Abstract
Registering clinical trials (CTs) in public domains enhances transparency, increases trust in research, improves participation and safeguards against publication bias. This work was done to study the profile of clinical research in Egypt in three CT registries with different scopes: the WHO International CT Registry Platform (ICTRP), the continental Pan-African CT Registry (PACTR) and the US clinicaltrials.gov (CTGR). In March 2014, ICTRP, PACTR and CTGR were searched for clinical studies conducted in Egypt. It was found that the number of studies conducted in Egypt (percentage) was 686 (0.30%) in ICTRP, 56 (11.3%) in PACTR and 548 (0.34%) in CTGR. Most studies were performed in universities and sponsored by university/organization, industry or individual researchers. Inclusion of adults from both genders predominated. The median number of participants per study in the three registries ranged between 63 and 155. The conditions researched differed among the three registries and study purpose was mostly treatment followed by prevention. Endpoints were mostly efficacy followed by safety. Observational:Interventional studies (i.e. clinical trials) represented 15.5%:84.5% in ICTRP, 0%:100% in PACTR and 16.4%:83.6% in CTGR. Most interventions were drugs or procedures. Observational studies were mostly prospective and cohort studies. Most CTs were phase 3 and tested drugs or procedures. Parallel group assignment and random allocation predominated. Blinding was implemented in many of trials and was mostly double-blind. We conclude that CTs from Egypt in trial registries are apparently low and do not accurately reflect clinical research conducted in Egypt or its potential. Development of an Egyptian CT registry is eagerly needed. Registering all Egyptian CTs in public domains is highly recommended.
Keywords: Clinical trial registry, Design, Interventional studies, Observational studies, Egypt
Introduction
Clinical research is the type of scientific research that involves human subjects and includes patient-oriented research, epidemiologic and behavioral studies, and outcomes research and health services research [1]. Clinical studies evaluate the effect of interventions or exposures on biomedical or health-related outcomes that include prevention, diagnosis and treatment of diseases. They are broadly classified into observational and interventional studies. Clinical trials (CTs) or interventional studies are clinical studies in which participants are assigned by researchers/investigators to receive one or more interventions to assess their safety and efficacy. In observational studies, participants are not assigned to interventions by the investigators. Clinical trials are classically classified into 5 phases (0–4). Phase 0 studies are exploratory studies involving very limited human exposure to an investigational new drug (IND) for example screening studies and microdose studies [2]. The primary aims of phase 0 studies are identifying, early in the process of drug development, viable candidates and eliminating those lacking promise with a potential reduction in costs and time-to-first-in-human testing. The endpoints include evaluation of analogs for lead selection, modulation of a molecular target in vivo, whole-body imaging for tissue distribution/target binding affinity, and agent pharmacokinetics [∗]. They are ethically acceptable [∗∗]. Phase 1 studies aim to find out the drug’s most frequent and serious adverse events and the drug metabolism and excretion. Phase 2 studies gather preliminary data on efficacy. Phase 3 studies gather more information about safety and effectiveness. The randomized controlled trial (RCT) is widely regarded as the gold standard for evaluating health care interventions. Phase 4 studies occur after marketing approval of a drug by authorities and aim to gather additional information about a drug’s safety, efficacy and optimal use [2].
Participation in clinical trials is a voluntary action after subjects are fully informed of the research and give their consent [3]. Without participants, no CT can conclude. Clinical trial registries (CTRs) facilitate the prospective registration of CTs and the accessibility of their information by patients, physicians, researchers and other interested stakeholders. This enhances transparency, increases participation in CTs and can eliminate publication bias that arises from publishing positive trial results more than the negative ones [4], [5]. Some countries mandate CT registration; others do not ask for registration, but often strongly encourage it. CT registration is mandated or recommended by many laws and policies including U.S. Federal law, Declaration of Helsinki, European Union Clinical Trials Directive, WHO Clinical Trials Directive, International Committee of Medical Journal Editors (ICMJE) [6]. Currently, there are many CTRs with a scale being global (e.g. WHO International Clinical Trials Registry Platform [ICTRP]) [4], continental/regional (e.g. EU Clinical Trials Register [EU-CTR] [7] and Pan-African Clinical Trials Registry [PACTR] [8]), country-specific (e.g. US clinicaltrials.gov [6]) or companies and associations (e.g. International Federation of Pharmaceutical Manufacturers Associations [IFPMA]) [9].
ClinicalTrials.gov registry (CTGR) is a trial registry hosted by the US National Institute of Health (NIH). It is a governmental website. In February 2000, it was made available to the public as a registry of clinical trials information on federally and privately funded trials conducted under investigational new drug (IND) applications to test the effectiveness of experimental drugs for serious or life-threatening diseases or conditions. In September 2008, more information on study participants and a summary of study outcomes, including adverse events were made available. By 17th of March 2014, CTGR contained 163,090 studies [10]. The idea of a global clinical trial registry rose in the year 2004. The WHO first established the requirements of CTRs and a trial registration data set. The focus then shifted to establishing the two key elements of the platform: the International Clinical Trials Registry Platform (the ICTRP Network) and a single point of access (the ICTRP Clinical Trials Search Portal (CTSP)). CTSP provides a single point of access for the identification of trials in many contributing registries. CTSP was launched in May 2007 and initially contained trial records provided by three CTRs: the Australian New Zealand Clinical Trials Registry (ANZCTR), CTGR and the International Standard Randomised Controlled Trial Number (ISRCTN) Registry. In addition to the above registries, the ICTRP Registry Network includes registries based in Brazil, China, Cuba, EU-CTR, Germany, India (CTRI), Iran, Korea, Japan, the Netherlands, PACTR, Sri Lanka, Thai and UK [11]. By 17th of March 2014, ICTRP contained 271,811 records for 229,638 trials [4].
In early 2007, the Pan-African Clinical Trials Registry (PACTR) was established by the South African Cochrane Centre, in partnership with the European and Developing Countries Clinical Trials Partnership and the Cochrane Infectious Disease Group. In the initial phase, PACTR registered trials in HIV/AIDS, tuberculosis and malaria to demonstrate proof of concept. Once established, the goal of PACTR is to become the registry of choice for any clinical trial conducted in Africa [8]. On 25th of September 2009, PACTR was officially launched as a member of the WHO Primary Registry Network in Abuja, Nigeria [12]. PACTR is presently the only African member of the WHO Network of Primary Registers and transfers all trial information to the WHO CTSP on a quarterly basis. As of 17th of March 2014, PACTR contains 300 trials [8].
The aim of this work is to study the profile of clinical trials in Egypt in three clinical trial registries with different scopes: the global ICTRP registry, the regional/continental PACTR registry and the US CTGR.
Methods
The US clinical trial registry “clinicaltrials.gov; CTGR” was searched for trials conducted in Egypt. In the “advanced search http://clinicaltrials.gov/ct2/search/advanced, “Egypt” was searched in the countries 1, 2 and 3 and the search yielded 548 entries. All entries were downloaded and manually scrutinized. The International Clinical Trials Registry Platform “ICTRP” Clinical Trials Search Portal (CTSP) (http://apps.who.int/trialsearch/AdvSearch.aspx) was searched for trials from Egypt and the search yielded 686 entries. The Pan-African Clinical Trials Registry “PACTR” (http://www.pactr.org/) in the advanced search and 73 entries for 56 studies were retrieved. All searches were done on the same day (10th of March, 2014). Analyses were done using Microsoft Excel® 2010 and IBM SPSS® version 21.
The ICTRP was selected because it is an international registry that accepts studies from several registries including country-specific, regional and continental ones. Thus, it should be exhaustive and includes almost all the registered studies. The clinicaltrials.gov was chosen because it is the most mature registry and it preceded the ICTRP and thus it may contain studies not listed in the latter registry. The PACTR was chosen because it is the only registry in the continent where Egypt lies (Africa). Studying this latter registry would help in the process of planning a proposed Egyptian registry.
Results
As of 10th March 2014, the total numbers of studies included in the ICTRP, CTGR and PACTRP were 229,638, 163,090 and 645, respectively. Within ICTRP, Egypt ranked the 55th with a total of 686 studies representing 0.30% of ICTRP total. Within CTGR, Egypt ranked the 115th with a total of 548 studies representing 0.34% of its total. Within PACTRP, Egypt ranked the 2nd (following South Africa) with a total of 73 entries for 56 studies representing 11.3% of PACTRP total (Table 1). CTGR and PACTRP are primary registries that receive data directly from registerers while ICTRP receives trial information from many primary registries. Main sources of Egypt’s studies in ICTRP were CTGR (72.3%), ANZCTR (13.6%), EU-CTR (7.7%), ISRCTN (4.2%) and CTRI (2%). Comparing study titles showed that 72.3% of studies in CTGR were repeated in ICTRP. However, none of PACTRP studies was repeated in CTGR or in ICTRP.
Table 1.
Total number, recruitment status and funding sources of Egyptian studies in 3 trial registries.
| CTGR | ICTRP | PACTR | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Registry total | 163,090 (100.0) | 229,638 (100.0) | 645 (100.0) |
| Egypt’s total | 548 (0.34) | 686 (0.30) | 56 (11.30) |
| Egypt’s rank | 115 | 55 | 2 |
| Recruitment | |||
| Recruiting | 150 (27.4) | 131 (19.1) | 17 (30.4) |
| Not recruiting | 85 (15.5) | 508 (74.1) | 18 (32.1) |
| Completed | 295 (53.8) | 0 (0) | 19 (33.9) |
| Terminated/Withdrawn | 18 (3.3) | 0 (0) | 2 (3.6) |
| Authorized | 0 (0) | 45 (6.6) | 0 (0) |
| Not available | 0 (0) | 2 (0.2) | 0 (0) |
| Results | |||
| Available | 44 (8.0) | –a | –a |
| Not available | 504 (92.0) | –a | –a |
| Funding source | |||
| University/Organization | 384 (53.8) | 262 (38.2) | 50 (89.3) |
| Industry | 273 (38.2) | 292 (42.6) | 0 (0) |
| Clinical Research Network | 25 (3.5) | 0 (0) | 0 (0) |
| US Government (excluding U.S. Federal) | 15 (2.1) | 0 (0) | 0 (0) |
| National Institutes of Health | 17 (2.4) | 0 (0) | 0 (0) |
| Individual (researcher) | 0 (0) | 70 (10.2) | 6 (10.7) |
| Other | 0 (0) | 62 (9.0) | 0 (0) |
CTGR: U.S. ClinicalTrials.gov Registry, ICTRP: WHO International Clinical Trials Registry Platform, PACTR: Pan-African Clinical Trials Registry.
Not included.
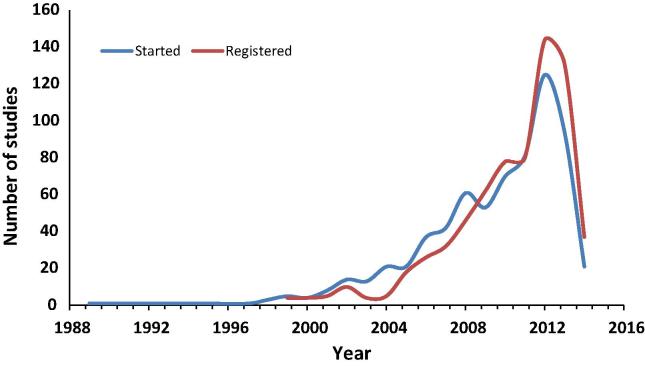
Registration of Egypt’s clinical studies to both CTGR and ICTRP started in 1999 and peaked in 2012 (Fig. 1, Fig. 2). Registration to PACTRP started in 2009 and peaked in 2013. Information on study completion was available in CTGR only.
Fig. 1.
Egyptian studies in Clinicaltrials.gov according to the registration, starting and completion years.
Fig. 2.
Egyptian studies in International Clinical Trials Registry Platform (ICTRP) according to the registration and starting years.
Recruitment status differed in the three registries. Active recruiting status was encountered in 27.4% in CTGR, 19.1% in ICTRP and 26% in PACRP. Completed status was encountered in 53.8% in CTGR and 33.9% in PACTRP and not mentioned in ICTRP. Study results were only available in CTGR and only 8% of Egypt’s studies have results (Table 1).
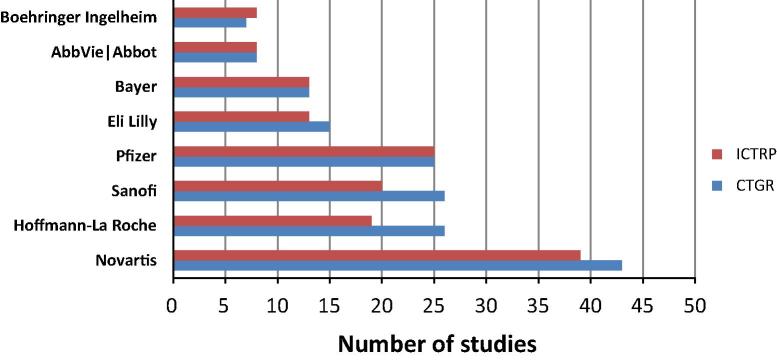
In PACTRP and CTGR, most studies were sponsored by universities/organizations followed by industry in CTGR or individual researcher in PACTRP. None of PACTRP trials were sponsored by industry. In ICTRP, industry sponsorship predominated. The most common Egyptian sites were Cairo University, Mansura University, Ain Shams University, Assiut University and Alexandria University (Fig. 3). The most common industrial sponsors were Novartis, Hoffman-La Roche, Sanofi, Pfizer, Eli Lilly and Bayer (Fig. 4).
Fig. 3.
Sites of conduct of Egyptian of studies registered to Clinicaltrials.gov (CTGR) and International Clinical Trials Registry Platform (ICTRP) and Pan-African Clinical Trials Registry platform (PACTRP).
Fig. 4.
Industrial sponsors of Egyptian studies registered to Clinicaltrials.gov (CTGR) and International Clinical Trials Registry Platform (ICTRP).
Interventional studies were the most common type of studies in both CTGR and ICTRP (∼84%) followed by observational studies. PACTRP contained interventional studies only (100%). Treatment was the most common study purpose (∼69%) in CTGR and PACTRP followed by prevention (Table 2). In CTGR, combined safety/efficacy was the commonest endpoint (49.1%) followed by efficacy alone (19.5%). In the three registries, inclusion of both genders and adults was the most common practice. The median number of enrolled subjects was 155 in CTGR, 150 in ICTRP and 63 in PACTRP.
Table 2.
Types, purposes, endpoints and enrollment of Egyptian studies in 3 trial registries.
| CTGR | ICTRP | PACTR | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| 548 | 686 | 56 | |
| Study types | |||
| Interventional | 458 (83.6) | 580 (84.5) | 56 (100.0) |
| Observational | 87 (15.9) | 104 (15.2) | 0 (0) |
| Expanded access | 3 (0.5) | 2 (0.3) | 0 (0) |
| Purpose | |||
| Treatment | 379 (69.2) | –a | 41 (73.2) |
| Prevention | 46 (8.4) | –a | 7 (12.5) |
| Supportive care | 9 (1.6) | –a | 5 (8.9) |
| Diagnostic | 6 (1.1) | –a | 2 (3.6) |
| Health services research | 5 (0.9) | –a | 0 (0) |
| Screening | 2 (0.4) | –a | 0 (0) |
| Education counseling training | 0 (0) | –a | 1 (1.8) |
| Others/not mentioned | 101 (18.4) | –a | 0 (0) |
| End point classification | |||
| Safety/Efficacy study | 269 (49.1) | –a | –a |
| Efficacy study | 107 (19.5) | –a | –a |
| Safety Study | 5 (0.9) | –a | –a |
| Pharmacokinetics study | 3 (0.5) | –a | –a |
| Bio-equivalence study | 1 (0.2) | –a | –a |
| Pharmacokinetics | 1 (0.2) | –a | –a |
| Pharmacokinetics/dynamics | 1 (0.2) | –a | –a |
| Others/not mentioned | 161 (29.4) | –a | –a |
| Gender | |||
| Both | 391 (71.4) | 442 (64.4) | 37 (66.1) |
| Female | 137 (25.0) | 176 (25.7) | 18 (32.1) |
| Male | 20 (3.6) | 25 (3.6) | 1 (1.8) |
| Not mentioned | 0 (0) | 43 (6.3) | 0 (0) |
| Age | |||
| Child (<18 year) | 37 (6.8) | 68 (9.9) | 4 (7.1) |
| Adult (18–65 year) | 174 (31.8) | 299 (43.6) | 35 (62.5) |
| Senior (>65 year in WHO) | 1 (0.2) | 1 (0.1) | 0 (0) |
| Child and adult | 35 (6.4) | 22 (3.2) | 2 (3.6) |
| Adult and senior | 233 (42.5) | 142 (20.7) | 12 (21.4) |
| Child, adult and senior | 68 (12.4) | 18 (2.6) | 3 (5.4) |
| Number of enrolled subjects | |||
| Median (IQR) | 155 (70–538) | 150 (60–532) | 63 (41–100) |
CTGR: U.S. ClinicalTrials.gov registry, ICTRP: WHO International Clinical Trials Registry Platform, PACTR: Pan-African Clinical Trials Registry, IQR: inter-quartile range.
Not included.
For interventional studies in CTGR and PACTRP, the most common intervention was drug use (61.9%) followed by procedure (12.8%). In the three registries, the commonest study phase was phase 3 followed by phase 4 and phase 2 (Table 3). The commonest model was parallel group assignment followed by single group assignment. The commonest allocation method was the randomized method; blinding was implemented in many of trials and was mostly double-blind. In CTGR and ICTRP, observational studies constituted almost 15% of the studies and were mostly prospective and cohort studies (Table 4). None of the studies in PACTRP was classified as observational.
Table 3.
Interventional studies from Egypt in 3 clinical trial registries.
| CTGR | ICTRP | PACTR | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Total | 458 | 580 | 56 |
| Intervention | |||
| Drug | 316 (69.0) | –a | 30 (53.6) |
| Procedure | 64 (14.0) | –a | 11 (19.6) |
| Biological | 22 (4.7) | –a | 0 (0) |
| Device | 16 (3.5) | –a | 0 (0) |
| Radiation | 11 (2.4) | –a | 5 (8.9) |
| Dietary supplement | 6 (1.3) | –a | 2 (3.6) |
| Behavioral | 3 (0.7) | –a | 0 (0) |
| Other | 20 (4.4) | –a | 8 (14.3) |
| Study phase | |||
| Phase 3 | 151 (33.0) | 119 (20.5) | 48 (85.7) |
| Phase 4 | 77 (16.8) | 83 (14.3) | 0 (0) |
| Phase 2 | 67 (14.6) | 67 (11.6) | 3 (5.4) |
| Phase 2|Phase 3 | 27 (5.9) | 26 (4.5) | 5 (8.9) |
| Phase 1|Phase 2 | 16 (3.49) | 21 (3.6) | 0 (0) |
| Phase 1 | 12 (2.6) | 13 (2.2) | 0 (0) |
| Phase 0 | 7 (1.5) | 6 (1.0) | 0 (0) |
| Phase 3/phase 4 | 0 (0) | 4 (0.7) | 0 (0) |
| Unknown | 101 (22.05) | 241 (41.6) | 0 (0) |
| Model | |||
| Parallel assignment | 320 (69.9) | 326 (56.2) | 50 (89.3) |
| Single group assignment | 106 (23.1) | 103 (22.4) | 3 (5.4) |
| Crossover assignment | 5 (1.1) | 2 (0.4) | 2 (3.6) |
| Factorial assignment | 4 (0.9) | 4 (0.7) | 1 (1.8) |
| Not mentioned | 113 (24.7) | 145 (25.0) | 0 (0) |
| Allocation | |||
| Randomized | 355 (77.5) | 380 (65.5) | 48 (85.7) |
| Non-randomized | 50 (10.9) | 66 (11.4) | 5 (8.9) |
| NM/Other | 53 (11.6) | 134 (23.1) | 3 (5.4) |
| Masking | |||
| Open label | 226 (49.3) | 220 (37.9) | 13 (23.2) |
| Double blind | 168 (36.7) | 182 (31.4) | 24 (42.9) |
| Single blind | 50 (10.9) | 77 (13.3) | 19 (33.9) |
| Unknown | 114 (24.9) | 101 (17.4) | 0 (0) |
CTGR: U.S. ClinicalTrials.gov registry, ICTRP: WHO International Clinical Trials Registry Platform, PACTR: Pan-African Clinical Trials Registry.
Not included.
Table 4.
Observational studies from Egypt in 3 trial registries.
| CTGR | ICTRP | PACTR | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| 87 | 104 | 0 | |
| Time perspective | |||
| Prospective | 68 (78.2) | 71 (68.3) | 0 (0) |
| Cross-sectional | 11 (12.6) | 11 (10.6) | 0 (0) |
| Retrospective | 5 (5.8) | 5 (4.8) | 0 (0) |
| Not mentioned | 3 (3.4) | 17 (16.4) | 0 (0) |
| Observational model | |||
| Cohort | 41 (47.1) | 44 (42.3) | 0 (0) |
| Case control/case only/case-crossover | 28 (32.2) | 25 (24.1) | 0 (0) |
| Cross-sectional | 0 (0) | 7 (6.7) | 0 (0) |
| Not mentioned | 18 (20.7) | 28 (26.9) | 0 (0) |
CTGR: U.S. ClinicalTrials.gov registry, ICTRP: WHO International Clinical Trials Registry Platform, PACTR: Pan-African Clinical Trials Registry.
a Not included.
Classification of the conditions being researched differed between the registries. Thus, no direct comparisons could be made. In CTGR, the most researched conditions were Digestive System Diseases, Cancers/Other Neoplasms, Symptoms/General Pathology (Fig. 5). In ICTRP, the most common conditions were Cancer, Hepatitis and Diabetes mellitus (Fig. 6). In PACTRP, diseases and conditions being researched were listed as individual conditions rather than grouped items. In PACTRP, pregnancy and infertility were the commonest conditions (n = 12; 21.4%) followed by surgical procedure (n = 9; 16.1%), cancer (12.5%), pains (10.7%), and others (39.3%).
Fig. 5.
Conditions researched in Clinicaltrials.gov.
Fig. 6.
Disease categories in International Clinical Trials Registry Platform (ICTRP).
Discussion
Egypt is the “Land of Civilizations” and is reputed worldwide for its distinct 7000-year-old record of civilization and immense wealth of knowledge [13]. Egyptian medicine was dominant from approximately 2850 BC to 525 BC. The Egyptian Imhotep was the first physician figure to rise out of antiquity. Imhotep was a known scribe, priest, architect, astronomer and magician. He performed surgery, practiced some dentistry, extracted medicine from plants and knew the position and function of the vital organs. Surgical instruments and surgical procedures such as circumcision are documented on the paintings on the temples [14], [15].
With its 82-million population, Egypt constitutes 1.2% of the total world’s population [16]. However, it contributes only 0.34% of records in US clinical trial registry (Clinicaltrials.gov; CTGR) and 0.30% in the WHO registry (ICTRP). It ranked 115th and 55th in both registries, respectively. We believe that trials in Egypt are far more than those in the registries. Egypt has 41 universities and 94 health-related faculties and medical schools [17], [18]. There are 24 Faculties of Medicine with up to 34 departments in each faculty [19], 18 Faculties of Dentistry with up to 12 departments in each faculty [20], 34 Faculties of Pharmacy with up to 9 departments in each faculty [21], 12 Faculties of Nursing with up to 7 departments in each faculty [22] and 6 Faculties of Physical Therapy with up to 8 departments in each faculty. In Egypt, there are more than 42,000 faculty members and 344,000 post-graduate students [17], [18], 140,000 physicians, 18,200 dentists, 37,500 pharmacists 176,000 nurses and 35,000 physical therapists [23]. Clinical research including clinical trials is an essential mandate for getting Master and Doctorate Degrees. Moreover, clinical research for publication is a mandate for promotion for faculty members. Thus, the output of about 94 health-related faculties, 1400 departments and the huge number of health care professionals and faculty members is definitely more than the registered studies.
The apparent low contribution of Egypt in trial registries may be related to multiple factors. There is no Egyptian national trial registry that hosts all trials running in the country. Many trials are not recorded in trial registries whether global e.g. WHO ICTRP, regional/continental e.g. PACTRP or other e.g. CTGR. There are no national mandates for trial registration. The reasons why some Egyptian trials are registered can be imputed from examining the trial sponsorship where most trials are sponsored by either pharmaceutical industry or academic universities. Many of the pharmaceutical industrial sponsors include an Egyptian site in addition to other non-Egyptian sites. Industrial sponsors have to comply with the international regulations that mandate trial registration [6]. In addition to innovation, great majority of research conducted in the Egyptian universities aims at publication in renowned international journals that both increases author citation h-index [24] and advances the university ranking in the university index [25], [26]. Based on ICMJE [6], most renowned journals ask author to provide details for trial registration prior to publication. Otherwise, many Egyptian investigators may be unaware of the presence of trial registries or the mandate of registration. They may lack the appropriate training on the detailed steps of registration. They may miss the support from administrative research offices/facilities that can carry out this job on their behalf. All of these factors contribute to the low number of Egyptian trials in registries.
Clinical trial registries serve many beneficial purposes [27]. It is mandated by many laws and recommended by many authorities and bodies [6]. Of the three studies registries, the most mature registry is CTGR followed by the ICTRP and lastly the PACTRP. There are many similarities between search fields in the three registries (Table 5). CTGR user-interface is simple and enables easy, effective and accurate search. CTGR allows searching by intervention, study phase, gender, age groups, date of last update, sponsors/collaborators, results, completion status. It displays the total number of studies and allows further work on the results within the website. PACTRP allows further search by study purpose, dates of start and end and principle investigator name and country. However, interface was less user-friendly and total results are not shown and could not be worked within the website.
Table 5.
Comparing advanced search in three trial registries and suggestions for the proposed Egyptian registry.
| Searchable items | CTGR | PACTRP | ICTRP | EGCRR |
|---|---|---|---|---|
| Simple interface | + | ± | ± | + |
| Study title/acronym or ID | + | + | + | + |
| Recruitment status | + | + | + | + |
| Study or trial type | + | + | − | + |
| Study purpose | − | + | − | + |
| Location (state and country list) | +(L) | +(FT) | +(L&FT) | +(L&FT) |
| Condition | + | + | + | + |
| Intervention | + | − | + | + |
| Study phase | + | − | − | + |
| Gender | + | − | − | + |
| Age | +(C & A & S) | +(Mn & Mx) | +(C) | + |
| Funding source | + | + | − | + |
| Dates of registration | + | + | + | + |
| Date of start | − | + | − | + |
| Date of end | − | + | − | + |
| Date of last update | + | − | − | + |
| Sponsor/collaborators | + | − | + | + |
| Study results | + | − | − | + |
| Completion status | + | − | − | + |
| Display total number of results | + | − | + | + |
| Principal Investigator Name and country | − | + | − | + |
| Results can be worked out further in the site | + | − | − | + |
CTGR: U.S. ClinicalTrials.gov registry, ICTRP: WHO International Clinical Trials Registry Platform, PACTR: Pan-African Clinical Trials Registry, EGCRR: Egyptian Clinical research registry (proposed), L: list, FT: free-test, C: children (<18 years), A: adults (18–65 years), S: seniors (>65 years), Mn & Mx: minimum & maximum, (+): present, (−): absent, (±): intermediate.
The most common diseases researched in the registries were digestive system diseases, cancers/neoplasms, hepatitis, DM and urogenital conditions. While most of these conditions are included in the top Egyptian biomedical research topics [28], yet the order is not perfectly matching. While hepatitis is the top research priority, it is the second in the registered trials. Similarly, tumors were the 7th research priority but came first in the registered trials. Schistosomiasis is the second research priority but the 4th in the registered studies. Reasons for the difference between the country’s research priorities and the actual research done can be many. Researchers may be unaware of these priorities. Mechanisms to enforce compliance may be lacking. Most of the research is funded by universities or Industry. Universities are not under the portage of the Ministry of Health (MOH) and thus are not mandated to comply with its set priorities. Industry enforces research topics that are within their main interest e.g. to get a drug’s approval. Additionally and as mentioned above, trials in the registries do not provide a comprehensive picture of the biomedical research in Egypt.
Most of the studies were funded by either academic universities/organizations (38–54%) or industry (38–43%) and most of the industries are multinational ones. Unfortunately, we could not get information on the amount of funding and the share of different stakeholders. In developed countries such as US, investment in health research comes mainly from Industry (64.4%), federal government (32.6%) and universities (3.0%) [29]. Industry focuses on research with the best return on investment (ROI). This may, or may not, coincide with the national priorities. Thus, in developing countries such as Egypt, governmental sponsorship should predominate to enforce the national priorities.
While the mentioned registries were set to host clinical trials, yet up to 17% of the entries refer to observational studies i.e. non-trial studies. With time, the number of observational studies in clinicaltirals.gov increased and the scope of registration widened. For example, the clinicaltrials.gov was initially set to host clinical trials on IND for life-threatening conditions, then it expanded to include other types of studies as well as their results [10]. This was because much of the rationale for the prospective registration of clinical trials applies to the registration of observational studies. Observational studies could provide valuable information not achieved even by the well conducted phase III clinical trials particularly related to drug safety. Thus, many authorities and medical journals now recommend registering observational studies. Making both observational and interventional studies available in a single registry will provide researchers and others with a more comprehensive view of the growing evidence base [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2952011/#!po=10.7143].
A limitation of the current study includes missing of some data. However, this relates to the source registries and the way they were designed (Table 5). For example, some registries ask specifically for some information not requested by the others making comparison unfeasible. Also, some of the information requested may be either optional or may not have exhaustive quality checks for what was entered. Optional fields may be left blank. Even, if some data were entered there were no exhaustive checks that read, understand and advise authors for the necessary changes.
Conclusions
Clinical trial registration is mandated both ethically and from a regulatory viewpoint. However, clinical trials from Egypt in three trial registries are apparently low and do not accurately reflect clinical research conducted in Egypt or its potential.
Recommendations
We recommend that Egyptian authorities immediately start a national Egyptian clinical research registry (EGCRR) that matches the well-developed registries particularly CTGR and hosts clinical trials as well as observational studies (Table 5). We propose that EGCRR be hosted by the Egyptian Ministry of Health, the Ministry of Higher Education or the Ministry of Scientific Research. We recommend that all Egyptian clinical research mandatorily get registered to this EGCRR immediately following ethical approval or exemption by the ethical committees, and that investigators and institutions receive appropriate training on research registration. In addition to the several advantages of registration e.g. transparency, enhanced participation and bias elimination, such Egyptian registry (EGCRR) would ensure that research is not repeated and that the country’s research activity can be assessed at any time.
To ensure compliance with registration, there should be some enforcement. This can be done at least at two levels; first funding may be linked to registration and second there should be some legislation to mandate registration. We are to see the first Egyptian Clinical Research Law that will regulate and advances clinical research as well as protect research participants’ rights, safety and well-being. This Law should address registration. To protect the interests of sponsors and investigators, they are highly encouraged to patent and copy-right their investigational agents and intellectual work prior to embarking on experimentation in humans and hence registration. The registry should not reveal the sensitive and secret aspects of an investigational agent e.g. its chemical structure. Rather, it may refer to the investigational agent through a coding process. Access to secret and sensitive information should be restricted and not publicly available.
We also recommend setting of research support offices/facilities that can carry out the job of registration or at least provide the support and guidance. It is highly recommended that Egypt sets a clear and frequently updates national research priorities. These priorities should be decided on with the involvement of all stakeholders including, but not limited to, MOH, academic institutions, politicians, researchers, funding agencies, pharmaceutical companies and community representatives. Priorities should be declared and enforced. Research funding should be linked to compliance with these priorities. To avoid skewing research toward the best interest of international pharmaceutical industry, governmental funding of research should increase to encourage implementing national priorities. National pharmaceutical industry should be encouraged to engage more in research.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Institute of Translational Health Sciences. Definitions of Clinical and Translational Research [Internet]; 2014. <https://www.iths.org/funding/definitions> [cited 16.03.14].
- 2.National Institutes of Health, US. Glossary of Common Site Terms – ClinicalTrials.gov [Internet]; 2014. <http://clinicaltrials.gov/ct2/about-studies/glossary> [cited 16.03.14].
- 3.World Medical Association. WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects [Internet]; 2013. <http://www.wma.net/en/30publications/10policies/b3/> [cited 16.03.14]
- 4.World Health Organization. WHO|About the WHO ICTRP [Internet]; 2014 <http://www.who.int/ictrp/about/en/> [cited 16.03.14].
- 5.Government of Canada HC. Registration and Disclosure of Clinical Trial Information – Drugs and Health Products – Health Canada [Internet]; 2005. <http://www.hc-sc.gc.ca/dhp-mps/prodpharma/proj/enreg-clini-info/index-eng.php> [cited 16.03.14].
- 6.National Institutes of Health, US. Why Should I Register and Submit Results? – ClinicalTrials.gov [Internet]; 2014. <http://clinicaltrials.gov/ct2/manage-recs/background> [cited 16.03.14].
- 7.European Medicines Agency. Clinical Trials register [Internet]; 2014. <https://www.clinicaltrialsregister.eu/ctr-search/search?query=&country=3rd> [cited 16.03.14].
- 8.Pan-African clinical trials registry. PACTR Registry [Internet]; 2014. <http://www.pactr.org/> [cited 16.03.14].
- 9.International Federation Of Pharmaceutical Manufacturers Associations. IFPMA Clinical Trials Portal – Quick and Easy Access to Clinical Trial Information – IFPMA Clinical Trials Portal [Internet]; 2014. <http://clinicaltrials.ifpma.org/clinicaltrials/no_cache/en/myportal/index.htm> [cited 16.03.14].
- 10.National Institutes of Health. ClinicalTrials.gov Background – ClinicalTrials.gov [Internet]; 2014. <http://clinicaltrials.gov/ct2/about-site/background> [cited 16.03.14].
- 11.Ghersi D., Pang T. From Mexico to Mali: four years in the history of clinical trial registration. J Evid-Based Med. 2009;2(1):1–7. doi: 10.1111/j.1756-5391.2009.01014.x. [DOI] [PubMed] [Google Scholar]
- 12.Abrams A., Siegfried N. The Pan African clinical trials registry: year one data analysis of the only African member of the world health organization network of primary registries. J Evid-Based Med. 2010;3(4):195–200. doi: 10.1111/j.1756-5391.2010.01099.x. [DOI] [PubMed] [Google Scholar]
- 13.Egypt State Information Service. Egypt: Land & People. [Internet]; 2014 [cited 17.03.14]. <http://www.sis.gov.eg/En/Story.aspx?sid=1> [accessed March 2014].
- 14.Thorwald J. Harcourt, Brace and World; New York: 1962. Science and secrets of early medicine. [Google Scholar]
- 15.Association of American Medical Colleges . Association of American Medical College; Washington, DC: 1999. Task force on clinical research 2000. 3p. [Google Scholar]
- 16.US Census Bureau. U.S. & World Population Clocks. [Internet]; 2010. <http://www.census.gov/main/www/popclock.html> [cited 17.03.14].
- 17.Supreme Council of Universities-Egypt. Egyptian Universities and High Institutes [Internet]; 2014. <http://www.scu.eun.eg/wps/portal/> [cited 25.03.14].
- 18.Supreme Council of Universities-Egypt. Statistical report on Higher Education in Egypt, 2014 [Internet]; 2014. <http://www.scu.eun.eg/wps/wcm/connect/3a2c4a00432a3cc6bef1fe4b78836778/%D9%85%D8%B7%D9%88%D9%8A%D8%A9++%D9%85%D8%A7%D8%B1%D8%B3+2014.pdf?MOD=AJPERES&CACHEID=3a2c4a00432a3cc6bef1fe4b78836778>.
- 19.Kasr Alainy. Department [Internet]; 2014. <http://www.medicine.cu.edu.eg/beta/index.php/en/departments> [cited 25.03.14].
- 20.List of medical schools in Egypt [Internet]. Wikipedia, the free encyclopedia; 2014 <http://en.wikipedia.org/w/index.php?title=List_of_medical_schools_in_Egypt&oldid=600658031> [cited 25.03.14].
- 21.Emam S. Progessional Associations rejects President decision on increasing numbers of students [author translation] [Internet]; 2013. <http://www.youm7.com/News.asp?NewsID=955762#.UzHA-YUsF4k> [cited 25.03.14].
- 22.Faculty of Nursing South Valley University. Egyptian faculties of Nursing [Internet]; 2014. <http://www.svu.edu.eg/faculties/nursing/colleges.html> [cited 25.03.14].
- 23.State Information Services. Health Care in Egypt [Internet]; 2014. <www.sis.gov.eg/home/society> [cited 25.03.14].
- 24.Hirsch J.E. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci USA. 2005;102(46):16569–16572. doi: 10.1073/pnas.0507655102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quacquarelli Symonds Limited. QS World University Rankings 2013/2014 [Internet]; 2014. <http://www.topuniversities.com/university-rankings/world-university-rankings/2013#sorting=rank+region=6+country=+faculty=+stars=false+search=> [cited 25.03.14].
- 26.ShanghaiRanking Consultancy. Academic Ranking of World Universities – 2013| Top 500 universities | Shanghai Ranking – 2013 | World University Ranking – 2013 [Internet]; 2013. <http://www.shanghairanking.com/ARWU2013.html> [cited 25.03.14].
- 27.Dickersin K., Rennie D. Registering clinical trials. JAMA, J Am Med Assoc. 2003;290(4):516–523. doi: 10.1001/jama.290.4.516. [DOI] [PubMed] [Google Scholar]
- 28.Scientific Committee for Health Research. Health research in Egypt – a country report [Internet]. Council on Health Research for Development; 2000. <http://www.cohred.org/downloads/689.pdf> [cited 02.05.14].
- 29.Research!America. Economic Impact of Health Research: United States. 2008 [Internet]; 2008. <http://www.researchamerica.org/econ_United_States> [cited 02.05.14].