Abstract
Amygdalin, D-mandelonitrile-β-D-glucoside-6-β-glucoside, belongs to aromatic cyanogenic glycoside group derived from rosaceous plant seed. Mounting evidence has supported the anti-cancer effects of amygdalin. However, whether amygdalin indeed acts as an anti-tumor agent against breast cancer cells is not clear. The present study aimed to investigate the effect of amygdalin on the proliferation of human breast cancer cells. Here, we show that amygdalin exerted cytotoxic activities on estrogen receptors (ER)-positive MCF7 cells, and MDA-MB-231 and Hs578T triple-negative breast cancer (TNBC) cells. Amygdalin induced apoptosis of Hs578T TNBC cells. Amygdalin downregulated B-cell lymphoma 2 (Bcl-2), upregulated Bcl-2-associated X protein (Bax), activated of caspase-3 and cleaved poly ADP-ribose polymerase (PARP). Amygdalin activated a pro-apoptotic signaling molecule p38 mitogen-activated protein kinases (p38 MAPK) in Hs578T cells. Treatment of amygdalin significantly inhibited the adhesion of Hs578T cells, in which integrin α5 may be involved. Taken together, this study demonstrates that amygdalin induces apoptosis and inhibits adhesion of breast cancer cells. The results suggest a potential application of amygdalin as a chemopreventive agent to prevent or alleviate progression of breast cancer, especially TNBC.
Keywords: Amygdalin, Apoptosis, Adhesion, Breast cancer
INTRODUCTION
Breast cancer has been estimated as one of most commonly diagnosed types of cancer among women (Siegel et al., 2014). In particular, triple-negative breast cancers (TNBCs) lacking the expressions of estrogen/progesterone receptors (ER/PR) and human epidermal growth factor receptor 2 (HER2) are correlated with poor prognosis (Sotiriou et al., 2003). Clinically effective drugs for the treatment of TNBC have not been reported to date (Hudis and Gianni, 2011).
Many natural chemopreventive compounds are shown to reduce the risk of cancer through induction of apoptosis, programmed cell death (Tanaka, 2013). Mitochondrial-mediated apoptosis is induced by multiple molecular events including decrease in B-cell lymphoma 2 (Bcl-2), increases in Bcl-2-associated X protein (Bax), and activation of caspase-3 (Chiarugi et al., 1994; Fernald and Kurokawa, 2013). Poly ADP-ribose polymerase (PARP), a nuclear enzyme involved in DNA repair, is a well-known substrate for caspase-3 in the apoptotic process (Lazebnik et al., 1994; D’Amours et al., 1998). The pro-apoptotic (such as JNK-1 and p38) and the anti-apoptotic (such as ERKs and JNK-2) signaling molecules have been shown to be involved in induction of cell death (Xia et al., 1995; Ruiter et al., 1999).
Tumor metastasis is a complex process involving extensive interactions between the tumor cells and host tissues including tumor cell dissociation, intravasation, extravasation, adhesion, and angiogenesis (Alizadeh et al., 2014). Cell adhesion plays a crucial role in cancer progression and metastasis (Albelda, 1993). Integrins are essential for forming cell-cell and cell-matrix bonds, regulating cell adhesion, extravasation and migration (Heyder et al., 2005).
Amygdalin, D-mandelonitrile-β-D-glucoside-6-β-glucoside, belongs to the aromatic cyanogenic glycoside group and is widely distributed in the rosaceous plant seed, for example, apricot, peach, apple, cherry, plum, etc. (Holzbecher et al., 1984; Santos Pimenta et al., 2014). Amygdalin is known for effective component of the traditional Chinese medicine and used as auxiliary medicine of cough expectorant agent and cancer therapy (Holland, 1982; Milazzo et al., 2011). A number of studies reported that amygdalin has various activities including anti-tussive, anti-asthmatic, anti-atherogenic, inhibition/prevention of fibrosis, anti-inflammatory, anti-ulcer and anti-cancer activities (Song and Xu, 2014).
Mounting evidence has supported that amygdalin induces apoptotic cell death of various cancer cells such as promyelocytic leukemia, prostate cancer, cervical and liver cancer cells (Kwon et al., 2003; Chang et al., 2006; Zhou et al., 2012; Chen et al., 2013; Song and Xu, 2014). Treatment with amygdalin increased expression of Bax, decreased expression of Bcl-2 and induced caspase-3 activation in human DU145 and LNCaP prostate cancer cells (Chang et al., 2006). Amygdalin induced apoptosis of HeLa cervical cancer cells mediated by endogenous mitochondrial pathway (Chen et al., 2013). A recent study showed that amygdalin reduced adhesion and migration of UMUC-3 and RT112 bladder cancer cells through activation of focal adhesion kinase (FAK) and modulation of β1 integrin (Makarević et al., 2014).
Despite many studies have demonstrated the anti-cancer effects of amygdalin on various cancer cells, the chemopreventive potential of amygdalin in breast cancer is poorly understood at present. In this study, we aimed to investigate the chemopreventive potential of amygdalin against breast cancer. To this end, we examined the anti-proliferative effect of amygdalin on ER-positive MCF7 human breast carcinoma cells and MDA-MB-231 and Hs578T TNBC cells. In addition, we investigated the effect of amygdalin on apoptosis and adhesion of Hs578T TNBC cells.
MATERIALS AND METHODS
Cell culture conditions
Hs578T and MDA-MB-231 breast cancer cells were purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). MCF7 cells were from American Type Culture Collection (ATCC, Manassas, USA). Hs578T and MCF7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 100 μg/mL penicillin-streptomycin as previously described (Koh et al., 2015). MDA-MB-231 cells were cultured in Roswell Park Memorial Institute medium (RPMI) supplemented with 10% FBS and 100 μg/mL penicillin-streptomycin.
Reagents
Amygdalin (depicted in Fig. 1) was purchased from Sigma Aldrich (St. Louis, MO, USA) and dissolved in serum-free media. MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide) was purchased from Sigma Aldrich (St. Louis, MO, USA). Z-VAD-FMK was purchased from R&D system, Inc. (Minneapolis, MN, USA).
Fig. 1.
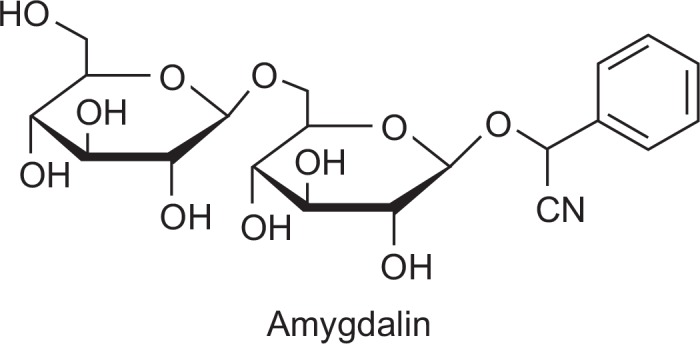
The structure of amygdalin.
MTT assay
Cells (1×104 cells) cultured in a 96-well plate were treated with amygdalin for 24 hr. After incubation, 25 μL of 5 mg/mL of MTT was added and incubated for 3 hr. Then the formazan was dissolved with 100 μL of DMSO and the optical density was measured at 540 nm using ELISA reader (Synergy 2; BioTek Instruments, Inc., Winooski, VT, USA).
Immunoblot analysis
The cells were cultured to 70% confluency and incubated in serum-free media containing various concentrations of amygdalin (0, 10, 20 and 40 mg/mL) for 24 hr. Immunoblot analysis was performed as described previously (Moon et al., 2000). Protein extracts in lysis buffer (50 mM Tris, 2% SDS, 1 mM EDTA, 0.1 M DTT, protease inhibitor cocktail) were subjected to immunoblot analysis. Rabbit polyclonal anti-Bax and mouse monoclonal anti-Bcl-2 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Mouse monoclonal anti-caspase-3 antibody was purchased from OncogeneTM research products (San Diego, CA, USA). Rabbit polyclonal anti-PARP, anti-phosphorylated p38 MAPK (anti-pp38 MAPK), anti-p38 MAPK and anti-integrin α5 antibodies were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). The enhanced chemiluminescence system was used for detection (Advansta Inc., Menlo Park, CA, USA).
Adhesion assay
Adhesion assays was performed on 96-well plates coated with 5 μg/mL collagen as previously described (Miao et al., 2005). Cells were added into each well and placed for 30 min at 37°C in 5% CO2 humidified air incubation. Non-adherent cells were removed by gently washing the wells three times with wash buffer. Adherent cells were fixed with 4% paraformaldehyde for 10 min at room temperature, followed by rinsing with wash buffer, and stained with 0.5% crystal violet for 10 min. After rinsing, the dye was released from the cells by addition of 2% sodium dodecyl sulfate (SDS), and the plates were read on a microplate reader (Synergy 2; BioTek Instruments, Inc., Winooski, VT, USA).
RESULTS
Amygdalin inhibits proliferation of MCF7, MDA-MB-231 and Hs578T cells
We investigated the cytotoxic effects of amygdalin on human breast carcinoma cells. We performed MTT assay upon treatment with various concentrations of amygdalin for 24 hr. As shown in Fig. 2, amygdalin inhibited proliferation of MCF7, MDA-MB-231 and Hs578T cells in a dose-dependent manner. The IC50 values of amygdalin in MCF7, MDA-MB-231 and Hs578T cells were 30.8, 48.5 and 52.9 mg/mL, respectively. The data demonstrate that amygdalin exerted cytotoxic effect on ER-positive MCF7 as well as MDA-MB-231 and Hs578T TNBC cells.
Fig. 2.
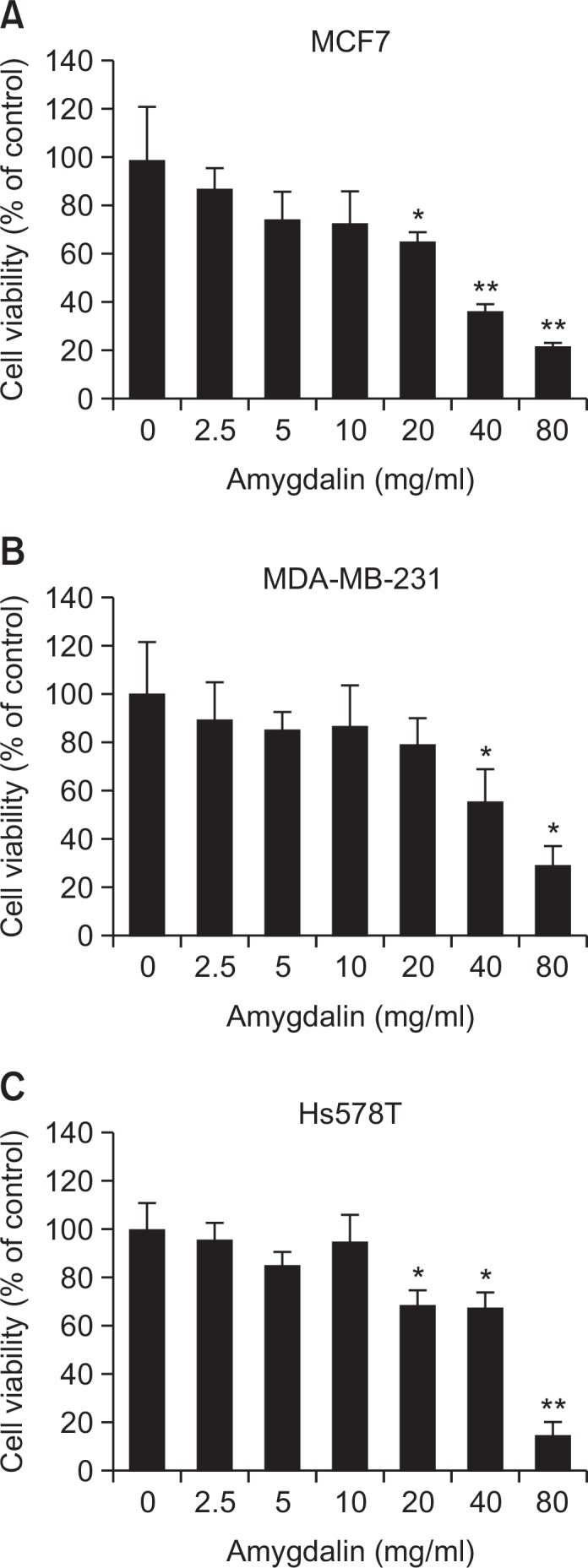
Amygdalin inhibits cell growth in breast carcinoma cells. The cells were treated with various concentrations of amygdalin for 24 hr and then subjected to an MTT assay in MCF7 (A), MDA-MB-231 (B) and Hs578T cells (C). The results represent mean ± SD for triplicates (*p<0.05 and **p<0.01 vs. control).
Amygdalin regulates apoptosis-related proteins and signaling molecules
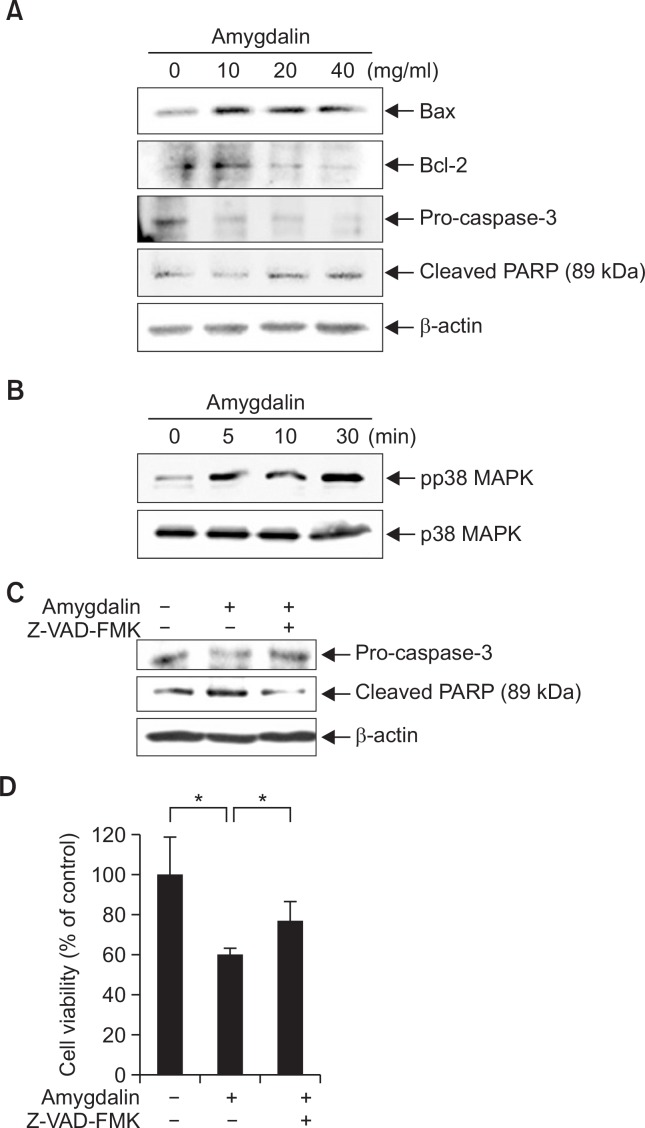
To evaluate if cytotoxic effect of amygdalin on breast cancer cells involves apoptosis, we determined the expression levels of apoptosis-related proteins in Hs578T TNBC cells. Cells were treated with amygdalin at various concentrations for 24 hr. The levels of Bcl-2, Bax, PARP and pro-caspase-3 were determined by immunoblot analysis. As shown in Fig. 3A, amygdalin increased the expression level of pro-apoptotic protein Bax and decreased that of anti-apoptotic Bcl-2. The level of pro-caspase-3 was decreased by amygdalin treatment. PARP cleavage was observed in Hs578T cells treated with amygdalin.
Fig. 3.
Amygdalin regulates apoptosis-related proteins. (A) Hs578T cells were treated with amygdalin at the indicated concentrations (0, 10, 20 and 40 mg/mL). The levels of Bax, Bcl-2, pro-caspase-3 and cleaved PARP were determined by immunoblot analysis using specific antibodies. (B) Hs578T cells were treated with 40 mg/mL amygdalin at the indicated time-points. The levels of phosphorylated and total p38 MAPK were determined by immunoblot analysis. (C) Hs578T cells were treated with 40 mg/mL amygdalin and 25 μM Z-VAD-FMK for 24 hr. The protein levels of cleaved PARP and pro-caspase-3 were determined by immunoblot analysis. (D) Hs578T cells were treated with 60 mg/mL amygdalin and 25 μM Z-VAD-FMK for 24 hr and then subjected to an MTT assay. The results represent mean ± SD for triplicates (*p<0.05 vs. control).
A kinetic study was performed to examine the effect of amygdalin on the activation of p38 MAPK which is known as a pro-apoptotic signaling molecule (Xia et al., 1995; Ruiter et al., 1999). pp38 MAPK was increased by amygdalin treatment in a time-dependent manner, while the expression level of total p38 MAPK was not increased (Fig. 3B). Taken together, these data demonstrate that amygdalin induced apoptosis in Hs578T cells in which signaling pathway through p38 MAPK may be involved.
To examine whether a caspase-3 inhibitor might attenuate amygdalin-induced cytotoxic effect, we treated the Hs578T cells with a caspase-3 inhibitor, 25 μM Z-VAD-FMK for 24 hr. Amygdalin-induced decrease in pro-caspase-3 level was recovered by Z-VAD-FMK. Increase in cleaved PARP by amygdalin was also recovered by Z-VAD-FMK (Fig. 3C). As shown in Fig. 3D, amygdalin-induced cytotoxicity was significantly attenuated by treatment with Z-VAD-FMK. These results suggest that amygdalin may induce apoptosis in Hs578T cells via caspase-3 pathway.
Amygdalin inhibits adhesion of Hs578T breast cancer cells
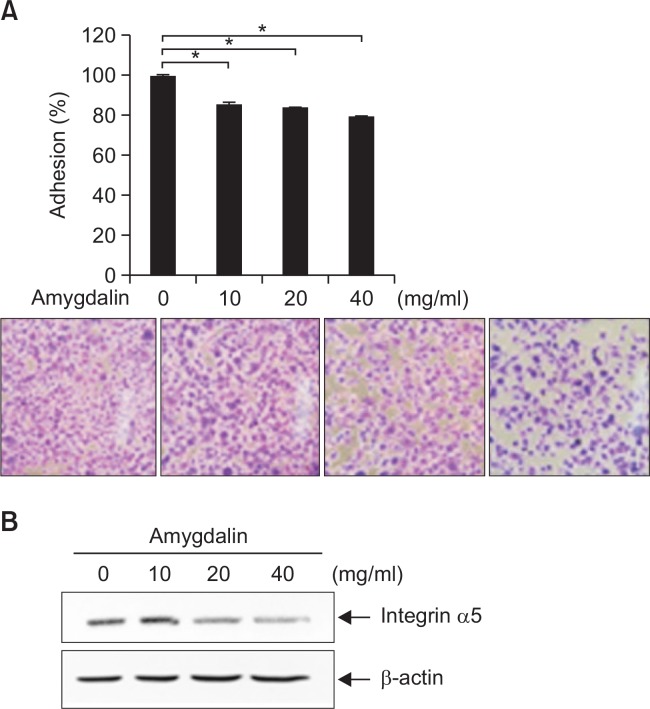
We next examined the effect of amygdalin on adhesive phenotype of Hs578T cells. The adhesion of Hs578T cells was significantly inhibited by treatment of amygdalin in a dose-dependent manner (Fig. 4A). The level of integrin α5 was decreased by amygdalin treatment (Fig. 4B). The results demonstrate that amygdalin effectively inhibited the adhesive phenotype of Hs578T breast cancer cells.
Fig. 4.
Amygdalin inhibits adhesion of Hs578T breast cancer cells. (A) Hs578T cells were treated with the 0, 10, 20 and 40 mg/ mL amygdalin for 24 hr. Adhesion assay was conducted. The results represent mean ± SD for triplicates (*p<0.05 vs. control). (B) Hs578T cells were treated with amygdalin at the indicated concentrations (0, 10, 20 and 40 mg/mL). The level of integrin α5 was determined by immunoblot analysis.
DISCUSSION
Compounds naturally derived from plants have provided a number of useful cancer chemotherapeutic drugs by wide variety of anti-tumor effects. The anti-tumor activity of amygdalin was reported in vitro using cancer cell lines such as bladder cancer, non-small cell lung cancer and liver cancer cell lines (Zhou et al., 2012; Makarević et al., 2014; Qian et al., 2015). In the present study, we showed that amygdalin inhibits proliferation of breast cancer cells. We further showed that amygdalin induces apoptosis and inhibits adhesion in Hs578T TNBC cells. Given that breast cancer is considered to be one of the most frequent malignancies in women (Jemal et al., 2007), our results may provide useful information on developing the anti-cancer strategy.
Reduction or resistance of apoptosis often leads to malignant progression of cancer (Evan and Vousden, 2001). Various signaling pathways may trigger the apoptotic process in human cancers (Townson et al., 2003). Amygdalin was previously demonstrated to induce apoptosis by increasing expression of Bax and decreasing expression of Bcl-2 and procaspase-3 in DU145 and LNCaP prostate cancer cells (Chang et al., 2006). The results of the present study strongly indicate that amygdalin induces apoptosis by increasing the expression of Bax and decreasing the expression of Bcl-2 in Hs578T breast cancer cells.
Integrins regulate cell adhesion to the extracellular matrix, a cellular process that mediates cell differentiation, metastasis and angiogenesis (Howe et al., 1998; Vellon et al., 2006; Bendas and Borsig, 2012). Recent study showed that integrin β1 and β4 involved in the anti-adhesive and anti-migratory effects of amygdalin on bladder cancer cells (Makarević et al., 2014). Amygdalin was shown to inhibit tumor cell adhesion through activation of FAK and modulation of integrin β1 in UMUC-3 and RT112 bladder cancer cells (Makarević et al., 2014). Our results demonstrate that amygdalin decreased cell adhesion in Hs578T human breast cancer cells, possibly via integrin α5.
Recent studies reported that various natural products inhibit tumor cell growth and metastasis, and induce apoptosis of cancer cells (Mantena et al., 2006; Milazzo et al., 2011; Tanaka, 2013), suggesting the potential application of these natural compounds as part of an alternative medical treatment of human cancer. The present study clearly showed that amygdalin inhibits proliferation of breast cancer cells through induction of apoptosis. Amygdalin inhibited adhesion of Hs578T TNBC cells. These results indicate that amygdalin is a potential pro-apoptotic and anti-adhesive agent that may merit future clinical research on breast cancer, especially TNBC.
Acknowledgments
The present study was supported by the Duksung Women’s University Research Grant 2013. The authors thank Dr. Eun-Sook Kim and Dr. Minsoo Koh at Duksung Women’s University (Seoul, Korea) for helpful discussion.
REFERENCES
- Albelda SM. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993;68:4–17. [PubMed] [Google Scholar]
- Alizadeh AM, Shiri S, Farsinejad S. Metastasis review: from bench to bedside. Tumour Biol. 2014;35:8483–8523. doi: 10.1007/s13277-014-2421-z. [DOI] [PubMed] [Google Scholar]
- Bendas G, Borsig L. Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. Int J Cell Biol. 2012;2012:676–731. doi: 10.1155/2012/676731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HK, Shin MS, Yang HY, Lee JW, Kim YS, Lee MH, Kim J, Kim KH, Kim CJ. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol Pharm Bull. 2006;29:1597–1602. doi: 10.1248/bpb.29.1597. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ma J, Wang F, Hu J, Cui A, Wei C, Yang Q, Li F. Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol Immunotoxicol. 2013;35:43–51. doi: 10.3109/08923973.2012.738688. [DOI] [PubMed] [Google Scholar]
- Chiarugi V, Magnelli L, Cinelli M, Basi G. Apoptosis and the cell cycle. Cell Mol Biol Res. 1994;40:603–612. [PubMed] [Google Scholar]
- D’Amours D, Germain M, Orth K, Dixit VM, Poirier GG. Proteolysis of poly (ADP-ribose) polymerase by caspase 3: kinetics of cleavage of mono (ADP-ribosyl)ated and DNA-bound substrates. Radiat Res. 1998;150:3–10. doi: 10.2307/3579638. [DOI] [PubMed] [Google Scholar]
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- Fernald K, Kurokawa M. Evading apoptosis in cancer. Trends Cell Biol. 2013;23:620–633. doi: 10.1016/j.tcb.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyder C, Gloria-Maercker E, Hatzmann W, Niggemann B, Zänker KS, Dittmar T. Role of the beta1-integrin subunit in the adhesion, extravasation and migration of T24 human bladder carcinoma cells. Clin. Exp. Metastasis. 2005;22:99–106. doi: 10.1007/s10585-005-4335-z. [DOI] [PubMed] [Google Scholar]
- Holland JC. Why patients seek unproven cancer remedies: A psychological perspective. CA Cancer J Clin. 1982;32:10–14. doi: 10.3322/canjclin.32.1.10. [DOI] [PubMed] [Google Scholar]
- Holzbecher MD, Moss MA, Ellenberger HA. The cyanide content of laetrile preparations, apricot, peach and apple seeds. J Toxicol Clin Toxicol. 1984;22:341–347. doi: 10.3109/15563658408992565. [DOI] [PubMed] [Google Scholar]
- Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/S0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16:1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Koh M, Woo Y, Valiathan RR, Jung HY, Park SY, Kim YN, Kim HR, Fridman R, Moon A. Discoidin domain receptor 1 is a novel transcriptional target of ZEB1 in breast epithelial cells undergoing H-Ras-induced epithelial to mesenchymal transition. Int. J. Cancer. 2015;136:E508–520. doi: 10.1002/ijc.29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HY, Hong SP, Hahn DH, Kim JH. Apoptosis induction of Persicae Semen extract in human promyelocytic leukemia (HL-60) cells. Arch Pharm Res. 2003;26:157–161. doi: 10.1007/BF02976663. [DOI] [PubMed] [Google Scholar]
- Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly (ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- Makarević J, Rutz J, Juengel E, Kaulfuss S, Tsaur I, Nelson K, Pfitzenmaier J, Haferkamp A, Blaheta RA. Amygdalin influences bladder cancer cell adhesion and invasion in vitro. PLoS One. 2014;9:e110244. doi: 10.1371/journal.pone.0110244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantena SK, Sharma SD, Katiyar SK. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol Cancer Ther. 2006;5:296–308. doi: 10.1158/1535-7163.MCT-05-0448. [DOI] [PubMed] [Google Scholar]
- Miao H, Strebhardt K, Pasquale EB, Shen TL, Guan JL, Wang B. Inhibition of integrin-mediated cell adhesion but not directional cell migration requires catalytic activity of EphB3 receptor tyrosine kinase. Role of Rho family small GTPases. J Biol Chem. 2005;280:923–932. doi: 10.1074/jbc.M411383200. [DOI] [PubMed] [Google Scholar]
- Milazzo S, Ernst E, Lejeune S, Boehm K, Horneber M. Laetrile treatment for cancer. Cochrane Database Syst Rev. 2011;11:CD005476. doi: 10.1002/14651858.CD005476.pub3. [DOI] [PubMed] [Google Scholar]
- Moon A, Kim MS, Kim TG, Kim SH, Kim HE, Chen YQ, Kim HR. H-ras, but not N-ras, induces an invasive phenotype in human breast epithelial cells: a role for MMP-2 in the H-ras-induced invasive phenotype. Int. J. Cancer. 2000;85:176–181. doi: 10.1002/(SICI)1097-0215(20000115)85:2%3C176::AID-IJC5%3E3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Qian L, Xie B, Wang Y, Qian J. Amygdalin-mediated inhibition of non-small cell lung cancer cell invasion in vitro. Int J Clin Exp Pathol. 2015;8:5363–5370. [PMC free article] [PubMed] [Google Scholar]
- Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M. Alkyl-lysopholipids activate the SAPK/JNK pathway and enhanceradiation-induced apoptosis. Cancer Res. 1999;59:2457–2463. [PubMed] [Google Scholar]
- Santos Pimenta LP, Schilthuizen M, Verpoorte R, Choi YH. Quantitative analysis of amygdalin and prunasin in Prunus serotina Ehrh. using 1HNMR spectroscopy. Phytochem Anal. 2014;25:122–126. doi: 10.1002/pca.2476. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Song Z, Xu X. Advanced research on anti-tumor effects of amygdalin. J Cancer Res Ther. 2014;10:3–7. doi: 10.4103/0973-1482.139743. [DOI] [PubMed] [Google Scholar]
- Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. Role of apoptosis in the chemoprevention of cancer. J Exp Clin Med. 2013;5:89–91. doi: 10.1016/j.jecm.2013.04.001. [DOI] [Google Scholar]
- Townson JL, Naumov GN, Chambers AF. The role of apoptosis in tumor progression and metastasis. Curr Mol Med. 2003;3:631–642. doi: 10.2174/1566524033479483. [DOI] [PubMed] [Google Scholar]
- Vellon L, Menendez JA, Lupu R. A bidirectional “alpha(v)beta(3) integrin-ERK1/ERK2 MAPK” connection regulates the proliferation of breast cancer cells. Mol Carcinog. 2006;45:795–804. doi: 10.1002/mc.20242. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis R, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Zhou C, Qian L, Ma H, Yu X, Zhang Y, Qu W, Zhang X, Xia W. Enhancement of amygdalin activated with β-D-glucosidase on HepG2 cells proliferation and apoptosis. Carbohydr Polym. 2012;90:516–523. doi: 10.1016/j.carbpol.2012.05.073. [DOI] [PubMed] [Google Scholar]