Abstract
In holoendemic Plasmodium falciparum transmission regions, malarial anemia is a leading cause of childhood morbidity and mortality. Identifying biomarkers of malaria disease severity is important for identifying at-risk groups and for improved understanding of the molecular pathways that influence clinical outcomes. We have previously shown that decreased cyclooxygenase (COX)-2-derived prostaglandin E2 (PGE2) levels are associated with enhanced clinical severity in cerebral malaria, malarial anemia, and malaria during pregnancy. Since children with malaria often have increased incidence of additional infections, such as bacteremia and HIV-1, we extend our previous findings by investigating COX-2 and PGE2 in children with falciparum malaria and co-infection with either bacteremia or HIV-1. Plasma bicyclo-PGE2/creatinine levels and peripheral blood COX-2 transcripts were significantly reduced in co-infected children relative to those with malaria mono-infection. Furthermore, suppression of circulating bicyclo-PGE2 was significantly associated with reduced hemoglobin levels in both mono- and co-infected children with malaria, suggesting that bicyclo-PGE2 may represent both a marker and mediator of malaria pathogenesis.
Keywords: PGE2, COX-2, HIV-1, Bacteremia, SMA, Anemia
1. INTRODUCTION
Plasmodium falciparum malaria is the most prevalent form of malaria in sub-Saharan Africa. Severe malaria in African children often presents as a diverse clinical spectrum, ranging from mild infections to life-threatening complications such as severe anemia, cerebral malaria, hypoglycemia, acute renal failure, and acidosis/respiratory distress [1–3]. In falciparum malaria holoendemic transmission regions of western Kenya, severe malarial anemia (SMA; Hb<6.0g/dL, with any density parasitemia) is the primary clinical manifestation of severe disease [4], often exacerbated by the presence of co-infections, including HIV-1, bacteremia, and upper respiratory tract viral infections [5–7].
Central to the pathogenesis of malarial anemia is the release of host innate immune mediators generated in response to parasite products [4]. Our laboratory has identified clinical predictors associated with enhanced pathogenesis of SMA [8] and inflammatory biomarkers of pediatric severe anemia [9]. Additional investigations revealed unique hematological predictors and inflammatory mediator patterns associated with the worsening anemia observed in children co-infected with falciparum malaria and HIV-1 [10,11]. Among the various effector molecules implicated in pathogenesis of severe malaria is cyclooxygenase (COX)-2-derived prostaglandin E2 (PGE2), a potential biomarker that is inversely associated with disease severity in cerebral malaria, malarial anemia, and malaria during pregnancy [12–14]. In addition, we recently discovered that suppression of COX-2-derived PGE2 in children with falciparum malaria was associated with reduced erythropoiesis and worsening anemia [15]. Given the important role of co-infections in contributing to severe malaria pathogenesis [5,7], the current study investigated the COX-2-PGE2 pathway in children with malaria that were co-infected with either bacteremia or HIV-1.
COX-2 (prostaglandin endoperoxide H2 synthase-2) is an inducible enzyme expressed in cells involved in inflammatory reactions [16,17], and when up-regulated by pro-inflammatory mediators, can generate high levels of PGE2 to modulate the host-immune response to infections [18–22]. Since PGE2 and its metabolites are unstable in vivo, levels of PGE2 are measured as bicyclo-PGE2 (the stable breakdown product of PGE2 and 13,14-dihydro-15-keto PGE2) and can be expressed relative to creatinine levels to account for differences in hydration status [23,24].
2. MATERIALS AND METHODS
2.1. Study site
The study was carried out at the Siaya District Hospital (SDH) in western Kenya. Although the last comprehensive malaria prevalence survey was conducted more than a decade ago and indicated ~83% infection in children between one and four years [25], from mid-2006 to date the area is characterized by an increase in pediatric malaria admissions [26]. In this holoendemic P. falciparum transmission region, malarial anemia is the primary cause of hospital-associated morbidity and mortality [27]. Information about the study site and malarial anemia in the pediatric population are described in our previous report [28].
2.2. Study participants
Parasitemic children (aged 3–36 months; n=101) were recruited at SDH after their parents/guardians provided informed written consent. All children were screened for HIV-1 infection using two rapid serological antibody tests and confirmed for HIV-1 positivity by pro-viral DNA according to our previous methods [5]. None of the children were receiving antiretroviral medication at the time of recruitment. Parents and/or guardians received pre- and post HIV-1/AIDS counseling. Bacteremia was determined according to our previous methods [7]. Based on screening results, children were grouped into three categories: those with falciparum malaria alone [Pf(+), n=74], malaria plus bacteremia [Pf(+)/Bac(+), n=19], or malaria plus HIV-1 [Pf(+)/HIV-1 (+), n=8]. Children were excluded from the study if they had mixed malaria species infections, prior hospitalization (for any reason), antimalarial and/or antipyretic treatment within two weeks prior to enrollment, and/or cerebral malaria. Patients were treated and provided supportive care according to the Ministry of Health (MOH)-Kenya guidelines. The study was approved by the Ethics Committees of the Kenya Medical Research Institute and University of New Mexico Institutional Review Board.
2.3. Laboratory evaluations
Venipuncture blood samples (<3.0 mL) were collected from enrolled participants before any treatment interventions. Complete blood counts were determined using the Beckman Coulter AcT diff2™ (Beckman-Counter Corporation, Miami, FL, USA). Asexual malaria trophozoites in thick and thin peripheral blood smears, and reticulocyte count were determined according to previous methods [29]. Inflammatory mediator patterns (cytokines and growth factors) were determined in children (n=62) grouped into [Pf(+), n=40], [Pf(+)/Bac(+), n=15], and [Pf(+)/HIV-1(+), n=7] using a Cytokine 25-plex Antibody Bead Kit, Human (BioSource™ International, CA, USA) as previously described [9,10]. Sickle cell trait (HbAS) and glucose-6-phosphate dehydrogenase (G6PD) deficiency were determined according to our published methods [30].
2.4. Determination of bicyclo-PGE2 and creatinine levels and COX-2 gene expression
Since PGE2 has a high turnover rate in peripheral circulation, the PGE2 metabolites (13,14-dihydro-15-keto PGA2 and 13,14-dihydro-15-keto PGE2) were converted to single derivatives (stable end product bicyclo-PGE2). Bicyclo-PGE2 levels were measured in plasma samples according the manufactures’ instructions (Cayman Chemical Company, MI, USA) and our recently reported methods [15].
Plasma creatinine levels were determined using the creatinine determination kit (Cayman Chemicals Company, MI, USA). Plasma samples were diluted 1:20 with ultra-pure water and creatinine was quantified by enzyme immunoassay (ELISA) according to the manufacturers’ protocol (Cayman Chemicals Company, MI, USA).
Total RNA was isolated from cryo-preserved white blood cell pellets (preserved in commercial RNAlater® RNA stabilization reagent [Qiagen, CA, USA]) by the acid guanidinium thiocyanate-phenol-chloroform extraction method [31]. Reverse transcription of RNA to complementary DNA (cDNA) was performed using the high capacity cDNA reverse transcription kit (Applied Biosystems, CA, USA) and our previously published reaction conditions [15]. Resulting complementary DNA (cDNA) was amplified for 30 cycles using oligonucleotides spanning the exon-intron junction in the COX-2 gene, with the sense (5′-GAC TCC CTT GGG TGT CAA AGG TAA-3′) and antisense (5′-GTG AAG TGC TGG GCA AAG AAT G-3′) sequence to generate a 138bp product according to our previous methods [15]. To normalize the amount of cDNA loaded per reaction, an internal control, the cyclophilin A (CYC-A) housekeeping gene was amplified in a 25 μL reaction, containing final concentrations of 0.3 μM each CYC-A sense oligo 5′-GTC TCC TTT GAG CTG TTT GC-3′ and antisense oligo 5′-AAG CAG GAA CCC TTA TAA CC-3′. Resulting fragments were resolved on 2% agarose gel stained with 0.5 mg/mL ethidium bromide (Sigma Chemicals Co. MO, USA) and visualized under UV (Sprectroline® Corporation, NY, USA). Electrophoretic gel films were analyzed using the ImageJ software [32] and PCR product mean band intensities quantified. The COX-2 mRNA expression mean values (arbitrary units; AU) were normalized by expressing them as ratios to CYC-A mRNA mean values.
2.5. Statistical Analyses
Analyses were computed using SPSS statistical software package 19 (IBM SPSS Inc., IL, USA). Proportions were compared using Pearson’s chi-square analysis, while across-group comparisons performed using Kruskal-Wallis test for median and ANOVA for mean levels. Bivariate analysis was performed using Mann-Whitney U test and Student’s t-tests for bicyclo-PGE2/creatinine levels and COX-2 mRNA transcripts, respectively. Correlation analysis was performed using Spearman’s rank test or Pearson’s zero-order rank test. Statistical significance was considered at P≤0.050.
3. RESULTS
3.1. Clinical and laboratory characteristics
Children enrolled in the study (n=101) were stratified into three groups: malaria alone [Pf(+); n=74], malaria plus bacteremia [Pf(+)/Bac(+); n=19], and malaria plus HIV-1 [Pf(+)/HIV-1(+); n=8]. As shown in Table 1, the demographic and clinical characteristics were comparable across groups. Similarly, hematological measures, erythropoietic and parasitological parameters, and genetic variants showed no significant across group differences. Although phagocytosis of P. falciparum hemozoin by monocytes is a driving factor for inflammatory mediator production [4], the percentage of pigment-containing monocytes was similar across the groups. Plasma bicyclo-PGE2 levels were decreased in children with co-infection (P<0.001), whereas plasma creatinine levels were elevated during co-infection (P=0.004), relative to those with malaria mono-infection.
Table 1.
Characteristics of study participants.
| Characteristics | Pf(+) | Pf(+)/Bac(+) | Pf(+)/HIV-1(+) | P |
|---|---|---|---|---|
| Sample size (n) | 74 | 19 | 8 | |
| Gender, n (%) | ||||
| Female | 29 (39.2) | 12 (63.2) | 5 (62.5) | 0.105a |
| Male | 45 (60.8) | 7 (36.8) | 3 (37.5) | |
| Age, months* | 10.5 (10.0) | 10.0 (12.0) | 7.0 (13.0) | 0.547b |
| Enrollment temperature, °C* | 37.7 (1.6) | 38.0 (1.9) | 38.0 (0.8) | 0.540b |
| Severe malarial anemia (Hb<5.0 g/dL), n (%) | 22 (29.7) | 5 (26.3) | 4 (50.0) | 0.448a |
| Severe malarial anemia (Hb<6.0 g/dL), n (%) | 36 (48.6) | 9 (47.4) | 5 (62.5) | 0.742a |
|
| ||||
|
Hematological Measures
| ||||
| Hemoglobin, g/dL* | 6.2 (3.3) | 6.0 (3.1) | 5.2 (3.5) | 0.847b |
| Hematocrit, % | 19.1 (9.8) | 19.7 (6.8) | 16.8 (12.2) | 0.921b |
| Red blood cells, ×106/μL* | 2.6 (1.4) | 2.9 (1.8) | 2.5 (1.8) | 0.737b |
| Red cell distribution width, %* | 21.3 (5.1) | 23.9 (5.7) | 21.7 (11.7) | 0.712b |
| Mean corpuscular volume, fL* | 70.7 (11.2) | 69.2 (10.9) | 73.2 (14.8) | 0.394b |
| Mean corpuscular hemoglobin, fL/cell* | 22.9 (4.0) | 22.0 (3.5) | 22.3 (5.5) | 0.248b |
| Mean corpuscular hemoglobin concentration, g/dL* | 32.3 (2.0) | 31.8 (3.5) | 30.3 (2.8) | 0.050b |
| White blood cells, ×109/L* | 11.1 (8.3) | 10.6 (8.5) | 10.4 (11.6) | 0.932b |
| Lymphocytes, ×103/μL* | 5.7 (4.2) | 5.8 (5.0) | 5.8 (7.5) | 0.850b |
| Monocytes, ×103/μL* | 1.2 (1.1) | 0.9 (0.9) | 1.2 (0.8) | 0.421b |
| Granulocytes, ×103/μL* | 4.6 (4.7) | 5.0 (4.4) | 4.0 (3.6) | 0.933b |
| Platelets, ×103/μL* | 0.12 (0.1) | 0.17 (0.2) | 0.12 (0.1) | 0.088b |
| Mean platelet volume, fL* | 8.1 (1.4) | 8.1 (1.4) | 9.3 (1.9) | 0.260b |
| Platelet distribution width, % | 17.6 (1.3) | 18.0 (1.7) | 17.6 (1.1) | 0.752b |
|
| ||||
|
Erythropoietic Parameters
| ||||
| Reticulocyte production index* | 0.83 (1.2) | 0.71 (1.4) | 0.91 (1.7) | 0.691b |
| RPI<2, n (%) | 66 (89.2) | 18 (94.7) | 6 (75.0) | 0.356a |
|
| ||||
|
Parasitological Parameters
| ||||
| Parasite density, MPS/μL* | 10,439 (33,027) | 6,514 (25,525) | 22,174 (37,660) | 0.345b |
| Geometric mean parasitemia, /μL | 9,564 | 5,227 | 15,301 | 0.687c |
| High density parasitemia (≥10,000/μL), n (%) | 38 (51.4) | 7 (36.8) | 6 (75.0) | 0.186a |
|
| ||||
|
Genetic Variants
| ||||
| Sickle cell trait, n (%) | 10 (13.5) | 5 (26.3) | 0 (0.0) | 0.176a |
| Glucose-6-phosphate dehydrogenase deficiency, n (%) | 5 (6.9) | 1 (5.9) | 1 (20.0) | 0.542a |
|
| ||||
|
Other Laboratory Parameters
| ||||
| PfHz containing monocytes, n (%) | 36 (48.6) | 8 (42.1) | 3 (37.5) | 0.761a |
| Plasma bicyclo-PGE2,pg/mL | 11.3 (8.8) | 4.9 (2.8) | 4.9 (4.4) | <0.001b |
| Plasma creatinine, mg/mL* | 0.45 (0.56) | 0.62 (0.23) | 0.75 (0.29) | 0.004b |
Data presented as median (interquartile range, IQR). Children were stratified into those with malaria [Pf(+)] alone, malaria plus bacteremia [Pf(+)/Bac(+)], and malaria plus HIV-1 [Pf(+)/HIV-1(+)], all with any density parasitemia.
Pearson’s χ2 test was used to determine differences in proportions.
Kruskal-Wallis test was used to compare differences across groups.
Analysis of variance (ANOVA) was used to compare geometric mean parasitemia. MPS – Malaria parasites.
3.2. Bicyclo-PGE2 and COX-2 transcript levels
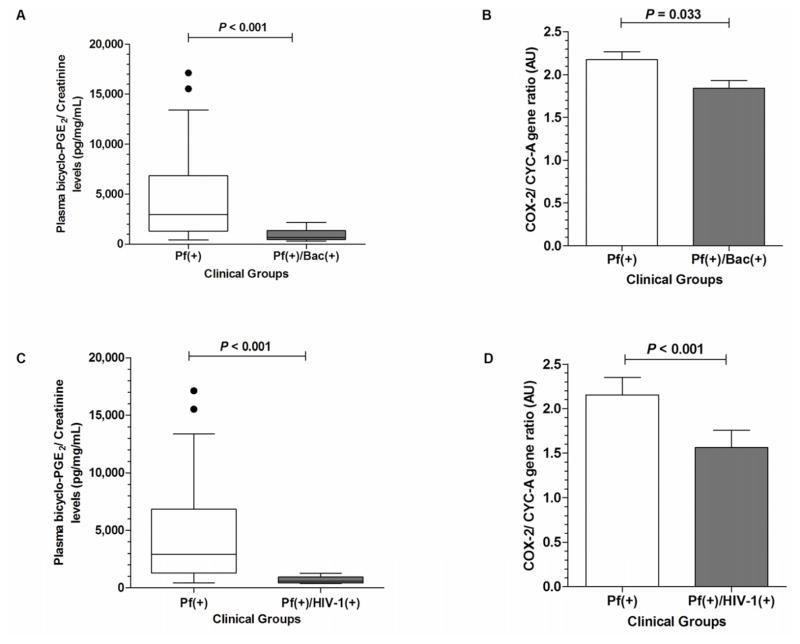
To determine the effect of co-infection on PGE2 production, we assayed the stable end-metabolite of PGE2 in circulation (bicyclo-PGE2, pg/mL) and normalized across the groups by expressing the levels relative to creatinine (bicyclo-PGE2/creatinine, pg/mg/mL). Analyses of bicyclo-PGE2/creatinine levels revealed that the Pf(+)/Bac(+) group had significantly decreased plasma levels compared to the Pf(+) group (P<0.001; Figure 1A). In addition, the Pf(+)/Bac(+) group had significantly lower (P=0.033) peripheral blood COX-2 transcripts relative to the Pf(+) group (Figure 1B). Analysis of PGE2 production in the Pf(+)/HIV-1(+) group revealed that co-infection was associated with decreased plasma bicyclo-PGE2/creatinine concentrations compared to children with malaria mono-infection (P<0.001, Figure 1C). In addition, the Pf(+)/HIV-1(+) co-infected group had lower COX-2 mRNA transcript levels relative to children with malaria mono-infection (P<0.001; Figure 1D).
Figure 1. Systemic bicyclo-PGE2 and COX-2 mRNA expression in children with malaria and co-infected with either bacteremia or HIV-1.
(A) Plasma bicyclo-PGE2/creatinine ratio in children with malaria alone [Pf(+)] versus malaria and bacteremia [Pf(+)/Bac(+)]. (B) Semi-quantitative COX-2 gene expression expressed relative to CYC-A in arbitrary units (AU) in children with malaria alone [Pf(+)] versus those co-infected with bacteremia [Pf(+)/Bac(+)]. COX-2 mRNA expression is presented as means, with the error bars showing the standard error of mean (SEM). (C) Plasma bicyclo-PGE2/creatinine ratio among children with malaria alone [Pf(+)] versus those with malaria plus HIV-1 [Pf(+)/HIV-1(+)]. (D) Semi-quantitative COX-2 gene expression expressed relative to CYC-A (AU) in children with malaria alone [Pf(+)] versus those with malaria plus HIV-1 [Pf(+)/HIV-1(+)]. Plasma levels were compared using Mann-Whitney U test. Differences in COX-2 gene expression between groups were computed using Student’s t-test.
3.3. Relationship between bicyclo-PGE2 and anemia
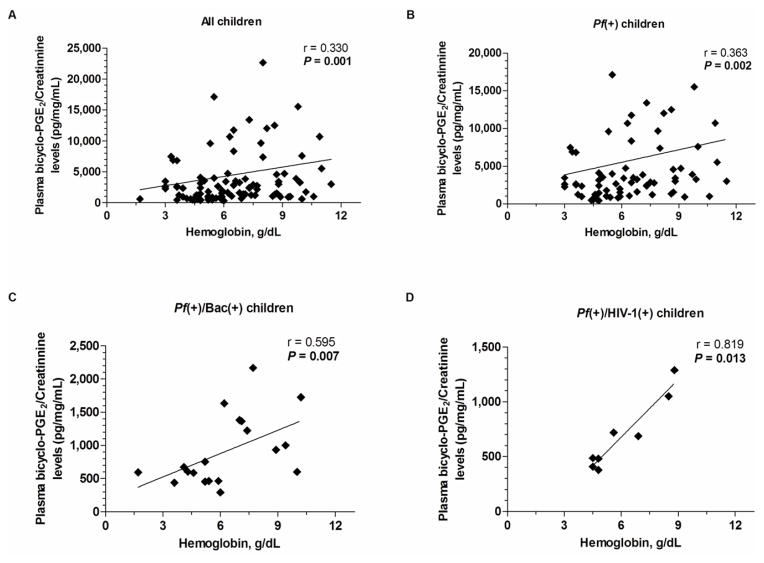
To determine if there was a relationship between PGE2 production and anemia, the association between circulating bicyclo-PGE2/creatinine levels and Hb were determined for the overall cohort, and for each of the groups separately. Spearman’s rank correlation analyses revealed a significant positive relationship between bicyclo-PGE2/creatinine and Hb concentrations in the overall cohort (r=0.330, P=0.001; Figure 2A), Pf(+) group (r=0.363, P=0.002; Figure 2B), Pf(+)/Bac(+) group (r=0.595, P=0.007; Figure 2C), and Pf(+)/HIV-1(+) group (r=0.819, P=0.013; Figure 2D). These results illustrate that circulating bicyclo-PGE2/creatinine levels are a valid biomarker for disease severity in children with malaria as a single infection and in the context of co-infection with either bacteremia or HIV-1.
Figure 2. Relationship between plasma bicyclo-PGE2/creatinine concentration and Hb levels in children with malaria and co-infection with either bacteremia or HIV-1.
Correlation between plasma bicyclo-PGE2/creatinine and Hb levels in the (A) overall cohort (n=101), (B) falciparum malaria alone [Pf(+)] (n=74), (C) malaria plus bacteremia [Pf(+)/Bac(+)] (n=19), (D) malaria plus HIV-1 [Pf(+)/HIV-1(+)] (n=8). The relationship between plasma bicyclo-PGE2/creatinine and Hb levels were computed using Spearman’s rank correlation analyses.
3.4. Relationship between bicyclo-PGE2 and inflammatory mediator production
During a malaria infection, there are counter-regulatory effects between COX-2-derived PGE2 and a number of cytokines including, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and interleukin (IL)-10 [4]. Investigation of this established immunological network revealed that plasma TNF-α levels were elevated in the Pf(+)/HIV-1(+) group (P=0.126), while IFN-γ was highest in Pf(+)/Bac(+) children (P=0.056), and that IL-10 was comparable across the groups (P=0.393) (Table 2). Further exploration using Pearson’s zero order correlation test demonstrated no significant relationships between circulating bicyclo-PGE2 levels and TNF-α (r=-0.047, P=0.716), IFN-γ (r=-0.114, P=0.265), and IL-10 (r=0.192, P=0.134). Since a multiplex was performed, additional exploratory analyses were performed to identify potential relationships between COX-2 and regulatory cytokines (Table 2). Only those found to be significant are presented here. There was a general trend of increasing levels of circulating IL-1 receptor antagonist (RA) (P=0.045), IL-8 (P=0.002), IL-12p40/70 (P=0.004), and monocyte chemoattractant protein (MCP)-1 (P=0.001) in co-infected children. Children co-infected with malaria and bacteremia had elevated levels of IFN-α (P=0.009), IL-4 (P=0.001), IL-7 (P<0.001), IL-15 (P<0.001), and IL-17 (P=0.004). Correlation analyses showed an inverse relationship between bicyclo-PGE2/creatinine and IL-4 (r=-0.287, P=0.024), IL-8 (r=-0.472, P<0.001) and MCP-1 (r=-0.389, P=0.002), with no other analyses revealing a significant association.
Table 2.
Inflammatory mediator profiles.
| Characteristics | Pf(+) | Pf(+)/Bac(+) | Pf(+)/HIV-1(+) | P |
|---|---|---|---|---|
| Sample size (n) | 74 | 19 | 8 | |
| Tumor necrosis factor-alpha (TNF-α), pg/mL | 13.8 (53.7) | 11.8 (20.0) | 56.6 (66.9) | 0.126 |
| Interferon-alpha (IFN-α), pg/mL | 25.3 (43.0) | 77.0 (79.0) | 26.4 (26.0) | 0.009 |
| Interferon-gamma (IFN-γ), pg/mL | 11.4 (24.8) | 18.2 (23.7) | 7.9 (21.9) | 0.056 |
| Monocyte chemoattractant protein (MCP)-1, pg/mL | 184.7 (203.7) | 446.5 (502.4) | 495.9 (843.4) | 0.001 |
| Interleukin (IL)-1Ra, pg/mL | 1,794.2 (3,615.5) | 4,399.7 (18,146.0) | 5,183.6 (7,194.9) | 0.045 |
| Interleukin (IL)-4, pg/mL | 5.2 (10.0) | 22.7 (49.7) | 14.5 (15.2) | 0.001 |
| Interleukin (IL)-7, pg/mL | 28.5 (41.2) | 88.6 (89.9) | 25.6 (24.4) | <0.001 |
| Interleukin (IL)-8, pg/mL | 13.9 (17.0) | 22.2 (29.4) | 20.7 (17.3) | 0.002 |
| Interleukin (IL)-10, pg/mL | 233.4 (793.6) | 165.5 (211.2) | 273.7 (805.3) | 0.393 |
| Interleukin (IL)-12p40/70, pg/mL | 335.9 (344.1) | 660.5 (649.6) | 691.8 (509.6) | 0.004 |
| Interleukin (IL)-15, pg/mL | 25.8 (25.3) | 199.1 (222.4) | 47.7 (105.3) | <0.001 |
| Interleukin (IL)-17, pg/mL | 20.4 (3.0) | 39.2 (60.9) | 20.4 (0.7) | 0.004 |
Data presented as median (interquartile range, IQR). Children were stratified into those with malaria [Pf(+)] alone, malaria plus bacteremia [Pf(+)/Bac(+)], and malaria plus HIV-1 [Pf(+)/HIV-1(+)], all with any density parasitemia. Kruskal-Wallis test was used to compare differences across groups.
4. DISCUSSION
Here, we report for the first time the profiles of circulating PGE2 levels in children with malaria co-infected with either bacteremia or HIV-1. Results presented here suggest that co-infection further suppresses the already reduced levels of COX-2-derived PGE2 present in children with malarial anemia [13,15]. Although we have previously shown that COX-2-derived PGE2 production is suppressed during severe malaria infections [13,14], this is the first report showing the influence of co-infection on the COX-2-PGE2 pathway. Based on the consistent findings showing decreased PGE2 in the context of enhanced disease severity in children [12,13,15] and pregnant women [14] with falciparum malaria, adults with vivax malaria [33], experimental models of murine malaria [34–36], and now in co-infected children with malarial anemia, measurement of PGE2 as a biomarker for severe malaria appears well justified.
We have previously shown in a larger cohort of children with malaria that both bacteremia and HIV-1 enhance the severity of pediatric anemia [5,7,10]. Based on the sample size in the current study in which bicyclo-PGE2/creatinine and the inflammatory mediators were available in only a subset of the larger population, logistic regression analyses were not performed. However, the progressive worsening of anemia in co-infected children, although non-significant, likely due to sample size issues, suggests that lowered bicyclo-PGE2/creatinine is indeed associated with enhanced anemia. This premise is supported by the significant positive relationship between circulating bicyclo-PGE2/creatinine and Hb in both mono- and co-infected children. Data showing that PGE2 is an important soluble factor for promoting efficient erythropoiesis [37–39], erythroid maturation, and Hb formation [40–44] lends further support to this hypothesis.
A number of previous studies have shown that PGE2 levels are elevated in HIV-1 [45–48] and HIV-1 and human papilloma virus co-infection [49], possibly accounting for the immunosuppressive effects witnessed in these virally infected patients. In addition, studies in primary human macrophages showed that the HIV-1 Tat protein increased COX-2 expression and PGE2 synthesis, and that a COX-2 inhibitor, as well as exogenous PGE2, promoted increased growth of Leishmania [50]. Although the reasons for a different trend reported here, in which COX-2 and PGE2 are suppressed in children co-infected with malaria and HIV-1, is unclear, it may be related to our previous findings demonstrating that malarial products ingested by circulating phagocytic cells suppress COX-2 mRNA and protein, and the subsequent production of PGE2, via up-regulation of IL-10 [51]. Thus, it appears that the suppressive effects that malaria has on the COX-2-PGE2 pathway cannot be overcome when infected with HIV-1, as evidenced by the even greater suppression during malaria and HIV-1 co-infection.
Decreased COX-2 expression and peripheral bicyclo-PGE2 in children with malaria and bacteremia co-infection parallels results reported in patients with sepsis, in which decreased PGE2 was associated with enhanced clinical severity [52]. Moreover, this investigation also revealed that the inducibility of COX-2-derived PGE2 in in vitro whole blood stimulation assays was markedly reduced in patients with severe sepsis [52]. Based on these results, the authors suggested that arachidonic acid metabolites were a valid biomarker for disease severity and clinical outcomes in patients with sepsis, an identical justification we propose in the context of malaria.
Although the inflammatory mediators typically associated with regulation of COX-2-derived PGE2 during malarial infections (e.g., TNF-α, IFN-γ, and IL-10) [12,51,53] were not significantly associated with bicyclo-PGE2 levels, there was a general trend towards increasing levels of inflammatory mediator production in co-infected children. In addition, there was a significant inverse correlation between bicyclo-PGE2, and IL-4, IL-8, and MCP-1. Additional analyses in a larger cohort of individuals will be required to determine if the observed relationship between PGE2, IL-8, and MCP-1 represents an important, yet unexplored, immunological network that influences disease outcomes in mono- and co-infected children.
Highlights.
Suppressed bicyclo-PGE2/creatinine in malaria-infected children with co-infection.
Decreased COX-2 transcripts in malaria-infected children with co-infection.
Suppressed bicyclo-PGE2 is associated with worsening malarial anemia.
Enhanced inflammatory mediators in co-infected children.
Inverse relationship between bicyclo-PGE2 and IL-4, IL-8, and MCP-1.
Acknowledgments
The study was funded by a National Institutes of Health (NIH) Grant 1 R01A151305 (DJP) and Fogarty International Center (FIC) Training Grant 1 D43TW05884 (DJP). Data presented are published with the permission and approval of the Director, Kenya Medical Research Institute. We offer our sincere gratitude and appreciation to all parents, guardians, and children from the Siaya District community for their participation in this study. We also thank the staff at University of New Mexico/KEMRI laboratories and the Siaya District Hospital management for their support during the study.
Footnotes
AUTHOR CONTRIBUTIONS
SBA, PK, and GCD performed the experiments, JBH assisted in the data analysis, DJP, JMO, and JMV designed and executed the study. SBA and DJP co-wrote the manuscript.
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 3.Zucker JR, Perkins BA, Jafari H, et al. Clinical signs for the recognition of children with moderate or severe anaemia in western Kenya. Bull World Health Organ. 1997;75(Suppl 1):97–102. [PMC free article] [PubMed] [Google Scholar]
- 4.Perkins DJ, Were T, Davenport GC, et al. Severe malarial anemia: innate immunity and pathogenesis. Int J Biol Sci. 2011;7:1427–42. doi: 10.7150/ijbs.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otieno RO, Ouma C, Ong’echa JM, et al. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS. 2006;20:275–80. doi: 10.1097/01.aids.0000200533.56490.b7. [DOI] [PubMed] [Google Scholar]
- 6.Waitumbi JN, Kuypers J, Anyona SB, et al. Outpatient upper respiratory tract viral infections in children with malaria symptoms in Western Kenya. Am J Trop Med Hyg. 2010;83:1010–3. doi: 10.4269/ajtmh.2010.10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Were T, Davenport GC, Hittner JB, et al. Bacteremia in Kenyan children presenting with malaria. J Clin Microbiol. 2011;49:671–6. doi: 10.1128/JCM.01864-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novelli EM, Hittner JB, Davenport GC, et al. Clinical predictors of severe malarial anaemia in a holoendemic Plasmodium falciparum transmission area. Br J Haematol. 2010;149:711–21. doi: 10.1111/j.1365-2141.2010.08147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong’echa JM, Davenport GC, Vulule JM, et al. Identification of inflammatory biomarkers for pediatric malarial anemia severity using novel statistical methods. Infect Immun. 2011;79:4674–80. doi: 10.1128/IAI.05161-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davenport GC, Hittner JB, Were T, et al. Relationship between inflammatory mediator patterns and anemia in HIV-1 positive and exposed children with Plasmodium falciparum malaria. Am J Hematol. 2012 doi: 10.1002/ajh.23200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davenport GC, Ouma C, Hittner JB, et al. Hematological predictors of increased severe anemia in Kenyan children coinfected with Plasmodium falciparum and HIV-1. Am J Hematol. 2010;85:227–33. doi: 10.1002/ajh.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins DJ, Hittner JB, Mwaikambo ED, et al. Impaired systemic production of prostaglandin E2 in children with cerebral malaria. J Infect Dis. 2005;191:1548–57. doi: 10.1086/429332. [DOI] [PubMed] [Google Scholar]
- 13.Perkins DJ, Kremsner PG, Weinberg JB. Inverse relationship of plasma prostaglandin E2 and blood mononuclear cell cyclooxygenase-2 with disease severity in children with Plasmodium falciparum malaria. J Infect Dis. 2001;183:113–8. doi: 10.1086/317660. [DOI] [PubMed] [Google Scholar]
- 14.Perkins DJ, Moore JM, Otieno J, et al. In vivo acquisition of hemozoin by placental blood mononuclear cells suppresses PGE2, TNF-alpha, and IL-10. Biochem Biophys Res Commun. 2003;311:839–46. doi: 10.1016/j.bbrc.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 15.Anyona SB, Kempaiah P, Raballah E, et al. Reduced systemic bicyclo-prostaglandin-E(2) and cyclooxygenase-2 gene expression are associated with inefficient erythropoiesis and enhanced uptake of monocytic hemozoin in children with severe malarial anemia. Am J Hematol. 2012 doi: 10.1002/ajh.23253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habib A, Creminon C, Frobert Y, et al. Demonstration of an inducible cyclooxygenase in human endothelial cells using antibodies raised against the carboxyl-terminal region of the cyclooxygenase-2. J Biol Chem. 1993;268:23448–54. [PubMed] [Google Scholar]
- 17.O’Banion MK, Winn VD, Young DA. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc Natl Acad Sci U S A. 1992;89:4888–92. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang M, Stolina M, Sharma S, et al. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58:1208–16. [PubMed] [Google Scholar]
- 19.Krysan K, Reckamp KL, Dalwadi H, et al. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 2005;65:6275–81. doi: 10.1158/0008-5472.CAN-05-0216. [DOI] [PubMed] [Google Scholar]
- 20.Pino MS, Nawrocki ST, Cognetti F, et al. Prostaglandin E2 drives cyclooxygenase-2 expression via cyclic AMP response element activation in human pancreatic cancer cells. Cancer Biol Ther. 2005;4:1263–9. doi: 10.4161/cbt.4.11.2138. [DOI] [PubMed] [Google Scholar]
- 21.Ramaswamy K, Kumar P, He YX. A role for parasite-induced PGE2 in IL-10-mediated host immunoregulation by skin stage schistosomula of Schistosoma mansoni. J Immunol. 2000;165:4567–74. doi: 10.4049/jimmunol.165.8.4567. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Torihashi S, Hori M, et al. Phagocytotic activation of muscularis resident macrophages inhibits smooth muscle contraction in rat ileum. J Vet Med Sci. 2007;69:1053–60. doi: 10.1292/jvms.69.1053. [DOI] [PubMed] [Google Scholar]
- 23.Granstrom E, Hamberg M, Hansson G, et al. Chemical instability of 15-keto-13,14-dihydro-PGE2: the reason for low assay reliability. Prostaglandins. 1980;19:933–57. doi: 10.1016/0090-6980(80)90127-6. [DOI] [PubMed] [Google Scholar]
- 24.Murphy RC, FitzGerald GA. Current approaches to estimation of eicosanoid formation in vivo. Adv Prostaglandin Thromboxane Leukot Res. 1994;22:341–8. [PubMed] [Google Scholar]
- 25.Bloland PB, Boriga DA, Ruebush TK, et al. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission II. Descriptive epidemiology of malaria infection and disease among children. Am J Trop Med Hyg. 1999;60:641–8. doi: 10.4269/ajtmh.1999.60.641. [DOI] [PubMed] [Google Scholar]
- 26.Okiro EA, Alegana VA, Noor AM, et al. Changing malaria intervention coverage, transmission and hospitalization in Kenya. Malar J. 2010;9:285. doi: 10.1186/1475-2875-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obonyo CO, Vulule J, Akhwale WS, et al. In-hospital morbidity and mortality due to severe malarial anemia in western Kenya. Am J Trop Med Hyg. 2007;77:23–8. [PubMed] [Google Scholar]
- 28.Ong’echa JM, Keller CC, Were T, et al. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area. Am J Trop Med Hyg. 2006;74:376–85. [PubMed] [Google Scholar]
- 29.Were T, Hittner JB, Ouma C, et al. Suppression of RANTES in children with Plasmodium falciparum malaria. Haematologica. 2006;91:1396–9. [PubMed] [Google Scholar]
- 30.Ouma C, Keller CC, Davenport GC, et al. A novel functional variant in the stem cell growth factor promoter protects against severe malarial anemia. Infect Immun. 2010;78:453–60. doi: 10.1128/IAI.00895-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 32.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonic International. 2004;11:36–42. [Google Scholar]
- 33.Andrade BB, Araujo-Santos T, Luz NF, et al. Heme impairs prostaglandin E2 and TGF-beta production by human mononuclear cells via Cu/Zn superoxide dismutase: insight into the pathogenesis of severe malaria. J Immunol. 2010;185:1196–204. doi: 10.4049/jimmunol.0904179. [DOI] [PubMed] [Google Scholar]
- 34.Deininger MH, Kremsner PG, Meyermann R, et al. Focal accumulation of cyclooxygenase-1 (COX-1) and COX-2 expressing cells in cerebral malaria. J Neuroimmunol. 2000;106:198–205. doi: 10.1016/s0165-5728(00)00187-9. [DOI] [PubMed] [Google Scholar]
- 35.Xiao L, Patterson PS, Yang C, et al. Role of eicosanoids in the pathogenesis of murine cerebral malaria. Am J Trop Med Hyg. 1999;60:668–73. doi: 10.4269/ajtmh.1999.60.668. [DOI] [PubMed] [Google Scholar]
- 36.Ball HJ, MacDougall HG, McGregor IS, et al. Cyclooxygenase-2 in the pathogenesis of murine cerebral malaria. J Infect Dis. 2004;189:751–8. doi: 10.1086/381503. [DOI] [PubMed] [Google Scholar]
- 37.Datta MC. Prostaglandin E2 mediated effects on the synthesis of fetal and adult hemoglobin in blood erythroid bursts. Prostaglandins. 1985;29:561–77. doi: 10.1016/0090-6980(85)90080-2. [DOI] [PubMed] [Google Scholar]
- 38.Ortega JA, Dukes PP, Ma A, et al. A clinical trial of prostaglandin E2 to increase erythropoiesis in anemia of end stage renal disease. A preliminary report. Prostaglandins Leukot Med. 1984;14:411–6. doi: 10.1016/0262-1746(84)90124-0. [DOI] [PubMed] [Google Scholar]
- 39.Lamikanra AA, Brown D, Potocnik A, et al. Malarial anemia: of mice and men. Blood. 2007;110:18–28. doi: 10.1182/blood-2006-09-018069. [DOI] [PubMed] [Google Scholar]
- 40.Boer AK, Drayer AL, Rui H, et al. Prostaglandin-E2 enhances EPO-mediated STAT5 transcriptional activity by serine phosphorylation of CREB. Blood. 2002;100:467–73. doi: 10.1182/blood.v100.2.467. [DOI] [PubMed] [Google Scholar]
- 41.Dukes PP, Shore NA, Hammond D, et al. Enhancement of erythropoiesis by prostaglandins. J Lab Clin Med. 1973;82:704–12. [PubMed] [Google Scholar]
- 42.Dupuis F, Desplat V, Praloran V, et al. Effects of lipidic mediators on the growth of human myeloid and erythroid marrow progenitors. J Lipid Mediat Cell Signal. 1997;16:117–25. doi: 10.1016/s0929-7855(97)00007-2. [DOI] [PubMed] [Google Scholar]
- 43.Lorenz M, Slaughter HS, Wescott DM, et al. Cyclooxygenase-2 is essential for normal recovery from 5-fluorouracil-induced myelotoxicity in mice. Exp Hematol. 1999;27:1494–502. doi: 10.1016/s0301-472x(99)00087-9. [DOI] [PubMed] [Google Scholar]
- 44.Oonishi T, Sakashita K, Ishioka N, et al. Production of prostaglandins E1 and E2 by adult human red blood cells. Prostaglandins Other Lipid Mediat. 1998;56:89–101. doi: 10.1016/s0090-6980(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 45.Abel PM, McSharry C, Galloway E, et al. Heterogeneity of peripheral blood monocyte populations in human immunodeficiency virus-1 seropositive patients. FEMS Microbiol Immunol. 1992;5:317–23. doi: 10.1111/j.1574-6968.1992.tb05916.x. [DOI] [PubMed] [Google Scholar]
- 46.Foley P, Kazazi F, Biti R, et al. HIV infection of monocytes inhibits the T-lymphocyte proliferative response to recall antigens, via production of eicosanoids. Immunology. 1992;75:391–7. [PMC free article] [PubMed] [Google Scholar]
- 47.Griffin DE, Wesselingh SL, McArthur JC. Elevated central nervous system prostaglandins in human immunodeficiency virus-associated dementia. Ann Neurol. 1994;35:592–7. doi: 10.1002/ana.410350513. [DOI] [PubMed] [Google Scholar]
- 48.Ramis I, Rosello-Catafau J, Gelpi E. In vivo transformation of arachidonic acid into 12-hydroxy-5,8,10,14-eicosatetraenoic acid by human nasal mucosa. J Chromatogr. 1992;575:143–6. doi: 10.1016/0378-4347(92)80515-r. [DOI] [PubMed] [Google Scholar]
- 49.Fitzgerald DW, Bezak K, Ocheretina O, et al. The effect of HIV and HPV coinfection on cervical COX-2 expression and systemic prostaglandin E2 levels. Cancer Prev Res (Phila) 2012;5:34–40. doi: 10.1158/1940-6207.CAPR-11-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barreto-de-Souza V, Pacheco GJ, Silva AR, et al. Increased Leishmania replication in HIV-1-infected macrophages is mediated by tat protein through cyclooxygenase-2 expression and prostaglandin E2 synthesis. J Infect Dis. 2006;194:846–54. doi: 10.1086/506618. [DOI] [PubMed] [Google Scholar]
- 51.Keller CC, Hittner JB, Nti BK, et al. Reduced peripheral PGE2 biosynthesis in Plasmodium falciparum malaria occurs through hemozoin-induced suppression of blood mononuclear cell cyclooxygenase-2 gene expression via an interleukin-10-independent mechanism. Mol Med. 2004;10:45–54. doi: 10.2119/2004-00035.perkins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruegel M, Ludwig U, Kleinhempel A, et al. Sepsis-associated changes of the arachidonic acid metabolism and their diagnostic potential in septic patients. Crit Care Med. 2012;40:1478–86. doi: 10.1097/CCM.0b013e3182416f05. [DOI] [PubMed] [Google Scholar]
- 53.Keller CC, Davenport GC, Dickman KR, et al. Suppression of prostaglandin E2 by malaria parasite products and antipyretics promotes overproduction of tumor necrosis factor-alpha: association with the pathogenesis of childhood malarial anemia. J Infect Dis. 2006;193:1384–93. doi: 10.1086/503047. [DOI] [PubMed] [Google Scholar]