Abstract
Lichens have been known to possess multiple biological activities, including anti-proliferative and anti-inflammatory activities. Vascular cell adhesion molecule-1 (VCAM-1) may play a role in the development of atherosclerosis. Hence, VCAM-1 is a possible therapeutic target in the treatment of the inflammatory disease. However, the effect of lobaric acid on VCAM-1 has not yet been investigated and characterized. For this study, we examined the effect of lobaric acid on the inhibition of VCAM-1 in tumor necrosis factor-alpha (TNF-α)-stimulated mouse vascular smooth muscle cells. Western blot and ELISA showed that the increased expression of VCAM-1 by TNF-α was significantly suppressed by the pre-treatment of lobaric acid (0.1–10 μg/ml) for 2 h. Lobaric acid abrogated TNF-α-induced NF-κB activity through preventing the degradation of IκB and phosphorylation of extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), and p38 mitogen activated protein (MAP) kinase. Lobaric acid also inhibited the expression of TNF-α receptor 1 (TNF-R1). Overall, our results suggest that lobaric acid inhibited VCAM-1 expression through the inhibition of p38, ERK, JNK and NF-κB signaling pathways, and downregulation of TNF-R1 expression. Therefore, it is implicated that lobaric acid may suppress inflammation by altering the physiology of the atherosclerotic lesion.
Keywords: Lobaric acid, Atherosclerosis, VCAM-1, MAPK, NF-κB, MOVAS-1
INTRODUCTION
Atherosclerosis is a chronic inflammatory disease, characterized by the accumulation of lipids and fibrous elements in the large arteries. Vascular smooth muscle cells (VSMCs) plays a major role in early phase of atherosclerosis (Lusis, 2000; Owens et al., 2004; Falk, 2006). As the atherosclerosis, VSMCs are physically interplay with inflammatory cells, which play a very important function in further aggravating the atherosclerosis (Braun et al., 1999). The adhesion of inflammatory to VSMCs during atherosclerosis is primarily interacted by cell adhesion molecules, including vascular cell adhesion molecule-1 (VCAM-1). Up-regulation of VCAM-1 has been shown to be expressed at atherosclerosis, implication an important role of adhesion molecules (Libby and Li, 1993; Jang et al., 1994). Additionally, VCAM-1 expression has been displayed to be induced in response to inflammatory cytokines, such as TNF-α in vascular cells (Huo and Ley, 2001). On the basis of this background, it may be primary for factor to affect the induction of endothelial cell adhesion molecules including VCAM-1 in regulating vascular inflammatory processes.
Lobaric acid, an ingredient of the lichen Stereocaulon aplinum, is one of the most biologically potent secondary metabolites (Fig. 1) (Thadhani et al., 2014). Various biological activities of lobaric acid have been previously reported, and include antitumor, anti-proliferative, anti-inflammatory, antioxidant, and antimicrobial effects (Gissurarson et al., 1997; Hidalgo et al., 2005; Morita et al., 2009). However, up to now, there is no solid evidence to show how lobaric acid regulates the expression of adhesion molecules in vascular smooth muscle cells.
Fig. 1.
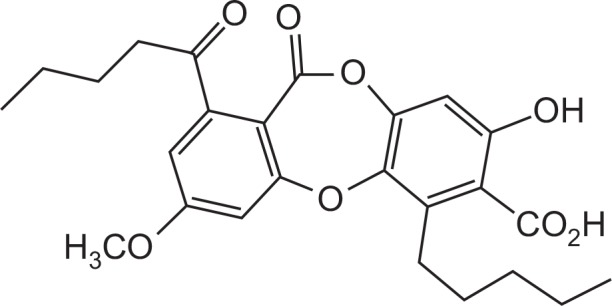
Structure of lobaric acid.
In the present study, we therefore examine the influence of lobaric acid on the expression of adhesion molecule to cultured mouse vascular smooth muscle cells. The results suggest that lobaric acid inhibits TNF-α-induced VCAM-1 expression through the inhibition of TNF-α receptor expression, and MAPK and NF-κB signaling pathways.
MATERIALS AND METHODS
Reagents
Unless otherwise indicated, all the chemicals used in this study were purchased from Sigma Chemical Co. (St Louis, MO, USA). Anti-VCAM-1 antibody was purchased from R&D Systems, USA. Lipofectamine Plus, DMEM medium, and fetal bovine serum were purchased from Life Technologies, Inc. (Carlsbad, CA, USA). The reporter plasmid pGL3-NF-κB used in the luciferase assay system was obtained from Promega (Madison, WI, USA), and pCMV-β-gal was obtained from Lonza (Walkersville, MD, USA). 3-amino-1,2,4-triazole was purchased from Calbiochem (La Jolla, CA, USA). Antibodies against IκB-α, p65, JNKs, phospho-JNK (p-JNK), ERK, phos-pho-ERK (p-ERK), phospho-38, p38, lamin A, TNF-α receptor 1 and β-actin were purchased from Abcam Inc, USA. Lobaric acid was acquired from Korea Polar Research Institute., Korea.
Plant material
Stereocaulon alpinum was collected from the Korean Antarctic Research Station site on King George Island (S62º 13.3′, W58º47.0′), Antarctica. The species was identified by Dr. Soon Gyu Hong by comparing morphological characteristics with those previously published (Ovstedal and Smith, 2001). The voucher specimen was deposited in the Polar Lichen Herbarium, Korea Polar Research Institute, KOPRI, Incheon, South Korea.
Extraction and isolation
The extraction of lobaric acid was performed using a modification of the technique described previously (Ingolfsdottir et al., 1996). Briefly, a dried sample of Stereocaulon alpinum was extracted with MeOH, and the resulting crude MeOH extract was subjected to C18 functionalized silica gel flash column chromatography, eluting with a stepwise gradient consisting of MeOH in H2O (10–100% MeOH with 10% increment for each step; 400 mL each). The fraction eluted at 80% MeOH was subjected to silica gel column chromatography followed by semi-preparative reversed-phase HPLC to yield. The isolated compound was identified as lobaric acid.
Cell culture
The vascular smooth muscle cell line (MOVAS-1) that has been utilized in various vascular studies (Charlmers et al., 2008; Choi et al., 2010; Mackenzie et al., 2011) was purchased from ATCC (Rockville, MD, USA) and grown in DMEM medium supplemented with 200 mg/ml G418, 100 IU/ml penicillin, 100 mg/ml streptomycin, and 10% heat-inactivated fetal bovine serum (FBS) in a humidified atmosphere containing 5% CO2 incubator at 37ºC. For sub-culturing, the cells were detached using 0.125% trypsin containing 0.01 M EDTA. All experiments were carried out with the same batch of MOVAS-1.
Cell proliferation assay
MOVAS-1 cells were seeded at a concentration of 5×104 cells/well in 96-well tissue culture plates (Nunc, Denmark) and incubated with different concentrations of lobaric acid (0.01, 0.1, 1, 10, 100 μg/ml) for 24 h. After treatment, cell proliferation was assessed by incubating the cells with 25 μg/ml of MTT (Sigma, St. Louis, MO, USA) for another 4 h. Then, the MTT-formazan produced by viable cells was dissolved in dimethyl sulfoxide (DMSO) and a Molecular Device microplate reader (Menlo Park, CA, USA) was used to measure absorbance at 560 nm. The blank control only contained cell culture medium and the absorbance of untreated cultures was set at 100%. At least three independent experiments were performed.
Determination of cell surface expression of VCAM-1 by ELISA
The cell surface expression of the adhesion molecules on the muscle cell monolayers was quantified by ELISA using a modification of the methods described previously (Mo et al., 2007). VSMCs were seeded at a concentration of 2×104 cells/well in 96-well gelatin-coated plates, cultured to confluence and pretreated with lobaric acid (0.1, 1, 10 μg/ml) for 2 h at 37ºC. These pretreated cells were then incubated with fresh growth medium containing TNF-α (10 ng/ml) for 8 h. After incubation, the cells were washed with phosphate buffer saline pH 7.4 (PBS) and fixed with 1.0% glutaraldehyde for 30 min at 4ºC. Bovine serum albumin (1.0% in PBS) was added to the cells to reduce non-specific binding. The cells were then incubated with monoclonal antibodies against either VCAM-1 or an isotype matched control antibody (0.25 g/ml, diluted in blocking buffer) overnight at 4ºC, washed with PBS, and incubated with alkaline phosphatase-conjugated goat anti-mouse secondary antibody (1 μg/ml, diluted in PBS). The cells were washed with PBS and exposed to the peroxidase substrate (p-nitorphenyl phosphate 1 mg/ml in 0.1 M glycine buffer, pH 10.4 containing 1 mM MgCl2, and 1 mM ZnCl2). The absorbance was measured at 405 nm using a Molecular Device microplate reader (Menlo Park, CA, USA). The absorbance values of the isotype matched control antibody were taken as the blank, which were subtracted from the experimental values.
Transient transfection and reporter assays
Cells (5×105 cells/well) were plated into each well of a 6-well plate. The cells were transiently co-transfected with the plasmids, pGL3-NF-κB and pCMV-β-gal, using LipofectAMINE Plus according to the manufacturer’s protocol. Briefly, a transfection mixture containing 0.5 μg pGL3-NF-κB and 0.2 μg pCMV-β-gal was mixed with the Lipofectamine plus reagent and added to the cells. After 4 h, the cells were pretreated with lobaric acid for 2 h followed by the addition of TNF-α for 4 h, and then lysed with 200 μl of lysis buffer (24 mM Tris-HCl (pH 7.8), 2 mM dithiothreitol, 2 mM EDTA, 10% glycerol, and 1% Triton X-100). Ten microliters of cell lysates were used for the luciferase activity assay. The luciferase and β-galactosidase activities were determined. The values shown represent an average of three independent transfections, which were normalized with β-galactosidase activity. Each transfection was carried out in triplicate and experiments were repeated three times.
Western blot analysis
Western blot analysis was performed by a modification of the technique described previously (Cho et al., 2003). The cells were pretreated with lobaric acid (0.1, 1, 10 μg/ml) for 2 h and incubated with fresh growth medium containing TNF-α (10 ng/ml) for 4 or 8 h. After treatment, the cells were washed twice in PBS and suspended in 70 μl of Buffer A [10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.5 mM PMSF and Protease Inhibitor Cocktail (Sigma)] and incubated on ice. After 15 min, 0.5% Nonidet P (NP)-40 was added to lyse the cells, which were vortexed for 10 sec. The cytosolic cell extracts were obtained after centrifuging at 1500×g for 10 min at 4ºC. The collected nuclei were resuspended in 50 μl of Buffer C [20 mM HEPES (pH 7.9), 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA, 25% v/v Glycerol, 0.5 mM PMSF and Protease Inhibitor Cocktail] and incubated on ice for 20 min with intermittent agitation. Nuclear cell extracts were recovered after centrifugation for 10 min at 13,000×g at 4ºC. Protein concentration was determined by using the Bio-Rad protein assay (Bio-Rad Lab, Hercules, CA, USA) with BSA as the standard. The cytosol lysates (20 μg) and nuclear extracts (40 μg) were resolved on a 7.5% SDS-polyacrylamide gel. The fractionated proteins were electrophoretically transferred to an immobilon polyvinylidene difuride membrane (Amersham, Arlington Heights, IL, USA) and probed with the appropriate antibodies. The blots were developed using an enhanced chemiluminescence (ECL) kit (Amersham). In all immunoblotting experiments, the blots were reprobed with an anti-β-actin antibody as a control for the protein loading.
Measurement of mRNA levels by quantitative real time polymerase chain reaction (RT-PCR)
The total RNA was extracted using a single-step guanidinium thiocyanate-phenol-chloroform method. The yield and purity of the RNA were confirmed by measuring the ratio of the absorbances at 260 and 280 nm. Quantitative RT-PCR was performed using VCAM-1-specific primers to identify cDNA. cDNA was amplified in 20 μl of PCR (8 μl of cDNA solution in water, 1 μl of forward primer and reverse primer, and 10 μl of PowerSYBR Green PCR Master Mix) in a quantitative Real-time PCR System, and fluorescence was monitored at each cycle. The VCAM-1 RT-PCR primers were 5′-CTCAGGTG-GCTGCACAAGTT-3′ (forward primer) and 5′-AGAGCTCAA-CACAAGCGTGG-3′(reverse primer). The GAPDH RT-PCR primers were 5′-TGCATCCTGCACCAA-3′ (forward primer) and 5′-TCCACGATGCCATTG-3′ (reverse primer).
Immunofluorescence for NF-κB p65 localization
MOVAS cells, grown on 22-mm diameter glass coverslips were pretreated with lobaric acid (10 μg/ml) for 2 h and incubated with fresh growth medium containing TNF-α (10 ng/ml) for 4 h. Cells were washed in PBS and fixed with 4% paraformaldehyde for 15 min at room temperature. The fixed cells were permeabilized with 0.5% Triton-X 100 in PBS for 10 min and blocked with a 5% bovine serum albumin in PBS. The anti-NF-κB p65 antibody was diluted 1:1000 and incubated overnight at 4°C. The cells were then incubated with Alexa 594-conjugated anti-rabbit antibody (red channel) for 1 h in 1% BSA/0.05% Triton X-100/PBS. Cells were washed twice with permeabilization buffer, incubated for 5 min in a Hoechst 33342-containing PBS solution, and washed with PBS. Coverslips were mounted to glass slides using ProLong Gold antifade agent and photographed with a confocal microscope (LSM 510 META; Carl Zeiss).
Statistical analysis
Results are represented as means ± S.E.M. All experiments were performed at least three times. For comparisons between two groups, the Student’s t test (SigmaPlot) was used. Multi-group comparisons of mean values were analyzed by one-way ANOVA (GraphPad program). Experimental differences were considered statistically significant when a p-value was less than 0.05.
RESULTS
Effects of lobaric acid on cell proliferation
We examined the anti-proliferative effect of lobaric acid on mouse vascular smooth muscle cell line, MOVAS-1 cells, by exposing them to lobaric acid for 24 h. Cell proliferation was determined by MTT assay. When MOVAS cells were exposed to lobaric acid (0.01–100 μg/ml), cell growth was inhibited at a concentration of 100 μg/ml (Fig. 2). Thus, the concentration selection for the present experiments was based on the cell proliferation results. In subsequent experiments, cells were treated with lobaric acid at concentrations of 0.1, 1, and 10 μg/ml.
Fig. 2.
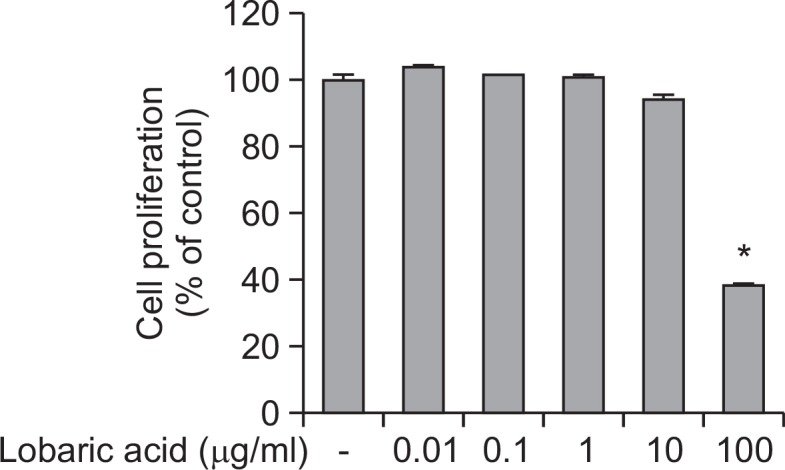
Effects of lobaric acid on MOVAS cell. Lobaric acid (0.01, 0.1, 1, 10, and 100 μg/ml) was incubated with MOVAS-1 cells for 24 h and cell proliferation was determined by MTT assay. The data shown represent percentages of viable cells relative to the control (means ± S.E.M of 96-wells of a representative experiment) *Significantly different from TNF-α-stimulated cells not treated with lobaric acid (*p<0.05).
Effect of lobaric acid on TNF-α-induced vascular cell adhesion molecule expression
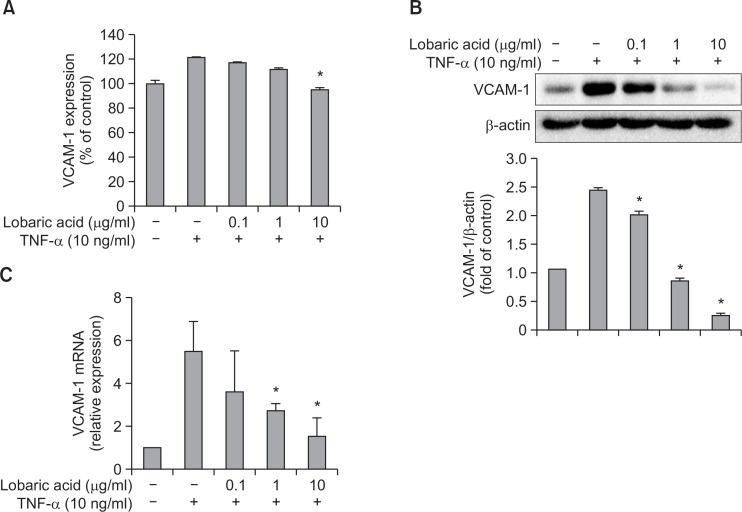
This experiment was conducted to determine the effect of lobaric acid on the expression of TNF-α-induced adhesion molecules. VSMCs were pretreated with or without various concentrations of lobaric acid for 2 h, followed by treatment with TNF-α (10 ng/ml) for 8 h. As detected by ELISA, pretreatment with lobaric acid significantly suppressed cell surface expression of TNF-α-induced VCAM-1 in a concentration-dependent fashion (Fig. 3A). Additionally, the expression of total cellular adhesion molecule on VSMCs in response to TNF-α stimulation and lobaric acid treatment was examined by Western blot analysis. The results showed a similar pattern of inhibition by lobaric acid at total cellular protein level (Fig. 3B). Thus, these results strongly suggest that lobaric acid is effective in blocking the expression of VCAM-1 induced by TNF-α.
Fig. 3.
Effect of lobaric acid on TNF-α-induced vascular cell adhesion molecule expression. (A) Expression of VCAM-1 in VSMCs after pre-incubation with lobaric acid was measured by ELISA. The data are expressed as a percentage of TNF-α-induced adhesion molecule expression. (B) The VCAM-1 protein levels were determined in MOVAS by Western blot assay. The β-actin protein level was considered as an internal control. The results illustrated are from a single experiment, and are representative of three separate experiments. The levels of VCAM-1 expression are in arbitrary units, and data are normalized to respective amount of β-actin protein. (C) Levels of the mRNA for adhesion molecules were determined by quantitative real time-PCR. GAPDH served as a housekeeping gene. The results illustrated are from a single experiment, and are representative of three separate experiments. Expression of VCAM-1 mRNA is in arbitrary units, and data are normalized to respective amount of GAPDH mRNA. The results are expressed as the mean ± S.E.M of three independent experiments.*Significantly different from TNF-α-stimulated cells not treated with lobaric acid (*p<0.05).
Next, we determined if lobaric acid interferes with the expression of TNF-α-induced adhesion molecules at the transcriptional level. To examine gene transcription, total cellular RNA was isolated from VSMCs and analyzed by real time-PCR. VSMCs were pretreated with various concentrations of lobaric acid for 2 h, followed by treatment with TNF-α for 4 h (Fig. 3C). Lobaric acid concentration-dependently attenuated VCAM-1 mRNA expression, suggesting that the effect of lobaric acid on TNF-α-induced VCAM-1 mRNA expression occurs at the level of RNA.
Inhibition of TNF-α-induced activation of NF-κB by lobaric acid
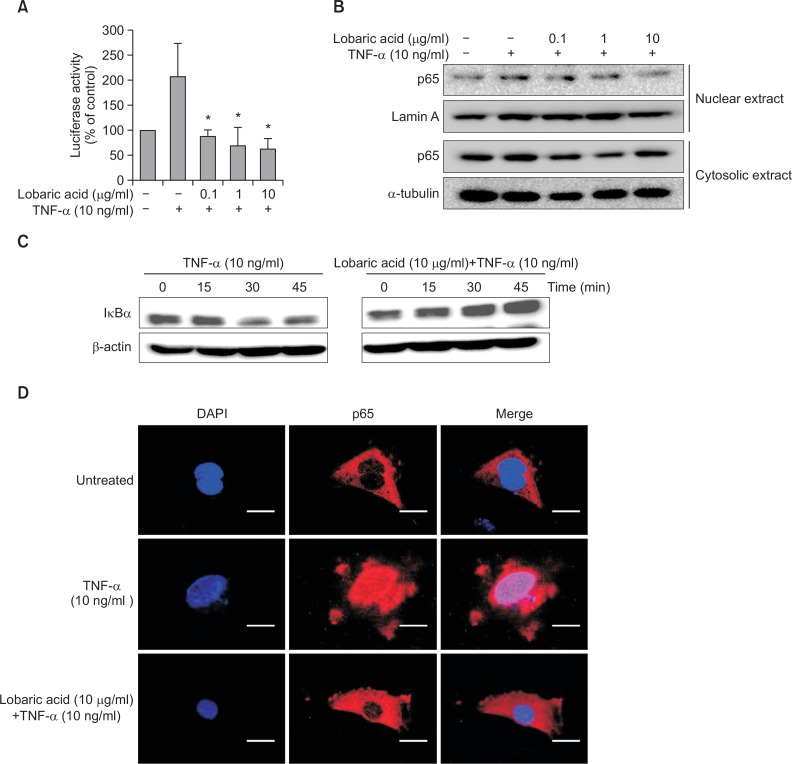
Because NF-κB activation in the inflammatory response may trigger upregulation of adhesion molecule, we examined the effect of lobaric acid on NF-κB transcriptional activation using Luciferase reporter assays. Cells were pretreated with various concentrations of lobaric acid for 2 h before stimulation with TNF-α for 4 h. Stimulation of the cells with TNF-α resulted in an approximately 2-fold increase in luciferase activity, and this increase was considerably suppressed by lobaric acid at 10 μg/ml (Fig. 4A). We also examined the effect of lobaric acid on the expression of p65 NF-κB protein (Fig. 4B). As shown in Fig. 4B, pre-incubation of VSMCs with lobaric acid decreased the nuclear translocation of p65 NF-κB. These data indicate that lobaric acid inhibits TNF-α-induced nuclear translocation of NF-κB.
Fig. 4.
Effect of lobaric acid on NF-κB activation and IκBα degradation. NE, nuclear extracts; CE, cytoplasmic extracts. (A) VSMCs (MOVAS-1) were transfected with a pGL3-NF-κB-Luc reporter plasmid and pCMV-β-gal, pretreated with various concentrations of lobaric acid for 2 h, and stimulated with TNF-α for 4 h. The results are mean ± S.E.M of 3 experiments. *p<0.05, significantly different from the group treated with TNF-α-induced MOVAS-1 cells. (B) and (C) were pre-incubated with or without various concentrations of lobaric acid for 2 h, and then treated with TNF-α for 4 h. The whole cell lysates of MOVAS were analyzed by Western blot with anti-IκBα antibody. Lamin A and α-tubulin were used as loading controls for nuclear and cytosolic protein fractions, respectively. (D) MOVAS cells were either pretreated or untreated with lobaric acid for 2 h and then exposed to TNF-α for 4 h. The p65-FITC translocation from cytosol to nucleus was observed under a fluorescent microscope at 400× magnification. The results illustrated are from a single experiment, and are representative of three separate experiments. Scale bars are 20 μm.
To examine whether lobaric acid affects TNF-α-induced degradation of IκBα, the expression of IκBα protein was determined by Western blot assay (Fig. 4C). Stimulation with TNF-α significantly degraded IκBα at 45 min as compared to untreated control cells, but TNF-α-induced cells pretreated with lobaric acid failed to degrade IκBα. Consistent with the protein expression, a significant inhibitory effect of lobaric acid on the TNF-α-induced NF-κB p65 nuclear translocation determined by immunofluorescence assay was observed (Fig. 4D). These results further demonstrate that lobaric acid inhibits TNF-α-induced NF-κB activation. Collectively, these results suggest that lobaric acid inhibits TNF-α-induced VCAM-1 expression by blocking the activation of NF-κB.
Effect of lobaric acid on the expression of MAP kinases in TNF-α-stimulated smooth muscle cells
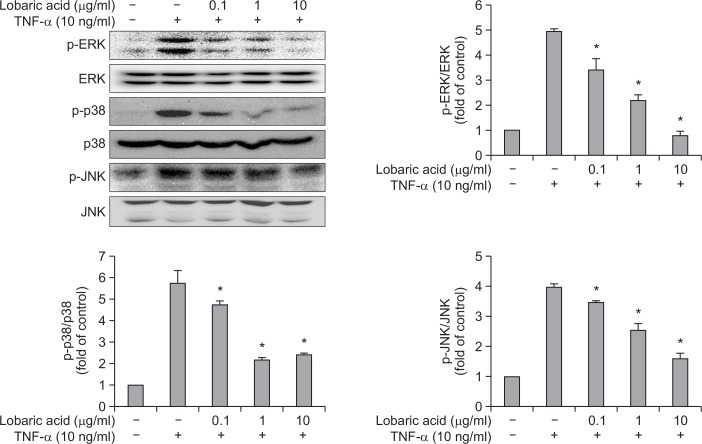
Many studies have demonstrated that MAPK plays a role in the induction of adhesion molecules by TNF-α (Ho et al., 2008) and our data also showed that lobaric acid suppressed VCAM-1 expression. Therefore, we investigated whether lobaric acid treatment resulted in the decreased activation of MAPK. As shown in Fig. 5, stimulation of cells with TNF-α increased an activity level of p38 MAPK, ERK1/2 and JNK, whereas TNF-α-induced MAPK activity was significantly inhibited by pretreatment with lobaric acid for 2 h. Thus, these results suggest that lobaric acid may inhibit TNF-α-induced VCAM-1 expression through the suppression of MAPK activation.
Fig. 5.
Effect of lobaric acid on the expression of MAP kinases in TNF-α-stimulated smooth muscle cells. VSMCs were pretreated with the indicated concentration of lobaric acid for 2 h and then incubated with TNF-α for 15 min. The whole cell lysates were analyzed by Western blot analysis. The relative intensities were expressed as the ratio of phospho-MAPK to total MAPK. The results are expressed as the mean ± S.E.M of three independent experiments. *p<0.05, significantly different from the group treated with TNF-α-induced VSMCs cells.
Inhibition of TNF-α-induced expression of TNF-R1 by lobaric acid
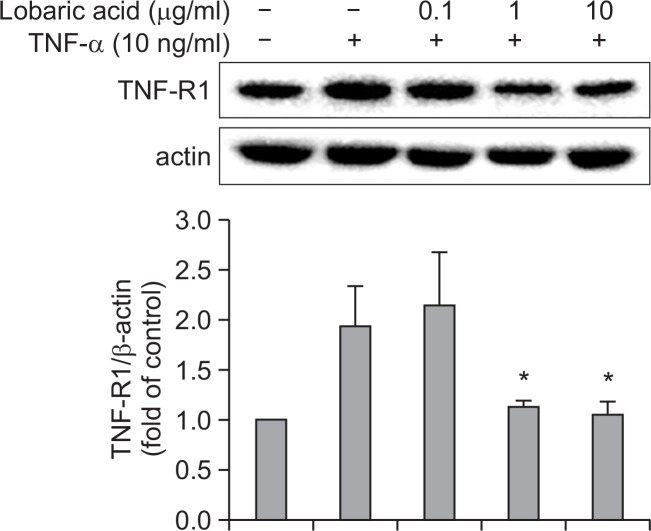
Since it has been known that TNF-α signaling through TNF-R1 could contributes to arterial inflammation and induction of VCAM-1 expression (Sawa et al., 2007; Kitagaki et al., 2012), we investigated the effect of lobaric acid on the expression of TNF-R1 (Fig. 6). The expression of TNF-R1 was increased in TNF-α-stimulated cells as compared to untreated cells. However, the expression of TNF-R1 was concentration-dependently inhibited by the pretreatment with lobaric acid for 2 h. These results suggest that lobaric acid suppresses TNF-α-induced VCAM-1 expression via the inhibition of TNF-R1 expression.
Fig. 6.
Effect of lobaric acid on the expression of TNF-R1. VSMCs were pretreated with the indicated concentration of lobaric acid for 2h and then incubated with TNF-α for 4 h. The cytoplasmic cell was analyzed by Western blot analysis. The results are mean ± S.E.M of 3 experiments. *p<0.05, significantly different from the group treated with TNF-α-induced VSMCs cells.
DISCUSSION
It has been reported that lichens synthesize and accumulate photoprotective compounds against UV radiation-induced damage in the photobiont (Hidalgo et al., 2005). Additionally, it has been known to have anti-proliferative, anti-inflammatory and immunomodulatory activities (Gissurarson et al., 1997; Ogmundsdóttir et al., 1998; Hidalgo et al., 2005; Vinitha et al., 2014). For this study, we examined the effect of lobaric acid on the expression of VCAM-1 in TNF-α stimulated mouse vascular smooth muscle cells.
Cytokines in the atherosclerosis could contribute to the expression of adhesion molecules (Huo and Ley, 2001; Lee et al., 2010). Vascular smooth muscle cells express the cellular adhesion molecules VCAM-1, which was associated with atherosclerotic plaques and was described with diseases (Kasper et al., 1996). Accordingly, therapeutic agents that suppress the expression of adhesion molecules have the potential to inhibit chronic inflammatory diseases, such as atherosclerosis. This is the first report indicating the inhibitory effect of lobaric acid on adhesion molecule expression in vascular smooth muscle cells.
Cytokines mediate the actions through interactions with high affinity surface receptors. Hence, interfering with ligand-receptor binding represents potential therapeutic approach. Various biological effects of TNF-α are mediated through two receptors, TNF-R1 and TNF-R2 (Armitage, 1994). TNF-R1 is the major signaling receptor in most cells and a critical mediator for upregulation of cellular adhesion molecules involved in atherosclerosis (Zhang L et al., 2007; Kitagaki et al., 2012). Intracellular mitogen-activated protein kinase (MAPK) signaling cascades play an important role in the signal transduction pathways that regulate cell adhesion molecules expressed on cells in response to external stimuli, including TNF-α (Ju et al., 2002; Ho et al., 2008). Additionally, TNF-R1 has been implicated in several signaling pathways including MAPK or NF-κB (Mackay et al,. 1993). In the present study, lobaric acid inhibited TNF-R1 expression by TNF-α. Moreover, our data demonstrate that the phosphorylation of MAPKs was inhibited by lobaric acid in a concentration-dependent manner. Therefore, these results suggest that lobaric acid inhibits TNF-R1 expression and this inhibition subsequently suppresses MAPK activation in TNF-α-stimulated VSMCs.
Activated MAPKs have been known to stimulate various transcription factors such as NF-κB, which has been shown to control inflammatory responses. In addition, activation of the transcription factor NF-κB is required for the transcriptional activation of cell adhesion molecules by TNF-α (Angel and Karin, 1991; Beg et al., 1993; Collins et al., 1995). Previous reports have been suggested that NF-κB activation is related to the phosphorylation and degradation of IκBα, and the nuclear translocation of p65 (Pahl, 1999; Waddick and Uckun, 1999). In addition, it has been suggested that NF-κB is essential to the gene expression of cell adhesion molecules, including VCAM-1 (Ledebur and Parks, 1995). Accordingly, the suppressed expression of adhesion molecules prompted us to examine the effect of lobaric acid on NF-κB activity. The present data showed that treatment with lobaric acid suppressed TNF-α-induced NF-κB activation through the inhibition of IκB kinase (IKK) activation. Further support for this conclusion comes from observation that lobaric acid could inhibit the TNF-α-induced NF-κB p65 nuclear translocation. Thus, our data implicated that MAPK and NF-κB pathways provide a novel insight into the mechanism of lobaric acid effects on VCAM-1 expression in VSMCs.
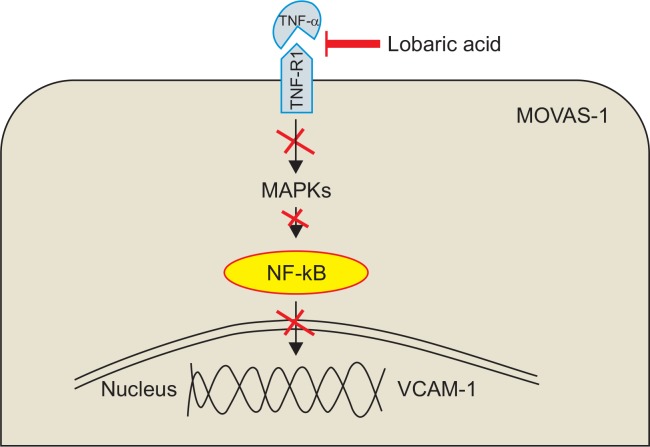
In summary, the results of the present study demonstrate that lobaric acid is capable of inhibiting the expression of VCAM-1 in VSMCs. This action results from suppression of MAPK pathways and NF-κB activation by blocking the expression of TNF-R1 (Fig. 7). The present data might account, at least in part, for the anti-inflammatory activities of lobaric acid. In addition, lobaric acid has been reported to have the anti-inflammatory effect by inhibiting the 5-lipoxygenase (Ogmundsdóttir et al., 1998).Thus, based on these findings, lobaric acid is proposed as an effective anti-inflammatory agent that may have potential therapeutic use in preventing the advancement of atherosclerotic lesions and other inflammatory diseases.
Fig. 7.
Inhibitory mechanism of VCAM-1 expression by lobaric acid.
Acknowledgments
This research was supported by the grant of the Ministry of Oceans and Fisheries’ R&D project (PM13030) and the Korea Polar Research Institute (KOPRI) project (PE13040).
REFERENCES
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Armitage RJ. Tumor necrosis factor receptor superfamily members and their ligands. Curr Opin Immunol. 1994;6:407–413. doi: 10.1016/0952-7915(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Beg AA, Finco TS, Nantermet PV, Baldwin AS., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993;13:3301–3310. doi: 10.1128/MCB.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M, Pietsch P, Schrör K, Baumann G, Felix SB. Cellular adhesion molecules on vascular smooth muscle cells. Cardiovasc Res. 1999;41:395–401. doi: 10.1016/S0008-6363(98)00302-2. [DOI] [PubMed] [Google Scholar]
- Chalmers JA, Martino TA, Tata N, Ralph MR, Sole MJ, Belsham DD. Vascular circadian rhythms in a mouse vascular smooth muscle cell line (MOVAS-1) Am J Physiol Regul Integr Comp Physiol. 2008;295:R1529–1538. doi: 10.1152/ajpregu.90572.2008. [DOI] [PubMed] [Google Scholar]
- Cho SJ, Kang NS, Park SY, Kim BO, Rhee DK, Pyo S. Induction of apoptosis and expression of apoptosis related genes in human epithelial carcinoma cells by Helicobacter pylori VacA toxin. Toxicon. 2003;42:601–611. doi: 10.1016/j.toxicon.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Choi KW, Park HJ, Jung DH, Kim TW, Park YM, Kim BO, Sohn EH, Moon EY, Um SH, Rhee DK, Pyo S. Inhibition of TNF-α-induced adhesion molecule expression by diosgenin in mouse vascular smooth muscle cells via downregulation of the MAPK, Akt and NF-κB signaling pathways. Vascul Pharmacol. 2010;53:273–280. doi: 10.1016/j.vph.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47:C7–12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Gissurarson SR, Sigurdsson SB, Wagner H, Ingolfsdottir K. Effect of lobaric acid on cysteinylleukotriene formation and contractile activity of guinea pig taenia coli. J Pharmacol Exp Ther. 1997;280:770–773. [PubMed] [Google Scholar]
- Hidalgo ME, Bascuñan L, Quilhot W, Fernández E, Rubio C. Spectroscopic and photochemical properties of the lichen compound lobaric acid. Photochem Photobiol. 2005;81:1447–1449. doi: 10.1562/2005-05-17-RA-530. [DOI] [PubMed] [Google Scholar]
- Ho AW, Wong CK, Lam CW. Tumor necrosis factor-alpha up-regulates the expression of CCL2 and adhesion molecules of human proximal tubular epithelial cells through MAPK signaling pathways. Immunobiology. 2008;213:533–544. doi: 10.1016/j.imbio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Huo Y, Ley K. Adhesion molecules and atherogenesis. Acta Physiol Scand. 2001;173:35–43. doi: 10.1046/j.1365-201X.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- Ingolfsdottir K, Gissurarson SR, Moller-Jakic B, Breu W, Wagner H. Inhibitory effects of the lichen metabolite lobaric acid on arachidonate metabolism in vitro. Phytomedicine. 1996;2:243–246. doi: 10.1016/S0944-7113(96)80049-3. [DOI] [PubMed] [Google Scholar]
- Jang Y, Lincoff AM, Plow EF, Topol EJ. Cell adhesion molecules in coronary artery disease. J Am Coll Cardiol. 1994;24:1591–1601. doi: 10.1016/0735-1097(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Ju JW, Kim SJ, Jun CD, Chun JS. p38 kinase and c-Jun N-terminal kinase oppositely regulates tumor necrosis factor alpha-induced vascular cell adhesion molecule-1 expression and cell adhesion in chondrosarcoma cells. IUBMB Life. 2002;54:293–299. doi: 10.1080/15216540215674. [DOI] [PubMed] [Google Scholar]
- Kasper HU, Schmidt A, Roessner A. Expression of the adhesion molecules ICAM, VCAM, and ELAM in the arteriosclerotic plaque. Gen Diagn Pathol. 1996;141:289–294. [PubMed] [Google Scholar]
- Kitagaki M, Isoda K, Kamada H, Kobayashi T, Tsunoda S, Tsutsumi Y, Niida T, Kujiraoka T, Ishigami N, Ishihara M, Matsubara O, Ohsuzu F, Kikuchi M. Novel TNF-α receptor 1 antagonist treatment attenuates arterial inflammation and intimal hyperplasia in mice. J Atheroscler Thromb. 2012;19:36–46. doi: 10.5551/jat.9746. [DOI] [PubMed] [Google Scholar]
- Ledebur HC, Parks TP. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J Biol Chem. 1995;270:933–943. doi: 10.1074/jbc.270.2.933. [DOI] [PubMed] [Google Scholar]
- Lee YW, Kim PH, Lee WH, Hirani AA. Interleukin-4, oxidative stress, vascular inflammation and atherosclerosis. Biomol Ther. 2010;18:135–144. doi: 10.4062/biomolther.2010.18.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Li H. Vascular cell adhesion molecule-1 and smooth muscle cell activation during atherogenesis. J Clin Invest. 1993;92:538–539. doi: 10.1172/JCI116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F, Loetscher H, Stueber D, Gehr G, Lesslauer W. Tumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. J Exp Med. 1993;177:1277–1286. doi: 10.1084/jem.177.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie NC, Zhu D, Longley L, Patterson CS, Kommareddy S, MacRae VE. MOVAS-1 cell line: a new in vitro model of vascular calcification. Int J Mol Med. 2011;27:663–668. doi: 10.3892/ijmm.2011.631. [DOI] [PubMed] [Google Scholar]
- Mo SJ, Son EW, Lee SR, Lee SM, Shin DH, Pyo S. CML-1 inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in endothelial cells through inhibition of IkBalpha kinase. J Ethnopharmacol. 2007;109:78–86. doi: 10.1016/j.jep.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Morita H, Tsuchiya T, Kishibe K, Noya S, Shiro M, Hirasawa Y. Antimitotic activity of lobaric acid and a new benzofuran, sakisacaulon A from Stereocaulon sasakii. Bioorg Med Chem Lett. 2009;19:3679–3681. doi: 10.1016/j.bmcl.2009.03.170. [DOI] [PubMed] [Google Scholar]
- Ogmundsdóttir HM, Zoëga GM, Gissurarson SR, Ingólfsdóttir K. Anti-proliferative effects of lichen-derived inhibitors of 5-lipoxygenase on malignant cell-lines and mitogen-stimulated lymphocytes. J Pharm Pharmacol. 1998;50:107–115. doi: 10.1111/j.2042-7158.1998.tb03312.x. [DOI] [PubMed] [Google Scholar]
- Ovstedal DO, Smith RL. Lichens of Antarctica and South Georgia a guide to their identification and ecology. Cambridge University Press; 2001. [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Sawa Y, Sugimoto Y, Ueki T, Ishikawa H, Sato A, Nagato T, Yoshida S. Effects of TNF-alpha on leukocyte adhesion molecule expressions in cultured human lymphatic endothelium. J Histochem Cytochem. 2007;55:721–733. doi: 10.1369/jhc.6A7171.2007. [DOI] [PubMed] [Google Scholar]
- Thadhani VM, Naaz Q, Choudhag MI, Mesaik A, Karunaratne V. Enzyme inhibitory and immunomodulatory activities of the depsidone lobaric acid extracted from the lichen Heterodermia sp. J. Natn. Sci. Foundation Sri Lanka. 2014;42:193–196. doi: 10.4038/jnsfsr.v42i2.6988. [DOI] [Google Scholar]
- Waddick KG, Uckun FM. Innovative treatment programs against cancer: II. Nuclear factor-kappaB (NF-kappaB) as a molecular target. Biochem Pharmacol. 1999;57:9–17. doi: 10.1016/S0006-2952(98)00224-X. [DOI] [PubMed] [Google Scholar]
- Zhang L, Peppel K, Sivashanmugam P, Orman ES, Brian L, Exum ST, Freedman NJ. Expression of tumor necrosis factor receptor-1 in arterial wall cells promotes atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:1087–1094. doi: 10.1161/01.ATV.0000261548.49790.63. [DOI] [PMC free article] [PubMed] [Google Scholar]