Abstract
Background
Oxidative stress plays a significant role in atherosclerosis development. HIV infection has been linked with heightened cardiovascular disease risk. HMG-CoA reductase inhibitors may reduce oxidative stress and subsequently subclinical vascular disease in HIV.
Design/methods
This is a randomized, placebo-controlled trial to evaluate the effect of rosuvastatin in HIV-infected adults on stable antiretroviral therapy with low-density lipoprotein less than 130 mg/dl and increased inflammation or T-cell activation on subclinical vascular disease. Changes over 48 weeks in oxidative stress markers, oxidized low-density lipoprotein (oxLDL) and F2-isoprostane/creatinine ratio (F2-Iso-P/Cr), were compared between groups. Spearman correlation and multivariable linear regression were used to evaluate relationships between changes in markers of oxidative stress, inflammation and monocyte activation and carotid intima media thickness (CIMT).
Results
One hundred and forty-seven adults enrolled (72 to rosuvastatin and 75 to placebo). In the rosuvastatin group, oxLDL decreased significantly over 24 weeks compared to placebo [mean absolute change in log-oxLDL for rosuvastatin −0.2 ± 0.468 log U/l (P < 0.001 within-group) vs. placebo −0.018 ± 0.456 log U/l (P = 0.83 within-group); P = 0.004 between groups] and this change was linked with changes in soluble CD14 and proportion of patrolling monocytes (CD14dimCD16+). Although oxLDL levels increased after initially declining and were not different from placebo at week 48, the early improvement in oxLDL was associated with improved CIMT at week 48. Changes in F2-IsoP/Cr were not significant between groups.
Conclusion
Rosuvastatin decreases oxLDL levels early after initiation and is associated with decreased monocyte activation. Early improvement in oxLDL is linked with improved CIMT in treated HIV infection.
Keywords: human immunodeficiency virus, inflammation, oxidative stress, oxidized low-density lipoprotein, rosuvastatin
Introduction
Chronic HIV infection leads to accelerated atherosclerosis and higher risk of myocardial infarction compared to the general population [1–3]. Traditional cardiovascular risk factors [4,5], antiretroviral therapy (ART) [6] and chronic inflammation [7,8] and immune activation, specifically monocyte activation [9–11], are thought to contribute to this heightened cardiovascular disease (CVD) risk. In addition, oxidative stress has been implicated in the development of early atherosclerotic lesions [12], and markers of oxidative stress are independent risk factors for CVD events in the general population [13–16]. HIV-infected individuals have higher levels of oxidized low-density lipoprotein (oxLDL) [17] and oxLDL has been linked with subclinical vascular disease with HIV infection [18].
HMG-CoA reductase inhibitors or statins reduce the risk of CVD events in patients with normal levels of low-density lipoprotein (LDL) [19]. Statins have several cholesterol-independent effects that may decrease CVD risk. We have previously shown that rosuvastatin decreases monocyte activation [soluble CD14 and the proportion of tissue factor-expressing patrolling monocytes (CD14dimCD16+ cells)], T-cell activation (proportion of CD38+HLA-DR+ T cells), systemic inflammation [cystatin C and interferon γ inducible protein-10 (IP-10)] and vascular inflammation (lipoprotein-associated phospholipase A2) in HIV [20–23]. Statins have antioxidant activity [24,25] and decrease markers of oxidative stress in some [26–28], but not all [29,30] disease states. To our knowledge, the effect of statins on oxLDL or other oxidative stress markers in the setting of chronic HIV infection has not been studied.
Therefore, the goal of this study was to evaluate the effect of rosuvastatin on oxidative stress markers including plasma oxLDL and urine F2-isoprostane/creatinine ratio (F2-IsoP/Cr) over 48 weeks in a randomized, placebo-controlled trial of HIV-infected adults on stable ART with LDL less than 130 mg/dl and evidence of heightened inflammation or immune activation. Additionally, we aimed to evaluate relationships between changes in oxLDL and changes in markers of systemic inflammation and monocyte activation, as well as changes in subclinical vascular disease. We have shown previously that rosuvastatin decreases monocyte activation and improves carotid intima media thickness progression (CIMT) [21,31]. Our hypotheses were that markers of oxidative stress would improve with rosuvastatin, that changes in monocyte activation would be linked with the change in oxLDL, and that changes in oxidative stress markers would be associated with favourable changes in CIMT over time.
Methods
The Stopping Atherosclerosis and Treating Unhealthy bone with Rosuvast-atiN (SATURN-HIV) study is a randomized, double-blind, placebo-controlled trial designed to study the effect of rosuvastatin 10 mg daily on markers of cardiovascular risk, skeletal health and immune activation in HIV-infected adults on ART. The results presented herein represent prespecified secondary analyses of the oxidative stress markers, oxLDL and F2-IsoP/Cr. The study is registered on clinicaltrials.gov (NCT01218802) and has been approved by the Institutional Review Board of the University Hospitals Case Medical Center, Cleveland, OH, USA. All participants provided written informed consent prior to enrolment.
The eligibility criteria for SATURN-HIV have been described [22]. In brief, all participants were at least 18 years of age, with chronic HIV-1 infection on stable ART for at least 3 months with cumulative ART duration of at least 6 months and HIV-1 RNA less than 1000 copies/ml. Additional entry criteria included fasting LDL less than 130 mg/dl and evidence of either heightened T-cell activation or systemic inflammation, defined as having proportion of CD8+ T cells that express CD38 and HLA-DR at least 19% or high sensitivity C-reactive protein (hsCRP) at least 2 mg/l, respectively. Notable exclusion criteria were known coronary artery disease or diabetes, pregnancy, immunomodulating, hormonal or anti-inflammatory medications, inflammatory condition including HIV or creatinine clearance less than 50 ml/min by Cockcroft–Gault. Participants were randomized on enrolment to rosuvastatin 10 mg daily or to matching placebo in a 1 to 1 ratio. Randomization was stratified by protease inhibitor use, by presence of coronary artery calcium on cardiac computed tomography and osteopenia on DEXA scan.
Study evaluations
For this oxidative marker analysis, participants were evaluated at 0, 24 and 48 weeks. Demographics and medical history were obtained by self-report. A targeted physical exam and 12-h fasting blood draw and urine collection were performed at each visit. From whole-blood samples collected in ethylenediaminetetraacetic acid–containing tubes, peripheral blood mononuclear cells (PBMCs) were separated by centrifugation with Ficoll-Hypaque. Additionally, plasma was isolated by centrifugation. Urine, PBMCs and plasma were cryopreserved at −80°C until analysed in batches.
Oxidative stress markers
Oxidized LDL levels were determined in plasma by quantitative sandwich ELISA (Mercodia, Uppsala, Sweden) based on the mouse monoclonal antibody 4E6, which is directed against the conformational epitope in oxidized ApoB-100 at Johns Hopkins University. F2-Isoprostane/creatinine ratios were quantified from urine using liquid chromatography-mass spectrometry at the Cleveland Heart Lab. Oxidized LDL and F2-IsoP/Cr levels were determined at 0, 24 and 48-week visits.
Inflammation and immune activation markers
Interleukin-6 (IL-6), soluble tumour necrosis factor receptors I and II (sTNF-RI and -RII) and interferon γ inducible protein-10 (IP-10) were measured by ELISA (R&D Systems, Minneapolis, Minnesota, USA). High sensitivity C-reactive protein was determined by particle enhanced immunonephelometric assay on a BNII nephelometer (Siemens, Indianapolis, Indiana, USA). These assays were performed at the Laboratory for Clinical Biochemistry Research at the University of Vermont with the exception of IP-10 which was performed at Case Western Reserve University. Soluble markers of monocyte activation [soluble CD14 (sCD14) and soluble CD163 (sCD163)] were measured by ELISA (R&D Systems) and were performed at Case Western Reserve University.
Monocytes were phenotyped from PBMCs by flow cytometry as previously described [22]. Monocyte subsets including CD14+CD16+ (inflammatory) and CD14dimCD16+ (patrolling) were quantified as a percentage of the overall monocyte population.
Subclinical vascular disease
At entry and week 48, all participants underwent high-resolution ultrasound scanning of the carotid arteries using a Philips iU22 with L9–3 MHz linear array transducer (Philips Healthcare; Andover, Massachusetts, USA) following the consensus protocol of the American Society of Echocardiography [32]. Common carotid artery intima media thickness (CCA IMT) was measured using semi-automated edge detection software (Medical Imaging Applications LLC, Coralville, Iowa, USA) as previously described [23].
Other measures
Insulin resistance was calculated from fasting glucose and insulin using the homeostatic model assessment of insulin resistance (HOMA-IR) [33]. CD4+ cell counts and HIV-1 RNA levels were measured as part of routine clinical care.
Statistical analysis
Demographics, HIV-related characteristics and cardiovascular risk factors are described overall and by group using mean ± standard deviation or SD for continuous variables and frequency (percentage) for categorical variables. All baseline variables were compared between groups using unpaired t-tests or Wilcoxon rank-sum tests as appropriate for continuous variables and by χ2 tests, Fisher’s exact tests or Pearson exact χ2 tests as appropriate for categorical variables. Between and within group changes in oxidative stress markers were tested using paired t-tests or Wilcoxon signed-rank tests and unpaired t-tests or Wilcoxon rank-sum tests as appropriate for the distribution of the variables, respectively. Absolute changes from weeks 0 to 24 and 0 to 48 were tested.
Next, Spearman correlation analysis was used to evaluate relationships between baseline oxidative stress markers, markers of systemic inflammation, immune activation and CCA IMT in all enrolled participants. Additionally, Spearman correlation analysis was used to determine associations between early changes in oxLDL (week 0 to 24); and early changes in markers of systemic inflammation and immune activation; and late (weeks 0 to 48) changes in CCA IMT in all participants and in the rosuvastatin group separately. Last, multivariable linear regression was used to determine variables independently associated with late changes in CCA IMT. Variables of interest in this analysis included early changes in oxLDL and soluble and cellular markers of monocyte activation. Baseline CCA IMT was included in all models. Models were checked to be sure the assumptions of linear regression were met. Because the markers of oxidative stress, systemic inflammation and monocyte activation and CCA IMT were skewed, each was transformed by taking the natural logarithm prior to analyses. Analyses of follow-up data were restricted to participants with full outcome information at each time point, that is, complete case analysis was used. Participants were analysed in the group to which they were randomized. All statistical tests were two-sided and considered significant if P < 0.05. Analyses were performed using SAS v. 9.4 (The SAS Institute, Carey, North Carolina, USA).
Results
From March 2011 to August 2012, 147 adults met entry criteria, were enrolled and randomized (72 to rosuvastatin, 75 to placebo) in the SATURN-HIV study. Follow-up through 48 weeks was completed by June 2013. Through 48 weeks, six participants on rosuvastatin and 13 participants on placebo withdrew consent or were lost to follow-up for reasons unrelated to the study medication, resulting in 136 participants (67 on rosuvastatin, 69 on placebo) at week 24, and 128 (66 on rosuvastatin, 62 on placebo) at week 48 with available data for analysis through these time points. There were no significant differences in the baseline characteristics of those participants who withdrew or were lost to follow-up and those who completed the study. (Participant flow chart through 48 weeks has been published previously [21].)
Baseline characteristics
Demographics, HIV-related characteristics and cardiovascular risk factors were balanced between groups at baseline (Table 1). Overall, 78% were men, 68% were African American, 29% were Caucasian and 12% had hepatitis C co-infection. The mean ±SD age was 45.4 ± 9.9 years and BMI was 28.1 ± 6.5 kg/m2. Sixty-three per cent were current smokers and an additional 16% were smokers in the past. The mean ± SD current and nadir CD4+ cell counts were 640 ± 300 and 200 ± 146 cells/μl, respectively. All participants were on ART by design. Protease inhibitor use (48 vs. 50% were on a protease inhibitor in the placebo vs. rosuvastatin groups, respectively; P = 0.81) and the various protease inhibitors/protease inhibitor combinations used [atazanavir alone (n = 3), ritonavir-boosted atazanavir (n = 41), ritonavir-boosted darunavir (n = 19), ritonavir-boosted lopinavir (n = 7) and ritonavir-boosted fosamprenavir (n = 2); P = 0.82] were balanced at baseline between groups: the mean ± SD cumulative duration of ART of 7.1 ± 5.2 years and known duration of HIV infection of 12.2 ± 6.9 years. Seventy-eight percentage of participants had HIV-1 RNA less than 50 copies/ml (range <20–600 copies/ml). Only three participants changed ART during the study: switched didanosine to abacavir; switched tenofovir/emtricitabine to abacavir/lamivudine switched lamivudine/zidovudine to emtricitabine/tenofovir + maraviroc. At baseline, oxLDL levels and F2-IsoP/Cr were similar between groups.
Table 1.
Baseline demographics, HIV-related characteristics and cardiovascular risk factors by randomization group.
| Rosuvastatin (n = 72) | Placebo (n = 75) | |
|---|---|---|
| Age, year | 45.4 ± 9.1 | 45.4 ± 10.7 |
| Men | 58 (81) | 57 (76) |
| Race | ||
| African American | 50 (69) | 50 (67) |
| Whites | 20 (28) | 23 (31) |
| Other | 2 (3) | 2 (3) |
| Chronic hepatitis C | 4 (6) | 5 (7) |
| Body mass index, kg/m2 | 28 ± 6.3 | 28.1 ± 6.7 |
| CD4+ cell count, cells/μl | 644 ± 287 | 636 ± 314 |
| Nadir CD4+ cell count, cells/μl | 210 ± 155 | 192 ± 137 |
| HIV RNA-1 < 50 copies/ml | 56 (78) | 58 (77) |
| Current smoker | 43 (60) | 50 (67) |
| HOMA-IR >2 | 29 (40) | 38 (51) |
| Low density lipoprotein, mg/dl | 92.2 ± 24.2 | 96.5 ± 25.6 |
| High density lipoprotein, mg/dl | 48.6 ± 16.4 | 48.7 ± 15.4 |
| Triglycerides, mg/dl | 155.4 ± 129.2 | 147.6 ± 88.4 |
| Oxidized low-density lipoprotein, U/l | 46.4 ± 20.5 | 51.2 ± 23.6 |
| F2-isoprostanes, pg/ml | 0.6 ± 0.41 | 0.58 ± 0.43 |
Results reported as mean ± standard deviation for continuous variables and frequency (percentage) for categorical variables. HOMA-IR, homeostatic model assessment of insulin resistance.
Changes in oxidative stress markers
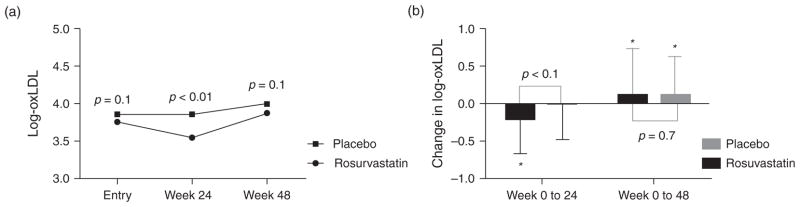
Figure 1 shows changes in oxLDL levels by randomization group. In the rosuvastatin group, oxLDL decreased significantly from week 0 to 24 compared to placebo [mean absolute change in log-oxLDL for rosuvastatin −0.2 ± 0.468 U/l (P < 0.001 within group) vs. placebo −0.018 ± 0.456 U/l (P = 0.83 within-group); P = 0.004 between-groups]. In both groups, oxLDL increased relative to baseline by 48 weeks and these changes were not different between groups. Whereas F2-IsoP/Cr increased in the rosuvastatin group over 24 weeks, changes were not different between groups [mean absolute change in log-F2-IsoP/Cr for rosuvastatin 0.174 ± 0.701 log pg/ml (P = 0.01 within group) vs. placebo 0.095 ± 0.655 log pg/ml (P = 0.23 within group); P = 0.6 between groups]. Week 0 to 48 changes in F2-IsoP/Cr were not significant within or between groups.
Fig. 1. Change in oxidized low density lipoprotein at each time point by randomization group.
(a) Mean log-transformed oxidized low density lipoprotein levels at each time point are shown. P-values are for between-group tests. (b) Mean absolute change in log transformed oxidized low density lipoprotein levels with standard deviation are shown. P-values are for between-group tests. *Denotes significant (P < 0.05) within-group change.
Oxidative stress marker correlation analysis
As shown in Table 2, baseline oxLDL levels positively correlated with hsCRP, but not with other markers of systemic inflammation or monocyte activation, HIV-related factors or baseline CCA IMT. Overall, early (week 0 to 24) changes in oxLDL were positively correlated with early changes in sCD14 and changes in the percentage CD14dimCD16+ monocytes, but not with changes in markers of systemic inflammation. This link between change in oxLDL and monocyte activation was also seen when the analysis was limited to those in the rosuvastatin group only. Last, early changes in oxLDL were positively correlated with later (week 0 to 48) changes in CCA IMT overall and within the rosuvastatin group. Baseline F2-IsoP/Cr positively correlated with percentage CD14+CD16+ monocytes (ρ=0.21; P = 0.01) and sCD14 (ρ=0.29; P < 0.001), but not with markers of systemic inflammation, HIV-related factors or CCA IMT. Changes in F2-IsoP/Cr were not correlated with changes in markers of systemic inflammation, monocyte activation or CCA IMT overall or in the rosuvastatin group.
Table 2.
Correlation analysis for week 0 oxidized low-density lipoprotein and week 0 to 24 change in oxidized low-density lipoprotein.
| Known duration of HIV | Week 0 log-oxLDL N = 147 | Earlya change in log-oxLDL N = 147 | Earlya change in log-oxLDL N = 72 (rosuvastatin only) −0.28 (P = 0.02) |
|---|---|---|---|
| HOMA-IR | 0.18 (P = 0.03) | ||
| Log-LDL | 0.52 (P < 0.0001) | ||
| Log-HDL | −0.24 (P < 0.01) | ||
| Log-triglycerides | 0.29 (P < 0.001) | ||
| Log-hsCRP | 0.19 (P = 0.02) | ||
| Log-sTNF-RI | −0.19 (P = 0.03) | −0.29 (P = 0.03) | |
| Log-IP-10 | −0.28 (P = 0.02) | ||
| Earlya changes in markers of inflammation and monocyte activation | |||
| Week 0 to 24 change in log-sCD14 | 0.2 (P = 0.02) | ||
| Week 0 to 24 change in log-sCD163 | 0.26 (P = 0.04) | ||
| Week 0 to 24 change in %CD14dimCD16+ | 0.25 (P < 0.01) | 0.32 (P < 0.01) | |
| Latea changes in carotid intima media thickness | |||
| Week 0 to 48 change in Log-CCA IMT | 0.26 (P < 0.01) | 0.28 (P = 0.02) | |
| Week 0 to 48 change in log-max CCA IMT | 0.19 (P = 0.04) | ||
Only variables with P < 0.05 are shown in the table. Baseline variables tested but not shown include the following: age, BMI, HIV-1 RNA level, current and nadir CD4+ cell counts, known duration of HIV, cumulative antiretroviral duration, interleukin-6, soluble tumour necrosis factor alpha-receptor-II, %CD14+CD16+ cells, %CD14dimCD16+ cells, soluble CD14, soluble CD163, common carotid artery intima media thickness, and max common carotid intima media thickness. Week 0 to 24 change in all markers of inflammation and monocyte activation were tested, but not all are shown. HOMA-IR, homeostatic model assessment of insulin resistance; hsCRP, high sensitivity C-reactive protein; IP-10, interferon γ inducible protein-10; sCD14, soluble CD14; sCD163, soluble CD163; sTNF-RI, soluble tumour necrosis factor alpha-receptor-I.
Early changes refer to week 0 to 24 changes and late changes refer to week 0 to 48 changes.
Multivariable linear regression
Informed by the correlation analysis linking early changes in oxLDL with later changes in CCA IMT and in-vitro data suggesting that oxLDL may be driving monocyte activation [34], we aimed to evaluate the factors that were independently associated with week 0 to 48 change in CCA IMT. In separate models adjusting for baseline CCA IMT only, both early changes in oxLDL (β=0.025; P = 0.03) and sCD14 (β=0.035; P = 0.04), but not proportion of CD14dimCD16+ monocytes (β=−0.001; P = 0.9), were associated with later changes in CCA IMT. When including each of these variables into the same model, only early change in oxLDL (β=0.03; 0.02) remained associated with later change in CCA IMT. This association between early change in oxLDL (β=0.028; P = 0.02) and later change in CCA IMT remained after further adjusting for clinically relevant baseline factors, including age, sex, race, smoking, BMI, systolic blood pressure, HOMA-IR, hepatitis C status, nadir CD4+ cell count and protease inhibitor use as well as when adjusting for baseline Framingham risk score (model not shown). The fact that early change in sCD14 was attenuated by inclusion of change in oxLDL into the model lends support to the hypothesis that oxLDL drives monocyte activation, and also that improvement in oxLDL may be in the causal pathway between improved monocyte activation and improved CCA IMT. Last, adjusting for baseline CCA IMT only, there was a trend towards randomization group (β=−0.02; P = 0.09) being associated with week 0 to 48 change in CCA IMT. The effect of randomization group (β=−0.011; P = 0.35) was also attenuated when including early change in oxLDL in the model signifying that change in oxLDL with rosuvastatin may be mediating the improvement in CCA IMT over time. Table 3 shows the stepwise multivariable modelling strategy used and the results of each model described.
Table 3.
Multivariable linear regression with week 0 to 48 change in log-common carotid artery intima media thickness as the outcome.
| Variable | Model 1 | Model 2 | Model 3 | Model 1+2+3 | Model 1+2+3a | Model 4 | Model 1+4 | Model 1+2+3+4 | Model 1+2+3+4a |
|---|---|---|---|---|---|---|---|---|---|
| Week 0 to 24 change in log-oxLDL | 0.025 (0.012); P = 0.03 | 0.03 (0.012); P = 0.02 | 0.028 (0.012); P = 0.02 | 0.023 (0.012); P = 0.05 | 0.026 (0.012); P = 0.03 | 0.027 (0.012); P = 0.03 | |||
| Week 0 to 24 change in log-sCD14 | 0.035 (0.017); P = 0.04 | 0.03 (0.016); P = 0.07 | 0.028 (0.016); P = 0.08 | 0.022 (0.016); P = 0.16 | 0.026 (0.012); P = 0.13 | ||||
| Week 0 to 24 change in log-%CD14dimCD16+ | −0.001 (0.011); P = 0.9 | −0.016 (0.01); P = 0.12 | −0.016 (0.01); P = 0.11 | −0.014 (0.01); P = 0.17 | −0.016 (0.01); P = 0.12 | ||||
| Group | −0.02 (0.012); P = 0.09 | −0.011 (0.011); P = 0.35 | −0.01 (0.012); P = 0.38 | −0.008 (0.012); P = 0.52 |
Values shown are parameter estimate (standard error). Each model is adjusted for baseline CCA IMT. HOMA-IR, hepatitis C status, nadir CD4+ cell count and protease inhibitor use.
These models are further adjusted for age, sex, race, smoking, BMI, SBP.
Discussion
In this randomized, placebo-controlled trial, we report for the first time that rosuvastatin 10 mg daily decreases oxLDL significantly over 24 weeks in HIV-infected adults on stable ART with LDL less than 130 mg/dl. Importantly, although the effect was not sustained, the early decrease in oxLDL was independently associated with later improvement in CCA IMT. Last, we have also shown that changes in oxLDL may be in the causal pathway between changes in monocyte activation and subclinical vascular disease in HIV.
Oxidized LDL is the proinflammatory form of LDL which plays a pivotal role in development of foam cells [35,36] and has been shown to contribute to smooth muscle cell proliferation and endothelial cell activation [37]. Each of these are steps important in the development of atherosclerotic plaque. Statins may decrease oxLDL by several mechanisms. First, by reducing LDL synthesis, there is less substrate for oxidation. In addition, statins decrease macrophage production of superoxide [38,39] and may decrease the susceptibility of the LDL present to be oxidized [40,41]. In our study, LDL decreased 25% from week 0 to 24 in the rosuvastatin group and week 0 to 24 changes in oxLDL were correlated with changes in LDL (ρ=0.48; P < 0.0001). This suggests that the effect of rosuvastatin on LDL reduction explains some of the effect on oxLDL. Further, from week 24 to 48, LDL increased 13% in the rosuvastatin group which is a possible explanation for why the reduction in oxLDL was not sustained. Short term, several studies have shown that statins reduce oxLDL outside of HIV [26,27,42–49]; however, few have follow-up to 48 weeks and the results are mixed [50–52]. In a study of adults with ischemic cardiomyopathy, the atorvastatin dose appeared to play a role in oxLDL reduction over 48 weeks [50]. In another study, reduction of oxLDL was different in the different LDL subfractions, that is, oxLDL was reduced mostly in medium density LDL [52]. Lipoprotein subfractions were not determined for our study.
Next, early decrease in oxLDL was linked with later improvement in CCAIMT. Outside of HIV, treatment with statins has been associated with improvement in measures of subclinical atherosclerosis and endothelial function [46,53] and risk of cardiovascular events [19]. There is limited data, however, linking improvement in oxLDL to improved outcomes. In adults with carotid stenosis, decrease in oxLDL correlated with reduction in stenosis and treatment with atorvastatin ameliorated restenosis after angioplasty [51]. Additionally, worsening oxLDL has been linked with CVD events [54] affirming the relationship of change in oxLDL with CVD outcomes in other populations. Interestingly, monocyte activation has been linked with CVD with HIV infection [9–11]. Both soluble and cellular markers of monocyte activation have been linked with high risk features in atherosclerotic plaques [10] and progression of coronary artery calcium [9] in HIV-infected adults on ART). Further, in a study led by one of the authors (N.T.F.), stimulation of whole blood samples with oxLDL, but not LDL, was shown to increase the proportion of inflammatory monocytes (CD14+CD16+) and the expression of tissue factor on classical and inflammatory monocytes suggesting a possible link between oxLDL and monocyte activation [34]. Our study supports this hypothesis as both early improvement in oxLDL and the monocyte activation markers CD14 were associated with later improvement in CCA IMT separately, but when both were included in a multivariable model, change in sCD14 was no longer significant, suggesting change in oxLDL is in the causal pathway between change in sCD14 and improved CCA IMT.
Last, F2-IsoP/Cr did not improve with rosuvastatin in our study. F2-isoprostanes are prostaglandin-like compounds produced by free radical-mediated peroxidation of arachidonic acid and are a marker of lipid oxidation [55]. They may also act as vasoconstrictors and promote platelet activation resulting in thrombus formation [56,57]. Measurement of F2-isoprostanes is widely considered the most accurate method to measure oxidant stress in vivo [58,59]. Although it is not clear why F2-IsoP/Cr did not decrease with rosuvastatin in our study, this is consistent with prior studies evaluating the effect of statins on this oxidative stress marker outside of HIV [29,60]. It is possible that HIV-related factors or ART may affect F2-IsoP levels so profoundly that statins are unable to improve these levels in the continued presence of HIV and/or ART effect.
Strengths of this study include the double-blind, placebo-controlled, randomized trial design, as well as the comprehensive evaluation of markers of inflammation and monocyte activation. However, some limitations deserve noting. We focused on a specific population of HIV-infected individuals with normal LDL-cholesterol levels, and, because of this, our findings may not be generalizable to all HIV-infected individuals. Also, given the decrease in oxLDL was not sustained, longer term follow-up would be necessary to determine the potential impact of the increase in oxLDL relative to baseline over 48 weeks in both groups. Next, over 48 weeks 13% of all participants (4% from the rosuvastatin group and 9% from the placebo group) withdrew consent or were lost to follow-up which may have resulted in the introduction of bias in determining the treatment effect. However, in the subset of participants that completed the week 48 visit, important baseline demographics, HIV-related factors and Framingham risk score remained balanced between the groups (data not shown) making this unlikely. Further, this rate of attrition is consistent with other randomized clinical trials reported in the literature and is not greater than would be expected [61]. Last, smoking status has been shown to confound the relationship between HIV and levels of markers of inflammation. In this study, smoking status was assessed at entry, week 24 and 48 visits. Through 48 weeks, 19 participants (10 in the placebo group and nine in the rosuvastatin group) changed smoking status from one of these study visits to another. Although this may have contributed to the results of this study, excluding participants who changed smoking status between week 0 and 24 (n = 14), the decrease in oxLDL over this time frame was still significant within the rosuvastatin group and between the rosuvastatin and placebo groups. Further, excluding participants whose smoking status was different between week 0 and 48 (n = 10) did not qualitatively change the results of the multivariable linear regression modelling.
In conclusion, rosuvastatin decreases oxLDL early after initiation and is associated with decreased monocyte activation. Importantly, early improvement in oxLDL is linked with improved CIMT in treated HIV infection. Further study is needed to determine if statins reduce clinical CVD events in HIV infection.
Acknowledgments
Role of each author: C.O.H. assisted in recruitment and study design relating to the statistical analysis, performed the statistical analysis and drafted the manuscript. R.T. with oversight by R.S. performed the oxidized LDL assays. N.F. performed the flow cytometry, inflammation and monocyte activation assays. G.M.C. conceived the study concept and design and oversaw all study procedures. All authors contributed to and approved the final manuscript.
This study was supported by the National Institutes of Health (grant number NR012642 to G.A.M., K23HL116209 to C.O.H., R00HL108743 to N.T.F. and R01 AG027012 and R01 HL111271 to R.D.S.).
Technical assistance was provided by the Center for AIDS Research, Case Western Reserve University (P30 AI36219).
Footnotes
Conflicts of interest
G.A.M. has received research grants from BMS, Gilead Sciences and GSK, has served as a consultant to BMS, GSK, Janssen, Merck and Gilead, as a speaker for BMS, GSK and Tibotec and on the DSMB for a Pfizer-sponsored trial. N.T.F. has served as a consultant for Gilead Sciences. All other authors report no conflicts.
References
- 1.Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68:209–216. doi: 10.1097/QAI.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen LD, Helleberg M, May MT, Afzal S, Kronborg G, Larsen CS, et al. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis. 2015;60:1415–1423. doi: 10.1093/cid/civ013. [DOI] [PubMed] [Google Scholar]
- 5.Stein JH, Brown TT, Ribaudo HJ, Chen Y, Yan M, Lauer-Brodell E, et al. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naive individuals with HIV infection. AIDS. 2013;27:929–937. doi: 10.1097/QAD.0b013e32835ce27e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 7.Hileman CO, Longenecker CT, Carman TL, McComsey GA. C-reactive protein predicts 96-week carotid intima media thickness progression in HIV-infected adults naive to antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;65:340–344. doi: 10.1097/QAI.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 8.Ross AC, Rizk N, O’Riordan MA, Dogra V, El-Bejjani D, Storer N, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009;49:1119–1127. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker JV, Hullsiek KH, Singh A, Wilson E, Henry K, Lichtenstein K, et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS. 2014;28:831–840. doi: 10.1097/QAD.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ, Jr, Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211:1219–1228. doi: 10.1093/infdis/jiu594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Horke S, Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237:208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Tsimikas S, Willeit P, Willeit J, Santer P, Mayr M, Xu Q, et al. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60:2218–2229. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- 14.Gomez M, Vila J, Elosua R, Molina L, Bruguera J, Sala J, et al. Relationship of lipid oxidation with subclinical atherosclerosis and 10-year coronary events in general population. Atherosclerosis. 2014;232:134–140. doi: 10.1016/j.atherosclerosis.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Trpkovic A, Resanovic I, Stanimirovic J, Radak D, Mousa SA, Cenic-Milosevic D, et al. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci. 2015;52:70–85. doi: 10.3109/10408363.2014.992063. [DOI] [PubMed] [Google Scholar]
- 16.Johnston N, Jernberg T, Lagerqvist B, Siegbahn A, Wallentin L. Oxidized low-density lipoprotein as a predictor of outcome in patients with unstable coronary artery disease. Int J Cardiol. 2006;113:167–173. doi: 10.1016/j.ijcard.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Duong M, Petit JM, Martha B, Galland F, Piroth L, Walldner A, et al. Concentration of circulating oxidized LDL in HIV-infected patients treated with antiretroviral agents: relation to HIV-related lipodystrophy. HIV Clin Trials. 2006;7:41–47. doi: 10.1310/7381-m1yd-rtv5-4ryt. [DOI] [PubMed] [Google Scholar]
- 18.Parra S, Coll B, Aragones G, Marsillach J, Beltran R, Rull A, et al. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 2010;11:225–231. doi: 10.1111/j.1468-1293.2009.00766.x. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 20.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving anti-retroviral therapy. J Infect Dis. 2014 doi: 10.1093/infdis/jiu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funderburg NT, Jiang Y, Debanne SM, Labbato D, Juchnowski S, Ferrari B, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68:396–404. doi: 10.1097/QAI.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, Clagett B, et al. Rosuvastatin treatment reduces markers of monocyte activation in hiv-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58:588–595. doi: 10.1093/cid/cit748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longenecker CT, Hileman CO, Funderburg NT, McComsey GA. Rosuvastatin preserves renal function and lowers cystatin C in HIV-infected subjects on antiretroviral therapy: the SATURN-HIV trial. Clin Infect Dis. 2014;59:1148–1156. doi: 10.1093/cid/ciu523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Profumo E, Buttari B, Saso L, Rigano R. Pleiotropic effects of statins in atherosclerotic disease: focus on the antioxidant activity of atorvastatin. Curr Top Med Chem. 2014;14:2542–2551. doi: 10.2174/1568026614666141203130324. [DOI] [PubMed] [Google Scholar]
- 25.Mihos CG, Pineda AM, Santana O. Cardiovascular effects of statins, beyond lipid-lowering properties. Pharmacol Res. 2014;88:12–19. doi: 10.1016/j.phrs.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Moon GJ, Kim SJ, Cho YH, Ryoo S, Bang OY. Antioxidant effects of statins in patients with atherosclerotic cerebrovascular disease. J Clin Neurol. 2014;10:140–147. doi: 10.3988/jcn.2014.10.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aydin MU, Aygul N, Altunkeser BB, Unlu A, Taner A. Comparative effects of high-dose atorvastatin versus moderate-dose rosuvastatin on lipid parameters, oxidized-LDL and inflammatory markers in ST elevation myocardial infarction. Atherosclerosis. 2015;239:439–443. doi: 10.1016/j.atherosclerosis.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Molcanyiova A, Stancakova A, Javorsky M, Tkac I. Beneficial effect of simvastatin treatment on LDL oxidation and antioxidant protection is more pronounced in combined hyperlipidemia than in hypercholesterolemia. Pharmacol Res. 2006;54:203–207. doi: 10.1016/j.phrs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Fassett RG, Robertson IK, Ball MJ, Geraghty DP, Coombes JS. Effects of atorvastatin on oxidative stress in chronic kidney disease. Nephrology (Carlton) 2015 doi: 10.1111/nep.12502. [DOI] [PubMed] [Google Scholar]
- 30.Tsikas D, Pham VV, Suchy MT, van de Ree MA, Huisman MV, Frolich JC, et al. No effects of atorvastatin (10 mg/d or 80 mg/d) on nitric oxide, prostacyclin, thromboxane and oxidative stress in type 2 diabetes mellitus patients of the DALI study. Pharmacol Res. 2015;94:1–8. doi: 10.1016/j.phrs.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Longenecker CT, Jiang Y, Debanne SM, Labbato D, Kinley B, Storer N, et al. Rosuvastatin arrests progression of carotid intima-media thickness in Treated HIV. Conference on Retroviruses and Opportunistic Infections; Seattle, Washington. Seattle, Washington: 2015. [Google Scholar]
- 32.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 34.Zidar DA, Juchnowski S, Ferrari B, Clagett B, Pilch-Cooper HA, Rose S, et al. Oxidized LDL levels are increased in hiv infection and may drive monocyte activation. J Acquir Immune Defic Syndr. 2015;69:154–160. doi: 10.1097/QAI.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itabe H, Suzuki K, Tsukamoto Y, Komatsu R, Ueda M, Mori M, et al. Lysosomal accumulation of oxidized phosphatidylcholine-apolipoprotein B complex in macrophages: intracellular fate of oxidized low density lipoprotein. Biochim Biophys Acta. 2000;1487:233–245. doi: 10.1016/s1388-1981(00)00098-6. [DOI] [PubMed] [Google Scholar]
- 36.Itabe H, Takano T. Oxidized low density lipoprotein: the occurrence and metabolism in circulation and in foam cells. J Atheroscler Thromb. 2000;7:123–131. doi: 10.5551/jat1994.7.123. [DOI] [PubMed] [Google Scholar]
- 37.Heery JM, Kozak M, Stafforini DM, Jones DA, Zimmerman GA, McIntyre TM, et al. Oxidatively modified LDL contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. J Clin Invest. 1995;96:2322–2330. doi: 10.1172/JCI118288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delbosc S, Morena M, Djouad F, Ledoucen C, Descomps B, Cristol JP. Statins 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J Cardiovasc Pharmacol. 2002;40:611–617. doi: 10.1097/00005344-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Giroux LM, Davignon J, Naruszewicz M. Simvastatin inhibits the oxidation of low-density lipoproteins by activated human monocyte-derived macrophages. Biochim Biophys Acta. 1993;1165:335–338. doi: 10.1016/0005-2760(93)90145-y. [DOI] [PubMed] [Google Scholar]
- 40.Rikitake Y, Kawashima S, Takeshita S, Yamashita T, Azumi H, Yasuhara M, et al. Antioxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 2001;154:87–96. doi: 10.1016/s0021-9150(00)00468-8. [DOI] [PubMed] [Google Scholar]
- 41.Yasuhara M, Suzumura K, Tanaka K, Takahashi M, Aoki S, Odawara A, et al. Fluvastatin, an HMG-CoA reductase inhibitor, protects LDL from oxidative modification in hypercholesterolemic rabbits. Biol Pharm Bull. 2000;23:570–574. doi: 10.1248/bpb.23.570. [DOI] [PubMed] [Google Scholar]
- 42.Mirjanic-Azaric B, Rizzo M, Jurgens G, Hallstroem S, Srdic S, Marc J, et al. Atorvastatin treatment increases plasma bilirubin but not HMOX1 expression in stable angina patients. Scand J Clin Lab Invest. 2015:1–8. doi: 10.3109/00365513.2015.1031691. [DOI] [PubMed] [Google Scholar]
- 43.Bostan C, Yildiz A, Ozkan AA, Uzunhasan I, Kaya A, Yigit Z. Beneficial effects of rosuvastatin treatment in patients with metabolic syndrome. Angiology. 2015;66:122–127. doi: 10.1177/0003319714522107. [DOI] [PubMed] [Google Scholar]
- 44.Tsai NW, Lee LH, Huang CR, Chang WN, Chang YT, Su YJ, et al. Statin therapy reduces oxidized low density lipoprotein level, a risk factor for stroke outcome. Crit Care. 2014;18:R16. doi: 10.1186/cc13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moutzouri E, Liberopoulos EN, Tellis CC, Milionis HJ, Tselepis AD, Elisaf MS. Comparison of the effect of simvastatin versus simvastatin/ezetimibe versus rosuvastatin on markers of inflammation and oxidative stress in subjects with hypercholesterolemia. Atherosclerosis. 2013;231:8–14. doi: 10.1016/j.atherosclerosis.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Akalin A, Temiz G, Akcar N, Sensoy B. Short term effects of atorvastatin on endothelial functions and oxidized LDL levels in patients with type 2 diabetes. Endocr J. 2008;55:861–866. doi: 10.1507/endocrj.k07e-121. [DOI] [PubMed] [Google Scholar]
- 47.Tavridou A, Efthimiadis A, Efthimiadis I, Manolopoulos VG. Simvastatin-induced changes in circulating oxidized low-density lipoprotein in different types of dyslipidemia. Heart Vessels. 2010;25:288–293. doi: 10.1007/s00380-009-1202-x. [DOI] [PubMed] [Google Scholar]
- 48.Azar RR, Badaoui G, Sarkis A, Azar M, Aydanian H, Harb S, et al. Effect of ezetimibe/atorvastatin combination on oxidized low density lipoprotein cholesterol in patients with coronary artery disease or coronary artery disease equivalent. Am J Cardiol. 2010;106:193–197. doi: 10.1016/j.amjcard.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Andreou I, Tousoulis D, Miliou A, Tentolouris C, Zisimos K, Gounari P, et al. Effects of rosuvastatin on myeloperoxidase levels in patients with chronic heart failure: a randomized placebo-controlled study. Atherosclerosis. 2010;210:194–198. doi: 10.1016/j.atherosclerosis.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 50.Huang B, Cheng Y, Xie Q, Lin G, Wu Y, Feng Y, et al. Effect of 40 mg versus 10 mg of atorvastatin on oxidized low-density lipoprotein, high-sensitivity C-reactive protein, circulating endothelial-derived microparticles, and endothelial progenitor cells in patients with ischemic cardiomyopathy. Clin Cardiol. 2012;35:125–130. doi: 10.1002/clc.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kougialis S, Skopelitis E, Gialernios T, Nikolaou S, Kroustalis A, Katsadorou E, et al. Atorvastatin therapy is associated with improvement of oxidized low-density lipoprotein cholesterol levels, which correlates with the degree of stenosis in patients with carotid atheromatosis with and without prior angioplasty. Int Angiol. 2010;29:338–347. [PubMed] [Google Scholar]
- 52.Homma Y, Michishita I, Hayashi H, Shigematsu H. Effects of low-dose simvastatin on the distribution of plasma cholesterol and oxidized low-density lipoprotein in three ultra-centrifugally separated low-density lipoprotein subfractions: 12-month, open-label trial. J Atheroscler Thromb. 2010;17:1049–1053. doi: 10.5551/jat.4077. [DOI] [PubMed] [Google Scholar]
- 53.Stojakovic T, Claudel T, Putz-Bankuti C, Fauler G, Scharnagl H, Wagner M, et al. Low-dose atorvastatin improves dyslipidemia and vascular function in patients with primary biliary cirrhosis after one year of treatment. Atherosclerosis. 2010;209:178–183. doi: 10.1016/j.atherosclerosis.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 54.Ajeganova S, de Faire U, Jogestrand T, Frostegard J, Hafstrom I. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis – an inception cohort study. J Rheumatol. 2012;39:1146–1154. doi: 10.3899/jrheum.111334. [DOI] [PubMed] [Google Scholar]
- 55.Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005;10 (Suppl 1):S10–23. doi: 10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- 56.Morrow JD, Minton TA, Roberts LJ., 2nd The F2-isoprostane, 8-epi-prostaglandin F2 alpha, a potent agonist of the vascular thromboxane/endoperoxide receptor, is a platelet thromboxane/endoperoxide receptor antagonist. Prostaglandins. 1992;44:155–163. doi: 10.1016/0090-6980(92)90077-7. [DOI] [PubMed] [Google Scholar]
- 57.Minuz P, Andrioli G, Degan M, Gaino S, Ortolani R, Tommasoli R, et al. The F2-isoprostane 8-epiprostaglandin F2alpha increases platelet adhesion and reduces the antiadhesive and antiaggregatory effects of NO. Arterioscler Thromb Vasc Biol. 1998;18:1248–1256. doi: 10.1161/01.atv.18.8.1248. [DOI] [PubMed] [Google Scholar]
- 58.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 59.Montuschi P, Barnes P, Roberts LJ., 2nd Insights into oxidative stress: the isoprostanes. Curr Med Chem. 2007;14:703–717. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- 60.Ky B, Burke A, Tsimikas S, Wolfe ML, Tadesse MG, Szapary PO, et al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J Am Coll Cardiol. 2008;51:1653–1662. doi: 10.1016/j.jacc.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 61.Hewitt CE, Kumaravel B, Dumville JC, Torgerson DJ. Assessing the impact of attrition in randomized controlled trials. J Clin Epidemiol. 2010;63:1264–1270. doi: 10.1016/j.jclinepi.2010.01.010. [DOI] [PubMed] [Google Scholar]