abcam), and fluorophore coupled secondary antibodies (Alexa Fluor 488, 555, 594, 647) (Invitrogen). To localize CSF1 in DRG neurons and in their processes, we used antibody to goat biotin IgG (Vector Laboratories) and streptavidin coupled to an Alexa Fluor 488 or 594 (Invitrogen). Images were collected with a Carl Zeiss LSM 700 microscope or a Zeiss Axio Image M2 (DRG overview images only) and were processed with Fiji/ImageJ (NIH). Corresponding images (e.g. ipsilateral vs. contralateral; CSF1 vs. PBS; wt vs. mutant) were processed in an identical manner. Each experiment was performed in at least 3 animals.
Extended Imaging Methods
All images were taken on a lsm 700 confocal microscope (Zeiss) equipped with 405 nm (5 mW fiber output), 488 nm (10 mW fiber output), 555 nm (10 mW fiber output) and 639 nm (5 mW fiber output) diode lasers, a main dichroic beam splitter URGB and a gradient secondary beam splitter for lsm 700 using a 10x EC Plan-Neofluar (10x/0.3) or a 20x Plan-Apochromat (20x/0.8) objective (Zeiss). Image acquisition was done with ZEN 2010 (Zeiss), and image dimensions were 1024×1024 or 2048×2048 pixels with an image depth of 8, 12 or 16 bit. Two times averaging was applied during image acquisition. Laser power and gain were adjusted to avoid saturation of single pixels. Adjustment of brightness/contrast, changing of artificial colors (LUT), and maximum projections of Z-stack images were done in Fiji/Image J.
Cell Counting
For cell counting of DRG neurons, we collected 14μm cryosections of the L5 DRG from 3 animals per group. The sections were directly mounted on Superfrost microslides. To avoid double counting of the same cell, we mounted, immunostained, and counted neurons in every fourth section of each ganglion. With this approach, at least 150 neurons were counted for each marker. To quantify the percentage of ATF3-immunoreactive DRG neurons that co-express CSF1, we counted at least 150 ATF3 positive neurons for each mouse, at each time point, and calculated the percentage of double-labeled ATF3/CSF1 immunoreactive neurons. To analyze BrdU incorporation in spinal cord microglia, we counted BrdU positive cells in the dorsal spinal cord from 3–4 mice/group in the 3 lumbar spinal cord sections containing the highest number of BrdU positive cells. Microglia identity was verified by double labeling with a microglia marker (Iba1, CD11b or CSF1R-GFP). The individual analyzing the images was blinded to the groups.
Image Quantification
For the quantification of signal intensities of CSF1R, CSF1R-GFP and Iba1 in dorsal horn microglia, we collected 30μm cryosection of the lumbar enlargement from 3–4 mice per group. Confocal images were taken from the 3 sections showing the highest microglia signals in each animal. The border of the dorsal horn was outlined, all microglia cells were identified using an independent microglia marker (Iba1 or CD11b), and signal intensities within this mask were analyzed using Fiji/Image J.
Microglia proliferation
Mice were injected with BrdU (100 μg/kg body weight, i.p.) 2h prior perfusion. Tissue sections were pretreated with 1M HCl (10 min, on ice), 2M HCl (10 min, room temperature), 2M HCl (20 min, 37°C), and 5 times in PBS before BrdU immunostaining.
Quantitative RT-PCR
We performed qRT-PCR as previously described53. In mice with a peripheral nerve injury, we collected ipsilateral and contralateral L4-6 DRGs and dorsal spinal cord. For the mice that received an intrathecal CSF1 injection, we collected the entire lumbar spinal cord. All primers were designed using the NCBI Primer-BLAST program. β-actin was used as the internal control for all the DRG samples, and Snap25 was used as the internal control for all spinal cord samples. The primer pair used are: Csf1 TGCTAAGTGCTCTAGCCGAG/ CCCCCAACAGTCAGCAAGAC, IL34 ACGTACAGCGGAGCCTCAT / CATGACCCGGAAGCAGTTGT, Csf1r ACACGCACGGCCACCATGAA / GCATGGACCGTGAGGATGAGGC, Tyrobp CCGAGGTCAAGGGACAGCGGA / TGCCTCTGTGTGTTGAGGTCACTGT, Cx3cr1 GCCTCTGGTGGAGTCTGCGTG / CGCCCAAATAACAGGCCTCAGCA, Itgam GAGTCTGCCTCCGTGTCCGC / TACGTGAGCGGCCAGGGTCT, CatS GGGGGCATAGAGGCAGACGCT / GGGCATCCTCGTCACCAAACGG, Bdnf CAGGTTCGAGAGGTCTGACG / AAGTGTACAAGTCCGCGTCC, P2X4 CGACTATGTGGTCCCAGCTC / GCGTCTGAATCGCAAATGCT, Irf8 GGGCAGCGTGGGAACC / GCTTCCAGGGGATACGGAAC, Irf5 TGGGGACAACACCATCTTCA / CTGGAAGTCACGGCTTTTGT, Trem1 ACTGCTGTGCGTGTTCTTTG / GCCTTCTGGCTGTTGGCATA, Trem3 CAAGATGTGGGGCTGTACCA/ AAGCCACACGTCAGAACGAT, β-actin CCACACCCGCCACCAGTTCG / TACAGCCCGGGGAGCATCGT, Snap25 AGCGGACAGCATCCTCCGGAG / GTCTGCGTCTTCGGCCATGGT, Tyrobp (rat) AAACAGCACATGGCTGAGAC / GCATAGGGTGGGTTCATCTGT, Snap25 (rat) ACCACTGACTTGCTGGCCCCG / CGACGGGTGCTTTCCAGGGAC
In situ hybridization
In situ hybridization (ISH) was performed using the Panomics’ QuantiGene ViewRNA tissue assay (Affymetrix/Panomics) as previously described55, with a probe set designed to cover all three variants of the mouse Csf1 coding sequence. The signal was detected using an alkaline phosphatase reaction with a fluorescent Fast Red substrate. For combine ISH with immunostaining of ATF3, 12 μm cryostat sections on glass slides were immersed in 10% (vol/vol) formalin in PBS for 10 min and then processed according to the manufacturer’s ISH protocol, with protease treatment for 12 min. The slides were then blocked in 5% (vol/vol) normal goat serum in PBS (without Triton X-100) for 1 h and then processed for immunohistochemistry.
Statistical Analysis
Student’s t test was used to compare means of two groups, and two-way ANOVA tests were used for multiple comparisons. The tests were two-sided, except for Supplementary Fig. 7, which is one-sided, and all our data met the assumptions of the tests. Data are presented as mean ± standard error (SEM). In the box plots, the box limits show the first and third quartile, the center line is the median and the whiskers represent the minimum and maximum values; statistical significance: * p≤ 0.05, ** p ≤0.01, *** p ≤0.001, **** p≤ 0.0001. No statistical methods were used to predetermine sample sizes, but our sample sizes are comparable to those reported in previous publications53,54. The data distribution was assumed to be normal and variances were assumed to be equal across groups, but this was not formally tested. In all the behavior studies, the animals were randomly assigned to test cylinders, with the person who performed the behavioral test blind to the animal assignment.
A supplementary methods checklist is available.
Supplementary Material
Acknowledgments
We thank Dr. Nirao Shah for providing Nestin-Cre mice and Drs. Daryl L. Davies and Francois Rassendren for providing the P2X4−/− mice. We also thank Dr. Elizabeth K. Unger for illustrating Supplementary Figure 13 and Jacqueline Leff for technical support. This work was supported by the Foundation for Anesthesia Education and Research (FAER) MRTG-BS-02/15/2010-G and NIH-K08NS078050 to ZG, DFG KU 3039/1-1 to JK, DE022001 to AIB/SL, NS14627 and a Wellcome Trust grant to AIB, AG045040 to SLW and AI068129 to LLL.
Footnotes
Author Contribution: ZG and AIB designed the experiments and with JAK, wrote the manuscript. ZG performed and organized experiments to which JAK, XW, CS, SV, AKG, ZE-R and JB contributed. JAK completed many of the neuroanatomical studies. BC performed RNA-Seq analysis. MD provided spinal cord tissue from HA and LA rats. SLA-W provided Csf1 fl/fl mice. LLL provided Tyrobp−/− mice. LLL and SL contributed to experimental design and interpretation of results.
References
- 1.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backonja M, Woolf CJ. Future directions in neuropathic pain therapy: closing the translational loop. Oncologist. 2010;15(Suppl 2):24–29. doi: 10.1634/theoncologist.2009-S502. [DOI] [PubMed] [Google Scholar]
- 4.Sieweke MH, Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342:1242974. doi: 10.1126/science.1242974. [DOI] [PubMed] [Google Scholar]
- 5.Salter MW, Beggs S. Sublime microglia: expanding roles for the guardians of the CNS. Cell. 2014;158:15–24. doi: 10.1016/j.cell.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Beggs S, Trang T, Salter MW. P2X4R+ microglia drive neuropathic pain. Nat Neurosci. 2012;15:1068–1073. doi: 10.1038/nn.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark AK, Malcangio M. Fractalkine/CX3CR1 signaling during neuropathic pain. Front Cell Neurosci. 2014;8:121. doi: 10.3389/fncel.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- 11.Zhuang ZY, et al. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2007;21:642–651. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Old EA, Malcangio M. Chemokine mediated neuron-glia communication and aberrant signalling in neuropathic pain states. Curr Opin Pharmacol. 2012;12:67–73. doi: 10.1016/j.coph.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Biber K, et al. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J. 2011;30:1864–1873. doi: 10.1038/emboj.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung H, et al. Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. J Neurosci. 2009;29:8051–8062. doi: 10.1523/JNEUROSCI.0485-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvo M, et al. Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury. J Neurosci. 2010;30:5437–5450. doi: 10.1523/JNEUROSCI.5169-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuda M, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 17.Ulmann L, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Echeverry S, Shi XQ, Zhang J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain. 2008;135:37–47. doi: 10.1016/j.pain.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Priller J, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 20.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 21.LaCroix-Fralish ML, Austin JS, Zheng FY, Levitin DJ, Mogil JS. Patterns of pain: meta-analysis of microarray studies of pain. Pain. 2011;152:1888–1898. doi: 10.1016/j.pain.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Perkins JR, et al. A comparison of RNA-seq and exon arrays for whole genome transcription profiling of the L5 spinal nerve transection model of neuropathic pain in the rat. Mol Pain. 2014;10:7. doi: 10.1186/1744-8069-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzumura A, Sawada M, Yamamoto H, Marunouchi T. Effects of colony stimulating factors on isolated microglia in vitro. J Neuroimmunol. 1990;30:111–120. doi: 10.1016/0165-5728(90)90094-4. [DOI] [PubMed] [Google Scholar]
- 24.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsujino H, et al. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- 28.Hokfelt T, Brumovsky P, Shi T, Pedrazzini T, Villar M. NPY and pain as seen from the histochemical side. Peptides. 2007;28:365–372. doi: 10.1016/j.peptides.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Burnett SH, et al. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol. 2004;75:612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 30.Harris SE, et al. Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects. Bone. 2012;50:42–53. doi: 10.1016/j.bone.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zurborg S, et al. Generation and characterization of an Advillin-Cre driver mouse line. Mol Pain. 2011;7:66. doi: 10.1186/1744-8069-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 33.Coull JA, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 34.Hickman SE, et al. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16:1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi M, Konishi H, Takai T, Kiyama H. A DAP12-dependent signal promotes pro-inflammatory polarization in microglia following nerve injury and exacerbates degeneration of injured neurons. Glia. 2015;63:1073–1082. doi: 10.1002/glia.22802. [DOI] [PubMed] [Google Scholar]
- 36.Bakker AB, et al. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13:345–353. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- 37.Devor M, Raber P. Heritability of symptoms in an experimental model of neuropathic pain. Pain. 1990;42:51–67. doi: 10.1016/0304-3959(90)91092-W. [DOI] [PubMed] [Google Scholar]
- 38.Bedard A, Tremblay P, Chernomoretz A, Vallieres L. Identification of genes preferentially expressed by microglia and upregulated during cuprizone-induced inflammation. Glia. 2007;55:777–789. doi: 10.1002/glia.20477. [DOI] [PubMed] [Google Scholar]
- 39.Otero K, et al. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat Immunol. 2009;10:734–743. doi: 10.1038/ni.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raivich G, et al. Regulation of MCSF receptors on microglia in the normal and injured mouse central nervous system: a quantitative immunofluorescence study using confocal laser microscopy. J Comp Neurol. 1998;395:342–358. doi: 10.1002/(sici)1096-9861(19980808)395:3<342::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto S, Nakajima K, Kohsaka S. Macrophage-colony stimulating factor as an inducer of microglial proliferation in axotomized rat facial nucleus. J Neurochem. 2010;115:1057–1067. doi: 10.1111/j.1471-4159.2010.06996.x. [DOI] [PubMed] [Google Scholar]
- 42.Dubois NC, Hofmann D, Kaloulis K, Bishop JM, Trumpp A. Nestin-Cre transgenic mouse line Nes-Cre1 mediates highly efficient Cre/loxP mediated recombination in the nervous system, kidney, and somite-derived tissues. Genesis. 2006;44:355–360. doi: 10.1002/dvg.20226. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 44.Raivich G, Moreno-Flores MT, Moller JC, Kreutzberg GW. Inhibition of posttraumatic microglial proliferation in a genetic model of macrophage colony-stimulating factor deficiency in the mouse. Eur J Neurosci. 1994;6:1615–1618. doi: 10.1111/j.1460-9568.1994.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 45.Elmore MR, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biber K, Boddeke E. Neuronal CC chemokines: the distinct roles of CCL21 and CCL2 in neuropathic pain. Front Cell Neurosci. 2014;8:210. doi: 10.3389/fncel.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 48.Masuda T, et al. IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep. 2012;1:334–340. doi: 10.1016/j.celrep.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masuda T, et al. Transcription factor IRF5 drives P2X4R+-reactive microglia gating neuropathic pain. Nat Commun. 2014;5:3771. doi: 10.1038/ncomms4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coull JA, et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 51.Sim JA, et al. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci. 2006;26:9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cavanaugh DJ, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci (USA) 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braz JM, et al. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74:663–675. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, et al. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron. 2013;78:312–324. doi: 10.1016/j.neuron.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solorzano C, et al. Primary afferent and spinal cord expression of gastrin-releasing peptide: message, protein, and antibody concerns. J Neurosci. 2015;35:648–657. doi: 10.1523/JNEUROSCI.2955-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.