Abstract
Paracoccidioides spp., a dimorphic pathogenic fungus, is the etiologic agent of paracoccidioidomycosis (PCM). PCM is an endemic disease that affects at least 10 million people in Latin America, causing severe public health problems. The drugs used against pathogenic fungi have various side effects and limited efficacy; therefore, there is an inevitable and urgent medical need for the development of new antifungal drugs. In the present study, we evaluated the transcriptional profile of Paracoccidioides lutzii exposed to argentilactone, a constituent of the essential oil of Hyptis ovalifolia. A total of 1,058 genes were identified, of which 208 were up-regulated and 850 were down-regulated. Cell rescue, defense and virulence, with a total of 26 genes, was a functional category with a large number of genes induced, including heat shock protein 90 (hsp90), cytochrome c peroxidase (ccp), the hemoglobin ligand RBT5 (rbt5) and superoxide dismutase (sod). Quantitative real-time PCR revealed an increase in the expression level of all of those genes. An enzymatic assay showed a significant increase in SOD activity. The reduced growth of Pbhsp90-aRNA, Pbccp-aRNA, Pbsod-aRNA and Pbrbt5-aRNA isolates in the presence of argentilactone indicates the importance of these genes in the response of Paracoccidioides spp. to argentilactone. The response of the P. lutzii cell wall to argentilactone treatment was also evaluated. The results showed that argentilactone caused a decrease in the levels of polymers in the cell wall. These results suggest that argentilactone is a potential candidate for antifungal therapy.
Author Summary
Paracoccidioidomycosis (PCM) is a neglected human systemic mycosis caused by Paracoccidioides spp. fungus that invades the host’s lungs and can disseminate to many other organs. Treatment usually involves amphotericin B, sulfadiazine, trimethoprim-sulfamethoxazole, itraconazole, ketoconazole or fluconazole for six months to two years. In this way, many adverse effects are associated with treatment, and patients can have many co-morbidities and difficulties in complying with treatment. For those reasons, more effective and less toxic drugs are needed. The discovery of a potentially bioactive molecule and its correlation with a biological target is an important step in the research and development of drugs. One of the ways in which cells adjust to environmental change is by changing the pattern of gene expression. Thus, the transcriptome is potential experimental strategy to elucidate the mode of action of bioactive molecules. Here, Paracoccidoides spp. altered the expression of genes, leading to a further understanding of the action of the compound argentilactone in the fungal cells. Argentilactone seems to be able to modulate cellular targets, to induce oxidative stress and to interfere with the biosynthesis of the P. lutzii cell wall.
Introduction
The genus Paracoccidioides, which comprises the species lutzii and brasiliensis, is the etiological agent of paracoccidioidomycosis (PCM), an important systemic mycosis in Latin America. The inhalation of mycelia fragments, infectious forms of the fungus, is the common route of infection that primarily affects the lungs [1]. PCM has been reported to affect individuals from northern Argentina to southern Mexico, with prevalence in Brazil, Colombia, Venezuela and Argentina. The most cases of PCM occur in men, rural workers and individuals between 30–50 years of age, although it affects individuals at any age [2]. In Brazil, PCM is responsible for over 50% of deaths caused by fungal infections [3].
Fungal infections are a serious threat to public health due to their association with high rates of morbidity and mortality [4]. Despite the existence of potent antifungal agents, the development of antifungal resistance by the fungal species, as well as cytotoxicity and collateral effects, has limited the use of current antifungals [5]. In addition, PCM treatment is a slow process that extends over months or years depending on the severity of the disease and the site of injury [6]. Given these facts, it is important to search for and identify novel antifungals.
New therapeutic approaches have been suggested for PCM [7]. In this way, our group has identified new antifungal targets, such as the enzymes 1,3-β-D-glucan synthase [8,9], malate synthase [10,11], isocitrate lyase [12] and (S)-adenosyl-L-methionine: C24 sterol methyl transferase [13], from Paracoccidioides spp. In addition, we have investigated new antifungal compounds, including thiosemicarbazide [14] and oenothein [15,16]. Here, as part of a continuing search for diverse chemicals from plants, we have examined argentilactone, a bioactive metabolite isolated from Hyptis ovalifolia, which is renowned for its wide range of anticancer, insecticidal and antimicrobial activities [17,18], including those against P. lutzii.
In our previous work, we have investigated the antifungal potential of argentilactone and its semi-synthetic derivatives on P. lutzii [19]. Argentilactone and the tetrahydro derivative inhibited native and recombinant isocitrate lyase from P. lutzii in the presence of glucose and acetate. Additionally, argentilactone and the tetrahydro derivative exhibited inhibitory activity against P. lutzii yeast cells and dose-dependently interfered with the dimorphic transition from the mycelium to the yeast phase. Argentilactone interfered with the viability of Paracoccidioides spp., but was not toxic to MRC5 cells at the IC50 concentration in the fungus. In silico studies showed that argentilactone and reduced argentilactone bound to the catalytic site of PbICL, and the amino acids involved in their binding were identified. The data obtained indicate that argentilactone is a potential candidate for antifungal therapy.
In this study, we investigated the transcriptional profile of P. lutzii yeast cells grown in the presence of argentilactone using the Illumina/Hiseq™2000 platform (Illumina, San Diego, CA, USA). A total of 1,058 genes were identified, of which 208 were up-regulated and 850 were down-regulated in response to argentilactone treatment. The genes identified were classified by the biological function of the encoded protein products. The main categories identified in the up-regulated genes were metabolism, cell rescue, defense and virulence, energy and cell cycle and DNA processing. The down-regulated gene categories were related to metabolism, transcription, protein fate and cell cycling and DNA processing.
Materials and Methods
Extraction of argentilactone (2H- Pyran- 2- one, 6- (1- heptenyl) - 5, 6- dihydro-, [R- (Z)])
The essential oil of H. ovalifolia was obtained as described previously, and the NMR data are consistent with the literature [18].
Culture conditions of Paracoccidioides spp.
P. lutzii (ATCC-MYA-826) was used in the experiments described in this study, except for the silenced mutant experiments, which were performed with P. brasiliensis strains ATCC 60855 and Pb339. The yeast phase was maintained at 36°C in Fava Netto’s semi-solid medium [20] containing 1% (w/v) peptone, 0.5% (w/v) yeast extract, 0.3% (w/v) proteose peptone, 0.5% (w/v) beef extract, 0.5% (w/v) NaCl, 4% (w/v) glucose, and 1.4% (w/v) agar, pH 7.2. For experiments, cells were transferred to Fava Netto’s liquid medium, where they remained for 72 h at 36°C under agitation at 150 rpm. Afterwards, the fungus was transferred into McVeigh Morton (MMcM) chemically defined liquid medium [21] and incubated for 16 h with agitation at 150 rpm.
The viability of P. lutzii cells grown in the absence or presence of 9 μg/mL argentilactone was determined using the trypan blue method [22], in which viable and non-viable cells are counted in a Neubauer chamber. Except for the transcriptional profiling of P. lutzii yeast cells, all experiments were done using yeast cells from three different seedings from different days.
Extraction and quantification of RNA
All procedures for extraction and manipulation of total RNA were performed in RNAse-free conditions. Total RNA from Paracoccidioides spp. yeast cells not treated or treated with 9 μg/mL argentilactone for 6 h at 36°C in MMcM liquid medium was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) according to the supplier's instructions. Each experiment (not treated and treated) was performed in triplicate and pooled. The mRNA was purified using the GenElute mRNA kit (Sigma Aldrich, St. Louis, MO, USA). Total RNA was quantified on a NanoDrop 8000 Spectrophotometer and stored at -80°C. Total RNA integrity was visualized using an agarose gel.
High-throughput mRNA sequencing (RNA-seq)
The cDNA libraries were prepared from poly(A)-fragment selected mRNA and processed on the Illumina HiSeq™2000 Sequencing System (http://www.illumina.com). The pipeline was performed as described previously [23]. Briefly, the sequencing reads were mapped to reference the P. lutzii genome (http://www.broadinstitute.org/annotation/genome/paracoccidioides_brasiliensis/Multiome.html) using the Bowtie 2 tool. Mapped read data were analyzed by the DEGseq package. Each read was allowed to align to just one site of the genome, and the reads were counted. The default parameters were used to perform the alignment. The number of mismatches allowed in seed alignment (-N) was 0, and the length of each seed (-L) was 20. The fold change selection method was used for differentially expressed gene selection using Fisher’s exact test, and a p-value of 0.001 was considered to select the genes. From the selected genes, a 1.5-fold change cut-off was considered. Genes with log2 (fold change) higher than 0.58 or less than -0.58 were selected and classified as up- and down-regulated genes, respectively. Genes’ identifications and annotations were determined from the P. lutzii genome database (http://www.broadinstitute.org/annotation/genome/paracoccidioides_brasiliensis/MultiHome.html). The biological processes were obtained using the Pedant on MIPS (http://pedant.helmholtz-muenchen.de/pedant3htmlview/pedant3view?Method=analysis&Db=p3_r48325_Par_brasi_Pb01), which provides a tool to browse and search the functional categories (FunCat) of proteins. Additionally, hypothetical proteins were annotated using the Blast program (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome).
Determination of the susceptibility of Paracoccidioides brasiliensis and Pbhsp90-aRNA, Pbccp-aRNA, Pbsod-aRNA, and Pbrbt5-aRNA isolates to argentilactone
The argentilactone sensitivity assay was performed from three independent experiments using P. brasiliensis, Pb339, Pb60855, Pb60855EV, Pb339EV and the silenced mutants for superoxide dismutase (SOD) protein (Pbsod-aRNA) [14], heat shock protein HSP90 (Pbhsp90-aRNA) [24], cytochrome C peroxidase protein (Pbccp-aRNA) [25] and hemoglobin ligand protein RBT5 (Pbrbt5-aRNA) [26] were grown in liquid Fava-Netto for 72 h with agitation at 150 rpm at 36°C, and then transferred to MMcM liquid medium, where they remained overnight. The cells were then washed with phosphate buffered saline (PBS 1X) and diluted to concentrations of 104, 105 and 106. The cells were plated in solid Fava-Netto medium supplemented with argentilactone at concentrations of 4.5 μg/mL, 9 μg/mL, 18 μg/mL and 36 μg/mL and incubated for 6 days at 36°C. The control was prepared in the absence of argentilactone.
Gene expression analysis by quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. The RNA was reverse transcribed using the high-capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA, USA). The cDNA was quantified by qRT-PCR using a SYBR green PCR master mix (Applied Biosystems Step One Plus PCR System). α-tubulin was used as endogenous control for data normalization, and its amplification was presented as relative expression in comparison to that of the experimental samples, whose value was set to 1. Data were expressed as the mean ± standard deviation of the biological triplicates of independent experiments. Standard curves were generated by diluting the cDNA solution 1:5. Relative expression levels of genes of interest were calculated using the standard curve method for relative quantification. Statistical comparisons were performed using Student’s t test and p-values < 0.05 were considered statistically significant. The specific sense and antisense primers are listed in S1 Table. Argentilactone-regulated transcripts were selected for qRT-PCR validation assays.
Protein extraction
The protein extraction was performed with cells grown in the presence and absence of argentilactone. After incubation with 9 μg/mL argentilactone for 6 h in MMcM liquid medium, the cells were centrifuged at 10,000 x g for 15 min at 4°C and protein was extracted using extraction buffer (20 mM Tris-HCl pH 8.8; 2 mM CaCl2) containing a mixture of protease inhibitors (GE Healthcare). After the addition of glass beads (0.45 mm), the cells were lysed in a bead-beater, followed by centrifugation at 10,000 x g for 15 min at 4°C. The supernatant was collected, and the protein concentrations were determined using Bradford reagent (Sigma-Aldrich) [27].
Assay of superoxide dismutase enzymatic activity
The superoxide dismutase activity was quantified using an SOD assay kit (Sigma-Aldrich) that determine to production of formazan dye upon reduction with O2- by colorimetric detection at 440 nm. The levels of SOD activity were quantified using 1 μg of total protein extract. Enzyme activity data were plotted as the mean of three independent experiments. The statistical analysis was performed using Student’s t-test and samples with a p-value ˂ 0.05 were considered statistically significant.
Fluorescence microscopy
Fluorescence microscopy assays were performed as previously described [28]. Calcofluor White (CFW) and Congo Red (CR) (Sigma-Aldrich) were used to label the cell wall of P. lutzii yeast cells. A total of 106 cells/mL P. lutzii yeast cells were inoculated in Fava Netto’s liquid medium and grown for 3 days with agitation at 150 rpm. Afterwards, the cultures were incubated in MMcM liquid medium overnight at 36°C with shaking for 16 h. The cells were centrifuged at 5,000 x g for 5 min and then transferred to MMcM liquid medium containing 9 μg/mL argentilactone for 6 h. Control cells were incubated in MMcM liquid medium without argentilactone. Cells were fixed in 100% methanol at -80°C for 20 min, and then at -20°C for 20 min, and finally they were washed and centrifuged. The collected cells were stained with 100 μg/mL CR and CFW in PBS for 15 min and washed with 1x PBS. The samples were analyzed under a fluorescence microscope at 345 nm and 500 nm for CR and CFW, respectively (Zeiss Axiocam MRc—Scope A1, Carl Zeiss, Jena, Germany).
Ethanol dosing
The level of ethanol was measured as previously described [23] using an enzymatic detection kit UV-test for ethanol (RBiopharm, Darmstadt, Germany). A total of 1 x 106 cells were grown in the presence and absence of the argentilactone. Afterwards, the protein extract was obtained after cell lysis using glass beads and a bead beater apparatus (BioSpec, Oklahoma, USA) in 5 cycles of 30 sec. The cell lysate was subjected to centrifugation for 15 min, at 4°C, at 10,000 × g. The enzyme assay was performed in triplicate using the supernatant according to the manufacturer's instructions.
Mitochondrial membrane potential measurement
The mitochondrial membrane was monitored using the rhodamine 123 fluorescent dye. A total of 106 cells/mL were treated with 9 μg/mL argentilactone for 6 h. After treatment, the cells were centrifuged and incubated with 20 μM rhodamine 123 for 20 min at room temperature. Afterwards, the cells were washed 2 times with 1X PBS and resuspended in 1 mL PBS for analysis using a guava easyCyte flow cytometer with excitation and emission wavelengths of 488 and 530 nm, respectively.
Results
Transcriptional response pattern of Paracoccidioides lutzii to argentilactone
Next-generation sequencing was used to produce the transcriptional profile. Approximately 46 million reads of 100-bp single-end sequences were obtained. The data were submitted in the ncbi.nlm.nih.gov, generating access number SRP064389. The reads were mapped using the reference genome of the P. lutzii genome database (http://www.broadinstitute.org/annotation/genome/paracoccidioides_brasiliensis/MultiHome.html) and were analyzed using the DEGseq package. For the global analysis, plotting graphs were prepared. The number of reads counted for each transcript in the presence or absence of argentilactone was represented by scattered dots. The transcripts are represented by dots, which could represent a different number of reads in each condition (S1A Fig). The statistical test was applied to identify differentially expressed transcripts, represented by red dots (S1B Fig).
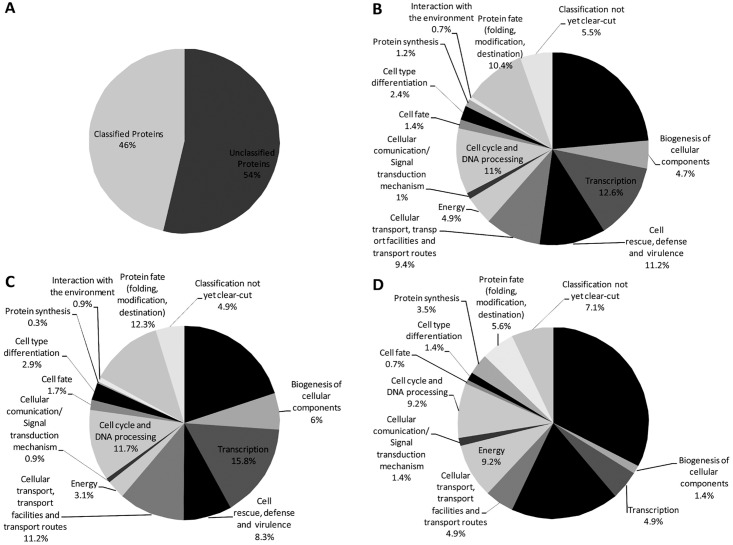
To determine the up- and down-regulated transcripts (S2 and S3 Tables, respectively), a cut-off of 1.5-fold change was used, resulting in 1,058 differentially expressed transcripts in P. lutzii yeast cells. A biological process classification was performed to gain a general understanding of the functional categories affected by argentilactone. A total of 54% (567 transcripts) were represented by proteins of unknown function (Fig 1A). After 6 h of exposure to argentilactone, transcripts associated with metabolism (23.6%) were the most represented. Other groups were also regulated, such as transcription (12.6%), cell rescue, defense and virulence (11.2%) and cell cycle and DNA processing (11%) (Fig 1B). The genes related to metabolism (20%), transcription (15.8%), protein fate (12.3%), cell cycle and DNA processing (11.7%) and cellular transport, transport facilities and transport routes (11.2%) (Fig 1C) were down-regulated. The up-regulated genes were mainly related to metabolism (32.4%), cell rescue, defense and virulence (18.3%), cell cycle and DNA processing (9.2%), energy (9.2%) and protein fate (5.6%) (Fig 1D).
Fig 1. Functional classification and abundance levels of transcripts regulated in Paracoccidioides lutzii in the presence of argentilactone obtained by RNAseq.
(A) Total transcripts represented by classified and unclassified categories; (B) Genes differentially expressed in the presence of argentilactone; (C) Down-regulated genes in the presence of argentilactone; (D) Up-regulated genes in the presence of argentilactone. Counting the number of reads for each gene in the genome annotated reference was applied to a statistical analysis using a Fisher's exact test for the identification of genes with differential expression.
Argentilactone seems interferes with urea synthesis and excretion in Paracoccidioides lutzii
From the transcriptional data obtained (S2 and S3 Tables), we could infer that, we could infer that P. lutzii down-regulated glutamine synthase, which catalyzes the condensation of glutamate and ammonia to glutamine, and glutamate dehydrogenase, which converts glutamate to α-ketoglutarate, and vice versa. In addition, two glutamate-1-semialdehyde 2,1-aminomutase, which converts glutamate 1-semialdehyde to 5-aminolevulinate, was down-regulated. Glutamate 1-semialdehyde is a molecule formed from glutamate and is a precursor to ornithine and proline. The cofactor of glutamate-1-semialdehyde 2,1-aminomutase is pyridoxal phosphate, which is produced from pyridoxal by pyridoxine kinase, was also down-regulated. On the other hand, in the presence of argentilactone, P. lutzii seems to use the amide group of xanthine, a purine base, in the synthesis of uric acid, which is excreted and metabolized into allantoic acid, allantoin and urea through an amphibian-like uricolytic pathway [29], as xanthine dehydrogenase, uricase and allantoinase were up-regulated. The production of putrescine and polyamines from ornithine is absent, as ornithine decarboxylase is down-regulated.
Effect of argentilactone on the metabolism of Paracoccidioides lutzii
Glycolysis is induced in P. lutzii in the presence of argentilactone, as class II aldolase and glyceraldehyde-3-phosphate dehydrogenase are up-regulated. It is noteworthy that several transporters, including sugar and amino acid transporters, were down-regulated. Although alcoholic fermentation is up-regulated in P. lutzii yeast cells [30], the presence of argentilactone repressed it because two alcohol dehydrogenases are down-regulated. The ethanol dosage assay confirmed these data, as ethanol is reduced in the presence of argentilactone (Fig 2).
Fig 2. Ethanol dosage in Paracoccidioides lutzii under the action of argentilactone.
A total of 106 cells were used for each sample, and the ethanol levels in the presence and absence of argentilactone were quantified using enzymatic detection. The data are expressed as the mean ± standard deviation of biological triplicates of independent experiments. Student's t-test was used. *, significantly different from the carbon condition at a p-value of ≤ 0.05.
Homogentisate 1,2-dioxygenase catalyzes the conversion of homogentisate to 4-maleylacetoacetate. Hydroxymethylglutaryl-CoA lyase, which converts β-hydroxy-β-methylglutaryl-CoA to acetoacetate and acetyl-CoA, and acetyl-CoA hydrolase, which converts acetyl-CoA to acetate, were up-regulated. P. lutzii utilizes β-oxidation to obtain energy, as acyl-CoA dehydrogenase, which converts fatty acyl-CoA into trans-2-enoyl-CoA, four enoyl-CoA hydratases, which convert trans-2-enoyl-CoA to L-β-hydroxy-acyl-CoA, and 3-ketoacyl-CoA thiolase B, which converts β-ketoacyl-CoA to acyl-CoA-fatty acid and acetyl-CoA, were up-regulated. The methylcitrate cycle, one of the major pathways for propionyl-CoA metabolism, is an alternative source of carbon through pyruvate production [31]. Here, 2-methylcitrate dehydratase, which participates in the methylcitrate pathway, was up-regulated.
Argentilactone reduces carbohydrate polymer levels in the Paracoccidioides lutzii cell wall
The P. lutzii cell wall seems to be affected by argentilactone, as several transcripts associated with the synthesis of chitin and glucan, the major components of the fungal cell wall, including that of Paracoccidioides [32,33], were down-regulated. Among the enzymes are endo-1,3(4)-β-glucanase, two glucan 1,3-β-glucosidases, β-glucan synthesis-associated protein and glucanase, which are related to the biosynthesis of glucan, and endochitinase, chitin deacetylase, UDP-N-acetylglucosamine pyrophosphorylase, UDP-N-acetylglucosamine transporter YEA4, chitin synthase B, chitin synthase regulator 3 and chitin synthase export chaperone, which are related to the biosynthesis of chitin.
To investigate the chitin and glucan levels in the cell wall, the cells were stained with CFW and CR and visualized by fluorescence microscopy. The fluorochrome CR and CFW interact with polysaccharides of cell wall [34] exhibiting strong affinity for chains of chitin [35,36]. P. lutzii yeast cells after treatment with argentilactone showed a decrease in fluorescence, indicating a reduction in the levels of this polymer (Fig 3), corroborating the transcriptional data.
Fig 3. Effect of argentilactone on the polymer levels of the Paracoccidioides lutzii cell wall.
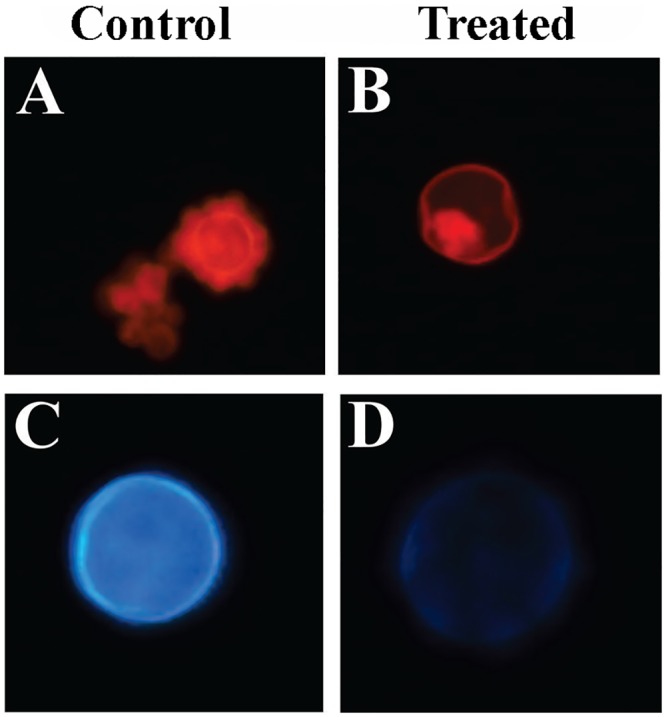
Fluorescence microscopy showing yeast cells stained by CFW and CR: (A) P. lutzii control stained with CR; (B) P. lutzii after treatment with argentilactone, stained with CR; (C) P. lutzii control stained with CFW; (D) P. lutzii after treatment argentilactone, stained with CFW.
Hypersensitivity of the Pbsod-aRNA, Pbhsp90-aRNA, Pbccp-aRNA and Pbrbt5-aRNA mutants to argentilactone
Several genes responding to stress were up-regulated. Among these genes are sod, rbt5, ccp, hsp90, heat shock protein 10 (hsp10) and heat shock protein SSC1 (hspssc1). The increased expression levels of hsp90, sod, rbt5 and ccp were confirmed by qRT-PCR in P. lutzii (Fig 4) and P. brasiliensis (S2 Fig), corroborating the transcriptional data.
Fig 4. Effect of argentilactone on transcript expression levels in Paracoccidioides lutzii yeast cells.
The expression levels of hsp90, ccp, sod and rbt5 genes in Paracoccidoides spp. yeast cells grown in MMcM liquid medium with or without argentilactone were analyzed. The data were normalized using the constitutive gene encoding α-tubulin as the endogenous control and are presented as relative expression in comparison to the experimental control cells, whose value was set to 1. Data are expressed as the mean ± standard deviation of the triplicates of independent experiments. *, significantly different from the control at a p-value of ˂ 0.05.
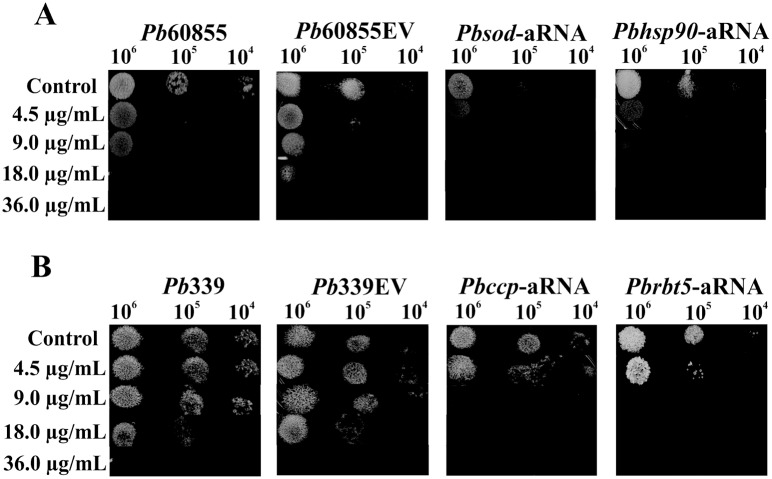
The growth of hsp90, sod, ccp and rbt5 silenced mutants was evaluated in the presence of argentilactone (Fig 5). Due the difficulty in obtaining mutants in P. lutzii, the mutants used here were obtained to P. brasiliensis from studies performed previously (14,24,25,26). Pb339, Pb60855, Pb339EV, Pb60855EV, Pbsod-aRNA, Pbhsp90-aRNA, Pbccp-aRNA and Pbrbt5-aRNA were grown for 6 days in the presence of 4.5, 9, 18 and 36 μg/mL argentilactone. All mutants presented greater sensitivity to argentilactone compared to wild-type cells and cells containing the empty vector. Argentilactone inhibits Pb60855 and Pb60855EV cells in the presence of 18 μg/mL of the compound, as a dose of 36 μg/mL was necessary to affect the cell growth of Pb339 and Pb339EV. For the silenced mutants Pbsod-aRNA, Pbhsp90-aRNA, Pbccp-aRNA and Pbrbt5-aRNA, 9 μg/mL argentilactone was sufficient to inhibit the growth of the Pbhsp90-aRNA-silenced mutant. These results suggest that overexpression of the hsp90, sod, ccp and rbt5 transcripts are important for the response of Paracoccidioides spp. to argentilactone and that the silencing of these genes directly influences the growth of the fungus in the presence of argentilactone.
Fig 5. Susceptibility of Paracoccidioides brasiliensis yeast cells exposed to argentilactone.
Samples containing 106, 105 and 104 Pbsod-aRNA, Pbhsp90-aRNA (A), Pbccp-aRNA and Pbrbt5-aRNA (B) yeast cells were spotted in solid Fava Netto’s supplemented with argentilactone at the concentrations of 4.5, 9, 18 and 36 μg/mL. Control cells, wild type (WT) and empty vector (EV) were assayed without argentilactone. The plates were incubated for 7 days at 36°C before photo documentation.
Argentilactone promotes the induction of oxidative stress and dysfunction of the mitochondrial membrane potential
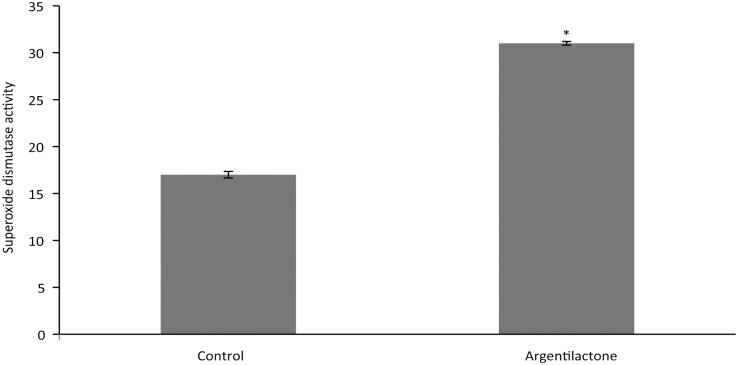
Due to the induction of genes related to oxidative stress, SOD enzymatic activity was investigated. The enzymatic activity was measured after growing P. lutzii for 6 h in the presence or absence of argentilactone. A significant increase of enzymatic activity was observed in the presence of argentilactone (Fig 6).
Fig 6. Superoxide dismutase activity.
Yeast cells were grown in the presence of argentilactone for 6 h, and total proteins were extracted and used to measure superoxide dismutase activity. Student’s t test was used for statistical comparisons, and the observed differences were statistically significant (p ˂ 0.05). The error bars represent the standard deviation of three biological replicates.
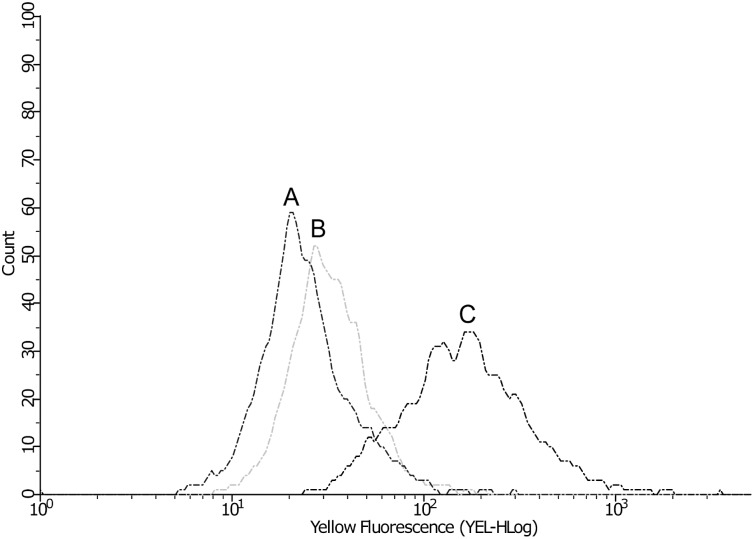
The ability of argentilactone to cause damage to the mitochondrial membrane potential was compared to antimycin A, a potent inhibitor of the electron transport chain. The mitochondrial membrane was monitored using rhodamine 123, which decreases its fluorescence when there is depolarization of the mitochondrial membrane potential. Flow cytometry data showed that argentilactone exerted a similar effect on cells as antimycin A. In the presence of argentilactone, the intracellular fluorescence due to rhodamine 123 was decreased (Fig 7).
Fig 7. Effect of argentilactone on the mitochondrial membrane potential of Paracoccidioides lutzii.
The mitochondrial membrane potential was determined by flow cytometry analysis of yeast cells treated with antimycin A (A) or argentilactone (B) for 6 h and stained with rhodamine 123. Control cells without treatment (C) were used in the test.
Discussion
The objective of this study was to analyze the transcriptional profile of P. lutzii in the presence of argentilactone. The regulation of transcripts and proteins from metabolic pathways by antifungals has been described for several microorganisms, including the antifungal itraconazole, which acts in a similar pathway as ergosterol [37,38,39]. The concentration of argentilactone utilized in the experiments affected global gene expression in P. lutzii. Here, argentilactone affected the metabolism of P. lutzii, as alcoholic fermentation was down-regulated, and glycolysis, β-oxidation and the methylcitrate cycle were up-regulated. In addition, argentilactone interfered with urea synthesis and excretion in P. lutzii. In treatment with argentilactone, the fungus seems to use the amphibian-like uricolytic pathway to excrete and metabolize amide groups. The utilization of different pathways to reach the destination of the amide groups has been reported in organisms. In Aedes aegypti, urea synthesis and excretion are regulated by a unique cross-talk mechanism, which is one of the biochemical mechanisms responsible for the success of the infection [40].
Chitin synthases are enzymes that play an important role in cellular integrity, growth and virulence of human fungal pathogens, including Candida albicans and Cryptococcus neoformans [41,42]. It has been observed that high ergosterol levels can inhibit chitin synthases, whereas C. albicans mutants with low ergosterol content showed increased levels of chitin synthesis [43]. Here, an ergosterol biosynthesis protein and sterol desaturase transcripts were up-regulated, and several transcripts related to the synthesis of chitin and glucan were down-regulated. In addition, the polymer levels of the cell wall of P. lutzii were decreased when treated with argentilactone. Studies have demonstrated the importance of genes related to cell wall biosynthesis in Paracoccidioides spp., including chitin synthase genes [9, 44–48].
We assumed that central carbon metabolism is inhibited by argentilactone, as we found a profile similar to the effects of pyrazole (an alcohol dehydrogenase inhibitor), which reduced the ethanol yield in Saccharomyces cerevisiae [49].
hsp90, a molecular chaperone that plays an important role in the assembly and regulation of several signaling systems in eukaryotes [50], is a highly conserved protein and represents about 2% of all cellular proteins in cells [51]. Furthermore, hsp90 is required for viability under stress conditions in eukaryotes [52] and plays an important role in fungi, including the physiology of P. lutzii [23], indicating its utility as a potential antifungal target [53,54]. In P. lutzii, the heat shock response is associated with pathogenesis because the change in temperature is responsible for dimorphic transition, which is essential to establish infection. hsp90 regulates the proliferation and adaptation of P. lutzii to different environmental conditions [23,55]. Here, transcriptional and qRT-PCR date showed that hsp90 was up-regulated in the presence of argentilactone. The plate sensitivity test using the mutant P. brasiliensis Pbhsp90-aRNA showed that hsp90 was recruited by P. brasiliensis growth in the presence of argentilactone.
Many organisms present a ROS defense system generated by aerobic respiration and substrate oxidation [56]. SOD is required for P. lutzii growth in the presence of argentilactone, as its activity was increased in this condition. Increased levels of SOD activity may represent a primary antioxidant defense against ROS in P. lutzii [57]. In addition, the P. brasiliensis mutant Pbsod-aRNA exhibited decreased growth in the presence of this compound, as found in C. neoformans [58]. Argentilactone seems to stimulate oxidative stress in Paracoccidioides spp. Therefore, the fungus seems to induce antioxidant enzymes, such as SOD, which act to reduce ROS, aiming to prevent cell damage. The induction of hsp90 and sod suggests that the primary heat shock stress could induce subsequent oxidative stress due to oxygen availability. Similar data have been previously described in S. cerevisiae [59,60]. Argentilactone seems to be acting on one of the main sources of ROS, the electron transport chain, as several transcripts related to electron transport, such as NADPH-adrenodoxin oxidoreductase, NADH-cytochrome b5 reductase and the indirectly related ccp, were up-regulated.
Peroxidases are enzymes that use various electron donors to reduce H2O2 to H2O. In C. neoformans, the growth of a ccp mutant was affected by the presence of H2O2 [58]. CCP catalyzes the reduction of hydrogen peroxide using cytochrome c as an electron donor. In S. cerevisiae, the increased expression of ccp seems to be caused by an increase of ROS produced during aerobic growth, thus confirming the biological role of this enzyme in cellular detoxification and the elimination of hydrogen peroxide [60]. We suggest here that the cellular oxidative stress caused by argentilactone results in an overexpression of sod, which is responsible for catalyzing the dismutation of superoxide into H2O2. The presence of H2O2 would induce the increased expression of ccp in an attempt to reduce hydrogen peroxide. Here, the increase in the ccp transcript level demonstrated in the transcriptional and qRT-PCR data and the decreased growth of Paracoccidioides Pbrbt5-aRNA in the presence of argentilactone show that ccp is required for the fungus in this condition.
ROS are universal products of aerobic metabolism, which can also be produced under stressful conditions. In eukaryotic cells, mitochondria are the main source of ROS [61]. Changes in the proton gradient resulting from the inhibition of electron transport can result in the production of reactive oxygen species (ROS) [61,62]. Thus, we assumed that argentilactone could have been acting on mitochondria, and our results confirmed this hypothesis.
In Coccidioides immitis [63] and S. cerevisiae [64], RBT5 is an antigenic protein anchored to the cell wall. rbt5 is important for the morphogenesis of the cell wall of C. albicans [65] and Coccidioides during infection [66]. In P. lutzii, rbt5 is a major surface antigen [67]. Here, the increase in the rbt5 transcript level exhibited in the transcriptional and qRT-PCR data and the decrease of Pbrbt5-aRNA growth in the presence of argentilactone suggest that rbt5 is required for the fungus in this condition. rbt5 could be increased in the presence of argentilactone in an attempt to maintain the morphogenesis of the cell wall, as several transcripts related to the biosynthesis of the cell wall, mainly to chitin biosynthesis, were down-regulated. Fluorescence microscopy data showing that the level of chitin and glucan polymers in the cell wall of P. lutzii yeast cells was lower in the presence than in the absence of argentilactone corroborate these results. Genes important to the maintenance and integrity of the cell wall were described to be down-regulated in fungi exposed to amphotericin B [68,69] or to investigatory antifungals, such as artemisinin [70] or oenothein B, an anti-Paracoccidioides [28]. In Aspergillus niger and S. cerevisiae, the transcription factor RlmA is required for the up-regulation of cell wall stress-induced genes [71] leading to increased chitin content. Here, RlmA is down-regulated, corroborating the down-regulation of genes related to chitin synthesis.
The considerable number of transcription factors regulated in the presence of argentilactone suggests a complex regulatory mechanism. Conversely, the high percentage of unclassified proteins indicates that additional studies must be performed to elucidate the mode of action of argentilactone on Paracoccidioides spp.
One of the limitations of the study was that all experiments were not performed on P. lutzii and P. brasiliensis due to the difficulty in obtaining mutants of P. lutzii. This analysis should be attempted in the future.
Conclusion
Argentilactone seems to be able to penetrate into Paracoccidioides spp. yeast cells and modulate cellular targets. Argentilactone seems to induce oxidative stress and interfere with the biosynthesis of the Paracoccidioides spp. cell wall. Given the overall stress caused by argentilactone in Paracoccidioides spp., other studies must be performed to better elucidate the mode of action of argentilactone in Paracoccidioides spp.
Supporting Information
Mapped reads were analyzed using the DEGseq package and plotting graphs were obtained. The transcripts are represented by dots. (A) Scatter plot shows the number of reads (log2) counts for each transcript P-Al (Presence of Argentilactone) and A-Al (Absence of Argentilactone) conditions. (B) MA-plot of P-Al versus A-Al conditions shows the intensity of the expression of identified transcripts (log2 of fold change) in the y axis [M] and the read counts (log2) for each transcript in the x axis [A]. In addition, the graph shows the number of differentially expressed transcripts obtained from FET (Fisher’s Exact Test) using a p-value of 0.001, as indicated in red.
(TIF)
The expression levels of hsp90, ccp, sod and rbt5 genes in Paracoccidoides brasiliensis. yeast cells grown in MMcM liquid medium with or without argentilactone were analyzed. The data were normalized using the constitutive gene encoding α-tubulin as the endogenous control and are presented as relative expression in comparison to the experimental control cells, whose value was set to 1. Data are expressed as the mean ± standard deviation of the triplicates of independent experiments. *, significantly different from the control at a p-value of ˂ 0.05.
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work performed at Universidade Federal de Goiás was supported by MCTI/CNPq (Ministério da Ciência e Tecnologia/Conselho Nacional de Desenvolvimento Científico e Tecnológico), FNDCT (Fundo Nacional de Desenvolvimento Científico e Tecnológico), FAPEG (Fundação de Amparo à Pesquisa do Estado de Goiás), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), FINEP (Financiadora de Estudos e Projetos), and INCT-IF (Instituto Nacional de Ciência e Tecnologia para Inovação Farmacêutica). Additionally, FSA and BRSN were supported by fellowship from CAPES. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brummer E, Castaneda E, Restrepo A. Paracoccidioidomycosis: an update. Clin Microbiol Rev. 1993; 6: 89–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marques SA. Paracoccidioidomycosis: epidemiological, clinical, diagnostic and treatment up-dating. An Bras Dermatol. 2013; 88: 700–711. 10.1590/abd1806-4841.20132463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prado M, Silva MB, Laurenti R, Travassos LR, Taborda CP. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: a review from 1996 to 2006. Mem Inst Oswaldo Cruz. 2009; 104: 513–521. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller M, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007; 20: 133–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhai B, Lin X. Recent progress on antifungal drug development. Current Pharm Biotechnol. 2011; 12: 1255–62. [DOI] [PubMed] [Google Scholar]
- 6.Bocca AL, Amaral AC, Teixeira MM, Sato PK, Shikanai-Yasuda MA, et al. Paracoccidioidomycosis: eco-epidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol 2013; 8: 1177–1191. 10.2217/fmb.13.68 [DOI] [PubMed] [Google Scholar]
- 7.Rittner GMG, Muñoz JE, Marques AF, Nosanchuk JD, Taborda CP, et al. Therapeutic DNA vaccine encoding peptide P10 against experimental paracoccidioidomycosis. PLoS Negl Trop Dis. 2012; 6: e1519 10.1371/journal.pntd.0001519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomazett PK, Castro NS, Lenzi HL, Soares CMA, Pereira M. Response of Paracoccidioides brasiliensis Pb01 to stressor agents and cell wall osmoregulators. Fung Biol. 2011;115: 62–69. [DOI] [PubMed] [Google Scholar]
- 9.Tomazett PK, Félix CR, Lenzi LH, Faria FP, Soares CMA, et al. 1,3-β-D-Glucan synthase of Paracoccidioides brasiliensis: recombinant protein, expression and cytolocalization in the yeast and mycelium phases. Fung Biol. 2010; 114: 809–816. [DOI] [PubMed] [Google Scholar]
- 10.Neto BRS, Silva JF, Mendes-Giannini MJ, Lenzi HL, Soares CMA, et al. The malate synthase of Paracoccidioides brasiliensis is a linked surface protein that behaves as an anchorless adhesion. BMC Microbiol. 2009; 24: 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zambuzzi-Carvalho PF, Cruz AH, Santos-Silva LK, Goes AM, Soares CMA, et al. The malate synthase of Paracoccidioides brasiliensis Pb01 is required in the glyoxylate cycle and in the allantoin degradation pathway. Med Mycol. 2009; 47: 734–744. 10.3109/13693780802609620 [DOI] [PubMed] [Google Scholar]
- 12.Cruz AH, Brock M, Zambuzzi-Carvalho PF, Santos-Silva LK, Troian RF, et al. ) Phosphorylation is the major mechanism regulating isocitrate lyase activity in Paracoccidioides brasiliensis yeast cells. FEBS J. 2011; 278: 2318–2332. 10.1111/j.1742-4658.2011.08150.x [DOI] [PubMed] [Google Scholar]
- 13.Pereira M, Song Z, Santos-Silva LK, Richards MH, Nguyen TT, et al. Cloning, mechanistic and functional analysis of a fungal sterol C24-methyltransferase implicated in brassicasterol biosynthesis. Biochim Biophys Acta. 2010; 1801:1163–1174. 10.1016/j.bbalip.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 14.do Carmo Silva L, Tamayo Ossa DP, Castro SV, Bringel Pires L, Alves de Oliveira CM, Conceição da Silva C, Coelho NP, Bailão AM, Parente- Rocha JA, Soares CM, Ruiz OH, Ochoa JG, Pereira M. Transcriptome Profile of the Response of Paracoccidoides spp. to a Camphene Thiosemicarbazide Derivative. PLos One. 2015; 26:10(6):e0130703 10.1371/journal.pone.0130703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zambuzzi-Carvalho PF, Tomazett PK, Santos SC, Ferri PH, Borges CL, Martins WS, de Almeida Soares CM, Pereira M. Transcriptional profile of Paracoccidioides induced by oenothein B, a potential antifungal agente from the Brazilian Cerrado plant Eugenia uniflora. BMC Microbiol. 2013, 13: 227 10.1186/1471-2180-13-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos GD, Ferri PH, Santos SC, Bao SN, Soares CM, Pereira M. Oenothein B inhibits the expression of PbFKS1 transcript and induces morphological changes in Paracoccidoides brasiliensis. Med Mycol. 2001; 45: 609–618. [DOI] [PubMed] [Google Scholar]
- 17.McNeil M, Facey P, Porter R. Essential oils from the Hyptis genus—a review (1909–2009). Nat Prod Commun. 2011; 6: 1775–96. [PubMed] [Google Scholar]
- 18.Oliveira CMA, Silva MRR, Kato L, Silva CC, Ferreira HD, et al. ) Chemical composition and antifungal activity of the essential oil of Hyptis ovalifolia Benth. (Lamiaceae). J Braz Chem Soc. 2004; 5:756–759. [Google Scholar]
- 19.Prado RS, Alves RJ, Oliveira CM, Kato L, Silva RA, et al. (2014) Inhibition of Paracoccidioides lutzii Pb01 Isocitrate Lyase by the Natural Compound Argentilactone and Its Semi-Synthetic Derivatives. PLoS One. 2014; 9: e94832 10.1371/journal.pone.0094832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fava-Netto C. Estudos quantitativos sobre a fixação de complemento na blastomicose sul-americana, com antigeno polissacarídico. Arq Cir Clin Exp. 1955; 18: 197–254. [PubMed] [Google Scholar]
- 21.Restrepo A, Jimenez BE. Growth of Paracoccidioides brasiliensis yeast phase in a chemically defined culture medium. J Clin Microbiol. 1980; 12: 279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001; Appendix 3:Appendix 3B. 10.1002/0471142735.ima03bs21 [DOI] [PubMed] [Google Scholar]
- 23.Lima Pde S, Casaletti L, Bailão AM, de Vasconcelos AT, Fernandes Gda R, et al. Transcriptional and proteomic responses to carbon starvation in Paracoccidioides. PLoS Negl Trop Dis. 2014; 8:e2855 10.1371/journal.pntd.0002855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamayo D, Muñoz JF, Torres I, Almeida AJ, Restrepo A, et al. Involvement of the 90 kDa heat shock protein during adaptation of Paracoccidioides brasiliensis to different environmental conditions. Fungal Genet Biol. 2013; 51: 34–41. 10.1016/j.fgb.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 25.Parente AF, Naves PE, Pigosso LL, Casaletti L, McEwen JG, Parente-Rocha JA, Soares CM. The response of Paracoccidioides spp. to nitrosative stress. Microbes Infect. 2015;17:575–585. 10.1016/j.micinf.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 26.Bailão EF, Parente JA, Pigosso LL, Castro KP, Fonseca FL, et al. Hemoglobin Uptake by Paracoccidioides spp. Is Receptor-Mediated. PLoS Negl Trop Dis. 2015; 8: e2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford MMA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 7: 248–254. [DOI] [PubMed] [Google Scholar]
- 28.Zambuzzi-Carvalho PF, Tomazett PK, Santos SC, Ferri PH, Borges CL, et al. Transcriptional profile of Paracoccidioides induced by oenothein B, a potential antifungal agent from the Brazilian Cerrado plant Eugenia uniflora. BMC Microbiol. 2013; 13: 227 10.1186/1471-2180-13-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scaraffia PY, Tan G, Isoe J, Wysocki VH, Wells MA, et al. Discovery of an alternate metabolic pathway for urea synthesis in adult Aedes aegypti mosquitoes. Proc Natl Acad Sci USA. 2008; 105: 518–23. 10.1073/pnas.0708098105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felipe MS, Andrade RV, Arraes FB, Nicola AM, Maranhão AQ, et al. Transcriptional Profiles of the Human Pathogenic Fungus Paracoccidioides brasiliensis in Mycelium and Yeast Cells. J Biol Chem. 2005; 280: 24706–24714. [DOI] [PubMed] [Google Scholar]
- 31.Brämer CO, Silva LF, Gomez JG, Priefert H, Steinbüchel A. Identification of the 2-methylcitrate pathway involved in the catabolism of propionate in the polyhydroxyalkanoate-producing strain Burkholderia sacchari IPT101(T) and analysis of a mutant accumulating a copolyester with higher 3-hydroxyvalerate content. Appl Environ Microbiol. 2002; 68: 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carbonell LM, Kanetsuna F, Gil F. Chemical morphology of glucan and chitin in the cell wall of the yeast phase of Paracoccidioides brasiliensis. J Bacteriol. 1970; 101: 636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanetsuna F, Carbonell LM, Azuma I, Yamamura Y. Biochemical studies on the thermal dimorphism of Paracoccidioides brasiliensis. J Bacteriol. 1972; 110: 208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood PJ. Specificty in the interaction of direct dyes with polysaccharides. Carbohydr Res. 1980; 81:271–287. [Google Scholar]
- 35.Roncero C, Durán A. Effect of Calcofluor white and Congo red on fungal cell wall morphogenesis: in vivo activation of chitin polymerization. J Bacteriol. 1985; 163: 1180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herth W (1980) Calcofluor white and Congo red inhibit chitin microfibril assembly of Poterioochromonas: evidence for a gap between polymerization and microfibril formation. J Cell Biol. 1980; 87: 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgiadou SP, Kontoyiannis DP. The impact of azole resistance on aspergillosis guidelines. Ann NY Acad Sci. 2012; 12721: 5–22 [DOI] [PubMed] [Google Scholar]
- 38.Hoehamer CF, Cummings ED, Hilliard GM, Rogers PD.Changes in the proteome of Candida albicans in response to azole, polyene, and echinocandin antifungal agents. Antimicrob Agents Chemother. 2010; 54: 1655–1664. 10.1128/AAC.00756-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neto BRS, Zambuzzi-Carvalho PF, Bailão AM, Martins WS, Soares CMA, et al. Transcriptional profile of Paracoccidioides spp. in response to itraconazole. BMC Genom. 2014; 15: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isoe J, Scaraffia PY. Urea Synthesis and Excretion in Aedes aegypti Mosquitoes Are Regulated by a Unique Cross-Talk Mechanism. PLoS. 2013; 8: e65393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munro CA, Winter K, Buchan A, Henry K, Becker JM, et al. Chs1 of Candida albicans is an essential chitin synthase required for synthesis of the septum and for cell integrity. Mol Microbiol. 2001; 39: 1414–1426. [DOI] [PubMed] [Google Scholar]
- 42.Banks IR, Specht CA, Donlin MJ, Gerik KJ, Levitz SM, et al. A chitin synthase and its regulator protein are critical for Chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot Cell. 2005; 4: 1902–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanden Bossche H. Biochemical targets for antifungal azole derivatives hypothesis on the mode of action. Curr Top Med Mycol. 1985; 1: 313–351. [DOI] [PubMed] [Google Scholar]
- 44.Villalobos-Duno H, San-Blas G, Paulinkevicius M, Sánchez-Martín Y, Nino-Vega G. Biochemical characterization of Paracoccidioides brasiliensis α-1,3-glucanase Agn1p, and its functionality by heterologous Expression in Schizosaccharomyces pombe. PLoS One.2013; 8: e66853 10.1371/journal.pone.0066853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camacho E, Sepulveda VE, Goldman WE, San-Blas G, Niño-Vega GA. Expression of Paracoccidioides brasiliensis AMY1 in a Histoplasma capsulatum amy1 mutant, relates an α-(1,4)-amylase to cell wall α-(1,3)-glucan synthesis. PLoS One. 2012; 7: e50201 10.1371/journal.pone.0050201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barreto L, Sorais F, Salazar V, San-Blas G, Niño-Vega GA. Expression of Paracoccidioides brasiliensis CHS3 in a Saccharomyces cerevisiae chs3 null mutant demonstrates its functionality as a chitin synthase gene. Yeast. 2010; 27: 293–300. 10.1002/yea.1748 [DOI] [PubMed] [Google Scholar]
- 47.Niño-Vega GA, Sorais F, San-Blas G. Transcription levels of CHS5 and CHS4 genes in Paracoccidioides brasiliensis mycelial phase, respond to alterations in external osmolarity, oxidative stress and glucose concentration. Mycol Res. 2009; 113: 1091–1096. 10.1016/j.mycres.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 48.San-Blas G, Niño-Vega G. Paracoccidioides brasiliensis: chemical and molecular tools for research on cell walls, antifungals, diagnosis, taxonomy. Mycopathol. 2008; 165: 183–195. [DOI] [PubMed] [Google Scholar]
- 49.Matsuda F, Shirai T, Ishii J, Kondo A. Regulation of central carbon metabolism in Saccharomyces cerevisiae by metabolic inhibitors. J Biosci Bioeng. 2013; 16: 59–64. [DOI] [PubMed] [Google Scholar]
- 50.Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, et al. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006; 440: 1013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Csermely P, Schnaider T, Sóti C, Prohászka Z, Narda G. The 90-kDa Molecular Chaperone Family: Structure, Function, and Clinical Applications. A Comprehensive Rev Pharm Ther. 1998; 79: 129–168. [DOI] [PubMed] [Google Scholar]
- 52.Thomas JG, Baneyx F. Roles of the Escherichia coli small heat shock proteins IbpA and IbpB in thermal stress management: comparison with ClpA, ClpB, and HtpG In vivo. J Bacteriol. 1998; 180: 5165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ki SW, Kasahara K, Kwon HJ, Ishigami K, Kitahara T, et al. Radicicol binding to Swo1/Hsp90 and inhibition of growth of specific temperature-sensitive cell cycle mutants of fission yeast. Biosci Biotechnol Biochem. 2001; 65: 2528–2534. [DOI] [PubMed] [Google Scholar]
- 54.Sreedhar AS, Mihály K, Pató B, Schnaider T, Steták A, et al. Hsp90 inhibition accelerates cell lysis. Anti-Hsp90 ribozyme reveals a complex mechanism of Hsp90 inhibitors involving both superoxide- and Hsp90-dependent events. J Biol Chem. 2003; 278: 35231–35240. [DOI] [PubMed] [Google Scholar]
- 55.Nicola AM, Andrade RV, Dantas AS, Andrade P, Arraes FBM, et al. The stress responsive and morphologically regulated hsp90 gene from Paracoccidioides brasiliensis is essential to cell viability. BMC Microbiol. 2008; 8: 158 10.1186/1471-2180-8-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15: 1583–1606. 10.1089/ars.2011.3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campos EG, Jesuino RSA, Dantas ADS, Brígido MDM, Felipe MSS. Oxidative stress response in Paracoccidioides brasiliensis. Genet and mol res. 2005;4: 409–429. [PubMed] [Google Scholar]
- 58.Giles SS, Batinic I, Perfect JR, Cox GM. Cryptococcus neoformans Mitochondrial Superoxide Dismutase : an Essential Link between Antioxidant Function and High-Temperature Growth. Eukaryot Cell. 2005; 4: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morano K, Grant CM, Moye-Rowley WS. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genet. 2012; 190: 1157–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon M, Chong S, Han S, Kim K. Oxidative stresses elevate the expression of cytochrome c peroxidase in Saccharomyces cerevisiae. Biochim Biophys Acta. 2003; 1623: 1–5. [DOI] [PubMed] [Google Scholar]
- 61.Suski JM, Lebiedzinska M, Bonora M, Pinton P, Duszynski J, Wieckowski MR. Relation between mitochondrial membrane potential and ROS formation. Meth Mol Biol. 2012; 810:183–205. [DOI] [PubMed] [Google Scholar]
- 62.Jezek P, Hlavatá L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organismo. J Biochem Cell Biol. 2005; 37: 2478–503. [DOI] [PubMed] [Google Scholar]
- 63.Zhu Y, Yang C, Magee DM, Cox RA. Coccidioides immitis antigen 2: analysis of gene and protein. Gen.1996; 181: 121–125. [DOI] [PubMed] [Google Scholar]
- 64.Moukadiri I, Armero J, Abad A, Sentandreu R, Zueco J. Identification of a mannoprotein present in the inner layer of the cell wall of Saccharomyces cerevisiae. J Bacteriol. 1997; 179: 2154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Braun BR, Head WS, Wang MX, Johnson AD. Identification and characterization of TUP1-regulated genes in Candida albicans. Genet. 2000; 156: 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johannesson H, Vidal P, Guarro J, Herr RA, Cole GT, et al. Positive directional selection in the proline-rich antigen (PRA) gene among the human pathogenic fungi Coccidioides immitis, C. posadasii and their closest relatives. Mol Biol Evol. 2004; 21: 1134–45. [DOI] [PubMed] [Google Scholar]
- 67.Castro NDS, Maia ZA, Pereira M, Soares CMA. Screening for glycosylphosphatidylinositol-anchored proteins in the Paracoccidioides brasiliensis transcriptome. Genet Mol Res. 2005; 4: 326–45. [PubMed] [Google Scholar]
- 68.Gautam P, Shankar J, Madan T, Sirdeshmukh R, Sundaram CS. Proteomic and transcriptomic analysis of Aspergillus fumigatus on exposure to amphotericin B. Antimicrob Agents Chemother. 2008; 52: 4220–4227. 10.1128/AAC.01431-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu L, Zhang W, Wang L, Yang J, Liu T, et al. Transcriptional profiles of the response to ketoconazole and amphotericin B in Trichophyton rubrum. Antimicrob Agents Chemother. 2007; 51: 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galal AM, Ross SA, Jacob M, ElSohly MA. Antifungal activity of artemisinin derivates. J Nat Prod. 2005; 68: 1274–1276. [DOI] [PubMed] [Google Scholar]
- 71.Damveld RA, Arentshorst M, Franken A, van Kuyk PA, Klis FM, et al. The Aspergillus niger MADS-box transcription factor RlmA is required for cell wall reinforcement in response to cell wall stress. Mol Microbiol. 2005; 58: 305–319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mapped reads were analyzed using the DEGseq package and plotting graphs were obtained. The transcripts are represented by dots. (A) Scatter plot shows the number of reads (log2) counts for each transcript P-Al (Presence of Argentilactone) and A-Al (Absence of Argentilactone) conditions. (B) MA-plot of P-Al versus A-Al conditions shows the intensity of the expression of identified transcripts (log2 of fold change) in the y axis [M] and the read counts (log2) for each transcript in the x axis [A]. In addition, the graph shows the number of differentially expressed transcripts obtained from FET (Fisher’s Exact Test) using a p-value of 0.001, as indicated in red.
(TIF)
The expression levels of hsp90, ccp, sod and rbt5 genes in Paracoccidoides brasiliensis. yeast cells grown in MMcM liquid medium with or without argentilactone were analyzed. The data were normalized using the constitutive gene encoding α-tubulin as the endogenous control and are presented as relative expression in comparison to the experimental control cells, whose value was set to 1. Data are expressed as the mean ± standard deviation of the triplicates of independent experiments. *, significantly different from the control at a p-value of ˂ 0.05.
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.