Abstract
Climate change will bring more drought periods that will have an impact on the irrigation practices of some crops like tomato, from standard water regime to deficit irrigation. This will promote changes in plant metabolism and alter their interactions with biotic stressors. We have tested if mild or moderate drought-stressed tomato plants (simulating deficit irrigation) have an effect on the biological traits of the invasive tomato red spider mite, Tetranychus evansi. Our data reveal that T evansi caused more leaf damage to drought-stressed tomato plants (≥1.5 fold for both drought scenarios). Mite performance was also enhanced, as revealed by significant increases of eggs laid (≥2 fold) at 4 days post infestation (dpi), and of mobile forms (≥2 fold and 1.5 fold for moderate and mild drought, respectively) at 10 dpi. The levels of several essential amino acids (histidine, isoleucine, leucine, tyrosine, valine) and free sugars in tomato leaves were significantly induced by drought in combination with mites. The non-essential amino acid proline was also strongly induced, stimulating mite feeding and egg laying when added to tomato leaf disks at levels equivalent to that estimated on drought-infested tomato plants at 10 dpi. Tomato plant defense proteins were also affected by drought and/or mite infestation, but T. evansi was capable of circumventing their potential adverse effects. Altogether, our data indicate that significant increases of available free sugars and essential amino acids, jointly with their phagostimulant effect, created a favorable environment for a better T. evansi performance on drought-stressed tomato leaves. Thus, drought-stressed tomato plants, even at mild levels, may be more prone to T evansi outbreaks in a climate change scenario, which might negatively affect tomato production on area-wide scales.
Introduction
Agricultural production faces the challenge to produce more food while constrained by a number of biotic and abiotic factors. Climate change is predicted to produce an increase in temperature and drought events in the next decades, especially in the mid-continental and Mediterranean climate areas where they are expected to be more frequent and intense [1]. Drought is by far the leading environmental stress in agriculture that limits the global productivity of major crops by directly reducing plant potential yield [2], but also by indirectly influencing their interactions with biotic factors, as a consequence, playing a critical role on the world´s food security.
Drought stress has been historically advocated as one key factor for herbivorous outbreaks [3, 4]. Yet, the relationship between arthropod outbreaks and drought is not consistent, depending on the timing, intensity and water stress phenology [5] and on the feeding guild that the herbivore belongs to [6]. It is widely accepted that drought stress triggers significant alterations in plant biochemistry and metabolism [7] that may alter the physiology of the host plant and modify the nutritional values, affecting herbivore performance [8]. There are several hypotheses concerning the response of the plant to drought stress and how herbivores adapt to those changes [5, 9, 10]. Drought induces metabolic changes in the plant, such as increased levels of free sugars and free essential amino acids, which according to the “Plant stress hypothesis” causes the plant to have a higher nutritional value for herbivores [6, 10, 11], and can play an important role in herbivore outbreaks [12, 13]. In contrast, drought is also associated with a reduction in growth and an increase in defense compounds making the plant less suitable for herbivores according to the “Plant Vigor Hypothesis” [9]. The resulting performance of phytophagous arthropods on drought-stressed plants will then depends on the access they have to an optimal balance of nutrients in the plant according to their feeding habit [5], and their adaptation to plant defense compounds according to their grade of specialization [14].
Climate change is expected to increase the incidence of water shortage in semi-arid environments. Then, deficit irrigation scheduling, yielding mild and moderate drought, might help to improve the efficiency with which water is used in major crops, such as tomato, widely cultivated in semi-arid regions. The tomato agro-ecosystem is threatened by a few major key pests, such as spider mites, and many minor or secondary pests [15]. The red tomato spider mite, Tetranychus evansi Baker & Pritchard was first recorded in Brazil, and has emerged as a serious invasive agricultural pest in invaded areas such as Africa and Europe [16]. In last decade, it has been considered one of the most important pests of solanaceous crops in Africa, causing high yield lossess in tomato in some African regions [17]. This species has been reported as highly tolerant to hot and dry conditions. As a result of climate change, the Mediterranean basin is the most threatened area for the potential spread of T. evansi [18]. In fact, outbreaks have been recorded in Europe, particularly around the Mediterranean basin where T. evansi has spread significantly in the last decades [18]. The high invasive potential of T. evansi and the severity of damage have prompted its addition to the alert list of the European and Mediterranean Plant Protection Organization [19].
When feeding on tomato leaves, T. evansi was found to suppress anti-mite plant defenses by down-regulating the expression of genes involved in the regulation of secondary metabolites and defense proteins, increasing the fitness of these mites in the absence of competitors [20, 21]. However, water deficit stress in tomato plants had been reported to increase some of these plant defenses, such as protease inhibitors and the oxidative enzymes polyphenol oxidases and peroxidases [6, 22, 23]. On the other hand, water deficit stress can elicit accumulations of nutrients in tomato plants. Among such nutrients, free amino acids, which are known to be instrumental in the maintenance of host plant osmotic balance, and free sugars are usually detected at elevated levels, and can therefore contribute to improve mite performance [24]. Moreover, free proline, a nonessential amino acid for arthropods that accumulates in most drought-stressed plants, has been reported to be a feeding stimulant for many phytophagous species [4]. However, there is little information on the combined effects of both biotic (mites) and abiotic (drought) stress on the tomato plant physiology and on how these changes influence T. evansi performance.
This study represents an attempt to establish if mild or moderate drought stress (simulating two scenarios of deficit irrigation) induces physiological changes (i.e. nutritional values and chemical defenses) in tomato plants, and if they have an effect on T. evansi behavior, development and physiology. This holistic approach provides insight into the bottom-up effects that may influence mite performance on drought-stressed tomato plants and the implications for outbreak risks of T. evansi, which might strongly affect tomato production on area-wide scales.
Materials and Methods
Plant material and mites rearing
A colony of T. evansi collected in Beausoleil (South of France) was provided by Dr. Maria Navajas (CBGP, France). Mites were maintained on detached tomato leaves (Solanum lycopersicum L., cv. Moneymaker) placed on ventilated plastic cages (22x30x15 cm) for about 30 generations. The petiole of the leaves was in contact with a thin layer of water in the bottom of the cages to avoid mites escaping and to maintain the leaf turgor. Tomato plants were grown from seeds in 40 wells trays. Plants with 3 expanded leaves were transferred to 2.5 liters pots (diameter: 16 cm, height: 15 cm) (Maceflor, Valencia, Spain) filled with 600 g of Universal growing medium `Compo sana´ (Compo GmbH, Münster, Germany) and watered to saturation. The mite colony and the tomato plants were maintained on a climate room at 25°C±1°C, 50±5% relative humidity and a 16 h light/8 h dark photoperiod.
Drought stress regime and experimental design
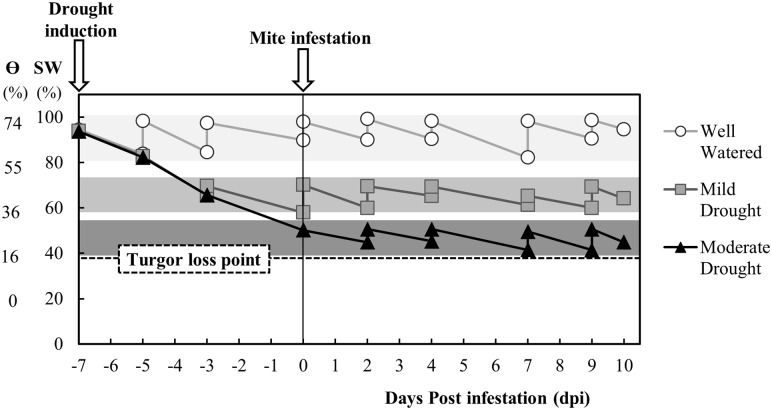
Water stress status of single tomato plants was quantified gravimetrically by frequent recording of pot weights (balance BSH 6000, PCE Iberica, Tobarra, Spain), and transpired water was replaced by intermittently watering them such that soil water content was maintained within a narrow range (Fig 1). Measurements of soil water status was determined by using two parameters: percentage of saturation weight [SW = (weight of 100% water saturated soil−soil weight)/weight of 100% water saturated soil]; and the volumetric soil water content [Ɵ = (soil weight−soil dry weight)/(H2O density*soil volume); soil dry weight was determined by drying 600 g of soil on stove at 70°C for 3 days]. Tomato plants with 4–5 expanded leaves were watered every 2–3 days to maintain the SW in the range of 80–95% (corresponding to Ɵ between 55–74%) for the control plants. We imposed two water stress regimes, defined as mild and moderate, by stopping irrigation for 4 or 7 days, respectively, and thereafter watering every 2–3 days to maintain the SW in the range of 60–73% (Ɵ = 36–50%) for mild stress and between 45–53% (Ɵ = 16–36%) for the moderate stress (Fig 1). Both water stress regimes were over the wilting point associated with severe drought stress [25] that was established at 40% of SW for Moneymaker in our experimental conditions.
Fig 1. Water stress status (well watered, mild and moderate drought) of tomato plant.
Drought induction was started 7 days before mite infestation and was continued until the end of the experiment [10 days post infestation (dpi)]. It is expressed as percentages of saturation weight (SW) and volumetric soil water content (Ɵ). The turgor loss point was established as 40% of SW.
Mild and moderate stressed plants were coupled with well-watered (control) plants in two independent experiments. In each experiment, plants were divided into eight different groups combining two different treatments: Drought stress (control or drought) and T. evansi infestation (infested or no infested); and two sampling times (4 or 10 days post infestation = dpi). Spider mites performance and leaf damage was assessed in both mild and moderate drought stress experiments. Chemical and biochemical analysis of plant material was only performed for moderate drought stress (the utmost applied).
Tomato plants (6–7 expanded leaves) were infested when steady stress conditions were reached (Fig 1) by placing 8 spider mites females on each of the two leaflets next to the apical one of the leaves 3, 4 and 5 (48 mites per plant). Mites were collected from the laboratory colony using a vacuum pump D-95 (Dinko S.A., Barcelona, Spain) with a sucking power of 10–50 mmHg connected to a modified Eppendorf. All plants were confined with a ventilated metacrylate cylinder fitting the pot diameter and set up in a climate chamber in a complete randomized block design. Temperature and humidity inside the cylinders was recorded introducing USB dataloggers Log 32 (Dostmann electronic GmbH, Wertheim, Germany), on average the relative humidity was 76±2% inside the well-watered cylinders, and 73±2%, and 56±2% for mild and moderate drought conditions, respectively. The average temperature was 24±1°C in all cases.
The stress intensity of all plants at infestation and at each sampling time (4 or 10 dpi) was assessed by measuring: a) stomatal conductance (gs) and b) variations in maximum quantum yield of photosystem II photochemistry (Fv/Fm), as they are reported to be good drought indicators [2, 26]. Between sampling times, the stress status was not recorded to avoid mite disturbance. Stomatal conductance was measured using a leaf porometer (SC-1 Decagon-T, Pullman, USA) and the Fv/Fm was estimated using a FluorPen FP 100 (PSI, Drasov, Czech Republic). Both parameters were measured on the 4th leaf, as preliminary experiments showed that it is representative of the plant status. Plant growth was estimated by measuring the stem length (distance between the soil and the terminal bud) and by weighting the aerial part of the plant (transformed to dry weight by using the water content data calculated as referred to below).
Performance of T. evansi and leaf damage
The performance of T. evansi was determined by estimating mite population growth at 4 and 10 dpi. At each time, 15 infested plants per treatment were analyzed in the moderate stress experiment, and 9 infested plants per treatment in the mild stress one. All leaves were detached from the plant, and the number of eggs and mobile mite stages (larvae, nymphs and adults) were counted under a stereomicroscope M125 (Leica Mycrosystem, Wetzlar, Germany). To determine the leaf damage area (mm2 of chlorotic lesions), damaged leaflets were scanned using hp scanjet (HP Scanjet 5590 Digital Flatbed Scanner series, USA). The scanned leaflets were analyzed by the program GIMP 2.8 (www.gimp.org), for which we created a new layer, and the damaged area was painted in black color, then the number of pixels in black was counted using the histogram-tool. Finally, the number of pixels was transformed to mm2 using a scanned ruler as reference.
Chemical and biochemical analysis of tomato plant material
Leaves from control and drought stress plants were collected and analyzed (6 plants/treatment). The collection was as follows: a) the left leaflets from leaves 3, 4 and 5 were collected together and frozen at -72°C; ground using a mortar and pestle in the presence of liquid nitrogen to a fine powder and stored for free amino acids, protein, protease inhibitors and oxidative enzymes analysis; and b) the right leaflets from the same leaves were collected together, dried in an oven at 70°C for 3 days, weighed before and after drying to assess the percentage of water and ground using a mortar and pestle to obtain a fine powder and stored for C, N and free sugar analysis. Unless otherwise specified, all chemical compounds used here and in the mite enzymatic activities assay were from Sigma-Aldricht (St Luis, USA).
Total C and N composition
Samples of 1 mg of dried leaf powder were analyzed to determine total nitrogen and carbon concentration at the Elemental Microelement Center of Complutense University (Madrid, Spain) by using a microelement analyzer LECO CHNS-932 (LECO, St Joseph, MI, USA).
Free sugars
Samples of 3 mg of dried leaf powder were homogenized in 650 μl of ethanol 95% (v/v), heated at 80°C for 20 min, centrifuged at 10000 rpm for 10 min, and the supernatant collected. The process was repeated two more times and the three supernatants were pooled. A volume of 750 μl of the mixture was dried on a SpeedVac Concentrator Savant SVC-100H (ThermoFisher scientific, Willmington, DE, USA) and redissolved in 500 μl of water. Soluble carbohydrate concentration was estimated by the anthrone method [27] using glucose as standard. In brief, 1 ml of anthrone reagent (0.2% v/v anthrone on 95% sulfuric acid) was added to the extract, heated at 90°C for 15 min, and the absorbance at 630 nm was measured using a VERSAmax microplate reader (Molecular Devices Corp., Sunnyvale, USA).
Free amino acids
The extraction of the free amino acids was done as described by [28]. Samples of 50 mg of leaf frozen powder were homogenized with 600 μl of water:chloroform:methanol (3:5:12 v/v/v). After centrifugation at 12000 rpm for 2 min, the supernatant was collected and the residue was re-extracted with 600 μl of the same mixture, pooling the two supernatants. A mixture of 300 μl of chloroform and 450 μl of water were added to the supernatants, and after centrifugation the upper water:methanol phase was collected and dried in a SpeedVac. The samples were dissolved on 100 μl of sodium citrate loading buffer pH 2.2 (Biochrom, USA) and 10 μl were injected on a Biochrom 30 Amino Acid Analyser (Biochrom, USA) at the Protein Chemistry Service at CIB (CSIC, Madrid, Spain).
Protein extracts
Samples of 100 mg of leaf frozen powder were homogenized in 500 μl of 0.15 M NaCl, ground with fine sand (Sigma, USA) in 1.5 ml tubes, the supernatant was frozen and stored at -20°C. The homogenate was centrifuged at 12000 rpm for 5 min at 4°C, and the total protein concentration was quantified using a Nanodrop 1000 spectophotometer (ThermoFisher scientific, Willmington, USA) with an absorbance ratio of A260/A280.
Protease inhibitors
The inhibitory activity of plant protein extracts was tested in vitro against commercial enzymes: papain (EC 3.4.22.2), cathepsin B from bovine spleen (EC 3.4.22.1), trypsin from bovine pancreas (EC 3.4.21.4) and α-chymotrypsin from bovine pancreas (EC 3.4.21.1). As substrates, Z-FR-AMC (N-carbobenzoxyloxy-Phe-Arg-7-amido-4-methylcoumarin) was used for papain, Z-RR-AMC (N-carbobenzoxyloxy-Arg-Arg-7-amido-4-methylcoumarin) for cathepsin B, Z-LA-AMC (Z-L-Arg-7-amido-4-methylcoumarin) for trypsin and SucAAPF-AMC (Suc-Ala-Ala-Pro-Phe-7-amido-4-methylcoumarin) for chymotrypsin, all of them purchased from Calbiochem (MerkMilipore, Billerica, USA). Samples of 20 μg of plant protein extracts were preincubated for 10 min with 100 ng of the commercial enzyme. The incubation was carried out at 28°C in 100 mM sodium phosphate buffer pH 6.0 (10 mM L-cysteine, 10 mM EDTA, 0.01% (v/v) Brij 35) for the papain and cathepsin B assays and at 35°C in 0.1 M Tris-HCl buffer, pH 7.5, for the trypsin and chymotrypsin assays. Subsequently, the substrates were added at a final concentration of 0.2 M and incubated for 1 h at 28°C or 35°C depending on the enzyme. Fluorescence was measured using an excitation filter of 350 nm and an emission filter of 465 nm (Tecan GeniusPro, Mannedorf, Switzerland). Double blanks were used to account for spontaneous breakdown of substrates and the plant protease activity, and all assays were done in duplicate. Results were expressed as a percentage of protease activity inhibited.
Oxidative enzymes
Samples of 100 mg of leaf frozen powder were ground in a 1.5 ml tube with sand and 500 μl of extraction buffer (0.1 M phosphate buffer, pH 7.0; 5% w:v polyvinylpolypyrrolidine). The homogenate was centrifuged at 12000 rpm for 10 min and the supernatant was collected and frozen until used. Polyphenol oxidase (PPO) activity was analyzed by incubating 20 μl of enzyme extract with cathecol (40 mM final concentration) in 160 μl of Tris-HCl pH 8.5 buffer at 30°C for 1 h. Absorbance was read at 420 nm. Peroxidase (POD) activity was determined incubating 20 μl of a 1:10 dilution of the enzyme extract with guaiacol (5 mM final concentration) and H2O2 (2.5 mM final concentration) in 150 μl of potassium phosphate pH 6 buffer at 30°C for 10 min. Absorbance was read at 470 nm. In both cases, a Varioskan Flash reader (ThermoFisher Scientific, Willmington, USA) was used.
Mite enzymatic activities
To analyze the effect of tomato drought-stressed plants on mite enzymatic activities an additional experiment was performed. Control and drought-stressed plants (6–7 leaves) were inoculated with 200 mites using the pump system previously described (6 replicates per treatment). Mites were collected at 10 dpi and frozen in liquid nitrogen. Mite protein extracts were obtained by homogenizing 1 mg of mites in 100 μl of 0.15M NaCl, centrifuged at 12000 rpm for 5 min and collecting the supernatant. Total protein content was determined according to the method of Bradford [29].
Cathepsin B-, cathepsin L- and legumain-like activities were assayed as described by [30], using Z-RR-AMC, Z-FR-AMC (Calbiochem, USA) and Z-VAN-AMC (N-carbobenzoxyloxy-Val-Ala-Asn-7-amido-4-methylcoumarin) (Bachem, Bubendorf, Switzerland) as substrates, respectively. The assays were performed by incubating 10 μl of mite protein extracts with 85 μl of 100 mM sodium citrate buffer (0.15 M NaCl, 1 mM DTT, pH 5.5 for cathepsin L- and B-like activities and pH 4.5 for legumain-like assay, containing 0.2 M of substrate) at 30°C for 15 min. Fluorescence was measured using an excitation filter of 350 nm and an emission filter of 465 nm on a Varioskan Flash reader (ThermoFisher Scientific, Willington, USA). A calibration curve was obtained with known amounts of AMC (7-amino-4-methylcoumarin) (Bachem, Swizerland).
Cathepsin D-like activity was determined using MocAc-GKPILFFRLK(Dnp)-D-R-NH2 (PeptaNova GmbH, Germany) as substrate The assays were performed incubating 5 μl of the mite protein extract with 85 μl of 100 mM sodium citrate buffer (0.15 M NaCl, 1 mM DTT, 10μM E-64, pH 3.5, containing 20 μM substrate) at 30°C for 15 min. Fluorescence was measured using an excitation filter of 328 nm and an emission filter of 393 nm on a Varioskan Flash reader (ThermoFisher Scientific, Willington, USA), and MCA (MoCAC-Pro-Leu-Gly) (Peptanova GmbH, Germany) was used as standard.
Leucine aminopeptidase-like activity was determined using LpNa (L-leucine p-nitroanilide) as substrate. Samples of 10 μl of mite extracts were incubated with 160 μl of 1mM LpNa in 0.1M Tris-HCl buffer (0.15M NaCl, 5mM MgCl2, pH 7.5) at 30°C for 4 h. The reaction was stopped with 100 μl of 30% acetic acid and the absorbance was measured at 410 nm, using a molar extinction coefficient of 8800 M-1 cm-1 for pNa.
α-Amylase activity was determined using starch as a substrate as described by [31]. Samples of 20 μl of mite extract were incubated with 80 μl of 0.1 M Tris-HCl buffer (40 mM CaCl2, 20 mM NaCl, pH 6.0) and 100 μl of 0.5% starch solution, at 30°C for 4 h. The reaction was stopped by adding 1 ml of lugol solution (0.02% I2; 0.2% KI). The mixture was centrifuged at 6000 rpm for 5 min and the supernatant’s absorbance was measured at 580 nm. A starch standard curve was used as a reference.
Esterase activity was determined using 1-naphthyl acetate (1-NA) as a substrate. Samples of 10 μl of a dilution 1/40 of the mite protein extract were incubated with 160 μl of 0.25 mM 1-NA in 0.1M Tris-HCl buffer (0.15M NaCl, 5mM MgCl2 pH 7.0) for 1 h at 30°C. The reaction was stopped by adding 80 μl of a solution (0.04% (w/v) of Fast blue salt BN (tetrazotized) and 1.5% (w/v) SDS) and incubating at room temperature for 1 h. A standard curve of 1-naphthol was used as a reference. Absorbance was measured at 600 nm.
Glutathione S-transferase (GST) activity was determined incubating 40 μl of mite protein extract with 220 μl of 0.1M Tris-HCl buffer (pH 8.0), containing 0.4 mM CDNB (1-chloro-2,4-dinitrobenzene) as substrate and 5 mM reduced glutathione as cofactor for 15 min at 30°C. The increment in absorbance at 340 nm was recorded every minute for 5 min, using a molar extinction coefficient of 9.6 mM–1 cm–1 for conjugated CDNB.
P450 activity was assayed by incubating 60 μl of mite protein extract with 120 μl of 100mM Tris-HCl buffer, (pH 7.0; containing the NADPH generating system [0.5 mM NADP, 2.5 mM glucose 6-phosphate and 0.3 units of glucose 6-phosphate dehydrogenase]) and 20 μl of a 50 μM cytochrome c solution at 30°C for 4 h. The reaction was stopped with 100 μl of methanol and the absorbance measured at 550 nm, using a molar extinction coefficient of 27.6 mM–1 cm–1 for cytochrome c reduced.
Hydrolytic activities were expressed as nmol of substrate hydrolyzed per min and mg of mite protein, GST activity as nmol of CDNB conjugated per min and mg of protein and P450 activity as nmol of cytochrome c reduced per min and mg of protein.
Proline feeding stimulant test
Assays were performed in plastic Petri dishes (30x90 mm), coated in their bottom half with about 30 ml of a 0.625% agar solution to prevent leaf desiccation. The Petri dishes had a hole in the top, and were covered with filter paper to allow ventilation and preventing the escape of mites. Tomato leaf disks (3.14 cm2) were cut from well-watered tomato plants with a cork borer (Ø: 2 cm) and weighed. The abaxial and the adaxial surfaces of the leaf disks were dispensed with 25 μl of an ethanol solution of L-proline (Sigma, St Louis, USA) or ethanol alone for the control, and allowed to dry before mite inoculation. Concentrations were chosen to simulate the L-proline induced by both drought and T evansi infestation on tomato plants at 4 and 10 dpi (see Results), corresponding to 0.070 mg and 0.202 mg of L-proline/g of fresh leaf weight, respectively. Twenty T. evansi adult females from the colony, replicated ten times, were placed in each leaf disk (one per plate), being the number of eggs laid and the leaf damaged area recorded after 48 h. All Petri dishes were kept in a growth chamber (Sanyo MLR-350-H, Sanyo, Japan) at 25°C±1°C, 70±5% relative humidity and a 16 h light/8 h dark photoperiod.
Statistical analysis
All plant and mite parameters analyzed were checked for the assumptions of normality and heteroscedasticity, and transformed if necessary. Stem length and stomatal conductance were log10(x) transformed, Fv/Fm was log10(x+1) transformed; and these two parameters and stem dry weight data were statistically analyzed using a three-way ANOVA (using as fixed factors drought treatment, T. evansi infestation and time). The number of T. evansi eggs and mobile forms and leaf damage area were log10(x) transformed and analyzed by a two way ANOVA (drought treatment, time). For stem length, plant aerial dry weight, stomatal conductance, Fv/Fm, mite population and leaf damage a Bonferroni post hoc test was performed to compare drought-stress treatments within each time. Percentage of nitrogen, protein, free amino acids and free sugars, as well as C:N ratio and protease inhibition were arcsen(squareroot(x)) transformed. These data and the oxidative enzyme activities were analyzed using a two-way ANOVA for each time separately using as fixed factors drought treatment and T. evansi infestation. A Newman-Keuls post hoc test was performed to see differences of mean between treatments. A Dunnet post hoc test was performed for every single amino acid data at 10 dpi, using as reference the control non-infested plant. Spider mite enzymatic activity data were analyzed by a t-student test. For the proline feeding assay, data were log10(x) transformed and analyzed by one-way ANOVA using proline concentration as a factor. A Bonferroni posthoc test was performed to determine the difference between treatments.
Results
Effects of drought on tomato plant growth, stomatal conductance and photosynthetic efficiency
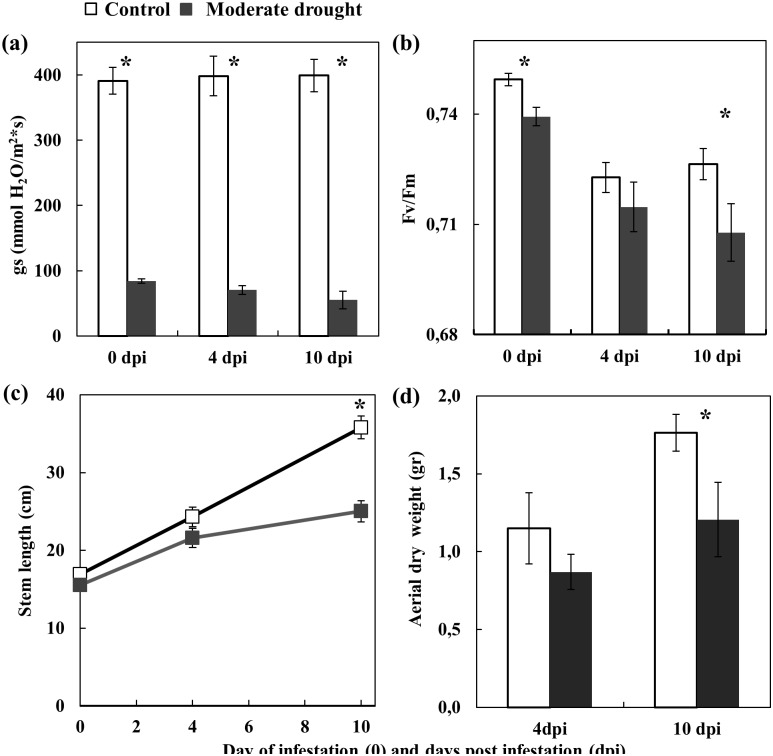
Data from both infested and non-infested plants were pooled as no significant differences were found between them (data no shown). Moderate drought stress had a strong effect on stomatal conductance, which was significantly reduced between 4 and 5 times with respect to the control plants at 4 and 10 dpi (Fig 2a). The Fv/Fm of moderate drought stress plants was also significantly smaller than the control at infestation and at 10 dpi (Fig 2b). Similar effects, but less pronounced were obtained with mild stressed plants, with the stomatal conductance progressively reduced from about 20% less than in control plants at mite infestation to about half at 10 dpi, whereas Fv/Fm was only significantly lower at 10 dpi (S1 Fig). Significant differences in the growing pattern (aerial dry weight and/or stem length) were obtained at 10 dpi for both drought levels (Fig 2c, 2d & S1 Fig), with the reductions more acute for moderate stressed plants.
Fig 2. Effect of moderate drought at mite infestation and at 4 and 10 days post infestation (dpi) on: a) tomato stomatal conductance (gs); b) maximum quantum yield of PSII photochemistry (Fv/Fm); c) stem length; d) plant aerial dry weight.
Data are mean ± SE. * indicates a statistically significant difference within each time (Three-way ANOVA, Bonferroni post hoc test, P<0.05).
Effects of drought on T. evansi population growth and leaf damage
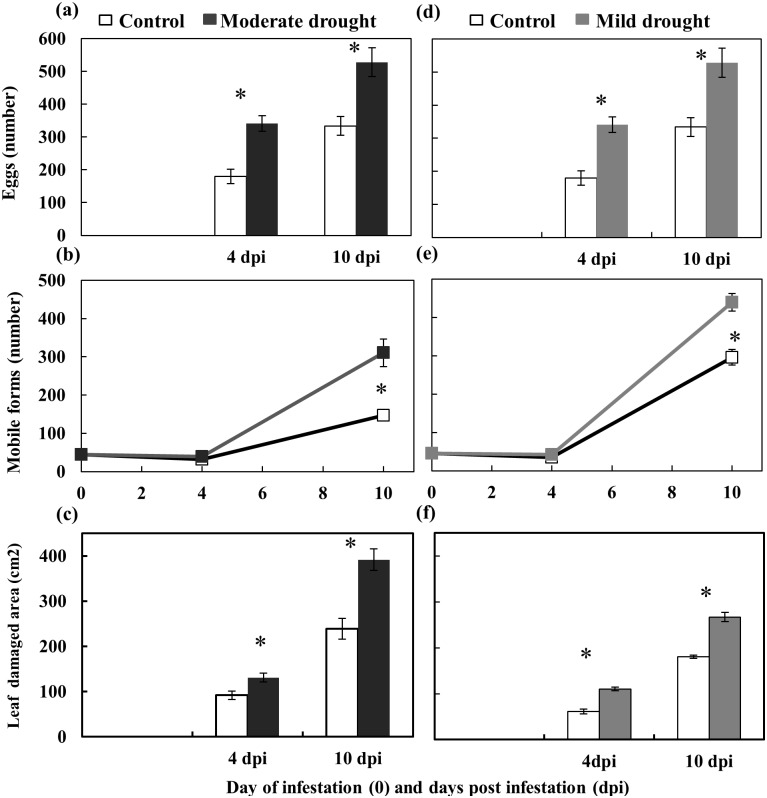
The population of T. evansi increased faster when feeding on both moderate and mild drought-stressed than on control tomato plants (Fig 3). The number of eggs laid under moderate stress was significantly higher at 4 (2 fold) and 10 dpi (1.6 fold) than on control plants (Fig 3a). No differences on mobile forms were observed at 4 dpi, as these individuals correspond to the surviving adult females (F0) that were used to infest the plant (Fig 3b). However, a significant increase (2.1 fold) was observed at 10 dpi, with most of these individuals from the resulting F1 progeny. Interestingly, the leaf damage area was 1.3 and 1.6 times significantly larger than on controls at both 4 dpi and 10 dpi, respectively (Fig 3c). When mites were reared on mild drought-stressed tomato plants, significant increases in eggs laid (Fig 3d), mobile forms (Fig 3e) and leaf damage area (Fig 3f) were also recorded.
Fig 3. Performance of T. evansi in the moderate (a,b&c) and mild (d,e,f) drought stress experiments.
The number of eggs (a&d), total mobile forms (b&e) and leaf damaged area (c&f) on control and drought stressed (mild or moderate) tomato plants at 4 and 10 days post infestation (dpi) were measured. Data are mean ±SE. * indicates a statistically significant difference within each time (Two-way ANOVA, Bonferroni post hoc test, P<0.05).
Effects of drought and T. evansi on plant nutritional composition and defense proteins
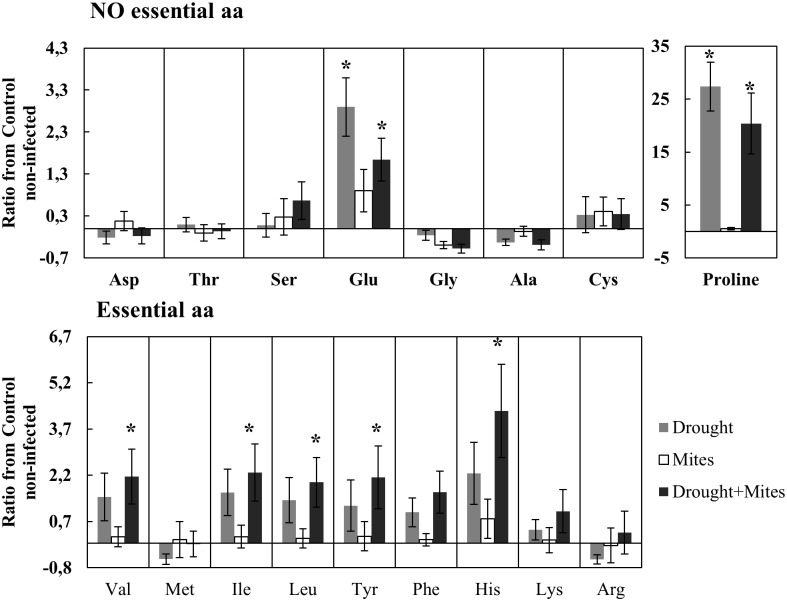
Differences in the levels of nutrients in the plants were found at 4 and 10 dpi, with drought stress the most significant factor (Table 1). At 4 dpi, drought, mite infestation and the combination of both factors produced a significant decrease of nitrogen and an increase of free sugars. Total protein was also reduced by drought at 4 dpi, but the difference was only significant in infested plants. At 10 dpi, a decrease in nitrogen and total protein, and an increase in free sugars were obtained with drought alone or in combination with mite infestation. Drought was also a significant factor for total free amino acids at 10 dpi, although no significant differences among treatments were found (Table 1). However, it could be demonstrated that drought drives the accumulation of specific amino acids (Fig 4). Proline was the amino acid more clearly induced by drought, increasing significantly by > 20 fold by drought alone or in combination with mite infestation (Fig 4). The levels of several essential (valine, isoleucine, leucine, tyrosine, histidine) and nonessential (glutamine) amino acids were also significantly induced by drought in combination with mites (Fig 4). The infestation by T. evansi did not have significant effects on any the amino acids analyzed at 10 dpi.
Table 1. Effect of moderate drought and T. evansi infestation on key nutrients in tomato leaves at 4 and 10 days post infestation.
| Non-infested | Infested | ANOVA (p<0.05) | |||
|---|---|---|---|---|---|
| Control | Drought | Control | Drought | ||
| 4 Days Post Infestation | |||||
| Nitrogen1 | 6.7±0.1a | 5.7±0.2b | 5.8±0.2b | 5.7±0.1b | D;I; D*I |
| Protein1 | 31±2ab | 26±3b | 35±2a | 25±2b | D |
| Free aa1 | 1.0±0.2 | 1.2±0.2 | 1.0±0.1 | 1.1±0.2 | - |
| C:N | 5.9±0.1 | 6.4±0.2 | 6.5±0.3 | 6.3±0.2 | - |
| Free Sugars1 | 3.3±0.1b | 3.9±0.3a | 4.2±0.1a | 4.3±0.2a | I |
| 10 Days Post Infestation | |||||
| Nitrogen1 | 6.3±0.2a | 5.7±0.1b | 5.9±0.2ab | 5.3±0.1b | D |
| Protein1 | 40±5a | 19±4b | 27±6ab | 15±2b | D |
| Total free aa1 | 0.9±0.2 | 1.7±0.3 | 1.2±0.3 | 1.8±0.4 | D |
| C:N | 6.3±0.1 | 6.4±0.1 | 6.1±0.2 | 6.6±0.2 | - |
| Free Sugars1 | 3.2±0.2c | 4.8±0.3a | 3.7±0.1cb | 4.3±0.3ab | D;D*I |
(1) Data, as % of dry weight, are mean ± SE. D (drought) and I (mites infestation) indicate significant factors in the Two-Way ANOVA. Different lower case letters within rows indicates significant differences (Newman-Keuls test at p<0.05).
Fig 4. Levels of free amino acids in tomato leaves subjected to moderate drought stress and T. evansi infestation at 10 days post infestation (dpi).
Data represent the ratio ± SE of the different treatments with respect to non-infested control plants at 10 dpi. = [(Treatment−Control 10dpi) / Control 10dpi]. The division between essential and nonessential amino acids is based on a study with the closely related species Tetranychus urticae [32]. * Indicates significant difference of the treatment with the control determined by Dunnet post hoc test p<0.05.
Tomato plant defense proteins were also affected by drought stress and mite infestation, but different responses were obtained depending on the post-infestation time (Table 2). At 4 dpi, the inhibitory activity of tomato leaf extracts against papain was reduced by mite infestation under both drought and control conditions, whereas the inhibitory activity against chymotrypsin was reduced by drought conditions on non-infested plants. By contrast, the inhibitory activity of tomato leaf extracts at 10 dpi was enhanced against papain and cathepsin B as a response to both drought and mite infestation, whereas the inhibitory activity against trypsin increased in response to drought. The specific activity of the oxidative enzymes PPO and POD was significantly increased in response to mite infestation, drought stress and a combination of both at 4 dpi, but only in response to mite infestation at 10 dpi.
Table 2. Effect of moderate drought and T. evansi infestation on plant defense proteins in tomato leaves at 4 and 10 days post infestation.
| Non-infested | Infested | ANOVA (p<0.05) | |||
|---|---|---|---|---|---|
| Control | Drought | Control | Drought | ||
| 4 Days Post Infestation | |||||
| Protease inhibitors (% Inhibition) | |||||
| Papain | 48±2ab | 64±2a | 31±5c | 45±5b | D;I |
| Cathepsin B | 49±3 | 62±6 | 63±8 | 66±4 | - |
| Trypsin | 40±8 | 44±2 | 49±8 | 43±2 | - |
| Chymotrypsin | 61±7a | 45±6b | 64±2a | 63±4a | I |
| Oxidative enzymes (specific activity) | |||||
| Polyphenol oxidases1 | 1.1±0.1b | 2.1±0.1a | 2.5±0.2a | 2.6±0.3a | D;I;D*I |
| Peroxidases2 | 2.7±0.2b | 5±0.5a | 6.8±1.1a | 5.1±0.2a | I; D*I |
| 10 Days Post Infestation | |||||
| Protease inhibitors (%Inhibition) | |||||
| Papain | 57±3c | 81±3a | 71±4b | 86±3a | D;I |
| Cathepsin B | 40±5c | 60±7b | 63±6b | 83±5a | D;I |
| Trypsin | 37±3ab | 54±4a | 31±8b | 52±5a | D |
| Chymotrypsin | 69±4 | 65±6 | 74±6 | 77±4 | - |
| Oxidative enzymes (specific activity) | |||||
| Polyphenol oxidases1 | 2.2±0.1b | 2.5±0.4b | 3.7±0.5a | 3±0.4ab | I |
| Peroxidases2 | 4.3±0.5b | 4±0.5b | 8.1±0.9a | 5.8±0.6b | D; I |
(1) PPO: nmol hydrolyzed Cathecol/ mg Protein*min
(2) POD: nmol hydrolyzed Guaiacol/ mg Protein*min.
Data are mean ± SE. D (drought) and I (mites infestation) indicate significant factors in the Two-Way ANOVA. Different lower case letters within rows indicates significant differences (Newman-Keuls test at p<0.05).
Enzymatic activities of T. evansi fed on drought-stressed and well-watered tomato plants
Hydrolytic and detoxification enzyme activities in T. evansi were analyzed after feeding for 10 days on moderate drought-stressed or control tomato plants (Table 3). A significant increase in total mite protein and a reduction in the specific activity of cathepsin D- and legumain-like proteases and of α-amylase were observed on mites fed on tomato plants exposed to drought stress. The activity of the other digestive proteases (cathepsin B- and L-like and aminopeptidase) and detoxification enzymes (esterase, GST and P450) analyzed were not affected by the drought status of the plant in which they fed.
Table 3. Enzymatic activities in T. evansi feeding on moderate drought-stressed or control tomato plants for 10 days.
| Control | Drought-stressed | |
|---|---|---|
| Protein1 | 55±2 | 69±5* |
| Digestive enzymes (specific activity) | ||
| Cathepsin B2 | 7.8±1.0 | 8.7±1.1 |
| Cathepsin L2 | 1.9±0.2 | 1.8±0.2 |
| Cathepsin D2 | 13±2 | 5.5±0.4* |
| Legumain 2 | 0.29±0.03 | 0.20±0.02* |
| Aminopeptidase2 | 23±3 | 24±3 |
| α-Amylase2 | 162±7 | 130±8* |
| Detoxification enzymes | ||
| Esterase2 | 1774±146 | 1397±216 |
| GST3 | 197±30 | 179±17 |
| P4504 | 0.2±0.02 | 0.2±0.01 |
Data are mean ± SE.
* indicates statistically significant difference between treatments (t-student, p<0.05).
(1) μg/mg fresh weight,
(2) nmol substrate hydrolyzed per min and mg of protein,
(3) nmol CDNB conjugated per min and mg of protein,
(4) nmol cytochrome c reduced per min and mg of protein.
Proline feeding stimulant assay
The proline feeding stimulant assay showed a significant increase of eggs laid (2.5 fold) and leaf damage area (1.8 fold) on leaf disk treated with L-proline at the highest concentration tested (equivalent to that estimated for drought- and mite-stressed tomato plants at 10 dpi). In contrast, the effect was not significant when disks were treated with a concentration of proline equivalent to that estimated for drought- and mite-stressed tomato plants at 4 dpi (Fig 5). This result suggests a phagostimulant effect on T. evansi at the highest concentration of proline tested.
Fig 5. Performance of T. evansi with regard to the number of eggs laid (a) and leaf damaged area (b), on leaf disks treated with L-proline.
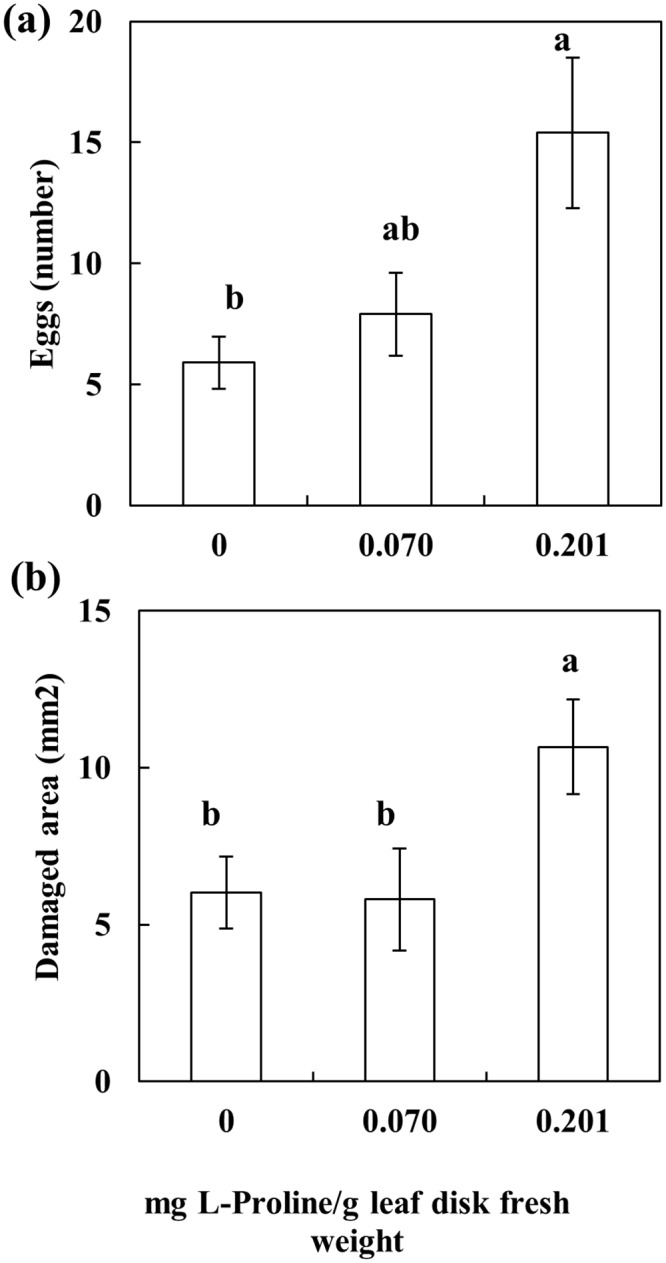
Concentrations applied are equivalent to the one estimated on drought-stressed tomato plants at 4 and 10 days post infestation. Data are mean ±SE. Different letters indicate significant differences (One-way ANOVA, Bonferroni post hoc test, P<0.05).
Discussion
Under a climate change scenario, invasive mite species, such as T. evansi, raise further concerns because they might expand to new geographical areas [33] and have more generations per year, causing severe damage on tomato [34]. Our data revealed that mild and moderate drought-stressed tomato plants trigger bottom-up effects on T. evansi, with these plants physiologically more suitable for mite development. Mite performance was enhanced on both mild and moderate drought-stressed tomato plants, as revealed by a significant increase in the number of eggs laid and of mobile forms, resulting in more leaf damage. It has been reported that plants under drought stress mobilize proteins into amino acids and carbohydrates into free sugars for osmotic adjustments [7]. We have found that both drought and T. evansi damage triggered the decline of foliar soluble protein and total nitrogen content, suggesting that mites and drought are accelerating leaf senescence and likely inducing the transference of nutrients to younger leaves [35], as reported in cotton for the combined effects of water-stress and T. urticae infestation [36]. In contrast, the amount of several free amino acids and free sugars increased significantly in drought-stressed tomato leaves, as already reported for tomato [37], with proline the amino acid most clearly induced by drought. Interestingly, we have demonstrated that L-proline, a known phago-stimulant for insects [4, 24], also has a significant feeding stimulant effect on T. evansi, increasing both eggs laid and leaf damage area when added to tomato leaf discs. In addition, mite-infested, drought-stressed tomato plants showed significantly increased concentrations of five essential amino acids (valine, isoleucine, leucine, tyrosine and histidine), which have been reported to enhance the fecundity of the closely-related species T. urticae on strawberry, cucumber and chrysanthemum plants [38, 39]. Therefore, T. evansi is probably taking advantage of the increase in these plant-derived essential amino acids to synthesize structural proteins and enzymes, reducing the metabolic costs associated with digestion that can be reallocated for growth and development. A similar role can be proposed for the increase in free sugars, since it has been reported that fecundity of T. urticae correlated with sugar content in apple leaves [40], and free sugars can also act as phagostimulants for some herbivores [24]. Altogether, our data suggests that significant increases of available free sugars and essential amino acids, jointly with their phagostimulant effects, created a favorable environment for a better T. evansi performance on drought-stressed tomato leaves.
Another important aspect concerning the palatability of plants for mites is the level of anti-nutritional plant defense proteins that accumulate in response to abiotic and biotic stresses. As reported for other species [41], drought consistently induced the inhibitory activity of tomato-leaf extracts against cysteine (papain and cathepsin B) and serine (trypsin) proteases. This response was obtained for both T. evansi infested and non-infested plants, indicating that the response was driven by drought stress. By contrast, chymotrypsin inhibitory activity and the activity of PPO and POD varied depending on the time of plant exposure to drought stress, which may be a consequence of the complex regulatory pathways for some of these inducible defense genes in plants [42]. It has been reported that T. evansi suppresses plant defenses in tomato by down-regulating the induction of both the salicylic acid and jasmonic acid signaling pathways [43]. In particular, this was reflected in a reduction in the expression of serine protease inhibitors and PPO [20, 21], which are normally induced in tomato leaves in response to the attack by other spider mites [44]. Likewise, we have shown that the inhibitory activity against serine proteases (trypsin and chymotrypsin) was not significantly induced in leaves attacked by T. evansi, when compared to non-infested tomato plants. However, we found that mite infestation induced the inhibitory activity against cysteine proteases (cathepsin B and papain), which were not considered in previous studies. These results are especially relevant, since proteolytic digestion in spider mites relies on cysteine proteases (including T. evansi, see below) and cysteine protease inhibitors have been shown to be harmful against the spider mite T. urticae, in vivo [45]. We have also found that the specific activities of both PPO and POD were significantly increased in response to T. evansi infestation. These results may be considered somewhat contradictory with those obtained by Alba et al. [21], who reported that T. evansi suppressed the expression of two PPO genes (PPO-D and PPO-F) in tomato. However, this suppression only occurred when compared with tomato plants infested with mites from a non-suppressor T. urticae strain, but the expression of these genes in plants infested with T. evansi was slightly but significantly higher than on non-infested tomato plants [21] Thus, further studies to determine the in vivo effect of tomato cysteine protease inhibitors and PPOs on T. evansi are required.
Plant-feeding arthropods have developed a remarkable diversity of physiological adaptations to respond to changes in the nutritional composition of their host plants and to counteract the adverse effects of plant defenses including the regulation of digestive and detoxification enzymes [46, 47, 48]. The hydrolysis of dietary proteins in spider mites relies on cysteine (cathepsin B-, cathepsin L- and legumain-like) and aspartyl (cathepsin D-like) proteases and aminopeptidases [30, 49]. Our results indicate that T. evansi has a similar digestive proteolytic profile, hydrolyzing specific substrates for cathepsin D, cathepsin B, cathepsin L and legumain proteases and aminopeptidases. In addition, we determined the presence of α-amylase activity for the hydrolysis of carbohydrates in T. evansi, as already reported in other tetranychid mite species [50]. We found that cathepsin D- and legumain-like proteases and α-amylase activities were significantly reduced when T. evansi mites were fed on drought-stressed plants, but this reduction did not seem to affect their performance. The decrease of these mite hydrolytic activities could be explained by the ingestion of hydrolytic enzyme inhibitors from tomato plants. However, cathepsin B- and L-like activities in T. evansi were not affected, despite the induction of specific inhibitors for these two types of proteases in drought-stressed plants. These results suggest that T. evansi is capable of circumventing the adverse effects of at least some plant protease inhibitors. An alternative explanation for the reduction of some digestive protease and α-amylase activities in T. evansi could be related to the higher availability of nutrients in a readily assimilated form (essential amino acids and free sugars) in tomato plants subjected to drought conditions. Tomato plants are known to induce an array of secondary metabolites during periods of water deficit [23], thereby potentially increasing the concentration of compounds involved in plant defense. However, no significant changes in esterase, GST or P450 specific activities were observed when T. evansi was fed on drought-stressed plants. Secondary metabolites were not analyzed in this study, but it has been reported that T. evansi suppresses the expression of genes involved in regulation of secondary metabolites [21] and the release of inducible volatiles [20]. This down-regulation of plant defenses may render unnecessary the induction of the mite detoxification enzymes, and the metabolic resources saved diverted toward growth and reproduction. Nevertheless, further studies will be needed to determine the combined effects of drought and T. evansi infestation in the regulation of secondary metabolites in tomato.
Conclusions
Our data reveal that both drought and T. evansi infestation induced significant changes in the nutritional quality of tomato plants, as more essential amino acids and free sugars are available. These changes trigger a bottom-up effect on key biological traits of T evansi causing a highly significant increase of leaf damage and mite performance. Plant defense proteins were also induced by drought and/or mite infestation, but the mite physiological responses suggest that T. evansi is capable of circumventing their potential adverse effects. These findings support the “Plant stress hypothesis” and suggest that drought-stressed tomato plants even at a mild level may be more prone to T evansi outbreaks in a climate change scenario, which might strongly affect tomato production on area-wide scales. Moreover, abiotic stresses can also exert a strong impact at higher tropic levels. Thus, it has been demonstrated recently that plant water status may also trigger bottom-up effects on the reproductive behavior and fitness of some natural enemies, especially the omnivorous predators [51, 52], which might shape the arthropod structure. Therefore, this complexity of food-web structures should be considered when using species distribution models to infer climate change effects on crop pests.
Supporting Information
Data are mean ± SE. * Indicates significant difference within each time (Three-way ANOVA, Bonferroni post hoc test, P<0.05).
(TIF)
Acknowledgments
We are grateful to Dr. M. Barón, Zaidín, CSIC, for her advice on drought plant techniques. Thanks are also due to Dr. P. Palukaitis for kindly editing this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by an INIA grant: GENOMITE, Proposal No 618105 FACCE Era Net Plus-Food security, Agriculture, Climate Change (new generation sustainable tools to control emerging mite pests under climate change).
References
- 1.IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge; 2013.
- 2.Mishra KB, Iannacone R, Petrozza A Mishra A, Armentano N, La Vecchia G, et al. Engineered drought tolerance in tomato plants is reflected in chlorophyll fluorescence emission. Plant Sci. 2012; 182: 79–86. 10.1016/j.plantsci.2011.03.022 [DOI] [PubMed] [Google Scholar]
- 3.English-Loeb GM. Plant drought stress and outbreaks of spider mites: a field test. Ecology. 1990; 71: 1401–1411. [Google Scholar]
- 4.Mattson W, Haack R. The role of drought in outbreaks of plant-eating insects. BioScience. 1987; 37: 110–118. [Google Scholar]
- 5.Huberty A, Denno R. Plant water stress and its consequences for herbivorous insects: a new synthesis. Ecology. 2004; 85: 1383–1398. [Google Scholar]
- 6.Inbar M, Doostdar H, Mayer RT. Suitability of stressed and vigorous plants to various insect herbivores. Oikos. 2001; 94: 228–235. [Google Scholar]
- 7.Hummel I, Pantin F, Sulpice R Piques M, Rolland G, Dauzat M, et al. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: An integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol. 2010; 154: 357–372. 10.1104/pp.110.157008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han P, Lavoir AV, Le Bot J, Amiens-Desneux E, Desneux N. Nitrogen and water availability to tomato plants triggers bottom-up effects on the leafminer Tuta absoluta. Scientific Reports 2014; 4: 4455 10.1038/srep04455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelissen T, Wilson Fernandes G, Vasconcellos-Neto J. Size does matter: Variation in herbivory between and within plants and the plant vigor hypothesis. Oikos. 2008; 117: 1121–1130. [Google Scholar]
- 10.White TCR. Plant vigour versus plant stress: A false dichotomy. Oikos. 2009; 118: 807–808. [Google Scholar]
- 11.White TCR. The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia. 1984; 63: 90–105. [DOI] [PubMed] [Google Scholar]
- 12.Guo H, Sun Y, Li Y, Tong B, Harris M, Zhu-Salzman K, et al. Pea aphid promotes amino acid metabolism both in Medicago truncatula and bacteriocytes to favor aphid population growth under elevated CO2. Glob Chang Biol. 2013; 19: 3210–3223. 10.1111/gcb.12260 [DOI] [PubMed] [Google Scholar]
- 13.Johnson SN, Ryalls JMW, Karley AJ. Global climate change and crop resistance to aphids: Contrasting responses of lucerne genotypes to elevated atmospheric carbon dioxide. Ann Appl Biol. 2014; 165: 62–72. [Google Scholar]
- 14.Gutbrodt B, Mody K, Dorn S. Drought changes plant chemistry and causes contrasting responses in lepidopteran herbivores. Oikos. 2011; 120: 1732–1740. [Google Scholar]
- 15.Lange WH, Bronson L. Insect pests of tomatoes. Annu Rev Entomol. 1981; 26: 345–371. [Google Scholar]
- 16.Meynard CN, Migeon A, Navajas M. Uncertainties in predicting species distributions under climate change: A case study using Tetranychus evansi (Acari: Tetranychidae), a widespread agricultural pest. Plos One. 2013; 8: e66445 10.1371/journal.pone.0066445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunyama IGM, Knapp M. Effects of pruning and trellising of tomatoes on red spider mite incidence and crop yield in Zimbabwe. African Crop Sci J. 2003; 11: 269–277. [Google Scholar]
- 18.Navajas M, de Moraes GJ, Auger P, Migeon A Review of the invasion of Tetranychus evansi: biology, colonization pathways, potential expansion and prospects for biological control. Exp Appl Acarol. 2013; 59: 43–46. 10.1007/s10493-012-9590-5 [DOI] [PubMed] [Google Scholar]
- 19.EPPO. European and Mediterranean Plant Protection Organization. Reporting Service no. 05–2004 Num. article: 2004/080. Available: https://gd.eppo.int/reporting/article-1601. 2004.
- 20.Sarmento RA, Lemos F, Bleeker PM, Schuurink RC, Pallini A, Oliveira MG, et al. A herbivore that manipulates plant defence. Ecol Lett. 2011; 14: 229–236. 10.1111/j.1461-0248.2010.01575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alba JM, Schimmel BCJ, Glas JJ, Ataide LM, Pappas ML, Villarroel CA, et al. Spider mites suppress tomato defenses downstream of jasmonate and salicylate independently of hormonal crosstalk. New Phytol. 2015; 205: 828–840. 10.1111/nph.13075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.English-Loeb G, Stout MJ, Duffey SS. Drought stress in tomatoes: Changes in plant chemistry and potential nonlinear consequences for insect herbivores. Oikos. 1997; 79: 456–468. [Google Scholar]
- 23.Ripoll J, Urban L, Staudt M, Lopez-Lauri F, Bidel LPR, Bertin N. Water shortage and quality of fleshy fruits—making the most of the unavoidable. J Exp Bot. 2014; 65: 4097–4117. 10.1093/jxb/eru197 [DOI] [PubMed] [Google Scholar]
- 24.Showler AT. Water Deficit Stress—Host Plant Nutrient Accumulations and Associations with Phytophagous Arthropods In: Vahdati K editor. Abiotic Stress—Plant Responses and Applications in Agriculture. 2013. 10.5772/53125 Available: http://www.intechopen.com/books/abiotic-stress-plant-responses-and-applications-in-agriculture/water-deficit-stress-host-plant-nutrient-accumulations-and-associations-with-phytophagous-arthropods. [DOI] [Google Scholar]
- 25.Fitter AH, Hay RKM. Environmental Physiology of Plants. 2002. Academic Press, London UK [Google Scholar]
- 26.Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006; 45: 523–539. [DOI] [PubMed] [Google Scholar]
- 27.Maness N. Extraction and analysis of soluble carbohydrates, In: Sunkar R editor. Plant Stress Tolerance, Methods in Molecular Biology, Springer Science+Business Media; 2010, pp. 341–370. [DOI] [PubMed] [Google Scholar]
- 28.Hacham Y, Avraham T, Amir R. The N-terminal region of Arabidopsis cystathionine γ-synthase plays an important regulatory role in methionine metabolism. Plant Physiol. 2002; 128: 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 30.Santamaría ME, González-Cabrera J, Martínez M, Grbic V, Castañera P, Díaz I, et al. Digestive proteases in bodies and faeces of the two-spotted spider mite, Tetranychus urticae. J Insect Physiol. 2015; 78: 69–77. 10.1016/j.jinsphys.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 31.Valencia A, Bustillo AE, Ossa GE, Chrispeels MJ. α-Amylases of the coffee berry borer (Hypothenemus hampei) and their inhibition by two plant amylase inhibitors. Insect Biochem Mol Biol. 2000; 30: 207–213. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez JG, Hampton RE. Essential amino acids determined in the two-spotted spider mite, Tetranychus urticae Koch (Acarina, Tetranychidae) with glucose-U-C14. J Insect Physiol. 1966; 12: 1209–1216. [Google Scholar]
- 33.Migeon A, Ferragut F, Escudero-Colomar LA, Fiaboe K, Knapp M, de Moraes GJ, et al. Modelling the potential distribution of the invasive tomato red spider mite, Tetranychus evansi (Acari: Tetranychidae). Exp Appl Acarol. 2009; 48: 199–212. 10.1007/s10493-008-9229-8 [DOI] [PubMed] [Google Scholar]
- 34.Azandémè-Hounmalon GY, Fellous S, Kreiter S, Fiaboe KKM, Subramanian S, Kungu M, et al. Dispersal behavior of Tetranychus evansi and T. urticae on tomato at several spatial scales and densities: Implications for integrated pest management. Plos One. 2014; 9: e95071 10.1371/journal.pone.0095071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mody K, Eichenberger D, Dorn S. Stress magnitude matters: Different intensities of pulsed water stress produce non-monotonic resistance responses of host plants to insect herbivores. Ecol Entomol. 2009; 34: 133–143. [Google Scholar]
- 36.Sadras VO, Wilson LJ, Lally DA. Water deficit enhanced cotton resistance to spider mite herbivory. Ann Bot. 1998; 81: 273–286. [Google Scholar]
- 37.Bauer D, Biehler K, Fock H, Carrayol E, Hirel B, Migge A, et al. A role for cytosolic glutamine synthetase in the remobilization of leaf nitrogen during water stress in tomato. Physiol Plant. 1997; 99: 241–248. [Google Scholar]
- 38.Tulisalo U. Free and bound amino acids of three host plant species and various fertilizer treatments affecting the fecundity of the two-spotted spider mite, Tetranychus urticae Koch (Acarina, Tetranychidae). Ann Entomol Fenn. 1971; 37: 155–163. [Google Scholar]
- 39.Dabrowski ZT, Bielak B. Effect of some plant chemical compounds on the behaviour and reproduction of spider mites (Acarina: Tetranychidae). Entomol Exp Appl. 1978; 24: 317–326. [Google Scholar]
- 40.Wermelinger B, Oertli JJ, Baumgartner J. Environmental factors affectig the life-tables of Tetranychus urticae (Acari: Tetranychidae). III. Host-plant nutrition. Exp Appl Acarol. 1991; 12: 259–274. [Google Scholar]
- 41.Mosolov VV, Valueva TA. Inhibitors of proteolytic enzymes under abiotic stresses in plants (review). Appl Biochem Microbiol. 2011; 47: 453–459. [PubMed] [Google Scholar]
- 42.Atkinson NJ, Jain R, Urwin PE. The response of plants to simultaneous biotic and abiotic stress, In: Mahalingam R editor. Combined Stresses in Plants. Springer International Publishing; Switzerland: 2015. pp: 181–201. [Google Scholar]
- 43.Kant MR, Jonckheere W, Knegt B, Lemos F, Liu J, Schimmel BCJ, et al. Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities. Ann Bot. 2015; 115: 1015–1051. 10.1093/aob/mcv054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kant MR, Ament K, Sabelis MW, Haring MA, Schuurink RC. Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol. 2004; 135: 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santamaria ME, Cambra I, Martinez M, Pozancos C, González-Melendi P, Grbic V, et al. Gene pyramiding of peptidase inhibitors enhances plant resistance to the spider mite Tetranychus urticae. Plos One. 2012; 7: e43011 10.1371/journal.pone.0043011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortego F. Physiological adaptations of the insect gut to herbivory In: Smagghe G, Diaz I editors. Arthropod-Plant Interactions—Novel Insights and Approaches for IPM. Series: Progress in Biological Control. Volume 14, Springer; 2012. pp: 75–88. [Google Scholar]
- 47.Zhu-Salzman K, Zeng R. Insect response to plant defensive protease inhibitors. Annu Rev Entomol. 2015; 60: 233–252. 10.1146/annurev-ento-010814-020816 [DOI] [PubMed] [Google Scholar]
- 48.Grbić M, Van Leeuwen T, Clark RM, Rombauts S, Rouzé P, Grbić V, et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature. 2011; 479: 487–492. 10.1038/nature10640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carrillo L., Martinez M, Ramessar K, Cambra I, Castañera P, Ortego F, et al. Expression of a barley cystatin gene in maize enhances resistance against phytophagous mites by altering their cysteine-proteases. Plant Cell Rep. 2011; 30: 101–112. 10.1007/s00299-010-0948-z [DOI] [PubMed] [Google Scholar]
- 50.Akimov IA, Barabanova VV. Morphological and functional characteristics of the digestive system in tetranychid mites (Trombidiformes, Tetranychoidea). Entomologicheskoe Obozrenie. 1997; 56: 912–922 [Google Scholar]
- 51.Han P, Dong YC, Lavoir AV, Adamowicz S, Bearez P, Wajnberg E, Desneux N. Effect of plant nitrogen and water status on the foraging behavior and fitness of an omnivorous arthropod. Ecology and Evolution. 2015. In press, 10.1002/ece3.1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seagraves MP, Riedell WE, Lundgren JG. Oviposition preference for water-stressed plants in Orius insidiosus (Hemiptera: Anthocoridae). J Insect Behav. 2011; 24: 132–143. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data are mean ± SE. * Indicates significant difference within each time (Three-way ANOVA, Bonferroni post hoc test, P<0.05).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.