Abstract
Importance
The growing rate of contralateral prophylactic mastectomy (CPM) among women diagnosed with breast cancer has raised concerns about potential for overtreatment. Yet, there are few large survey studies of factors that affect women’s decisions for this surgical treatment option.
Objective
To determine factors associated with use of CPM in a population-based sample of breast cancer patients.
Design, Setting and Participants
A longitudinal survey of 2290 women newly diagnosed with breast cancer and reported to the Detroit and Los Angeles SEER registries from 6/05-2/07 and again 4 years later (6/09-2/10) merged with SEER registry data (n=1536). Multinomial logistic regression was used to evaluate factors associated with type of surgery. Primary independent variables included clinical indications for CPM (genetic mutation and/or strong family history), diagnostic MRI, and patient extent of worry about recurrence at the time of treatment decision making.
Main outcome measure
Type of surgery received from patient self-report, categorized as CPM, unilateral mastectomy (UM) or breast conservation surgery (BCS).
Results
Of the 1443 women in the analytic sample, 19% strongly considered CPM and 7.6% received it. Of those who strongly considered CPM, 45.8% ultimately received UM and 22.8% received BCS. The majority (69%) of patients who received CPM had no major genetic or familial risk factors for contralateral disease. Multivariate regression showed that receipt of CPM (vs. either UM or BCS) was significantly (P<0.01) associated with genetic testing (positive or negative), a strong family history of breast/ovarian cancer, receipt of MRI, higher education, and greater worry about recurrence.
Conclusions
Many women considered CPM and a substantial number received it, although few had clinically significant risk of contralateral breast cancer. Receipt of MRI at diagnosis contributed to receipt of CPM. Worry about recurrence appeared to drive decisions for CPM though the procedure has not been shown to reduce recurrence risk. More research is needed about the underlying factors driving utilization of CPM.
INTRODUCTION
A patient’s decision to undergo contralateral prophylactic mastectomy (CPM) as part of initial treatment for breast cancer is a growing challenge in the management of the disease. Removal of the unaffected breast in most patients diagnosed with breast cancer has not been shown to prolong survival.1 Additionally, the widespread use of adjuvant therapy even for small node negative breast cancers has resulted in a decrease in the incidence of contralateral breast cancer of approximately 3% per year since 1985.2 Subgroups of breast cancer patients at increased risk for development of contralateral cancer, and thus in whom having the non affected breast removed could improve survival, have been identified. Indeed, the Society for Surgical Oncology suggests that CPM should be considered in the minority of patients at higher than average risk for developing a contralateral breast cancer, specifically those patients with either: 1) a genetic mutation of BRCA1 or BRCA2 or other known mutation; or 2) a strong family history of at least two first degree relatives with breast or ovarian cancer with no demonstrable mutations.3 It is estimated that less than 10% of women with newly diagnosed unilateral breast cancer have one or both of these clinical indications.4–6 Despite the cautious approach to CPM outlined in these recommendations, rates have been steadily increasing over the past decade.4,7–10
This situation has raised concerns about overtreatment and questions about why women are choosing the procedure.4,9 The growing use of magnetic resonance imaging (MRI) as part of the diagnostic workup in breast cancer patients has contributed to these concerns, as it may detect occult lesions for which treatment is not likely to improve outcomes for patients.11–13 Two review papers have noted that unnecessary CPM is one of the potential harms possible from the use of preoperative MRI.12–13 Studies that have examined factors associated with receipt of CPM provide insight regarding the decision making process, but are limited by relatively select and homogenous single-institution clinic populations.14–16 Larger studies using population-based registry data or large, multi-institutional convenience samples are limited by lack of information about use of preoperative MRI and about patient attitudes.7–8,10
We used data from a large survey of a diverse population-based sample of patients to evaluate factors associated with receipt of CPM. The objectives were: 1) to describe rates of CPM compared with unilateral mastectomy (UM) and breast conserving surgery (BCS); and 2) to evaluate factors associated with receipt of CPM, including key clinical indicators of an increased risk of contralateral cancer development, use of MRI, and patient worry about recurrence.
METHODS
Study Population
We conducted a population-based survey of women aged 20–79 years at diagnosis with a first incident case of primary ductal carcinoma in situ (DCIS) or invasive breast cancer (stage I–IIIa), reported to the Surveillance and Epidemiology and End Results (SEER) registries of Los Angeles (LA) or Detroit metropolitan areas from 6/1/05-2/1/07.17 Details have been published elsewhere.18–25 We oversampled Latina patients in LA and African American (AA) patients in Detroit and LA. Asian women in LA were excluded because they were being recruited for another SEER study. Patients were excluded if they had stage IV breast cancer or could not complete a questionnaire in English or Spanish.
Data Collection
Patients were identified via rapid case ascertainment and surveyed a mean of 9 months (Time 1) and again approximately 4 years (Time 2) later. The Dillman method26 was used to encourage response, including a small cash incentive. In LA, study packets were sent in both English and Spanish to those with Spanish surnames.27
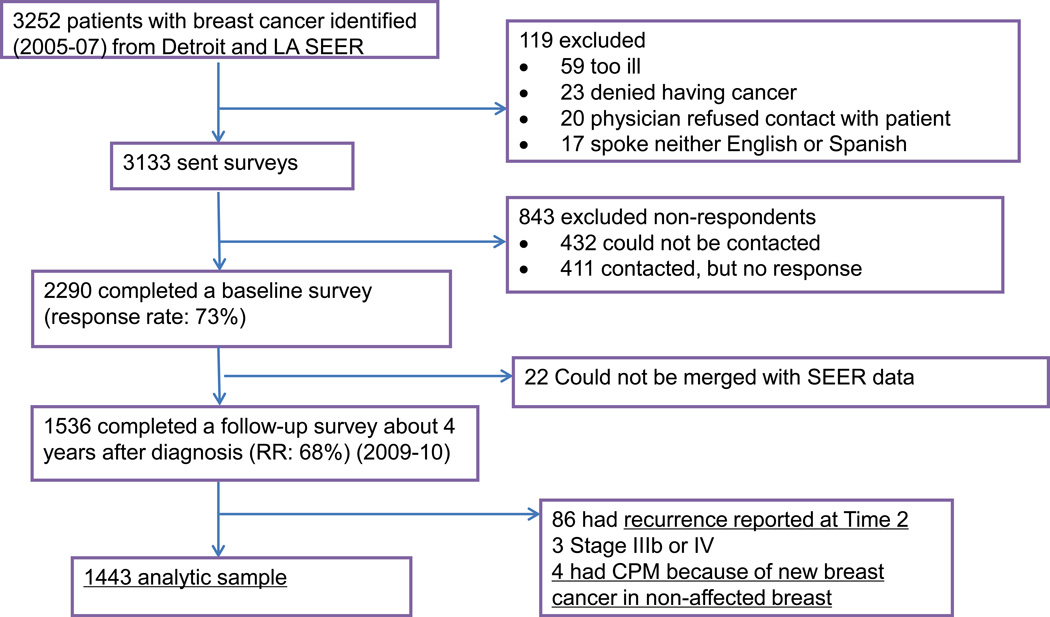
Time 1 and Time 2 datasets were combined and merged with SEER data to create a single dataset. The evolution of the sample is detailed in Figure 1.
Figure 1.
Study Flow Diagram
The study protocol, including all human subject involvement, was approved by the institutional review boards of the University of Michigan, the University of Southern California, and Wayne State University. All participants received information about the study’s purpose, the risks and benefits of participation, and patient confidentiality. A waiver of documentation of signed informed consent was obtained from participating sites.
Main Outcome Measures
Questionnaires were developed based on theoretical models, including measures previously developed to assess relevant constructs. The primary outcome variable was the initial surgical treatment the patient received obtained from patient self-report; UM and BCS were collected at Time 1 and CPM at Time 2. Women who indicated their double mastectomy was done because of a new breast cancer were excluded (N=4). We also assessed whether women had considered CPM.
Independent variables
The primary independent variables were measures of the two main clinical indications for CPM, obtained at Time 2, including a positive genetic test result indicating a BRCA1 or BRCA2 mutation or a family history of two or more first degree relatives with breast or ovarian cancer.3,28–29 Genetic testing was described in the survey, and respondents were asked if they had undergone a test. Response options included having had no test, or having had a test with a negative result, an unclear or unknown result, or a deleterious BRCA1 or BRCA2 mutation (i.e., a positive result). Respondents were asked to indicate their family history for breast and ovarian cancers with response options of none, one first degree relative, two or more first degree relatives (defined as a strong family history for analysis). Genetic testing and family history were evaluated separately in models, however for some analyses, respondents who had a positive genetic test and/or a strong family history were defined as having a clinical indication(s) for CPM, while respondents without these factors were considered not to have a clinical indication.
To assess worry about recurrence, we evaluated two questions from Time 1 asking respondents to rate how important two issues were in making their surgical decision (from not at all to very): 1) keeping them from worrying about the cancer coming back; and 2) reducing the chances of the cancer coming back. These questions were averaged then dichotomized to create a binary variable reflecting the overall importance of worry (less vs. very important) at the time of treatment decision making20. The MRI test was described in the survey, and its use as part of the diagnostic workup assessed by asking, “Did you have an MRI when you were first diagnosed with breast cancer?” (yes/no/don’t know). Breast size was assessed through self-reported bra cup size at Time 2 (small: A or B cup, large: C or larger cup).
We controlled for patient reported demographic factors from Time 1, including age at diagnosis (<49, 50–64 and >65), education level (< high school graduate, > some college), marital status, annual household income (< $49,000, $50–89,000, ≥$90,000, and unknown/missing), and race/ethnicity ( Latina, African American, white, or “other”). Tumor stage was obtained from SEER.
Statistical Analysis
We generated descriptive statistics for all variables and evaluated associations between the primary outcome variable (BCS, UM or CPM) and independent variables. Chi-square tests were used to test for differences in surgical treatment and categorical independent variables, and analysis of variance (ANOVA) for continuous variables, with Wald (F) tests uses for group variables. We compared receipt of CPM by clinical indication(s) using chi-square tests. All statistical tests were two-sided, and a P value less than 0.05 was considered statistically significant.
We conducted multinomial logistic regression (MNL) to evaluate factors associated with our 3-category outcome measure and to generate relative risk ratios (RRRs). The MNL method is recommended in cases with categorically distributed dependent variable that are not naturally ordered, and allowed us to compare factors associated with receipt of CPM to both BCS and UM.30 The first model used BCS as the base category against which we compared UM and CPM. 30 To allow for comparison of CPM to UM, we ran a second MNL model using UM as the base category. Each model controlled for all demographic and clinical factors.
We used the results of the two MNL models to generate predicted probabilities for each type of surgery for women with each category of genetic testing, family history, and both levels of worry about recurrence. All analyses done in STATA 11.0 and were weighted using survey procedures to account for differential probabilities of sample selection and non-response.
RESULTS
Description of the sample
The sample had a mean age of 59.1 (range: 25–79) and was racially and ethnically diverse. Slightly over half were married/partnered (57%) and had at least some college (58.8%). About half of the respondents (57.8%) received BCS, one third (34.5%) received UM, and 7.7% received CPM (Table 1). Approximately 19% of patients who received any mastectomy elected to undergo CPM. Many more women considered CPM than ultimately received it: 18.9% of the full sample of respondents reported considering CPM “quite a bit or very strongly.” Of those who strongly considered CPM, 45.8% ultimately received UM and 22.8% received BCS. Eighty percent of women who received CPM indicated it was done “to prevent breast cancer from developing in my other breast.” Most women who opted for CPM received breast reconstruction (86% vs. 54% of those who received UM, P<0.001).
Table 1.
Description of the sample (N=1443)
| Variable | Weighted % (N)1 | Weighted % with CPM | p-value |
|---|---|---|---|
| Outcome variables | |||
| Type of surgery | |||
| CPM | 7.9 (106) | --- | |
| UM | 34.4 (458) | --- | |
| BCS | 57.6 (879) | --- | |
| Considered CPM | |||
| Not at all/a little/somewhat | 81.2 (1147) | 2.1 | P<0.001 |
| Quite a bit/very strongly | 18.9 (251) | 32.2 | |
| Patient demographic and clinical factors | |||
| Age at diagnosis | |||
| ≤ 49 | 25.9 (370) | 12.3 | |
| 50–64 | 44.8 (660) | 7.1 | P<0.001 |
| ≥ 65 | 29.3 (417) | 4.9 | |
| Race/ethnicity | |||
| White | 46.8 (672) | 11.6 | |
| African American | 21.0 (321) | 3.4 | |
| Latina-low acculturation | 15.6 (221) | 3.0 | P<0.001 |
| Latina-high acculturation | 14.6 (201) | 8.7 | |
| Other | 2.0 (32) | 3.1 | |
| Education | |||
| High school graduate or less | 41.1 (546) | 2.7 | P<0.001 |
| Some college or more | 58.8 (901) | 11.8 | |
| Marital status | |||
| Married/partnered | 57.1 (615) | 6.3 | P=0.151 |
| Not married | 42.9 (832) | 9.3 | |
| Income (annual family) | |||
| ≤ $49,000 | 42.7 (611) | 5.4 | |
| $50,000–$89,999 | 20.7 (314) | 8.2 | P=0.001 |
| ≥$90,000 | 17.3 (262) | 14.9 | |
| Missing/don’t know | 19.2 (261) | 6.9 | |
| Cancer stage | |||
| Stage 0 (DCIS) | 19.3 (365) | 6.9 | |
| Stage I | 34.6 (525) | 6.9 | |
| Stage II | 32.9 (400) | 8.3 | P=0.067 |
| Stage IIIa | 7.1 (85) | 12.4 | |
| Unknown | 6.1 (72) | 9.3 | |
| Receipt of MRI | |||
| Yes | 41.5 (588) | 10.9 | P=0.001 |
| No/don’t know | 58.5 (840) | 5.3 | |
| Worry about recurrence | |||
| Low worry | 21.9 (309) | 2.7 | P=0.001 |
| High worry | 78.1 (1,085) | 10.0 | |
| Breast size (based on bra cup size) | |||
| Small | 31.3 | 7.6 | P=0.122 |
| Large | 68.7 | 8.0 | |
| Genetic testing | |||
| Not tested | 80.5 (1,056) | 5.4 | |
| Tested-positive result | 1.8 (22) | 51.4 | P<0.001 |
| Tested-negative result | 12.5 (160) | 20.8 | |
| Tested-unknown result | 5.2 (68) | 6.8 | |
| Family history of breast/ovarian cancer | |||
| No first degree relatives | 63.7 (919) | 6.6 | |
| One first degree relative | 27.6 (398) | 7.4 | P=0.001 |
| Two or more first degree relatives | 8.6 (124) | 20.5 | |
| Clinical indication(s) for CPM (positive genetic test and/or 2+ first degree relatives with breast or ovarian cancer) |
|||
| Yes | 9.4 (136) | 24.3 | P<0.001 |
| No | 90.6 (1,314) | 5.8 | |
All N’s do not add to 1443 due to missing values on some questions
About 10% of respondents had a clinical indication for CPM. Most women (78.1%) indicated worry about recurrence was very important at the time of treatment decision making. The bivariate comparisons found significant (P<0.05) differences in receipt of CPM according to patient age, race/ethnicity, education, income, genetic testing, strong family history, receipt of MRI, and greater worry about recurrence. Of the 106 women who received CPM, 31.1% had a clinical indication(s), while the remaining majority of women (68.9%) did not (P<0.001).
Factors associated with receipt of surgery for breast cancer
Table 2 shows the MNL results comparing CPM to UM (column 2) and CPM to BCS (column 3), and UM to BCS (column 4). Compared to UM, women who received CPM had higher educational attainment (RRR: 5.04; 95% CI 2.37–10.7), had a positive or negative genetic test (RRR: 10.5; 95% CI: 3.71–30.5 and RRR: 2.17; 95% CI 1.13–4/15, respectively), had a strong family history of breast cancer (RRR: 5.19; 95% CI 2.34–11.6), had received a diagnostic MRI (RRR: 2.07; 95% CI: 1.21-3.52), and worry about recurrence was very important (RRR: 2.81; 95% CI: 1.14-6.88). All these factors were also statistically significantly associated with receipt of CPM relative to BCS (see Table 2 for RRRs), however African American women were significantly less likely to receive CPM vs. BCS relative to white women (RRR: 0.25; 95% CI 0.11-0.56). We ran two sensitivity analyses with different MNL model specifications. The first excluded patients with stage IIIa cancer, to account for the possibility that some of those women may have been recommended mastectomy. The second allowed for a broader definition of family history (1+ first degree relative). Neither analyses showed any substantive differences from the results presented in Table 2. Our MNL model also produced results comparing UM vs. BCS which are presented in Table 2.
Table 2.
Multinomial Logistic Regression Model Results of factors associated with surgery (N=1447)
| CPM vs. UM RRR; 95% CI |
CPM vs. BCS RRR; 95% CI |
UM vs. BCS RRR; 95% CI |
|
|---|---|---|---|
| Age | |||
| ≤49 | 1.56 (0.67–3.61) | 2.42 (1.08–5.44) | 1.55 (1.07–2.33) |
| 50–64 | 1.11 (0.50–2.47) | 1.31 (0.60–2.84) | 1.18 (0.84–1.69) |
| ≥65 | REF | REF | REF |
| Wald F-test (P-value) | 1.31 (P=0.143) | 9.74 (P=0.008) | 13.80 (P=0.001) |
| Race/ethnicity | |||
| White | REF | REF | REF |
| African American | 0.34 (0.11–1.02) | 0.25 (0.11–0.56) | 2.65 (1.68–4.17) |
| Latina | 0.39 (0.19–0.97) | 0.81 (0.28–2.31) | 1.25 (0.86–3.94) |
| Other | 0.16 (0.05–4.92) | 0.19 (0.02–2.42) | 1.26 (0.61–3.28) |
| Wald F-test (P-value) | 15.37 (P=0.004) | 13.08 (P=0.011) | 24.18 (P<0.001) |
| Education (some college or more vs. high school or less) |
5.04 (2.37–10.71) | 4.38 (2.07–9.29) | 0.87 (0.62–1.12) |
| Marital status (not married vs. married/partnered) |
1.02 (0.58–1.81) | 0.87 (0.51–1.53) | 0.86 (0.63–1.16) |
| Income | |||
| < $49,000 | REF | REF | REF |
| $50-000–$89,999 | 0.99 (0.45–2.15) | 0.69 (0.33–1.45) | 0.70 (0.46–1.05) |
| >$90,000 | 0.95 (0.42–2.16) | 0.97 (0.45–2.12) | 1.02 (0.71–1.60) |
| Missing/don’t know | 1.23 (0.54–2.82) | 1.32 (0.59–2.96) | 1.07 (0.76–1.66) |
| Wald F-test (P-value) | 0.76 (P=0.521) | 3.32 (P=0.764) | 4.71 (P=0.247) |
| Cancer stage | |||
| Stage 0 | REF | REF | REF |
| Stage I | 0.66 (0.33–1.32) | 0.56 (0.29–1.07) | 0.85 (0.59–1.22) |
| Stage II | 0.52 (0.25–1.07) | 0.93 (0.45–1.79) | 1.75 (1.22–2.53) |
| Stage IIIa* | 0.51 (0.21–1.27) | 2.21 (0.91–5.40) | 4.28 (2.32–7.89) |
| Unknown | 0.32 (0.04–2.31) | 0.58 (0.07–4.54) | 1.82 (0.86–4.27) |
| Wald F-test (P-value) | 0.72 (P=0.462) | 14.38 (P=0.006) | 45.50 (P<0.001) |
| Worry about recurrence (high vs. low/moderate) |
2.81 (1.14–6.88) | 4.24 (1.80–9.98) | 1.50 (1.07–2.14) |
| MRI receipt (yes vs. no/DK) | 2.07 (1.21–3.52) | 2.14 (1.28–3.58) | 1.04 (0.79–1.38) |
| Breast size (larger vs. smaller) | 1.59 (0.94–2.70) | 1.08 (0.65–1.78) | 0.66 (0.51–0.90) |
| Genetic testing | |||
| Not tested | REF | REF | REF |
| Tested-positive result | 10.48 (3.61–30.48) | 19.10 (5.67–56.41) | 1.81 (0.50–6.42) |
| Tested-negative result | 2.17 (1.13–4.15) | 2.26 (1.25–4.07) | 1.05 (0.66–1.69) |
| Tested-unknown result | 0.72 (0.22–2.39) | 1.32 (0.41–4.23) | 2.10 (1.00–4.10) |
| Wald F-test (P-value) | 15.95 (P=0.004) | 28.74 (P<0.001) | 6.60 (P=0.084) |
| Family history of breast/ovarian |
|||
| No family history | REF | REF | REF |
| One first degree relative | 1.40 (0.78–2.51) | 0.98 (0.57–1.71) | 0.70 (0.51–0.98) |
| 2+ first degree relatives^ | 5.19 (2.34–11.56) | 4.24 (1.80–9.88) | 1.00 (0.58–1.65) |
| Wald F-test (P-value) | 19.32 (P<0.001) | 25.93 (P<0.001) | 5.21 (P=0.131) |
When models were run with stage IIIa cancer excluded (N=1362), there were no substantial changes to the results presented above.
When models were run considering a broader definition of family history, there were no substantial changes to the results presented above. Family history of 1+ first degree relative remained associated with CPM relative to UM (RRR:2.03; 95% CI;1.24–3.42) and to BCS (RRR:1.62; 95% CI: 1.05–2.76)
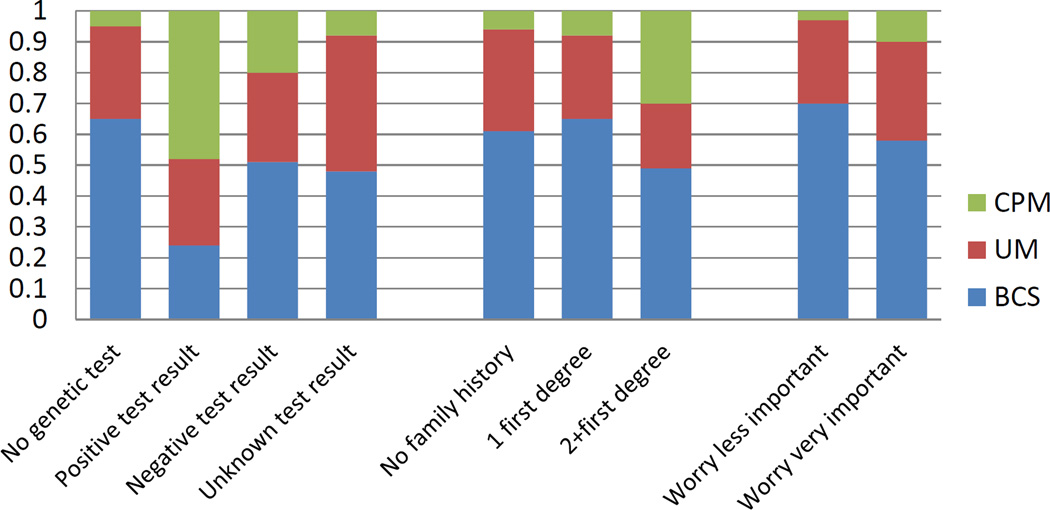
Figure 2 shows the predicted probabilities of receipt of type of surgery (CPM, UM and BCS) separately according to each clinical indication and worry about recurrence, adjusted for demographic and clinical factors. Not all women at higher than average risk for a contralateral breast cancer opted for CPM: the probability of BCS or UM among those with a positive genetic test was 24% and 28%, respectively. Among those with a strong family history, the probability of BCS or UM was 49% and 21%, respectively. Figure 2 also shows that the probability of surgery among by importance placed on worry about recurrence; among those reporting it was very important, the probability of BCS was 58.1%, followed by 32% for UM and 10% for CPM.
Figure 2.
Predicted probabilities of receipt of treatments by clinical indications and worry about recurrence
Adjusted for age, race/ethnicity, education, income, stage and study site
Associations between consideration of CPM and receipt of surgery
Of the 251 women who strongly considered CPM, we found that those who ultimately got CPM (N=81) were significantly different from those who ultimately received UM or BCS (N=170). The former group was significantly (P<0.01) more often white (71.6% vs. 22.9%), highly educated (88.9% vs. 44.9%), and were very worried about recurrence (93.8% vs. 80.1%). This concordant group also more often had a clinical indication for CPM (27.2% vs. 6.5%, P<0.001).
COMMENT
Rates of CPM have been increasing over the past decade, despite the fact that very few women with a new diagnosis of breast cancer are likely to experience a survival benefit from electing this procedure. We found that many women in our population-based sample from two geographic areas reported that they strongly considered having their non-affected breast removed as part of their initial treatment for breast cancer. Consistent with others, we found that about 8% of newly diagnosed patients (18.7% of mastectomy-treated patients) actually received CPM,7–8 and that this rate was higher for women with more education.10,16 Reflective of concerns about the impact of testing on overtreatment, women in our sample who had received an MRI at diagnosis more often received CPM than other surgeries. While other studies have suggested that increased MRI use during diagnosis may contribute to higher rates of CPM,11–13 to our knowledge this is the first population-based study to confirm this based on the reports of breast cancer patients themselves.
Our finding that clinical indications that elevate the risk of developing a new primary breast cancer (i.e., positive genetic mutation or a strong family history) in the non-affected breast were associated with receipt of CPM is consistent with other studies.14–16 Yet our results also distinctly contribute to the CPM literature. First, we found that most women who received CPM (nearly 70%) did not have either of the clinical indications evaluated and in fact some (20.8%) had a negative test result. Perhaps even more interestingly, nearly a fifth of our sample strongly considered CPM, yet many who did so ultimately received either UM (45.8%) or BCS (22.8%). In addition, although those women who strongly considered but did not receive CPM less often had clinical indications, they more often had higher worry about recurrence. These results suggest that both clinical and non-clinical factors motivate many patients to consider the operation.
One such prevalent and powerful non-clinical factor illustrated in our results and those of others is fear of disease recurrence.15,31 A patient’s decision to undergo CPM based on a strong fear of recurrence in the absence of clinical indications presents an important clinical challenge for surgeons.32 Patients at average risk for developing a contralateral cancer who are considering CPM should clearly understand the potential adverse consequences of CPM, including lengthy recovery time and the increased risk for serious operative complications,33–36 and to weigh that against the lack of empirical evidence that the procedure improves disease free survival from the cancer which is already present.36 There is a growing literature that supports the notion that patients have a difficult time assessing and interpreting their own risk, and that fear and anxiety related to disease recurrence often supersede accurate risk perceptions to drive health decisions.37–39
Our results provide evidence that decisions about CPM represent a clear case where better strategies to increase patient knowledge about their own risk of developing contralateral cancer, as well as the net benefit of treatment are needed, and should only be made after patients are accurately informed about these issues.40 Educational materials and decision tools for average-risk patients making initial breast cancer treatment decisions typically do not include information about CPM, detailed information about actual risk of contralateral breast cancer, or about interpretation of genetic test results. Such information could be useful for women making these decisions.31,40 However, our findings that CPM was strongly associated with higher educational attainment suggests that improved knowledge may not be sufficient to address patient factors, such as worry about recurrence, motivating strong consideration of the procedure. Furthermore, the association found between diagnostic MRI and receipt of CPM indicates a need to consider strategies for educating both patients and clinicians about the impact of extensive testing on treatment decision making. Strategies should also include ensuring clinicians have better understanding about the strong role of patient attitudes, including worry about recurrence, in choice of treatment.32
Some limitations of the study merit comment. Although population-based, our data came from two urban geographic areas and likely cannot be generalized to other areas. Many of our variables were obtained from patient self-report and may be subject to recall bias. In particular, inaccurate patient recall of genetic testing results could have under-estimated the proportion of patients with positive tests who underwent CPM and over-estimated the proportion with negative tests who received CPM. We cannot be sure if the timing of patient reports of genetic testing happened prior to or following surgery. Although we excluded women who reported CPM was done because of a new breast cancer, we cannot be totally sure that other women who received CPM did not have bilateral breast cancer. Additionally, we did not evaluate history of radiotherapy to the chest region or the finding of atypia on benign breast biopsies which are known to increase breast cancer risk, nor did we determine if the use of CPM varied with estrogen receptor status. Although we had information about receipt of breast reconstruction, we were not able to assess whether women decided to get CPM in order to have bilateral reconstruction. Finally, although response rates were high, we lost respondents from the baseline to follow survey which may have influenced the results.
CONCLUSION
A woman’s decision to have her non-affected breast removed at the same time as her affected breast represents the most extensive surgical option available for breast cancer patients, since most women who undergo CPM also receive bilateral breast reconstruction. Indeed, our study shows that many women who opted for CPM were candidates for breast conservation. The growing rate of CPM has motivated some surgeons to question whether it’s justified to perform an extensive operation which is not clinically indicated to reduce the fear of disease recurrence.35 Increased attention by surgeons coupled with decision tools directed at patients to aid in the delivery of risk and benefit information and to facilitate discussion could reduce the possibility of overtreatment in breast cancer.
ACKNOWLDEGEMENT
This work was funded by two grants from the National Institutes of Health, R01 CA109696 and R01 CA088370 to the University of Michigan. The funding agency had no involvement in the design or implementation of the study or in the analysis of the results. The authors would like to acknowledge the patients who completed the surveys at both time points for this study. Drs. Hawley and Katz had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis presented in this manuscript and take responsibility for the integrity of the data and the accuracy of the analysis.
Footnotes
None of the authors have conflicts of interest to disclose.
References
- 1.Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2010;(11):CD002748. doi: 10.1002/14651858.CD002748.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Nichols HB, Berrington de González A, Lacey JV, Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29(12):1564–1569. doi: 10.1200/JCO.2010.32.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giuliano AE, Boolbol S, Degnim A, et al. Society of Surgical Oncology: position statement on prophylactic mastectomy. Approved by the Society of Surgical Oncology Executive Council, March 2007. Ann Surg Oncol. 2007;14(9):2425–2427. doi: 10.1245/s10434-007-9447-z. Epub 2007 Jun 28. [DOI] [PubMed] [Google Scholar]
- 4.Tuttle TM, Abbott A, Arrington A, Rueth N. The increasing use of prophylactic mastectomy in the prevention of breast cancer. Curr Oncol Rep. 2010;12:16–21. doi: 10.1007/s11912-009-0070-y. [DOI] [PubMed] [Google Scholar]
- 5.Herrinton LJ, Barlow WE, Yu O, et al. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a cancer research network project. J Clin Oncol. 2005;23:4275–4286. doi: 10.1200/JCO.2005.10.080. [DOI] [PubMed] [Google Scholar]
- 6.van Sprundel TC, Schmidt MK, Rookus MA, et al. Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. Br J Cancer. 2005;93:287–292. doi: 10.1038/sj.bjc.6602703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25(33):5203–5209. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 8.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27(9):1362–1367. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 9.Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(Suppl 3):S108–S110. doi: 10.1016/S0960-9776(11)70306-X. [DOI] [PubMed] [Google Scholar]
- 10.Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998–2007. Ann Surg Oncol. 2010;17(10):2554–2562. doi: 10.1245/s10434-010-1091-3. [DOI] [PubMed] [Google Scholar]
- 11.Miller BT, Abbott AM, Tuttle TM. The influence of preoperative MRI on breast cancer treatment. Ann Surg Oncol. 2012;19(2):536–540. doi: 10.1245/s10434-011-1932-8. [DOI] [PubMed] [Google Scholar]
- 12.Jatoi I, Benson JR. The case against routine preoperative breast MRI. Future Oncol. 2013;9(3):347–353. doi: 10.2217/fon.12.186. [DOI] [PubMed] [Google Scholar]
- 13.Brennan ME, Houssami N, Lord S, et al. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: systematic review and meta-analysis of incremental cancer detection and impact on surgical management. J Clin Oncol. 2009;27(33):5640–5649. doi: 10.1200/JCO.2008.21.5756. [DOI] [PubMed] [Google Scholar]
- 14.Arrington AK, Jarosek SL, Virnig BA, et al. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol. 2009;16:2697–2704. doi: 10.1245/s10434-009-0641-z. [DOI] [PubMed] [Google Scholar]
- 15.Yi M, Hunt KK, Arun BK, et al. Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer Prev Res. 2010;3:1026–1034. doi: 10.1158/1940-6207.CAPR-09-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29(16):2158–2164. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- 17.SEER Training Module. Cancer Registration and Surveillance modules. U.S. National Institutes of Health, National Cancer Institute; [Accessed March 1, 2013]. http://training.seer.cancer.gov/. [Google Scholar]
- 18.Hawley ST, Fagerlin A, Janz NK, et al. Racial/ethnic disparities in knowledge about risks and benefits of breast cancer treatment: does it matter where you go? Health Services Research. 2009;43(4):1366–1387. doi: 10.1111/j.1475-6773.2008.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawley ST, Janz NK, Hamilton A, et al. Latina patient perspectives about informed treatment decision-making for breast cancer. Patient Educ Couns. 2008;73(2):363–370. doi: 10.1016/j.pec.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawley ST, Griggs JJ, Hamilton AS, et al. Decision Involvement and Receipt of Mastectomy Among Racially and Ethnically Diverse Breast Cancer Patients. J Natl Cancer Inst. 2009;101(19):1337–1347. doi: 10.1093/jnci/djp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janz NK, Mujahid MS, Hawley ST, et al. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008;113(5):1058–1067. doi: 10.1002/cncr.23660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton AS, Hofer T, Hawley ST, et al. Latinas and Breast Cancer Outcomes: Population-based sampling, Ethnic identity and Acculturation Assessment. Cancer Epidemiology, Biomarkers & Prevention. 2009;18(7):2022–2029. doi: 10.1158/1055-9965.EPI-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawley ST, Janz NK, Lillie SE, et al. Perceptions of care coordination in a population-based sample of diverse breast cancer patients. Patient Educ Couns. 2010;81(Suppl):S34–S40. doi: 10.1016/j.pec.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302(14):1551–1556. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jagsi R, Abrahamse P, Morrow M, et al. Postmastectomy radiotherapy for breast cancer: patterns, correlates, communication, and insights into the decision process. Cancer. 2009;115(6):1185–1193. doi: 10.1002/cncr.24164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dillman DA. Mail and Telephone Surveys. New York, NY: John Wiley and Sons Inc; 1978. [Google Scholar]
- 27.Word D, Perkins JR. Building a Spanish surname list for the 1990’s—a new approach to an old problem. U.S. Census Bureau; 1996. technical working paper 13. [Google Scholar]
- 28.Haffty BG, Harrold E, Khan AJ, et al. Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet. 2002;359(9316):1471–1477. doi: 10.1016/S0140-6736(02)08434-9. [DOI] [PubMed] [Google Scholar]
- 29.Metcalfe KA, Lynch HT, Ghadirian P, et al. The risk of ovarian cancer after breast cancer in BRCA1 and BRCA2 carriers. Gynecol Oncol. 2005;96(1):222–226. doi: 10.1016/j.ygyno.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 30.Agresti A. Categorical Data Analysis. New York: Wiley-Interscience; 2002. [Google Scholar]
- 31.Brewster AM, Parker PA. Current knowledge on contralateral prophylactic mastectomy among women with sporadic breast cancer. Oncologist. 2011;16(7):935–941. doi: 10.1634/theoncologist.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz SJ, Morrow M. The challenge of individualizing treatments for patients with breast cancer. JAMA. 2012;307(13):1379–1380. doi: 10.1001/jama.2012.409. [DOI] [PubMed] [Google Scholar]
- 33.Miller ME, Czechura T, Martz B, Hall ME, Pesce C, Jaskowiak N, Winchester DJ, Yao K. Operative Risks Associated with Contralateral Prophylactic Mastectomy: A Single Institution Experience. Ann Surg Oncol. 2013 Jul 19; doi: 10.1245/s10434-013-3108-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Osman F, Saleh F, Jackson TD, Corrigan MA, Cil T. Increased Postoperative Complications in Bilateral Mastectomy Patients Compared to Unilateral Mastectomy: An Analysis of the NSQIP Database. Ann Surg Oncol. 2013 Jul 12; doi: 10.1245/s10434-013-3116-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Katz SJ, Morrow M. Contralateral Prophylactic Mastectomy for Breast Cancer: Addressing Peace of Mind. JAMA. 2013 Aug 1; doi: 10.1001/jama.2013.101055. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Tuttle T, Habermann E, Abraham A, Emory T, Virnig B. Contralateral prophylactic mastectomy for patients with unilateral breast cancer. Expert Rev Anticancer Ther. 2007 Aug;7(8):1117–1122. doi: 10.1586/14737140.7.8.1117. [DOI] [PubMed] [Google Scholar]
- 36.Zikmund-Fisher BJ, Fagerlin A, Ubel PA. Risky feelings: why a 6% risk of cancer does not always feel like 6% Patient Educ Couns. 2010 Dec;81(Suppl):S87–S93. doi: 10.1016/j.pec.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blank T, Graves K, Sepucha K, Llewellyn-Thomas H. Understanding treatment decision making: contexts, commonalities, complexities, and challenges. Ann Behav Med. 2006 Dec;32(3):211–217. doi: 10.1207/s15324796abm3203_6. [DOI] [PubMed] [Google Scholar]
- 38.Klein WM, Stefanek ME. Cancer risk elicitation and communication: lessons from the psychology of risk perception. CA Cancer J Clin. 2007 May-Jun;57(3):147–167. doi: 10.3322/canjclin.57.3.147. [DOI] [PubMed] [Google Scholar]
- 39.Barry M, Sacchini V. When is contralateral mastectomy warranted in unilateral breast cancer? Expert Rev Anticancer Ther. 2011;11(8):1209–1214. doi: 10.1586/era.11.100. [DOI] [PubMed] [Google Scholar]