Graphical abstract
Keywords: Magnesium battery, Non-aqueous liquid electrolyte, Ionic conductivity, Magnesium ion transference
Abstract
Nonaqueous liquid electrolyte system based dimethyl sulfoxide DMSO and magnesium bromide (MgBr2) is synthesized via ‘Solvent-in-Salt’ method for the application in magnesium battery. Optimized composition of MgBr2/DMSO electrolyte exhibits high ionic conductivity of 10−2 S/cm at ambient temperature. This study discusses different concentrations from 0 to 5.4 M of magnesium salt, representing low, intermediate and high concentrations of magnesium salt which are examined in frequency dependence conductivity studies. The temperature dependent conductivity measurements have also been carried out to compute activation energy (Ea) by least square linear fitting of Arrhenius plot: ‘log σ − 1/T. The transport number of Mg2+ ion determined by means of a combination of d.c. and a.c. techniques is ∼0.7. A prototype cell was constructed using nonaqueous liquid electrolyte with Mg anode and graphite cathode. The Mg/graphite cell shows promising cycling.
Introduction
Due to gasoline high cost and probability of oil depletion, demands for renewable energy sources increased. Rechargeable batteries are the most important sources of renewable energy especially batteries to run electric cars. But the dream of the spread of the electric car is still far because we need battery systems suitable for load leveling applications. They have to be large, safe, and made of cheap and abundant components. Most importantly, it is mandatory that they have a very prolonged lifecycle [1], [2].
The lithium (Li) battery used as a power source because of its high specific power and high energy density. However, high demand for Li battery tends to make increase in Li price due to geographically limitedness in the earth crust [1]. As an alternative to lithium, magnesium has been foregrounded. Magnesium batteries have recently attracted great interest due to their high energy density and environmentally friendly components, coupled with magnesium’s low cost (∼$2700/ton for Mg compared to $64,000/ton for Li) and abundance in the earth’s crust (∼13.9% Mg compared to ∼0.0007% of Li) [3], [4], [5].
The kinetically sluggish Mg intercalation and diffusion in cathode materials and the incompatibility between anode and electrolyte due to the high polarizing ability of the Mg2+ cation are the major obstacles that prohibit Mg batteries from commercialization [6], [7], [8]. Thus, the great challenge in the commercialization of Mg batteries is the development of an electrolyte which is stable in contact with the electrode materials, does not form a blocking layer, low cost and has a wide electrochemical window [9].
The development of magnesium electrolytes is considered the most important challenge for the commercial application of rechargeable magnesium (Mg) batteries because electrolyte properties govern battery performance and determine the class of cathodes utilized [10]. Aurbach et al. [8] developed a prototype Mg cell using Mo6S8 Chevrel phase cathode, Mg(AlCl2EtBu)2/tetrahydrofuran electrolyte with a little fade of capacity. However, the development of rechargeable Mg batteries still hindered by using electrolyte suffers from the use of very volatile solvents such as THF, and organohaloaluminate electrolytes with highly corrosive nature, high cost, high air sensitivity, and low anodic stability which limit the choice of cathodes [11], [12].
Magnesium bromide (MgBr2) is the fast conducting salt in a number of crystalline and amorphous materials. Furthermore, Dimethyl sulfoxide (DMSO) is an organosulfur compound with Melting point 19 °C and Boiling point 189 °C and the formula (CH3)2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. Recently, Peng et al. [13] report that a Li-O2 cell composed of a DMSO-based electrolyte and a NPG electrode can sustain reversible cycling, retaining 95% of its capacity after 100 cycles and having >99% purity of Li2O2 formation at the cathode, even on the 100th cycle, and its complete oxidation on charge. Although, to date, there is no works on DMSO-based electrolyte for magnesium batteries have been done. This prompted us to try the test dimethyl sulfoxide/MgBr2 as an electrolyte for magnesium battery.
Upon the above considerations, a key obstacle to obtain the better performance, new electrolyte system for rechargeable magnesium batteries should be developed. Therefore, in this article, new classes of non-aqueous liquid ‘Solvent-in-Salt’ electrolytes, MgBr2/DMSO are discussed. The electrolytes were characterized by using XRD and impedance spectroscopy. Generally, this work mainly focuses on studies of ionic conductivity and Mg2+ ion transference with the aim of gaining understanding in ionic conduction of magnesium in this electrolyte system. With non-aqueous electrolyte optimum composition, Mg/Graphite cell is assembled, and its cycling performances will be briefly examined to evaluate the applicability of the electrolyte to solid-state magnesium batteries.
Experimental
All tested electrolytes consisted of a magnesium bromide (492 ppm of moisture, Sigma) with different concentrations, x = 0.0, …, 0.55 M in dimethyl sulfoxide (Loba Chemie). The resulting solutions were stirred for 2 h at room temperature.
The XRD patterns of MgBr2, MgBr2/DMSO precipitated solid product were taken using Rigaku diffractometer type RINT-Ultima IV/S. The diffraction system based with Cu tube anode with voltage 40 kV and current 40 mA.
Conductivity measurements of MgBr2/DMSO liquid electrolyte were performed using impedance method. Electrolyte samples were put into a conductivity cell between two similar aluminum electrodes. The whole assembly was placed in a furnace monitored by a temperature controller. The rate of heating was adjusted to be 2 K min−1. Impedance measurements were performed on Gwinstek LCR-811OG in the frequency ranging from 20 Hz to 10 MHz at different temperatures.
Slurry was obtained by mixing 1 g graphite, 0.1 g magnesium sulfate and 0.2 g PVA binder using magnetic stirrer hot plate (60 °C) for 2 h. The cathode (reduced electrode) is prepared by coating pole of stainless steel by 0.1 g of this slurry in dry atmosphere at 100 °C for two hours. Entek PE membrane separator has been used. The whole assembly was shown in Fig. 1. The tube cell was discharged at room temperature on a multi-channel battery test system (NEWARE BTS-TC35) with the charge/discharge time of 2/2 h and 10 min rest. The current density was 10 μA/cm2.
Fig. 1.
Schematic design of the laboratory cell.
Results and discussion
Plausible reaction model between MgBr2 and DMSO is shown in Fig. 2a. In the hexahydrate (MgBr2·6H2O) the magnesium cation is surrounded only by water molecules, forming distinct octahedral [14]. By dissolving MgBr2·6H2O in DMSO two water molecules are leaving the structure being replaced by DMSO molecules with Mg atom bridging two oxygen atoms. The magnesium-ion transference numbers were obtained by combining alternating-current (AC) impedance and direct-current (DC) polarization measurements using a symmetric Mg/electrolyte/Mg cell. First, AC impedance test was performed to obtain a total resistance Rcell. Then DC polarization was carried out to obtain a stable current IDC. The magnesium-ion transference number was calculated by the formulas (tMg = Rcell/RDC and RDC = VDC/IDC) [15]. The transport number of Mg2+ ion determined by means of a combination of d.c. and a.c. techniques is ∼0.7. The mechanism of ion transport likely proceeds via structure diffusion (exchange of the bridging (O–Mg–O)) or via vehicular transport (movement of the metal cations along with the first solvation shell) [16]. The prediction scheme of Mg2+ ion transports in operating Mg-cell is shown in Fig. 2b.
Fig. 2.
(a) Proposed reaction scheme of DMSO/MgBr2 electrolyte. (b) Expected schematic design of Mg2+ ion transport in Mg-cell.
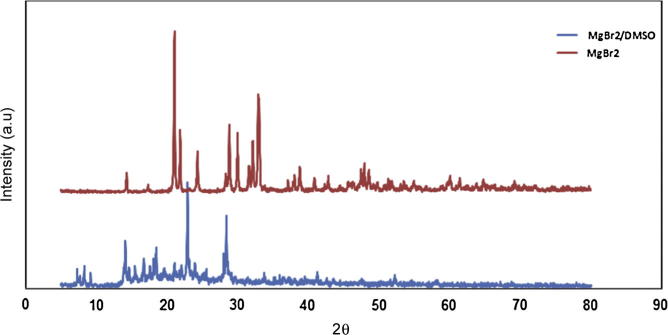
The reacting behavior of magnesium bromide and DMSO was studied by using XRD powder diffraction. Fig. 3 shows XRD pattern of pure MgBr2 compound, and the MgBr2/DMSO solid product precipitated from super-saturated solution. It can be seen that there are significant differences between them. The intensity of the peaks decreased due to the reaction of MgBr2 with DMSO. The data of pure MgBr2 matched with JCPDS file No. 74-1040 for MgBr2 hexahydrate with preferential orientation of the (1 1 1) direction, 2θ = 22° ((2 1) and (1 1 2) at 2θ = 32.9°). XRD data of MgBr2/DMSO solid product precipitated from super-saturated solution exhibited low intensity peaks. New peaks are observed at 2θ = 18° and 22.2°. These peaks may be associated with structure perturbation due to the dehydration features [14], where two water molecules are leaving the structure being replaced by DMSO molecules with Mg atom bridging two oxygen atoms. Chemical reactivity between salt and solvent is a crucial issue. In fact, significant reactivity can induce the formation of new structure phases promote the ionic transfer.
Fig. 3.
XRD pattern of MgBr2 and DMSO/MgBr2 solid product.
The crystallite size D of pure MgBr2 compound, and the MgBr2/DMSO solid product was calculated from X-ray data using Scherrer equation as given below [17]:
| (1) |
where 0.9 is the Scherrer constant, λ is the wavelength of X-ray, B is the breadth of the pure diffraction profile and θ is the incidence angle of the X-ray. Using this formula, we have calculated the particle size of pure MgBr2 compound, and the MgBr2/DMSO solid product in the range 50–70 nm as shown in Table 1.
Table 1.
XRD results of MgBr2 and DMSO/MgBr2 solid product.
| 2Theta | FWHM (B) | d | Particle size (nm) |
|---|---|---|---|
| Structure parameters of MgBr2 | |||
| 21.05 | 0.12 | 4.21 | 67.30 |
| 32.94 | 0.12 | 2.71 | 69.00 |
| 33.06 | 0.12 | 2.70 | 69.02 |
| 28.82 | 0.12 | 3.09 | 68.32 |
| 29.98 | 0.18 | 2.97 | 45.67 |
| 21.86 | 0.15 | 4.06 | 53.91 |
| Structure parameters of MgBr2/DMSO | |||
| 22.91 | 0.12 | 3.87 | 67.52 |
| 28.40 | 0.12 | 3.13 | 68.26 |
| 14.12 | 0.09 | 6.26 | 88.91 |
| 18.47 | 0.12 | 4.79 | 67.04 |
| 28.05 | 0.09 | 3.17 | 90.94 |
| 16.72 | 0.15 | 5.29 | 53.51 |
Impedance spectroscopy is an excellent tool for characterizing many of the electrical properties of materials and their interfaces with electronically conducting electrodes. The frequency response analyses were carried out over a wide frequency range from 20 Hz to 10 MHz. Complex impedance plot of MgBr2/DMSO electrolyte at different MgBr2 concentrations and temperatures is shown in Fig. 4a. The complex plot shows semicircle which corresponds to the bulk resistance Rb with parallel combination of the frequency dependent capacitance Cg, Fig. 4a (inset). The diameter of the semicircle decreases with increasing concentrations and temperatures. The bulk resistance value Rb is determined from the low frequency intercepts on the x-axis of the complex impedance plots. The ionic conductivity is calculated using the equation , where L is the thickness of the electrolyte, A is the surface area of the film.
Fig. 4.
(a) Cole–Cole plots of DMSO/MgBr2 electrolyte. (b) Variation of ionic conductivity of DMSO/MgBr2 electrolyte as a function of MgBr2 concentration.
Fig. 4b shows the variation of ionic conductivity (σ) of MgBr2/DMSO electrolyte with respect to the content of MgBr2. The maximum conductivity of pure DMSO is found to be ∼10−6 S cm−1 at room temperature (∼25 °C). A gradual increase in conductivity is observed, when the MgBr2 salt is added in the DMSO and maximum conductivity around ∼10−4–10−2 S cm−1 was achieved at x = 0 > 0.16 M of MgBr2 for bulk and ac conductivity(1 kHz), respectively. On further addition of MgBr2 a slight decrease in conductivity is observed, and the electrolyte is not dimensionally stable, showing glue like nature beyond the addition of 0.16 M of MgBr2. General expression of ionic conductivity is illustrated as below:
| (2) |
where n is the number of charge carriers, q is the charge of ions type, and μ is the mobility of ion pairs. Based on the equation above, the quantity and mobility of the charge carriers are the main factors that could affect the ionic conductivity. Therefore, the possible reason of enhancement in conductivity at low concentration of MgBr2 is due to generation/introduction of mobile charged species, namely Mg2+ and Br−. The decrease in conductivity, observed after ∼0.16 M of MgBr2, is consistent with the higher viscosities of the more concentrated salt mixtures, and thus restricted free cation mobility (i.e., decrease of μ), as a result, lower ionic conductivity is observed [16], [18], [19].
Fig. 5a and b shows the frequency dependence of the total conductivity for MgBr2/DMSO electrolyte at different concentrations and temperatures, respectively. The conductivity of samples is found to increase with increasing frequency, as well as temperature. As compared to the high frequency region, the electrolyte shows a lower conductivity at low frequency. Slow periodic reversal of the applied electric field is the main contributor for this phenomenon. The low frequency dispersion region is due to the interfacial resistance or electrode polarization (EP). It refers to the electrodes being polarized as charge accumulates due to the slow periodic reversal of the electric field at low frequency. As the frequency decreases, more and more charges are accumulated at the electrodes and electrode interfaces, which leads to a decrease in the number of free mobile ions and eventually a drop in conductivity at lower frequency. At higher frequency, the conductivity increases with frequency as the greater mobility of charge carriers and faster hopping of ions. As a result, the ion exchange process occurs effectively at higher frequency. An almost frequency-independent region is observed thereafter. It implies that the conductivity is equal to the bulk ionic conductivity. Above 1 MHz, the conductivity drops down (excluding pure DMSO) with the frequency. It is ascribed to the short period of the applied electric field for the charging to occur. The onset of conductivity drop decreases with increasing temperature. As the frequency increases, more and more (>1 MHz) charges return to accumulate at the electrodes and electrode interfaces due to ions not being able to follow the field variation at higher frequencies which leads to a decrease in the number of free mobile ions and eventually a drop in conductivity at higher frequency [20], [21], [22].
Fig. 5.
(a) Frequency-conductivity dependence of DMSO/MgBr2 electrolyte at different MgBr2 concentrations. (b) Frequency-conductivity dependence of DMSO/MgBr2 (x = 0.4 M) electrolyte at different constant temperatures.
It can be noticed that the behavior follows universal power law [23]
| (3) |
where σdc is the dc conductivity (the extrapolation of the plateau region to zero frequency), A is the frequency independent pre-exponential factor, ω is the angular frequency and n is the frequency exponent. The values of the exponent n have been obtained using the least square fitting of Eq. (3) for two regions are listed in Table 2. For the first region (20–800 Hz), the values of n1 lie within the range of 0.4 < n < 0.8. The values of n, predict the domination of hopping conduction in MgBr2/DMSO electrolyte. It can be noticed that the values of n for the second region (1 kHz–1 MHz) n2 ∼0, frequency independent. The theoretical approaches of this behavior may be attributed to that the carriers transport takes place through infinite percolation path that can be explained by ‘percolation’ model [24].
Table 2.
Electrical parameters of DMSO/MgBr2 (x = 0.4 M) electrolyte at room temperatures.
| MgBr2 concentration × M | Region 1 n1 | Region 2 n2 | Frequency ω (Hz) | Activation energy E (eV) |
|---|---|---|---|---|
| Conductivity–power exponent dependence | Frequency-activation energy dependence | |||
| 0 | 0.38 | 1.28 | 0 | 0.01 |
| 0.06 | 0.64 | 0.02 | 1 kHz | 0.15 |
| 0.20 | 0.79 | 0.07 | 10 kHz | 0.18 |
| 0.27 | 0.75 | 0.05 | 100 kHz | 0.19 |
| 0.50 | 0.75 | 0.05 | 1 MHz | 0.13 |
| 0.54 | 0.74 | 0.05 | 10 MHz | 0.004 |
In the present study, the effects of temperature on the ionic conductivity of the selected electrolyte (0.4 M) were studied. The temperature dependence of dc conductivity (the extrapolation of the plateau region to zero frequency), and ac conductivity of MgBr2/DMSO electrolyte is of the Arrhenius type:
| (4) |
where σo in Eq. (4) is a pre-exponential factor, Ea the activation energy, K is the Boltzmann constant and T is the temperature in Kelvins. Fig. 6 shows ln(σ) versus 1000/T plots at different constant frequencies. The regression values of all three chosen samples are near to unity, indicating that the temperature-dependent ionic conductivity for this system obeys Arrhenius rule. The results are tabulated in Table 2. The values of activation energy decrease with increasing frequency. This reflects the role of frequency to initiate ion transfer.
Fig. 6.
Temperature-conductivity dependence of DMSO/MgBr2 (x = 0.4 M) electrolyte at different constant frequencies.
To further examine the usability of the electrolyte for a full cell with a magnesiation/de-magnesiation cathode, tube type cell using typical graphite cathode and Mg ribbon anode was assembled, and the charge–discharge properties were investigated at room temperature. Fig. 7 presents the typical potential time profile with the discharge and charge time limits of 2/2 h. The cell demonstrates reversible cycling behavior at current density ∼10 μA/cm2 with stable profile. The chemical reaction that probably takes place at the cathode is
That is, during the first discharge, Mg2+ ion is inserted into graphite structure from MgBr2/DMSO electrolyte, and deserted from graphite to electrolyte during the recharge.
Fig. 7.
Charge–discharge profiles of Mg/Graphite tube-cell at charge/discharge time of 2/2 h and 10 min rest.
Conclusions
Nonaqueous liquid electrolyte containing Mg2+ ions have been prepared and characterized by impedance techniques. Three different types of impedance spectra have been identified and differentiated by magnitude of ionic conductivity. Its trend increases almost proportional to the content of magnesium salt, and reaches highest ionic conductivity of ∼10−2 S/cm at >0.16 M of MgBr2 salt. This can be related to the increase of charge carriers and amorphous phase from low to high level of dopant salt content. The Conductivity is found to be dependent on both temperature and frequency. From the results obtained, it can be observed that this non-aqueous liquid electrolyte system already shows great potential. It is worthy to be further investigated with incorporation of other additives, such as plasticizers, or ionic liquids. Nonaqueous liquid electrolyte system based dimethyl sulfoxide DMSO and magnesium bromide (MgBr2), opens the door for the further development of electrolytes for the high energy magnesium batteries.
Conflict of interest
The author has declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgment
The author thanks Dr. Mostafa Nassar, Chemistry Department, Benha University for help in drawing chemical structure.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Yoo H., Markevich E., Salitra G., Sharon D., Aurbach D. On the challenge of developing advanced technologies for electrochemical energy storage and conversion. Mater Today. 2014;17:110–121. [Google Scholar]
- 2.Saha P., Datta M., Velikokhatnyi O., Manivannan A., Alman D., Kumta P. Rechargeable magnesium battery: current status and key challenges for the future. Prog Mater Sci. 2014;66:1–86. [Google Scholar]
- 3.Park J., Kim J., Park J., Nam T., Kim K., Ahn J. Discharge mechanism of MoS2 for sodium ion battery: electrochemical measurements and characterization. Electrochim Acta. 2013;92:427–432. [Google Scholar]
- 4.Lv D., Xu T., Saha P., Datta M.K., Gordin M.L., Manivannan A. A scientific study of current collectors for Mg batteries in Mg(AlCl2EtBu)2/THF electrolyte. J Electrochem Soc. 2013;160:A351–A355. [Google Scholar]
- 5.Peng B., Liang J., Tao Z., Chen J. Magnesium nanostructures for energy storage and conversion. J Mater Chem. 2009;19:2877–2883. [Google Scholar]
- 6.Li W., Li C., Zhou C., Ma H. Metallic magnesium nano/mesoscale structures: their shape-controlled preparation and Mg/air battery applications. Angew Chem. 2006;45:6009–6012. doi: 10.1002/anie.200600099. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y., Jiao L., Wu Q., Du J., Zhao Y., Si Y. Sandwich-structured graphene-like MoS2/C microspheres for rechargeable Mg batteries. J Mater Chem A. 2013;1:5822–5826. [Google Scholar]
- 8.Aurbach D., Lu Z., Schechter A., Gofer Y., Gizbar H., Turgeman R. Prototype systems for rechargeable magnesium batteries. Nature. 2000;407:724–727. doi: 10.1038/35037553. [DOI] [PubMed] [Google Scholar]
- 9.Zhao-Karger Z., Mueller J., Zhao X., Fuhr O., Jacob T., Fichtner M. Novel transmetalation reaction for electrolyte synthesis for rechargeable magnesium batteries. RSC Adv. 2014;4:26924–26927. [Google Scholar]
- 10.Gamal R., Sheha E., Shash N., El-Shaarawy M. Preparation and characterization of Mg2+-ion conducting composite based on poly(vinyl alcohol) with various concentrations of Li2O. Mater Express. 2014;4:293–300. [Google Scholar]
- 11.Ha S., Lee Y., Woo S., Koo B., Kim J., Cho J. Magnesium(II) bis(trifluoromethane sulfonyl) imide-based electrolytes with wide electrochemical windows for rechargeable magnesium batteries. ACS Appl Mater Int. 2014;6:4063–4073. doi: 10.1021/am405619v. [DOI] [PubMed] [Google Scholar]
- 12.Saha P., Jampani P., Datta M., Okoli C., Manivannan A., Kumta P. A convenient approach to Mo6S8 Chevrel phase cathode for rechargeable magnesium battery. J Electrochem Soc. 2014;161:A593–A598. [Google Scholar]
- 13.Peng Z., Freunberger S., Chen Y., Bruce P. A reversible and higher-rate Li-O2 battery. Science. 2012;337:563–566. doi: 10.1126/science.1223985. [DOI] [PubMed] [Google Scholar]
- 14.Dinnebier R., Runcevski T., Sugimoto K. Dehydration of magnesium bromide hexahydrate studied by in situ X-ray powder diffraction. Z Anorg Allg Chem. 2013;639:59–64. [Google Scholar]
- 15.Suo L., Hu Y., Li H., Armand M., Chen L. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat Commun. 2013;4:1481. doi: 10.1038/ncomms2513. [DOI] [PubMed] [Google Scholar]
- 16.Jeremias S., Giffin G., Moretti A., Jeong S., Stefano Passerini, Passerini S. Mechanisms of magnesium ion transport in pyrrolidinium bis(trifluoromethanesulfonyl)imide-based ionic liquid electrolytes. J Phys Chem C. 2014;118:28361–28368. [Google Scholar]
- 17.Kitazawa S., Choi Y., Yamamoto S., Yamaki T. Rutile and anatase mixed crystal TiO2 thin films prepared by pulsed laser deposition. Thin Solid Films. 2006;515:1901–1904. [Google Scholar]
- 18.Sheha E. Preparation and physical properties of (PVA)0.75(NH4Br)0.25(H2SO4)xM solid acid membrane. J Non-Cryst Solids. 2010;356:2282–2285. [Google Scholar]
- 19.Polu A., Kumar R. Effect of Al2O3 ceramic filler on PEG-based composite polymer electrolytes for magnesium batteries. Adv Mater Lett. 2013;4:543–547. [Google Scholar]
- 20.Kumar Y., Hashmi S., Pandey G. Ionic liquid mediated magnesium ion conduction in poly(ethylene oxide) based polymer electrolyte. Electrochim Acta. 2011;56:3864. [Google Scholar]
- 21.Ramesh S., Liew C., Arof A. Ion conducting corn starch biopolymer electrolytes doped with ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate. J Non-Cryst Solids. 2011;357:3654–3660. [Google Scholar]
- 22.Ramesh S., Lu S., Morris E. Towards magnesium ion conducting poly(vinylidene-fluoride hexafluoropropylene)-based solid polymer electrolytes with great prospects: ionic conductivity and dielectric behaviours. J Taiwan Inst Chem E. 2012;43:806–812. [Google Scholar]
- 23.Jonscher A. The universal dielectric response. Nature. 1977;267:673–679. [Google Scholar]
- 24.Karlsson L., Mcgreevy R. Mechanisms of ionic conduction in Li2SO4 and LiNaSO4: paddle wheel or percolation. Solid State Ionics. 1995;76:301–308. [Google Scholar]