Abstract
Triclosan is an antimicrobial or sanitizing agent used in personal care and household products such as toothpaste, soaps, mouthwashes and kitchen utensils. There are increasing evidence of the potentially harmful effects of triclosan in many systemic and cellular processes of the body. In this study, we investigated the effects of triclosan in the survivability of cultured rat neural stem cells (NSCs). Cortical cells from embryonic day 14 rat embryos were isolated and cultured in vitro. After stabilizing the culture, triclosan was introduced to the cells with concentrations ranging from 1 μM to 50 μM and in varied time periods. Thereafter, cell viability parameters were measured using MTT assay and PI staining. TCS decreased the cell viability of treated NSC in a concentration-dependent manner along with increased expressions of apoptotic markers, cleaved caspase-3 and Bax, while reduced expression of Bcl2. To explore the mechanisms underlying the effects of TCS in NSC, we measured the activation of MAPKs and intracellular ROS. TCS at 50 μM induced the activations of both p38 and JNK, which may adversely affect cell survival. In contrast, the activities of ERK, Akt and PI3K, which are positively correlated with cell survival, were inhibited. Moreover, TCS at this concentration augmented the ROS generation in treated NSC and depleted the glutathione activity. Taken together, these results suggest that TCS can induce neurodegenerative effects in developing rat brains through mechanisms involving ROS activation and apoptosis initiation.
Keywords: Triclosan, Apoptosis, Oxidation, Rat neural stem cells, MAPK signaling
INTRODUCTION
Triclosan [5-chloro-2-(2,4-diclorophenoxy)phenol; TCS] is a commonly used antimicrobial agent and preservative in personal care products, household items, medical devices, toys, plastic materials and textiles, among others (Glaser, 2004). From the total amount of triclosan used in industries per annum, 85% were allocated to personal care products including cosmetics, soaps, toothpastes and deodorants, and 10% were used in plastics and food contact materials which both have direct exposure to consumers; while the other 5% were used in textiles (SCCS, 2010). More prominent uses of triclosan in personal care products are found in soaps and toothpastes where they have dermal and oral entries, respectively. With the maximum allowable concentration of triclosan at 0.3% in cosmetics products, maximum use for each single product will not be expected to cause harm. However, the accumulation of exposure from many different products containing triclosan will most probably pose a risk to health according to the European Commission (SCCS, 2010).
Although much is known about the benefits of triclosan in various products, recent concerns are raised about its possible bioaccumulation effects in the environment which could affect various organisms, especially algae and aquatic resources (Brausch and Rand, 2011; Bedoux et al., 2012). Some studies also emphasized that there is no concrete evidence for the beneficial effect of triclosan in some products. For example, soaps that contain triclosan were not more effective than regular soaps in getting rid of microbes from the skin (Aiello et al., 2007). Furthermore, the possibility of developing bacterial resistance after triclosan exposure may pose a significant risk to individuals (Suller and Russell, 2000). More importantly, the increasing evidence of the cellular, metabolic, hormonal and teratogenic effects of triclosan in vitro and in vivo (Wang et al., 2004; Jacobs et al., 2005; Veldhoen et al., 2006; Chen et al., 2007; Ahn et al., 2008; Paul et al., 2010; Rodriguez and Sanchez, 2010) may offer hints that it may actually occur in humans, especially with regular exposures to various products with direct contact or entry to the body.
Triclosan was found in the plasma and milk in nursing mothers regularly consuming products containing this ingredient (Allmyr et al., 2006). In addition, triclosan can bioaccumulate in the liver and fat tissues, and possibly the brain at a low potential (Geens et al., 2012). With the potential that triclosan can accumulate in the brain, and with the lack of in vitro study in this area, we were prompted to investigate the outcome of triclosan exposure using the cultured rat neural stem cells (NSCs). Embryonic period is highly crucial for the development of the immature fetus and various environmental factors had been blamed for the increasing prevalence of many neurodevelopmental disorders. Here, we questioned whether triclosan has an effect on cell viability of NSCs. We aimed to identify molecular changes that could be affected by triclosan. We also explored the possible mechanisms involved for these effects.
The main objective of this study is to evaluate the potentially harmful effect of triclosan in cultured rat NSCs as an in vitro model. To meet this objective, we performed cell viability assay and assessed the apoptotic activity of the triclosan-treated cells. We also explored for possible mechanisms commonly involved in cell damage and protection such as ROS production, antioxidation, and the MAPK and PI3K signaling modulations. The findings of this study will offer a significant contribution on the possibility that triclosan accumulation in the brain may have deleterious effects, especially during the period of embryonic development.
MATERIALS AND METHODS
Materials
Triclosan was obtained from Sigma (St. Louis, MO, USA) while Dimethyl sulfoxide (DMSO), Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12), B-27 supplement and FGF were purchased from Invitrogen (Carlsbad, CA, USA). EGF was purchased from Sigma-Aldrich Co (St. Louis, MO, USA). Penicillin/streptomycin, 0.25% trypsin-EDTA, Tween® 20, and ECLTM Western blotting detection reagents were obtained from Amersham Life Science (Arlington Heights, IL, USA).
Antibodies were purchased from the following companies: anti-β-actin from Sigma (St. Louis, MO, USA); caspase3, cleaved caspase 3, ERK, phosphor-ERK, JNK, phosphor-JNK, PI3K, phosphor-PI3K, p38, phosphor-p38, Akt and phosphor-Akt from cell signaling (Boston, MA, USA); Bax from BD PharmingenTM (BD biosciences, USA); Bcl-2 and cytochrome C from Santa Cruz (CA, USA).
Neural Stem Cell (NSC) Culture
Neural stem cells were cultured from embryonic day 14 (E14) brain of Sprague-Dawley rat. In brief, cortices were dissociated into single cells by mechanical trituration, and cells were incubated in DMEM/F12 supplemented with B27-serum free supplement and growth factor (10 ng/ml FGF and 20 ng/ml EGF) in a 5% CO2 incubator. EGF and FGF were added every day and the cells grew into floating neurospheres, which were dissociated into single cells using trypsin-EDTA (0.1%) and plated into poly-L-ornithine pre-coated plates using B27-medium without growth factors for differentiation.
Drug treatment
Neural stem wells were treated in vitro with triclosan at 1, 10, 20, 30, 50 and 100 μM concentrations and applied into the culture media at different lengths of exposure (3, 6, 8, 12 and 24 h) to determine the dose and time-dependent effects. In other experiments, treatment concentrations and exposure lengths were purposely selected. All drug treatments and experiments were conducted during the 4th day of in vitro culture (DIV4) of NSCs.
Western blot analysis
Cells were washed twice with phosphate-buffered saline (PBS) and lysed with a lysis buffer. The total proteins for each sample were determined through BCA assay and then each sample was equalized to a certain level of protein concentration. The equalized aliquots were loaded in a 12% SDS-polyacrylamide gel for 120 min and the proteins were electrically transferred to the nitrocellulose membranes for 90 min. The blot was blocked with PVA (diluted at 1:1000 in TBST) at room temperature and incubated overnight at 4°C with each primary antibody and concentration (β-Actin, 1:5000; BCl2, 1:1000; Caspase3, 1:2000; Cleaved caspase3, 1:2000; Bax, 1:2000; ERK, 1:2000; phospho-ERK, 1:2000; JNK, 1:2000; phospho-JNK, 1:2000; AKT, 1:2000; phospho-AKT, 1:2000; p38, 1:2000; phospho-p38, 1:2000; PI3K, 1:2000; phospho-PI3K 1:2000) which were also diluted in TBST. The next day, the membranes were incubated further with horseradish peroxidase (HRP)-conjugated secondary antibody (Life technologies, USA) at room temperature for 2 h, with washing before and after incubation. Finally, the blots were imaged through enhanced chemiluminescence method (Amersham, Buckinghamshire, UK) for quantification.
PI staining
To evaluate neuronal cell death in culture triclosan treatment, propidium iodide (PI; Sigma) staining was performed. Neural stem cells on poly-L-ornithine hydrobromide pre-coated cover glasses were fixed with 4% paraformaldehyde (PFA) before incubated at room temperature for 15 min with PI (1:1000 diluted in DPBS) and DAPI (1:10,000 diluted in DPBS). Then, samples were washed and mounted using GEL/MOUNTTM (Bio Neda, CA, USA). Cellular images were taken through fluorescence microscopy (TSC-SP, Leica, Heidelberg, Germany).
Measurement of cell viability
An MTT assay was used to examine whether triclosan treatment can influence NSC proliferation. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, 500 μg/ml, Sigma, St. Louis, MO, USA) is a water-soluble tetrazolium salt that can be transformed to colored and water-insoluble formazan salt through a reaction with metabolically viable cells. In brief, NPCs were incubated for 2 h in the dark with an MTT reagent. The medium was then removed and replaced with DMSO (Duksan, Korea). Absorbance was read using an ELISA reader (TECAN, Austria) at 570 nm. The percentage of neuronal survival was calculated and compared from the absorbance of vehicle-treated cells (Kwon et al., 2011).
Determination of ROS and GSH content
Intracellular ROS formation in cultures was measured according to fluorescence using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH2-DA; molecular probe). After drug treatment, cells were washed with DPBS and loaded with 50 μM of DCFH2-DA (for ROS) or 20 μM of mBCl (for GSH) for 20 min at 37°C. The DCF fluorescence was observed under Inverted Fluorescence Microscope (LSM10; Carl Zeiss, Dublin, CA, USA). GSH fluorescence was analyzed at an excitation of 380 nm and an emission of 450 nm through a fluorescence plate reader (Spectramax Gemini EM, Molecular Devices, Palo Alto, CA, USA).
FACS analysis
The NSCs dissociated into single cells were used to detect the ratio of ROS by fluorescence activated cell sorting (FACS) analysis. NSCs were incubated with dihydroethidium (DHE, 100 μM) then trypsinized followed by the addition of DMEM/F12 containing 1% FBS. The suspension was centrifuged at 1,300 rpm for 3 min and the supernatant was completely removed without disturbing the pellet. The suspended cells were fixed with 70% ethanol in PBS and were incubated for 20 min, followed by centrifugation. The supernatants were again removed same with the previous step and the remaining cells were incubated with 100 μg/ml ribonuclease A (Sigma, St. Louis, MO, USA) in 500 μl PBS. ROS were measured by flow cytometry (FACS Calibur System, BD Biosciences, San Jose, CA, USA).
Statistical analysis
All data are quantified and presented as the mean ± the standard error of the mean (SEM). Statistical analyses were conducted using one-way analysis of variance (ANOVA) with Tukey’s test as the post hoc analysis. All statistical analyses were conducted using Graph Pad Prism (Version 5, California USA). Differences were considered statistically significant when the p-value was less than 0.05 (p<0.05).
RESULTS
Triclosan induces cell death in cultured rat NSCs
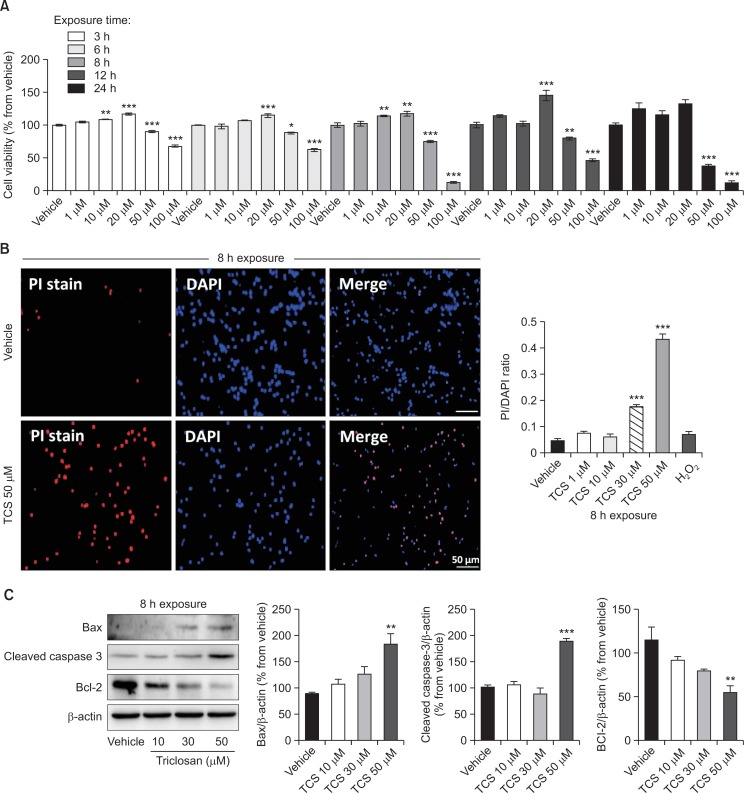
To determine the effect of triclosan in cultured rat NSCs with regards to dosage and timing of treatment, cell viability measurement was performed. In all time points, triclosan treatment up to 20 μM did not induce reduction of cell viability (Fig. 1A). Instead, it triggered a dose-dependent increase in cell viability at 10 and 20 μM, and in almost all time points. Intriguingly, the treatment of 50 and 100 μM triclosan to the cultured NSCs initiated the decreases in cell viability in dose and time dependent manners, except at 8 h when 100 μM triclosan decreased cell viability similar to 24 h (Fig. 1A). Since we found a negative effect of triclosan in neural stem cells starting at 50 μM, we then used this as the highest concentration in the next experiments.
Fig. 1.
Cell viability of cultured rat NSCs treated with triclosan. Cell viability was assessed from the different concentrations and lengths of exposure of vehicle or triclosan treatment in culture through MTT reduction assay (A). PI staining and visualization were done to detect the spread of apoptosis in triclosan-treated cells exposed for 8 h (B). Bar size=50 μm. Western blot analysis was also conducted after 8 h exposure of NSCs to triclosan to detect the expression of cleaved caspase-3, Bax and Bcl2 proteins quantified by densitometry reflected as bar graphs (C). β-actin was used as the loading control. All experiments were individually performed during the 4th day of NSC culture in vitro (DIV 4). The results are shown as the percentage of proteins relative to the vehicle or the PI to DAPI ratio. Values are expressed as the mean ± S.E.M. *p<0.05, **p<0.01, ***p<0.001 vs. vehicle.
After cell viability assessment of triclosan-treated NSCs, we assessed the apoptotic markers using PI stain. In line with the previous result, triclosan triggered apoptosis at 30 and 50 μM concentrations as evidenced by the increased PI staining (Fig. 1B).
Further investigation for the cell death assessment of NSCs treated with triclosan includes the quantification of apoptosis-related proteins through Western blot analysis. These proteins are cleaved-caspase3 known as the executioner of apoptosis, Bax which also activates apoptosis and Bcl-2 as a blocker of apoptosis in cells. As a result, cleaved caspase3 and Bax proteins were significantly increased while Bcl-2 was decreased in the 50 μM triclosan-treated cells compared to the vehicle group (Fig. 1C). Although the 10 and 30 μM concentrations have tendencies to dose-dependently increase or decrease Bax and Bcl-2 proteins, respectively, they did not reach statistically significant effects (p<0.05). All these experiments verify that high concentrations starting 50 μM can significantly induce cell death in cultured rat NSCs.
Triclosan at 50 μM induces ROS generation in cultured rat NSCs
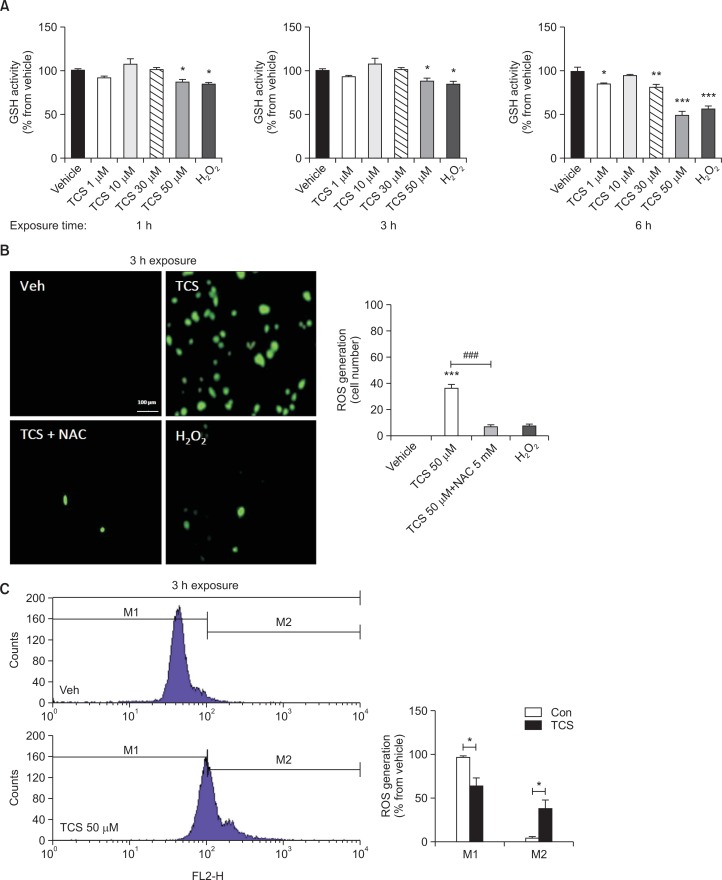
To further determine the effect of triclosan in cultured rat NSCs, the GSH activity and ROS generation were measured. Similar various concentrations were employed at 3 different time-lengths of exposures (1, 3 and 6 h). Remarkably, 50 μM triclosan decreased the GSH activity in cultured NSCs at all lengths of exposure similar to the positive control, H2O2 (Fig. 2A). Of note, 30 μM triclosan only decreased the GSH activity when exposed up to 6 h but not 3 h or shorter. In line with these results, the ROS generation of cultured NSCs was increased by 50 μM triclosan to about 40% (Fig. 2B). In addition, treatment with NAC, an ROS inhibitor, decreased the effect of triclosan. Using FACS analysis, it was confirmed that ROS production was augmented in the triclosan-treated NSCs showing a shift of the ROS levels from M1 to M2 wherein the M1–M2 ratio was decreased (Fig. 2C).
Fig. 2.
ROS generation profiles of cultured rat NSCs after triclosan treatment. GSH activity was measured in cells treated with vehicle or triclosan at different concentrations and time of exposures using the ROS assay (A). H2O2 was used as a positive control. ROS generation was also detected as a GFP using H2 DCF-DA assay after 3 h treatment of triclosan (B). NSCs were treated with either 50 μM TCS, 50 μM TCS+5 μM NAC or 200 μM H2O2 for 3 h. Histograms show the result of FACS analysis to further detect the levels of ROS generation in triclosan-treated rat NSCs (C). All experiments were individually performed during the 4th day of NSC culture in vitro (DIV 4). The results are shown as the percentage relative to the vehicle or the actual cell number. Values are expressed as the mean ± S.E.M. *p<0.05, **p<0.01, and ***p<0.001 vs. vehicle; ###p<0.001.
Triclosan at 50 μM affects the MAPK signaling in cultured rat NSCs
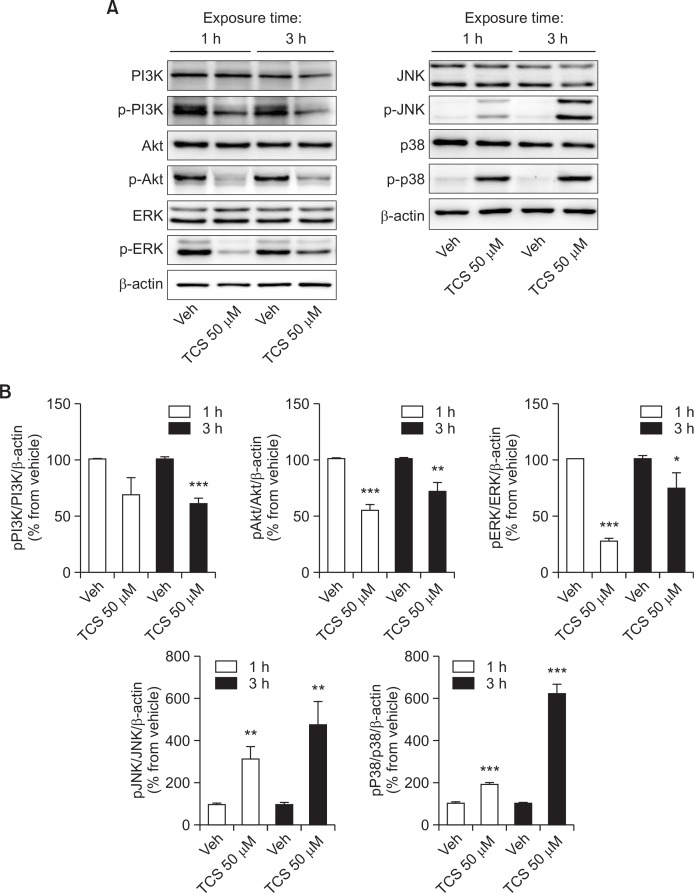
To explore the mechanism underlying the effects of triclosan in neural stem cells, we measured the protein levels of markers related to MAPK signaling. Triclosan at 50 μM did not affect the expressions of MAPK signaling proteins per se (Fig. 3). However, it differentially induced the increased expressions of both phosphorylated p38 and JNK proteins, which are implicated in the induction of cell death. On the contrary, it decreased the expressions of phosphorylated ERK, Akt and PI3K proteins, which are regarded as cell survival and anti-apoptotic signals.
Fig. 3.
The levels of phosphorylated MAPK and PI3K signaling proteins treated with triclosan. Western blot analyses were done to measure the protein levels of signaling molecules related to MAPK and PI3K after 1 or 3 h treatment with 50 μM triclosan in cultured rat NSCs. All experiments were individually performed during the 4th day of NSC culture in vitro (DIV 4). The results are shown as the percentage relative to the vehicle. Values are expressed as the mean ± S.E.M. *p<0.05, **p<0.01, and ***p<0.001 vs. vehicle.
DISCUSSION
This study highlights the effect of triclosan in vitro using cultured rat NSCs which demonstrated a negative effect on cell viability leading to induction of cell death or apoptosis. The augmented ROS generation along with reduced GSH activity could play a direct role for the apoptotic effects. In addition, phosphorylations of MAPK signaling proteins were dysregulated which could have driven the immature apoptosis of cells. To our knowledge, this is a pioneering work exploring the potential effect of triclosan in the mammalian brain through in vitro studies using NSCs. This investigation was prompted by the possibility that triclosan may cross and accumulate in the brain (Geens et al., 2012), especially with regular and potentially excessive dose from the use of different products containing this ingredient.
The apoptotic effect of triclosan in cultured rat NSC raises more concern for its safety knowing that it is widely used for human consumption. The activation of cleaved caspase3 and Bax expressions as well as the suppression of Bcl2 both complement to induce apoptosis in the triclosan-treated cells. Remarkably, it is interesting to note that the apoptotic effect of triclosan is more prominent starting at 50 μM concentration with lesser extent at 30 μM; while the doses 20 μM below does not affect the apoptotic levels but rather dose-dependently increased the cell viability in the cells. Thus, triclosan can induce its harmful effects when it is beyond a certain limit dosage. In personal care products, the addition of triclosan is limited only up to 0.3% as regulated by the European Commission Scientific Committee on Consumer Safety (SCCS, 2010). However, the Committee also emphasized that multiple and repeated exposures of various products containing triclosan would go beyond the limit of safety and could be harmful to consumers.
The apoptotic effect of triclosan in vitro using the neocortical neurons of Swiss mice was recently reported by Szychowski et al. (2015). They highlighted the apoptotic effect of triclosan by showing activation of caspase markers (8, 9, and 3) and Fas receptors. Our study was able to replicate the activation of the caspase-3 marker in triclosan-treated NSCs at 50 μM concentration and additionally showed the pro-apoptotic expressions of Bax and Bcl2 proteins. We further reflected the cell viability status of the triclosan-treated NSCs in congruence to the expression of pro-apoptotic markers. Apparently, both studies use different animal species as well as cell types representing different stages of neural development in each experiment (terminally differentiated neuron in the former and NSC in ours). While Szychowski et al. used Swiss mice neocortical neurons, we used the rat neural stem cells. Interestingly, the apoptotic effect of triclosan in both species and cell types was consistent, suggesting the broad activity of triclosan to induce similar effects across various stages of cell development. Indeed, our study not only extends the recent report but also newly introduces the possible mechanisms underlying the effects of triclosan in the early cortical cell populations. Especially, the results from the present study suggest that exposure to triclosan during early developmental periods may induce developmental aberration which may correlate with the manifestation of developmental disorders such as autism spectrum disorder, ADHD, although it should be investigated further in the future.
A balance between the production of ROS and the counteraction of antioxidants is needed to maintain cellular homeostasis and prevent cell damage. Any factor that would alter this equilibrium will bring about unrestrained oxidative stress leading to oxidative damage to many cellular components, eventually initiating cell death (Zhou et al., 2014). The pluripotent stem cells during embryonic development are special cell populations which are fated to differentiate into all types of adult cells. ROS regulation in the stem cell population plays an important role for the maintenance of quiescence or the activation of cellular processes for cell division (Ito and Suda, 2014). Interestingly, it was suggested that neural stem cells utilize high endogenous ROS levels in cell signaling to trigger self-renewal and neurogenesis (Le Belle et al., 2011). However, loss of antioxidant function in stem cells could initiate uncontrolled ROS utilization leading to transient hyperproliferation and overgrowth, then ultimately pose the cells to ROS intoxication and cell death (Paik et al., 2009; Renault et al., 2009). Our findings follow this pattern where the decreased GSH regulation in the triclosan-treated cells resulted to an uncontrolled ROS activity that disrupted the viability of rat NSCs. In addition, the ROS inhibitor NAC normalized the ROS levels of triclosan-stimulated NSCs, confirming the direct activation of ROS generation by triclosan.
Furthermore in our experimental conditions, triclosan increased the phosphorylation of p38 and JNK but decreased the phosphorylation of PI3K, Akt and ERK proteins in cultured rat NSCs. In some contrast, a recent study of triclosan stimulation to mouse epidermis-derived JB6 Cl 41-5a cells demonstrated the increased phosphorylation of ERK, JNK, p38 and Akt proteins (Wu et al., 2014). These somewhat conflicting results could be explained by the different concentrations of triclosan between the two studies as well as the difference in cell types. While we used 50 μM of triclosan to find the effects, the other study only used up to 8 μM concentration. Nevertheless, both studies have established that triclosan can influence cellular signals to affect cell activity and viability. Interestingly, JNK-p38 activation with ERK inhibition has been implicated in apoptosis of rat PC-12 pheochromocytoma cell culture (Xia et al., 1995). Indeed, these three known members of the MAPK family work along each other with distinct roles to modulate apoptosis (Johnson and Lapadat, 2002). In addition, the PI3/Akt signaling pathways are promoters of cell survival, especially the neurons by regulating the expression of genes implicated in cell death (Brunet et al., 2001). Our results thus conform to the mechanisms involved in the expression of cell death and survival markers affected by triclosan. Follow-up studies should be focused on cultured human cells types to open the possibility of triclosan effect in clinical settings.
The effects of triclosan on both ROS generation and MAPK signaling in our study may have a direct connection. For example, in hematopoietic stem cells (HSCs), increased ROS production phosphorylates the p38 MAPK to cause disruption of their quiescence which were reversed with an antioxidant or a p38 MAPK inhibitor (Ito et al., 2006). Similar to those findings, we demonstrated that triclosan stimulation in cultured rat NSCs upregulated the phosphorylation of p38 MAPK and JNK proteins, which could have resulted from the increased ROS generation and decreased antioxidant capacity. In the other study with mice, high ROS levels activate the PI3/Akt/mTOR pathway to initiate NADPH oxidase neural stem cell regulation (Le Belle et al., 2011). However, we observed decreased phosphorylation of these signals although we also found increased ROS generation in our experiment. Thus, we could infer that the normal regulation of ROS and its related signals in neural stem cells could be disrupted by external factors leading to loss of ROS and antioxidant balance.
Triclosan can remain unchanged for several days in the body after intake and before it is excreted via urine or feces (Sandborgh-Englund et al., 2006). From a biomonitoring survey in the U.S., triclosan was present in nearly 75% of the participants showing that the consumption of products containing this ingredient is widespread (Calafat et al., 2008). Moreover, the young adult population was the highest exposed among age groups with the mean of 12.7 μg/L from the latest survey between 2011–2012 (Prevention, 2015). In the Chinese survey, 93% of the sample population were detected with triclosan in the urine with a geometric mean of 3.77 μg/L (Li et al., 2013). So far, no concrete evidence has yet been reported pointing a toxic effect of triclosan in humans despite pieces of evidence in animal and cellular studies (Orvos et al., 2002; Ishibashi et al., 2004; Oliveira et al., 2009). This could mean that the levels of triclosan use in the general population are relatively low or within the regulated limits. Recently, Chen et al demonstrated that triclosan at 50 μM can disrupt the expression of pluripotency markers of mouse embryonic stem cells as well as zebrafish embryos, which could later affect their developmental architectures (Chen et al., 2015). The same is true in our findings where the negative effects of triclosan were demonstrated starting at 50 μM and not on doses from 20 μM and below. We can understand from these results that the deleterious effects of triclosan in various cell and animal populations would be at higher risk in certain large doses but not at lower doses. It is obvious that dysregulated (usually increased) apoptosis would hamper normal development and differentiation of nervous system, but is should also be emphasized that increased cell survival or neural stem cell proliferation may adversely affect the normal physiology of the brain. For example, we reported that in utero exposure to valproic acid induces prolonged and increased proliferation of NSCs, which might be responsible for the induction of macrocephaly and autism-like behaviors such as social deficits and increased seizure susceptibility in offspring rats (Go et al., 2012; Kim et al., 2014) In this regard, the role of triclosan in the regulation of brain architecture, such that it increased MTT signals at low concentration (Fig. 1) and vice versa at higher concentration, should be investigated further in the future.
We tested triclosan to the cultured rat NSC which were extracted from embryonic day 14 of gestation. We chose this cell population to identify the possible effects of triclosan during embryonic development, which is a high-risk period for the immature offspring. Various environmental factors have been blamed for the development of a wide array of neurodevelopmental disorders which affects the quality of life of the newborn and the family. Intriguingly, studies in rats have shown that exposure to triclosan during pregnancy and after birth can affect both the dam and the offspring commonly by disrupting thyroid homeostasis although the doses were relatively high at 50 and 300 mg/kg/day (Paul et al., 2010; Rodriguez and Sanchez, 2010). Indeed, the widespread exposure of humans to this ingredient, especially during gestation, is a crucial point wherein the safety of its use needs to be ensured. Based on the results of the previous and current study, we recommend that the exposure to triclosan should be specially monitored and limited during pregnancy and early after birth.
Acknowledgments
This research is supported by Korea Food & Drug Administration (14172 Cosmetics 975).
REFERENCES
- Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, Lasley B, Pessah IN, Kultz D, Chang DP. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens. Environ Health Perspect. 2008;116:1203–1210. doi: 10.1289/ehp.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello AE, Larson EL, Levy SB. Consumer antibacterial soaps: effective or just risky? Clin Infect Dis. 2007;45:S137–S147. doi: 10.1086/519255. [DOI] [PubMed] [Google Scholar]
- Allmyr M, Adolfsson-Erici M, McLachlan MS, Sandborgh-Englund G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci Total Environ. 2006;372:87–93. doi: 10.1016/j.scitotenv.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Bedoux G, Roig B, Thomas O, Dupont V, Le Bot B. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ Sci Pollut Res. 2012;19:1044–1065. doi: 10.1007/s11356-011-0632-z. [DOI] [PubMed] [Google Scholar]
- Brausch JM, Rand GM. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere. 2011;82:1518–1532. doi: 10.1016/j.chemosphere.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/S0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL. Urinary concentrations of triclosan in the US population: 2003–2004. Environ Health Perspect. 2008;116:303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ahn KC, Gee NA, Gee SJ, Hammock BD, Lasley BL. Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol Appl Pharmacol. 2007;221:278–284. doi: 10.1016/j.taap.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Xu B, Han X, Mao Z, Chen M, Du G, Talbot P, Wang X, Xia Y. The effects of triclosan on pluripotency factors and development of mouse embryonic stem cells and zebrafish. Arch Toxicol. 2015;89:635–646. doi: 10.1007/s00204-014-1270-2. [DOI] [PubMed] [Google Scholar]
- Geens T, Neels H, Covaci A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere. 2012;87:796–802. doi: 10.1016/j.chemosphere.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Glaser A. The ubiquitous triclosan. A common antibacterial agent exposed. Pestic. You. 2004;24:12–17. [Google Scholar]
- Go HS, Kim KC, Choi CS, Jeon SJ, Kwon KJ, Han S-H, Lee J, Cheong JH, Ryu JH, Kim C-H, Ko KH, Shin CY. Prenatal exposure to valproic acid increases the neural progenitor cell pool and induces macrocephaly in rat brain via a mechanism involving the GSK-3β/β-catenin pathway. Neuropharmacology. 2012;63:1028–1041. doi: 10.1016/j.neuropharm.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, Ishibashi Y, Takao Y, Arizono K. Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat Toxicol. 2004;67:167–179. doi: 10.1016/j.aquatox.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15:243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MN, Nolan GT, Hood SR. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR) Toxicol Appl Pharmacol. 2005;209:123–133. doi: 10.1016/j.taap.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kim KC, Lee DK, Go HS, Kim P, Choi CS, Kim JW, Jeon SJ, Song M-R, Shin CY. Pax6-dependent cortical glutamatergic neuronal differentiation regulates autism-like behavior in prenatally valproic acid-exposed rat offspring. Mol Neurobiol. 2014;49:512–528. doi: 10.1007/s12035-013-8535-2. [DOI] [PubMed] [Google Scholar]
- Kwon KJ, Kim JN, Kim MK, Lee J, Ignarro LJ, Kim HJ, Shin CY, Han SH. Melatonin synergistically increases resveratrol-induced heme oxygenase-1 expression through the inhibition of ubiquitin-dependent proteasome pathway: a possible role in neuroprotection. J Pineal Res. 2011;50:110–123. doi: 10.1111/j.1600-079X.2010.00820.x. [DOI] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ying GG, Zhao JL, Chen ZF, Lai HJ, Su HC. 4-Nonylphenol, bisphenol-A and triclosan levels in human urine of children and students in China, and the effects of drinking these bottled materials on the levels. Environ Int. 2013;52:81–86. doi: 10.1016/j.envint.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Oliveira R, Domingues I, Koppe Grisolia C, Soares AM. Effects of triclosan on zebrafish early-life stages and adults. Environ Sci Pollut Res Int. 2009;16:679–688. doi: 10.1007/s11356-009-0119-3. [DOI] [PubMed] [Google Scholar]
- Orvos DR, Versteeg DJ, Inauen J, Capdevielle M, Rothenstein A, Cunningham V. Aquatic toxicity of triclosan. Environ Toxicol Chem. 2002;21:1338–1349. doi: 10.1002/etc.5620210703. [DOI] [PubMed] [Google Scholar]
- Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, Chae SS, Zheng H, Ying H, Mahoney J. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, DeVito MJ, Crofton KM. Developmental triclosan exposure decreases maternal and neonatal thyroxine in rats. Environ Toxicol Chem. 2010;29:2840–2844. doi: 10.1002/etc.339. [DOI] [PubMed] [Google Scholar]
- Prevention, C. f. D. C. a. Fourth National Report on Human Exposure to Environmental Chemicals. 2015 http://www.cdc.gov/exposurereport/.
- Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PE, Sanchez MS. Maternal exposure to triclosan impairs thyroid homeostasis and female pubertal development in Wistar rat offspring. J Toxicol Environ Health A. 2010;73:1678–1688. doi: 10.1080/15287394.2010.516241. [DOI] [PubMed] [Google Scholar]
- Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J. Toxicol. Environ. Health A. 2006;69:1861–1873. doi: 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- SCCS . Opinion on triclosan (antimicrobial resistance) European Commission; 2010. [Google Scholar]
- Suller M, Russell A. Triclosan and antibiotic resistance in Staphylococcus aureus. J Antimicrob Chemother. 2000;46:11–18. doi: 10.1093/jac/46.1.11. [DOI] [PubMed] [Google Scholar]
- Szychowski KA, Sitarz AM, Wojtowicz AK. Triclosan induces Fas receptor-dependent apoptosis in mouse neocortical neurons in vitro. Neuroscience. 2015;284:192–201. doi: 10.1016/j.neuroscience.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Veldhoen N, Skirrow RC, Osachoff H, Wigmore H, Clapson DJ, Gunderson MP, Van Aggelen G, Helbing CC. The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquat Toxicol. 2006;80:217–227. doi: 10.1016/j.aquatox.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Wang L-Q, Falany CN, James MO. Triclosan as a substrate and inhibitor of 3′-phosphoadenosine 5′-phosphosulfate-sulfotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab Dispos. 2004;32:1162–1169. doi: 10.1124/dmd.104.000273. [DOI] [PubMed] [Google Scholar]
- Wu Y, Beland FA, Chen S, Fang JL. Extracellular signal-regulated kinases 1/2 and Akt contribute to triclosan-stimulated proliferation of JB6 Cl 41-5a cells. Arch Toxicol. 2014:1–15. doi: 10.1007/s00204-014-1308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Zhou D, Shao L, Spitz DR. Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res. 2014;122:1–67. doi: 10.1016/B978-0-12-420117-0.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]