Abstract
To determine the protective effect of aloe-emodin (AE) from high glucose induced toxicity in RIN-5F (pancreatic β-cell) cell and restoration of its function was analyzed. RIN-5F cells have been cultured in high glucose (25 mM glucose) condition, with and without AE treatment. RIN-5F cells cultured in high glucose decreased cell viability and increased ROS levels after 48 hr compared with standard medium (5.5 mM glucose). Glucotoxicity was confirmed by significantly increased ROS production, increased pro-inflammatory (IFN-γ, IL-1β,) & decreased anti-inflammatory (IL-6&IL-10) cytokine levels, increased DNA fragmentation. In addition, we found increased Bax, caspase 3, Fadd, and Fas and significantly reduced Bcl-2 expression after 48 hr. RIN-5F treated with both high glucose and AE (20 μM) decreased ROS generation and prevent RIN-5F cell from glucotoxicity. In addition, AE treated cells cultured in high glucose were transferred to standard medium, normal responsiveness to glucose was restored within 8hr and normal basal insulin release within 24 hr was achieved when compared to high glucose.
Keywords: Aloe-emodin, Glucotoxicity, RIN-5F cells, Apoptosis, ROS
INTRODUCTION
Regulation of pancreatic β-cell mass is important for insulin secretion and glucose homeostasis that is involved in a variety of physiological and pathological conditions, such as apoptosis, autoimmunity, glucotoxicity and insulin resistance (Bernard et al., 1999). Permanent damage and pancreatic β cells apoptosis leads to a disruption in glucose homeostasis, which plays a critical role in the pathogenesis of diabetes (Winnay et al., 2014). Chronic hyperglycemia impairs β cell function, leading to the concept of glucotoxicity (Maedler et al., 2002). Piro et al. (2002) also confirmed that chronic exposure of high glucose or free fatty acid (FFA) levels may induce apoptosis of pancreatic β cells. Moreover, elevated glucose concentrations induce β cell apoptosis in cultured islets from humans (Federici et al., 2001); but high concentrations of glucose only cause β cell apoptosis in rodent islets (Efanova et al., 1998; Donath et al., 2001). Various molecular mechanisms have been proposed to underlie glucose-induced β cell dysfunction, such as formation of advanced glycation end products (Tajiri et al., 1977), impairment of insulin gene transcription (Robertson et al., 1994). Maedler et al. (2001) have reported that glucose induced Fas expression and its role in β cell apoptosis in human islets, but intermittent glucose mediator and mechanism are remain unknown.
Increasing evidence suggests that high glucose-induced β-cell toxicity primarily results from oxidative stress (Lee et al., 2014). Intermittent high glucose can induce apoptosis in pancreatic β cells, which is linked to increased formation of reactive oxygen species (ROS) (Zhang et al., 2015). ROS has the ability to oxidize or nitrify proteins, lipids and DNA by direct chemical modification and activate signaling pathways that cause cell death (Baines et al., 2005). Pancreatic β-cell mass is reduced in type 2 diabetic patients compared with nondiabetic subjects (Saito et al., 1979; Gepts and Lecompte, 1981); it has been suggested that high glucose-induced β cell apoptosis involves generation of ROS (Mauricio and Mandrup-Poulsen, 1998; Park and Hans, 2014). Inhibition of ROS is a promising strategy for blocking high glucose-triggered apoptosis in β cells (Park and Hans, 2014).
In the present study, we aimed to evaluate the effect of Aloe-emodin (AE) an anthraquinone (Fig. 1) on prevention of RIN-5F cell from glucotoxicity and restoration of its function. AE was originally isolated from leaves of Aloe vera (Hamman, 2008). Zhang et al. (2015) reported that AE have up-regulated glucose metabolism, decreased lipolysis and attenuated inflammation in 3T3-L1 preadipocytes. In addition, AE have been reported for its anticancer effects in various cancer cells (Wasserman et al., 2002; Fenig et al., 2004), liver cancer cell lines (Hep G2 and Hep 3B) and human promyelocytic leukemic cells (HL-60) (Chen et al., 2004). AE glycosides stimulate glucose transport and glycogen storage through PI3K-dependent mechanism in L6 myotubes and inhibit adipocyte differentiation in 3T3L1 adipocytes (Anand et al., 2010). Previously, we have reported the possible inhibitory effect of AE on human mesenchymal stem cell to adipocyte differentiation (Subash-Babu and Alshatwi, 2012). In the present study, our finding raises the possibility that AE may confer protection against high glucose-induced cytotoxicity in RIN-5F cells. We therefore explored the effect of AE on β-cell survival and apoptosis upon exposure to high glucose. In addition, both high glucose and AE treated cells on normal responsiveness to glucose and normal basal release of insulin were analyzed.
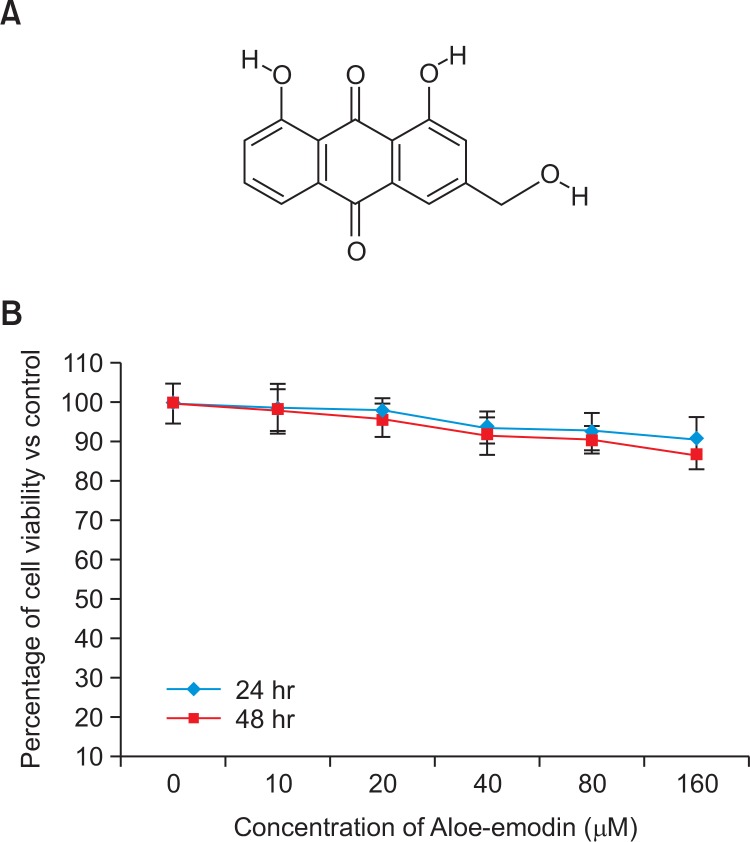
Fig. 1.
Structure of the bioactive compound Aloe-emodin, Molecular formula-C21H22O10; Molecular mass-434 (Fig. 1A). Cytotoxic effect of AE on RIN-5F cells at 24 hr and 48 hr (Fig. 1B). Each value is mean ± SD for 6 replicates.
MATERIALS AND METHODS
Chemicals and cell culture
Aloe-emodin was originally isolated from leaves of Aloe vera (Hamman, 2008). In the present study, we purchased Aloe-emodin from Sigma Chemical Co., St. Louis, MO, USA. All other chemicals used in this study were of the molecular biology grades and commercially available.
RIN5F and L6 myotubes were obtained from American Type culture collections (ATCC, Manassas, VA 20108 USA). Cell culture materials such as, RPMI-1640 (AG Biochrom, Germany), Fetal bovine serum (FBS) (Hiclone, Germany) and streptomycin (AG Biochrom, Germany. All spectrophotometric measurements were carried out using UV2010 Spectrophotometer (Hitachi, Germany). The cell line was maintained and the experiments were carried out according to the guidelines of CMRC ethical committee of KKUH, Saudi Arabia.
In vitro culture of RIN-5F cell and cytotoxicity study
RIN-5F cells derived from rat pancreatic β-cells were obtained from American Type Culture Collection (ATCC) and maintained in RPMI-1640 supplemented with 10% (v/v) FBS, streptomycin (100 μg/ml) and penicillin-G (100 U/ml) under an atmosphere of 5% CO2 and 95% humidified air at 37°C.
Initially, whether aloe-emodin (AE) produce any toxic to RIN-5F cells was analyzed by treating with increasing concentration of AE (such as, 0, 5, 10, 20, 40, 80 and 160 μmol) to RIN-5F cells cultured in standard medium. The cytotoxicity was analyzed after 24 hr and 48 hr incubations, respectively using MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide) assay as described by Mosmann (1983); the optical density was measured at 570 nm using 96-well microplate-reader (Bio-Rad, Model 680, Hercules, CA). The percentage of toxicity was calculated, using the formula:
Determination of glucotoxicity in RIN-5F cells
To determine high glucose induced toxicity to RIN-5F pancreatic β-cells, we cultured RIN-5F cells in two different concentration of glucose containing medium such as, standard medium (5.5 mM) and high glucose (25 mM) medium; the cytotoxicity was analyzed after 24 hr and 48 hr incubations, respectively.
The dose of AE (5, 10 and 20 μmol) has been selected based on our cytotoxicity study; to study the protective effect of AE from high glucose induced toxicity in RIN-5F cells. Briefly, RIN-5F cells cultured in high glucose medium was treated with AE and the cytotoxicity was analyzed after 24 hr and 48 hr incubations, respectively. The time and dose dependent cytotoxic effect was compared with respective untreated cells cultured in high glucose and standard (Normal) glucose medium.
Measurement of intracellular ROS
The level of intracellular reactive oxygen species (ROS) in RIN-5F cells cultured in high glucose was measured using 2′, 7′-dichlorofluorescin diacetate (DCFH-DA) (Wang and Joseph, 1999). DCFH-DA passively enters the cell where it reacts with ROS to form the highly fluorescent compound, dichlorofluorescein (DCF). Briefly, 10 mM DCFH-DA stock solution (in methanol) was diluted 500-fold in HBSS without serum or other additives to yield a 20 μM working solution. After 24 hr exposure with AE (5, 10&20 μmol), NAC (5 μmol) and in combination, RIN-5F cells in 24-well plates were washed twice with HBSS and then incubated in 2 ml of 20 μM DCFH-DA at 37°C for 30 min. In a separate experiment, the reference drug N-acetyl cysteine (20 mM) was treated with RIN-5F cells cultured in high glucose and compared with AE treated groups. Fluorescence was then determined at 485-nm excitation and 520-nm emission using a microplate reader.
Propidium iodide staining
The nuclear morphology was analyzed with 20 μmol AE treated RIN-5F cells after 48 hr using bright-field microscopy. Control cells were grown in the same manner without AE. The cells were trypsinized and fixed with ethanol. Then, cell nuclei were stained by adding 1 mg/ml propidium iodide (BD Biosciences, USA) at 37°C for 15 min in the dark. Characteristic apoptotic morphological changes were determined by PI staining as described by Leite et al. (1999). Briefly, a drop of cell suspension was placed on a glass slide and cover-slip was laid over to reduce light diffraction. At random 300 stained cells were examined under an inverted fluorescence microscope (Carl Zeiss, Jena, Germany) fitted with a 530/620 nm filter and observed at 400× magnification; in addition the percentage of cells showing pathological changes were calculated manually. Data were collected as four replicates and used to calculate the mean and standard deviation.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay
Terminal deoxynucleotidyl transferase-mediated dUTP end labeling (TUNEL) assay was performed to visualize DNA damage in cells. 1×104 cells were seeded into the wells of Permanox chamber slides (Thermo Scientific, Rochester, NY, USA). Next day, the media were exchanged for fresh RPMI-1640 medium. The cells were treated with high glucose and 20 μmol of AE for 48 hrs. Respective negative control also maintained without AE treatment. RIN-5F cells were fixed with 4% methanol-free paraformaldehyde in PBS for 10 min at room temperature. After fixation, wells were washed with PBS, permeabilized with a 0.2% Triton X-100 solution for 5 min, and washed twice in phosphate-buffered saline, then 100 μL of equilibration buffer was added at room temperature and incubated for 5–10 min. Samples were washed with PBS and incubated with terminal deoxynucleotidyl transferase, recombinant (rTdT) buffer at 37°C for 60 min inside the humidified chamber according to the manufacturer’s protocol (Promega). Reaction was terminated by adding 100 μL of SSC for 15 min. The wells were washed thrice, using PBS for 5 min to remove unincorporated fluorescein-12-dUTP nucleotides. Fragmented DNA was examined under inverted fluorescence microscope (Carl Zeiss). For each sample, the total number of cells and the number of TUNEL-positive cells were quantified in 10 representative fields. The results were presented as a representation from a series of three separate experiments.
Determination of inflammatory markers
The AE and their respective control samples were used to determine the amount of inflammatory mediators, such as IFN-γ, IL-1β, IL-6 and IL-10 in RIN-5F cells cultured in high glucose, using high-sensitivity ELISA-kits method (Quantikine, R&D Systems, Minneapolis, MN, USA). This assay analyzes the soluble and receptor-bound proteins, which gives a measurement of total concentration of inflammatory mediator proteins. The values were expressed as pg/mg protein for IFN-γ, IL-1β, IL-6 and IL-10.
qPCR analysis of oxidative stress and apoptosis responsive genes
Oxidative stress and apoptosis related genes expression was analyzed with quantitative reverse transcription-PCR (RT-PCR; Applied Biosystems 7500 Fast, Foster City, CA) using a real-time SYBR Green/ROX gene expression assay kit (QIAGEN). cDNA was directly prepared from cultured cells using a Fastlane® Cell cDNA kit (QIAGEN, Germany); and mRNA levels of CYP1A&TNF-α (oxidative stress related genes); apoptotic genes such as, Bax, Bcl-2, FAS, FADD and caspase 3 as well as the reference gene, GAPDH, were analyzed using gene-specific SYBR Green-based QuantiTect® Primer assays (QIAGEN, Germany). qPCR was performed in a reaction volume of 25 μL according to the manufacturer’s instructions. Briefly, 12.5 μL of master mix, 2.5 μL of assay primers (10×) and 10 μL of template cDNA (100 ng) were added to each well. After a brief centrifugation, PCR plate was subjected to 35 cycles under the following conditions: (i) PCR activation at 95°C for 5 minutes, (ii) denaturation at 95°C for 5 seconds and (iii) annealing/extension at 60°C for 10 seconds. All samples and controls were run in triplicate on an ABI 7500 Fast Real-Time PCR system. The quantitative RT-PCR data were analyzed using a comparative threshold (Ct) method, and the fold inductions of samples were compared with the untreated samples. GAPDH was used as an internal reference gene to normalize the expression of specific genes. The Ct cycle was used to determine the expression level in control and AE treated with RIN-5F cells after 48 hrs. The gene expression level was then calculated as previously described by Yuan et al. (2006). To determine the relative expression levels, the following formula was used: ΔΔCt=ΔCt (Treated)-ΔCt (Control). Thus, the expression levels are expressed as n-fold differences relative to the reference gene. The value was used to plot the expression of genes using the expression of 2−ΔΔCt.
Assay of insulin secretion activity
RIN-5F cells which were clones derived from rat pancreatic beta cells, were used to evaluate insulin secretion activity. The cells at a concentration of 2.0×105 of the cells/well in 24-well plates were seeded in RPMI-1640 medium. After 24 h incubation, the medium in each well was exchanged 1mL of the fresh medium and the cells were incubated for another 48 hr. The medium in the wells were removed and the cells were washed with the fresh medium (supplemented with 1% FBS) containing normal glucose (5.5 mM) and high glucose (25 mM). Different concentrations of AE (5, 10 & 20 μmol) were treated to the respective wells for 48 hrs, and then the concentration of insulin in the mediums was determined by ELISA system. In addition, after 48 hrs, both high glucose and AE treated cells were transferred to standard medium, normal responsiveness to glucose was measured after 8 hr and normal basal release of insulin was measured after 24 hr, respectively.
The activity was evaluated by an increase of the concentration of insulin-release comparing with the control experiment without test compounds. As a reference control, quercetin (20 μmol) was compared with the insulin secretory effect. Each experiment was done in triplicate and the results are presented as means SD. Group of data was compared using student’s t-test.
Statistical analysis
All the grouped data were statistically evaluated using SPSS/11.5 software package. The values were analyzed by one way analysis of variance (ANOVA) followed by Tukey’s test. All the results were expressed as mean ± SD with sufficient (six) replicates in each group. For all comparisons, differences were considered statistically significant at p≤0.05, p≤0.01 and p≤0.001 (Duncan, 1957).
RESULTS
In vitro cytotoxic effect of AE in RIN-5F cells
The tested concentrations of Aloe-emodin (0 to 160 μmol) did not produce toxicity to RIN-5F cells cultured in standard medium, also there were no significant decline in the viability of RIN-5F cells cultured in standard medium compared with the control after 24 hr or 48 hr (Fig. 1B).
High glucose induced cytotoxicity in RIN-5F cell
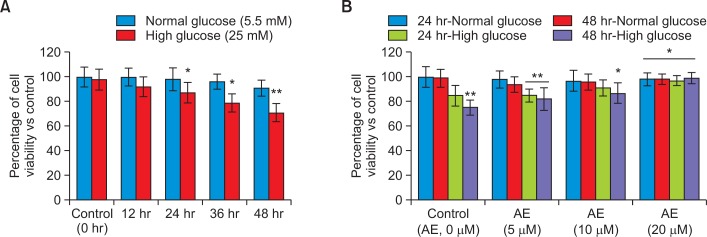
Fig. 2A shows the results of high glucose induced time dependent cytotoxic effect in RIN-5F cells, were cells cultured in standard (5.5 mM) and high (25 mM) glucose containing media for 24 hr and 48 hr. We found significant reduction of viability in RIN-5F cells cultured in high glucose such as, 13% reduction in 24 hrs (p≤0.05) and 29% reduction in 48 hrs (p≤0.001). Interestingly, there was no significant reduction in cell viability in tested standard (normal) glucose medium in 24 hr or 48 hr.
Fig. 2.
High glucose induced cytotoxic effect in (Fig. 2A) RIN5F cells with different time intervals; Fig. 2B showing protective effect of Aloe-emodin in RIN5F cells from high glucose toxicity after 24 hr and 48 hrs. Each value is mean ± SD for 6 replicates. *p≤0.05, RIN-5F cells cultured in high glucose compared with AE-treated high glucose. **p≤0.001, compared between high glucose and normal glucose.
Protective effect of AE from glucotoxicity using RIN-5F cells
In Fig. 2B, we have shown the protective effect of AE on high glucose induced toxicity using RIN-5F cells. AE at a dose of 5, 10 and 20 μmol significantly improved cell viability in a dose and time dependent manner. We found, RIN-5F cell cultured in high glucose medium significantly (p≤0.05) decreased the viability after 48 hrs. RIN-5F cells cultured in high glucose medium treated with 20 μmol of AE protect the cells and viability was significantly increased. We found the viability was reduced to 86% (24 hrs) and 73% (48 hrs), respectively in untreated control (Fig. 2B). But, AE treated RIN-5F cell the viability was significantly increased time dependently, such as 96 % in 48 hrs and 92% in 24 hrs. Dose-dependent increase in the viability of RIN-5F cells was ranged 72–79% cells are viable in 5 μmol, 80–86% (p≤0.05) in 10 μmol and 90–98% (p≤0.001) in 20 μmol of AE treatment to RIN-5F cells cultured in high glucose medium after 48 hrs. Viability of RIN-5F cells in 20 μmol dose was significantly high as compared to lower doses.
High glucose induced ROS generation level
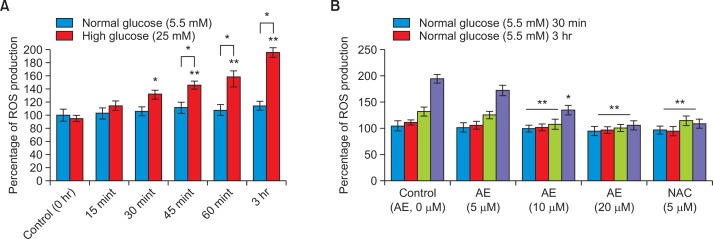
RIN-5F cells cultured in high glucose (25 mM) significantly (p≤0.001) increased ROS generation when compared to standard glucose condition (Fig. 3A). Significant increase of intracellular ROS production (p<0.001) was observed after 1 hr and increased till 6hr of incubation of pancreatic cells with high glucose as compared to normal glucose. Moreover, the increase in peroxides amounts generated by RIN-5F cells was time-dependent, being significantly higher (p≤0.001) in the later treatment (30 mint–3 hr) in comparison to beginning time (0–15 mint). Actually, peroxides levels were approaching higher after 1hr and 3hr exposure in RIN-5F cells to high glucose compared to normal glucose. We found an increased ROS generation may be affecting the cell growth significantly (p≤0.001).
Fig. 3.
Intracellular ROS generation (Fig. 3A) in RIN5F cells with different time intervals; Fig. 3B showing ROS quenching effect of Aloe-emodin. Each value is mean ± SD for 6 replicates. *p≤0.05, Comparison between normal glucose and high glucose. **p≤0.001, RIN-5F cells cultured in high glucose compared with AE-treated high glucose.
Fig. 3B shows the time-course effect of AE on quenching of intracellular peroxide levels in RIN-5F cells. After treatment with AE (20 μmol), we found significant reduction of ROS and cellular stress in RIN-5F cells cultured in high glucose compared with untreated control. The decrease in peroxide amount generated by RIN-5F cells was time and dose dependent, being significant higher (p≤0.001) at the lower doses (5 and 10 μmol) in comparison to relative higher dose (20 μmol) for 30 min after the addition of DCFH-DA. We observed a significant (p≤0.05) decrease in reactive oxygen species (peroxide) generation in 20 μmol AE treated cells as compared with untreated control and reference drug, NAC (Fig. 3B).
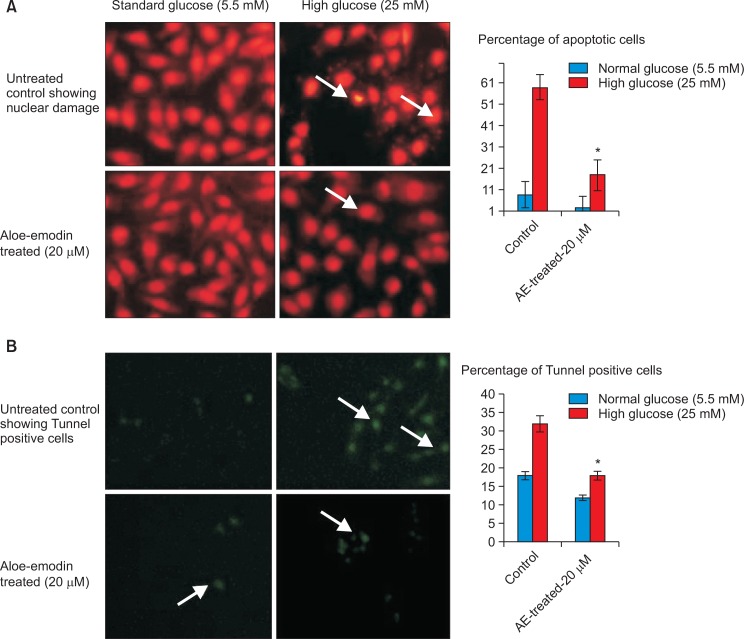
Nuclear morphology and DNA damage induced by high glucose
Cell and nuclear morphology have been evaluated using PI staining and DNA damage was analyzed using TUNNEL assay; and the results have been presented in Fig. 4. PI staining of RIN-5F cells cultured in high glucose medium resulted in significant morphological changes such as, abnormal nuclei, horseshoe-shaped nucleus, specially indicating fragmented nuclei/chromatin when compared to untreated control cells after 48 hr. Staining allows the clear discrimination between unaffected cells, apoptotic and necrotic cells (Fig. 4A). PI staining of RIN-5F cells cultured in high glucose showing 52% of cells with abnormal shape, nuclear condensation, may be cause apoptotic/necrotic stage after 48 hr. After treatment with AE, protect the cells and showing clear cellular and nuclear morphology (Fig. 4A). The increased cells viability may be due to the decreased ROS generation and oxidative stress. The increased cell viability was dose-dependent, being significant in high dose (p≤0.01) at 20 μmol in comparison to 5 and 10 μmol after 48 hr.
Fig. 4.
Propidium iodide staining and TUNNEL assay for RIN-5F cells cultured in high glucose medium and Aloe-emodin treated cells after 48 hrs. (A) Propidium iodide staining of control and AE treated RIN-5F cells. (B) Tunnel assay of control and AE treated RIN-5F cells.*p≤0.05, RIN-5F cells cultured in high glucose compared with AE-treated high glucose.
In TUNEL assay confirmed the presence of terminal DNA damage in high glucose treated RIN-5F cells (Fig. 4B) compared to normal cells. Increased green florescence intensity indicates the degree of DNA damage induced by high glucose induced oxidative stress. The results confirm RIN-5F cells were undergone oxidative stress induced cell death by apoptosis/necrosis. Also the manual count of PI shows 52% of cells were shown apoptosis, 9% of cells are in necrotic stage (Fig. 4A) and tunnel positive cells shown in Fig. 4B. Our observation clearly showed that high glucose induced ROS production take part in highly organized cellular signaling, signal transduction and apoptosis.
Effect of aloe-emodin on inflammatory cytokines
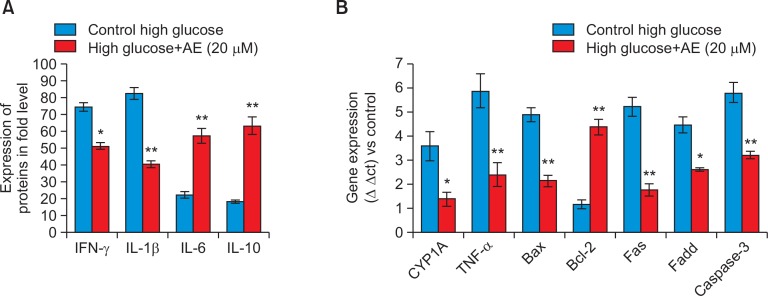
The levels of pro- and anti-inflammatory cytokines were significantly increased in RIN-5F cells cultured in high glucose, such as IL-1β (2.13 fold), IFN-γ (3.05) was increased and IL-6 (3.15) and IL-10 (2.95) were decreased when compared with untreated control (Fig. 5A). After AE treatment, the altered cytokine such as, IL-1β and IFN-γ levels were decreased, whereas IL-6 and IL-10 levels were significantly increased to two fold.
Fig. 5.
Changes of pro-inflammatory markers (Fig. 5A), oxidative metabolic stress and apoptosis related gene expression (Fig. 5B) analysis during RIN-5F cells exposed to high glucose medium and AE treatment after 48 hr. Each value is mean ± SD for 6 replicates. *p≤0.05, AE treated group compared with untreated RIN-5F cells cultured in high glucose. **p≤0.001 AE treated group compared with untreated RIN-5F cells cultured in high glucose.
Gene expression analysis
We analyzed oxidative metabolic stress related genes such as CYP1A and TNF-α in control and AE (20 μmol) treated RIN-5F cells cultured in high glucose medium for 48 hr. The relative quantitation of oxidative stress related genes such as, CYP1A and TNF-α, were shown in Fig. 5B. The expression of CYP1A and TNF-α was down regulated two fold significantly (p≤0.01) in AE (20 μmol) treated cells when compared to untreated RIN-5F cells grown in high glucose medium.
In addition Fig. 5B, shows the alterations in Bax, Bcl-2, Fas, Fadd and caspase 3 gene expressions between control and AE treated RIN-5F cells grown in high glucose medium for 48 hr. A marked two fold increased (p≤0.001) in the mRNA levels of Bax, Fas, Fadd, caspase-3 and at the same time Bcl-2 expression significantly decreased a fold in RIN-5F cells. After 20 μmol of AE treatment significantly decreased the apoptotic genes and increased Bcl-2 expression.
Basal and Glucose stimulated Insulin secretory (GSIS) effect in RIN5F cells
Table 1 shows the influence of AE on baseline and GSIS in RIN-5F cells cultured in high glucose medium. Insulin secretion (ng/well) was significantly increased in AE treated RIN-5F cells compared to untreated controls after 48 hr. We observed the GSIS was directly proportional to the severity of glucose and dose-dependently; and significant (p≤0.001) GSIS were seen at 10 and 20 μmol in high glucose medium compared to standard glucose medium after 48 hr. In addition, AE treated cells further cultured in standard medium, showed normal responsiveness on insulin secretion after 8 hr. However, in high glucose treated cells basal insulin release was reached normal after 24 hr in AE treated cells, compared to untreated control cells. Compared to quercetin (20 μmol), AE treatment significantly (p≤0.001) increased basal insulin secretion.
Table 1.
Glucose stimulated insulin secretion (GSIS) level of AE treated RIN-5F cells after 48 hrs; normal responsiveness and basal insulin release by AE in RIN5F cells
| Groups | GSIS of AE treated RIN-5F cells after 48 hrs (ng/well) | Basal insulin releasing level (ng/well) of RIN-5F cells (¥) in standard medium | ||
|---|---|---|---|---|
|
|
|
|||
| Normal glucose (5.5 mM) | High glucose (25 mM) | 8 hr | 24 hr | |
| Control (AE, 0 μmol) | 6.4 ± 0.9 | 4.5 ± 0.4 | 10.2 ± 0.9 | 5.2 ± 0.6 |
| AE (5 μmol) | 7.2 ± 0.8 | 11.4 ± 0.8* | 10.5 ± 1.2 | 8.6 ± 0.8 |
| AE (10 μmol) | 12.5 ± 1.6 ** | 15.9 ± 1.6** | 12.9 ± 0.9** | 11.6 ± 1.7* |
| AE (20 μmol) | 14.2 ± 1.1** | 21.3 ± 1.3**,* | 13.5 ± 1.4** | 12.9 ± 0.5** |
| Quercetin (20 μmol) | 13.6 ± 1.5* | 16.2 ± 0.9* | 9.5 ± 0.6* | 7.1 ± 1.3* |
Normal responsiveness and basal insulin release by Aloe-emodin, in high glucose treated RIN-5F cells transferred to standard medium. Each value is mean ± SD for 6 replicates.
p≤0.05, AE treated group compared with untreated RIN-5F cells cultured in high glucose. Comparison between high and normal glucose medium,
p≤0.001 AE treated group compared with untreated RIN-5F cells cultured in high glucose.
DISCUSSION
During the progression of type 2 diabetes, glucotoxicity is an important factor that contributes to advancing pancreatic β-cell failure and development of diabetes (Maedler et al., 2002). High glucose-induced β cell apoptosis involves generation of ROS (Park and Hans, 2014). Persistent hyperglycemia further accelerates the production of ROS in pancreas and enhances cellular stress. Inhibition of ROS is a promising strategy for blocking high glucose-triggered apoptosis in β cells (Park and Hans, 2014). We found in our study, AE significantly impaired high glucose-induced ROS formation in RIN-5F cells. It may be suggest that the protection of RIN-5F cells by AE is associated with suppression of ROS generation in β cells. Alteration of ROS production may represent an important mechanism for AE mediated regulation of cellular behaviors. In our previous study, we have evidenced that inhibition of ROS generation in RIN-5F cells by nymphayol is possible and basal insulin secretion also restored (Subash-Babu et al., 2015).
The generation of ROS in response to the high concentrations of glucose also cause mitochondrial dysfunction and trigger β-cells apoptosis (Hodgin et al., 2013). In addition, previous reports suggest that β-cells failure and diabetes is associated with inflammatory response; mitochondrial oxidative stress and induction of apoptosis are interrelated (Jialal et al., 2002). Increased IL-1β is an indicator of the toxic effects of elevated glucose concentrations. In addition, it was confirmed that IL-1β impairs insulin release and induce Fas expression, which enable Fas-triggered apoptosis in human islets (Giannoukakis et al., 1999). Most interestingly, we found in our study, the IL-1 β and IFN-γ was increased might be due to the high glucose induced immune response. Maedler et al. (2002) also demonstrate that high concentrations of glucose induce IL-1β production and secretion in human β cells, leading to Fas receptor upregulation, NF-κB activation; β cell apoptosis and dysfunction. Together, the above findings led us to hypothesize that high glucose may induce IL-1β secretion from β cells in the absence of an autoimmune process.
Mitochondrial dependent apoptotic pathway involves reduction of the mitochondrial potential, leakage of cytochrome C from mitochondria (Ly et al., 2003). RIN-5F cells cultured in high glucose up regulated TNF-α, Fas and its adaptor protein FADD in death receptor-dependent pathways. It may be due to the increased Bax/decreased Bcl-2 expression, which release cytochrome C via up regulating CYP1A, subsequently activated extrinsic pathway. Those observations indicate that chronic exposure to elevated glucose levels increases apoptosis in pancreatic islets and these cytotoxic effects could be mediated by oxidative stress. However, AE treatment significantly quenching the ROS generation and possibly down regulate the intrinsic and extrinsic apoptotic signaling gene expression, which decrease the high glucose induced toxicity to RIN-5F cells.
Noteworthy that AE treatment to RIN-5F cells produce significantly higher insulin secretion in glucose stimulated conditions, relatively low generation of ROS. The stimulation of insulin secretion by AE depends on the severity of hyperglycemic condition, which was not related to cellular stress or altered mitochondrial dynamics. In our previous study, we have confirmed that nymphayol possibly improve early phase glucose stimulated insulin secretion and restoring cellular insulin sensitivity in vitro (Subash-Babu et al., 2015).
In conclusion, Aloe-emodin protects RIN-5F pancreatic β cells from glucotoxicity. Also AE restored the normal responsiveness to glucose and basal insulin secretion in hyperglycemic condition. Aloe-emodin may be a therapeutic agent to overcome β-cell failure that occurs most of the chronic type 2 diabetic patients.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research, King Saud University for its funding of this research through the Research Group Project No. RG-1435-045.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Anand S, Muthusamy VS, Sujatha S, Sangeetha KN, Bharathi Raja R, Sudhagar S, Poornima Devi N, Lakshmi BS. Aloe emodin glycosides stimulate glucose transport and glycogen storage through PI3K dependent mechanism in L6 myotubes and inhibits adipocyte differentiation in 3T3L1 adipocytes. FEBS Lett. 2010;584:3170–3178. doi: 10.1016/j.febslet.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Bernard C, Berthault MF, Saulnier C, Ktorza A. Neogenesis vs. apoptosis as main components of pancreatic beta cell ass changes in glucose-infused normal and mildly diabetic adult rats. FASEB J. 1999;13:1195–1205. doi: 10.1096/fasebj.13.10.1195. [DOI] [PubMed] [Google Scholar]
- Chen HC, Hsieh WT, Chang WC, Chung JG. Aloe-emodin induced in vitro G2/M arrest of cell cycle in human promyelocytic leukemia HL-60 cells. Food Chem Toxicol. 2004;42:1251–1257. doi: 10.1016/j.fct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Donath MY, Gross DJ, Cerasi E, Kaiser N. Hyperglycemia induced beta-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes. 2001;48:738–744. doi: 10.2337/diabetes.48.4.738. [DOI] [PubMed] [Google Scholar]
- Duncan BD. Multiple range test for correlated and heteroscedastic means. Biometrics. 1957;13:164–176. doi: 10.2307/2527799. [DOI] [Google Scholar]
- Efanova IB, Zaitsev SV, Zhivotovsky B, Köhler M, Efendić S, Orrenius S, Berggren PO. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration. J Biol Chem. 1998;273:33501–33507. doi: 10.1074/jbc.273.50.33501. [DOI] [PubMed] [Google Scholar]
- Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, Usellini L, Nano R, Bonini P, Bertuzzi F, Marlier LN, Davalli AM, Carandente O, Pontiroli AE, Melino G, Marchetti P, Lauro R, Sesti G, Folli F. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50:1290–1301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- Fenig E, Nordenberg J, Beery E, Sulkes J, Wasserman L. Combined effect of aloe-emodin and chemotherapeutic agents on the proliferation of an adherent variant cell line of Merkel cell carcinoma. Oncol Rep. 2004;11:213–217. [PubMed] [Google Scholar]
- Gepts W, Lecompte PM. The pancreatic islets in diabetes. Am J Med. 1981;70:105–114. doi: 10.1016/0002-9343(81)90417-4. [DOI] [PubMed] [Google Scholar]
- Giannoukakis N, Rudert WA, Ghivizzani SC, Gambotto A, Ricordi C, Trucco M, Robbins PD. Adenoviral gene transfer of the interleukin-1 receptor antagonist protein to human islets prevents IL-1betainduced beta-cell impairment and activation of islet cell apoptosis in vitro. Diabetes. 1999;48:1730–1736. doi: 10.2337/diabetes.48.9.1730. [DOI] [PubMed] [Google Scholar]
- Hamman JH. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13:1599–1616. doi: 10.3390/molecules13081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgin JB, Nair V, Zhang H, Randolph A, Harris RC, Nelson RG, Weil EJ, Cavalcoli JD, Patel JM, Brosius FC, Kretzler M. Identification of cross-species shared transcriptional networks of diabetic nephropathy in human and mouse glomeruli. Diabetes. 2013;62:299–308. doi: 10.2337/db11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jialal I, Devaraj S, Venugopal SK. Oxidative stress, inflammation, and diabetic vasculopathies: the role of alpha tocopherol therapy. Free Radic Res. 2002;36:1331–1336. doi: 10.1080/1071576021000038531. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jung IR, Choi SE, Lee SM, Lee SJ, Han SJ, Kim HJ, Kim DJ, Lee KW, Kang Y. Toxicity generated through inhibition of pyruvate carboxylase and carnitine palmitoyl transferase-1 is similar to high glucose/palmitate-induced glucolipotoxicity in INS-1 beta cells. Mol Cell Endocrinol. 2014;383:48–59. doi: 10.1016/j.mce.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Leite M, Quinta-Costa M, Leite PS, Guimmaraes JE. Critical evaluation of techniques to detect and measure cell death-study in a model of UV radiation of the leukaemic cell line HL60. Anal Cell Pathol. 1999;19:139–151. doi: 10.1155/1999/176515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (Δ ψm) in apoptosis; an update. Apoptosis. 2003;8:115–128. doi: 10.1023/A:1022945107762. [DOI] [PubMed] [Google Scholar]
- Maedler K, Spinas GA, Lehmann R, Sergeev P, Weber M, Fontana A, Kaiser N, Donath MY. Glucose induces b-cell apoptosis via upregulation of the Fas-receptor in human islets. Diabetes. 2001;50:1683–1690. doi: 10.2337/diabetes.50.8.1683. [DOI] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1 beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI200215318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauricio D, Mandrup-Poulsen T. Apoptosis and the pathogenesis of IDDM. A question of life and death. Diabetes. 1998;47:1537–1543. doi: 10.2337/diabetes.47.10.1537. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Park MH, Han JS. Padina arborescens extract protects high glucose-induced apoptosis in pancreatic β cells by reducing oxidative stress. Nutr Res Pract. 2014;8:494–500. doi: 10.4162/nrp.2014.8.5.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piro S, Anello M, Di Pietro C, Lizzio MN, Patan G, Rabuazzo AM, Vigneri R, Purrello M, Purrello F. Chronic exposure to free fatty acids or high glucose induces apoptosis in rat pancreatic islets: possible role of oxidative stress. Metabolism. 2002;51:1340–1347. doi: 10.1053/meta.2002.35200. [DOI] [PubMed] [Google Scholar]
- Robertson RP, Olson LK, Zhang HJ. Differentiating glucose toxicity from glucose desensitization: a new message from the insulin gene. Diabetes. 1994;43:1085–1089. doi: 10.2337/diab.43.9.1085. [DOI] [PubMed] [Google Scholar]
- Saito K, Yaginuma N, Takahashi T. Differential volumetry of alpha, beta and delta cells in the pancreatic islets of diabetic and non diabetic subjects. Tohoku J Exp Med. 1979;129:273–283. doi: 10.1620/tjem.129.273. [DOI] [PubMed] [Google Scholar]
- Subash-Babu P, Alshatwi AA. Aloe-emodin inhibits adipocyte differentiation and maturation during in vitro human mesenchymal stem cell adipogenesis. J Biochem Mol Toxicol. 2012;26:291–300. doi: 10.1002/jbt.21415. [DOI] [PubMed] [Google Scholar]
- Subash-Babu P, Ignacimuthu S, Alshatwi AA. Nymphayol increases glucose-stimulated insulin secretion by RIN-5F cells and GLUT4-mediated insulin sensitization in type 2 diabetic rat liver. Chem Biol Interact. 2015;25:72–81. doi: 10.1016/j.cbi.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Tajiri Y, Moller C, Grill V. Long-term effects of aminoguanidine on insulin release and biosynthesis: evidence that the formation of advanced glycosylation end products inhibits B cell function. Endocrinology. 1977;138:273–280. doi: 10.1210/endo.138.1.4851. [DOI] [PubMed] [Google Scholar]
- Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/S0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Wasserman L, Avigad S, Beery E, Nordenberg J, Fenig E. The effect of aloe emodin on the proliferation of a new Merkel carcinoma cell line. Am J Dermatopathol. 2002;24:17–22. doi: 10.1097/00000372-200202000-00003. [DOI] [PubMed] [Google Scholar]
- Winnay JN, Dirice E, Liew CW, Kulkarni RN, Kahn CR. p85α deficiency protects β-cells from endoplasmic reticulum stress-induced apoptosis. Proc Natl Acad Sci USA. 2014;111:1192–1197. doi: 10.1073/pnas.1322564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang R, Lv P, Yang J, Deng Y, Xu J, Zhu R, Zhang D, Yang Y. Emodin up-regulates glucose metabolism, decreases lipolysis, and attenuates inflammation in vitro. J. Diabetes. 2015;7:360–368. doi: 10.1111/1753-0407.12190. [DOI] [PubMed] [Google Scholar]