Abstract
A new class of injectable, biocompatible and biodegradable hydrogel is reported. This hydrogel is derived from N-succinyl chitosan (SCS) mixed with water-soluble dialdehyde starch (DAS) without using a conventional chemical crosslinker. The hybrid hydrogel is formed owing to the Schiff’s base reaction between amine groups of SCS and dialdehyde groups of DAS to form —CH N— group. SCS, DAS, and SCS–DAS hybrid hydrogels were synthesized and then characterized by FTIR analysis spectroscopy. The influence of SCS:DAS ratio in hybrid polymers solution on physicochemical properties of resultant hydrogels (e.g. gelation time, gel fraction (%) and equilibrium swelling ratio), surface morphology, in vitro weight loss (%), and mechanical stability was examined. The results demonstrated that SCS content has a profound role for forming tighter crosslinked hybrid hydrogels, where the increase of SCS content reduces the time for hydrogel forming. Also, the water uptake and hydrolytic weight loss decrease. Meanwhile, the DAS content increases, and mechanical properties of SCS–DAS hybrid hydrogels decrease. Curcumin release profile and adhered HGF cells on hydrogel surface sharply influenced the SCS portion in hybrid hydrogel composition. The SCS–DAS hybrid hydrogel properties afforded a possible opportunity to be used as a covalent in situ forming hybrid hydrogels in biomedical applications such as, tissue engineering and cartilage repair.
Keywords: Dialdehyde starch, N-succinyl chitosan, Schiff-base reaction, Hybrid hydrogel, Physiochemical properties
Introduction
Many biodegradable hydrogels have been employed as in situ forming scaffolds for a range of biomedical applications for example, drug delivery, cell encapsulation, or scaffolds for tissue engineering, which facilitate to incorporate cells or drugs without change in the size or shape of formed hydrogels [1], [2], [3]. Recently, a lot of attempts have been exerted for fabrication of injectable and in situ forming hydrogels, including photopolymerization [4], [5], chemical crosslinker such as, carbodiimide, glutaraldehyde and genipin [6]. However, special photo-sensitizers and prolonged irradiation were required for the photopolymerization which limits somewhat its use in critical applications. Also, chemical crosslinking agents are completely restricted and banned for the use as injectable in situ forming polymer scaffolds, due to their incompatibility and their toxicity for living cells [7]. Presently, limited attempts have utilized the Schiff-base reaction for crosslinking the functional amine and aldehyde groups, suggesting biomaterials with non-cytotoxicity effects compared to performed studies used traditional harmful chemical crosslinkers [8], [9]. Several polysaccharides such as dextran [4], [10], hydroxyethyl starch [5], [11], gum Arabic [12], sodium alginate [13], and hyaluronic acid [9], have been previously employed for biomedical application such as drug delivery and wound dressing polymeric materials. Nevertheless, few studies have used dialdehyde starch for the preparation of cell carrier hydrogels in tissue engineering [14]. Here, we show a new in situ self-forming biodegradable hydrogel is derived from water-soluble chitosan derivative and dialdehyde starch, without using any additional chemical crosslinker.
Chitosan, a copolymer of glucosamine and N-acetyl glucosamine units linked by 1-4 glucosidic bonds, is derived partially by de-acetylated of chitin. Chitosan is one of the most abundant natural amino polysaccharides which has a reported pH-sensitive property. Chitosan derivatives have a variety of applications in industrial pharmacy, and biotechnology, and it is commonly used as wound dressing materials [15], [16]. It has a huge biocompatibility, biodegradability, hemostatic, and brilliant antibacterial activity. High molecular weight’s chitosan is insoluble in water and has low solubility in physiological fluids too, caused by its strong-intermolecular hydrogen bonding. However, it can be dissolved in the water–acetic acid solution. The problem is revolving around hydrogels that can be fabricated from chitosan water–acetic solution, often need a consecutive washing cycles to neutralize and remove the excess of acid, which greatly restricts somewhat its use in the medical applications [16].
N-succinyl chitosan (SCS) is a fully water-soluble chitosan derivative in the mild conditions. SCS is obtained by inclusion of succinyl groups onto the N-terminal of the glucosamine units of chitosan. The demand on SCS will attract much attention as polymeric drug delivery compared to a pristine chitosan. This is owing to its appealing and intrinsic properties such as, simple solubility in different pHs without specific acidified medium need, high hydrophilicity, biocompatibility, and its antibacterial activity as existing in the pristine chitosan. SCS was previously synthesized and crosslinked with other polymers such as, hyaluronic acid, alginate [17], [18], [19], lactosaminate [20], and low-density lipoprotein for biomedical applications. Similarly, SCS was incorporated previously and covalently crosslinked with hyaluronic acid as scaffolds for soft and cartilage tissue engineering and repair [17], [19]. Dialdehyde starch (DAS) is a starch derivative, which is derived from the oxidation of raw starch using periodic acid or sodium periodate as oxidants to oxidize 2,3-o-dihydroxy starch into dialdehyde starch. DAS has a good biological degradation, and intrinsic biochemical characteristics such as, semi-alkali solubility, containing many aldehyde function groups, strong bonding, naturally abundant, low cost as compared with other biodegradable polymers, and easy crosslinking branches [21], [22]. Thus, for the aforementioned features of DAS, it has been chosen for crosslinking with SCS.
Herein, this work aimed to prepare and assess nontoxic in situ-forming biodegradable SCS–DAS hybrid hydrogels via advantageous Schiff-base covalent bonding without using any extraneous chemical crosslinkers. The influence of SCS:DAS ratio variations on the physicochemical properties of obtained hybrid hydrogels e.g. gelation time, gel fraction (%), equilibrium swelling, morphological microstructure, in vitro weight loss (%), mechanical stability, in vitro release profile of curcumin and cell adhesion behavior was examined and evaluated for biomedical purposes.
Material and methods
Materials
Chitosan (de-acetylating degree: <86%, Mn ∼4.5 × 105) was obtained from Ruji Biotech Development Co., Ltd. (Shanghai, China). Succinic anhydride, sodium periodate, acetone, l-lactic acid (>98%), and curcumin (from curcuma longa powder, 99%) were purchased from Sigma–Aldrich Chemie GmbH, Steinheim, Germany. Soluble starch was obtained from Shanghai Reagent Co., China. Distilled and deionized water were used throughout this research. DMEM was purchased from Biochrom GmbH, Germany (10% FBS, 120 μg mL−1 penicillin streptomycin, 5% CO2). All other chemicals were used without any further purification.
Preparation of dialdehyde starch (DAS) polymer
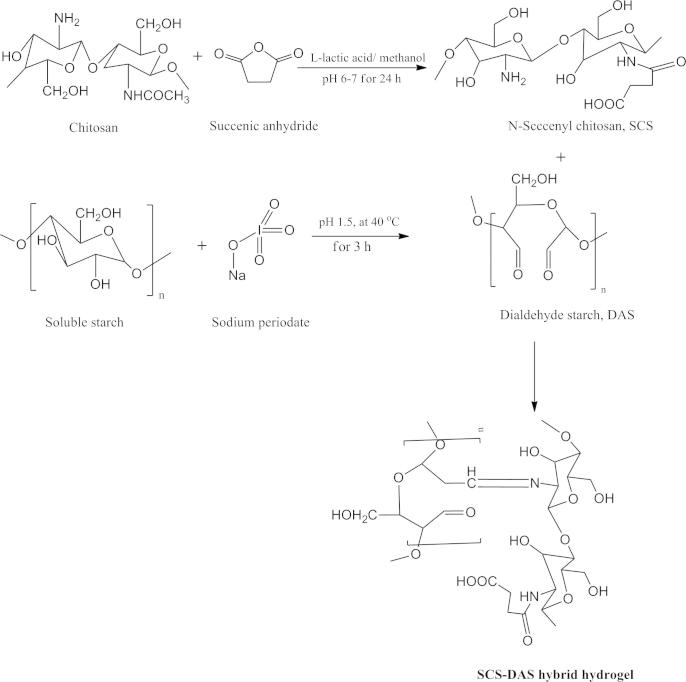
DAS polymer was synthesized according to the procedure of Lu et al. [23]. Typically, sodium periodate solution (0.7 M, pH 1.5) was added to the solution of soluble starch (1.0 g in 100 mL deionized water) with proportion of sodium periodate to starch that is 1:1, under continuous stirring at 40 °C for 6 h. DAS polymer was then precipitated by addition of dry-acetone. DAS precipitate was further purified by washing three times with mixture of deionized water–acetone (1:1). Thereafter, DAS was vacuum-dried (vacuum oven model no. 3618P, Thermo Scientific Co., Germany) at 40 °C for 24 h and kept under desiccators till use, Scheme 1.
Scheme 1.
Schematic representation of the synthetic route of N-succinyl chitosan (SCS, up), dialdehyde starch (DAS, middle) and SCS–DAS hybrid hydrogel (down).
Preparation of N-succinyl chitosan (SCS) polymer
SCS was synthesized according to reported procedure elsewhere, with slight modification [24]. 0.5 g of chitosan was dissolved in 20 mL (5%, v/v) l-lactic acid solution, followed by addition of 80 mL methanol to dilute the solution. 1.5 g of succinic anhydride was added to the last solution with a continuous stirring at room temperature for 24 h. SCS was isolated using precipitation process by adjusting the pH of chitosan mixture solution ∼6–7. The resultant SCS, white precipitate was further washed off in ethanol to remove any unreacted lactic acid, followed by filtration, re-dissolved in distilled water, and then dialysis for 2 days to eliminate the methanol traces and un-reacted lactic acid. The obtained pure SCS polymer was then freeze-dried and the resultant fluffy SCS polymer was stored at −4 °C till use (Scheme 1). The succinylation reaction was verified through the condensation between the amino group of SCS and the electrophilic carbonyl groups of the succinic anhydride , forming an amidic bond with opening of the anhydride ring [17].
Preparation of SCS–DAS hybrid polymer hydrogels
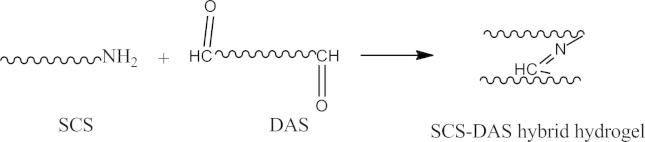
DAS–SCS hybrid hydrogel was prepared by dissolving SCS and DAS with different proportions in six ratios (9:1, 5:1, 3:1, 1:1, 1:3, and 1:5) with a total used polymer concentration of 20 wt.%, respectively in phosphate buffer solution (Scheme 2). The mixture solution was reacted in polyethylene vials as molds.
Scheme 2.
Schematic representation of the interaction between SCS–DAS hybrid hydrogel compositions via Schiff’s base crosslinking reaction.
Instrumental characterizations
Fourier transform infrared (FT-IR)
All samples were prepared for instrumental characterizations according to our previous and reported preparation methods [4], [5], [11], [13]. Vacuum-dried samples of SCS, DAS and SCS–DAS xerogels were analyzed by FT-IR on an EQUINOX 55 instrument (BRUKER, Germany). KBr transparent discs were prepared by grinding the dried sample with infrared grade KBr, followed by pressing. The FTIR spectrums were obtained by recording 64 scans between 4000 and 400 cm−1 with a resolution of 2 cm−1. All samples were pressed by applying a force 105 N into transparent disk (maximum disk weight = 145 mg, with a diameter 13 mm). KBr sample discs were measured in the transmittance mode (T %).
Scanning electron microscope (SEM)
The surface and internal structure of the SCS–DAS xerogel samples were investigated by SEM (JEOL, JSM-6360LA-Japan) with 20 kV. The xerogel was first dehydrated by freeze-dryer unit and coated with Au using an ion sputter coater (model: 11430, USA), combined with vacuum base unit or SPi module control (model: 11425, USA) [4], [5].
Determination of physicochemical properties of SCS–DAS hydrogels
The physicochemical properties of obtained SCS–DAS hybrid hydrogels e.g. gelation time, equilibrium swelling ratio and gel fraction were determined. The gelation time is detected when the polymer mixture solution loses its fluidity at room temperature and transferred visually from viscous to elastic/rubbery or solid state. The gelation rate is determined under the ambient conditions.
The obtained SCS–DAS hydrogels were first dried in a laminar airflow chamber under sterile conditions for 24 h, then dried again at 50 °C in a vacuum oven for 24 h and weighted (W0). The dried xerogel samples were soaked in 50 mL of distilled water for 24 h up to an equilibrium swelling weight (Ws) for removing the leachable or soluble DAS parts from xerogel. The xerogels are then dried directly at 50 °C in an oven and weighted again (We). The gel fraction (GF %) was calculated by the following equation [16]:
| (1) |
In order to measure the swelling ratio of SCS–DAS hybrid hydrogel, hydrogel disc samples (2.5 cm diameter and 0.8 cm thickness) were dried at 50 °C in vacuum oven for 24 h, and the weight of dried sample was determined (We). The dried samples were soaked in distilled water (50 mL), maintained and incubated at 37 °C, then weighted (Ws) at specific interval times that the sample reaches through to the equilibrium swelling state. The water uptake of SCS–DAS hydrogels was determined using the following equation [16]:
| (2) |
Determination of weight loss (%) of SCS–DAS hydrogels
The in vitro hydrolytic degradation of SCS–DAS hydrogels was estimated with respect to the weight loss in gram. SCS–DAS hybrid hydrogels have been dried under vacuum oven at 50 °C for 24 h. Dried hydrogel samples with dimension (1.8 cm diameter and ∼0.5 cm thickness), were weighted and immersed in 25 mL phosphate buffered saline (PBS) (0.1 M, pH 7.4, at 37 °C). The samples were removed at specific timed intervals and then wiped gently with soft paper to remove the water at sample surface. The weight loss was determined in PBS as a function of the incubation time. The weight loss ratio is given as follows [17]:
| (3) |
where W0 is initially weighed hydrogels and Wt is hydrogel which removed from PBS and weighed at function of specific incubation time intervals of hydrolytic degradation for hydrogels. All experiments were done in duplicate.
Dynamic mechanical stability
The dynamic mechanical stability of SCS–DAS hydrogel samples was performed with a dynamic mechanical rheostress (model: RS-100-HAAKE Instrument, Karlsruhe, Germany). The oscillation shear flow measurement was examined at 25 °C, using plate–plate geometry (PP20 Ti), and frequency range from 0.1 to 10 Hz. Dynamic mechanical analysis was used to analyze storage modulus of crosslinked SCS–DAS hybrid hydrogels, which have been crosslinked in situ at Ti-plate mold in dimension (22 mm diameter, and 4 mm thickness) [5]. All curves were obtained in storage modulus values as a function of oscillation frequency sweep (0–10 Hz) at 25 °C, where the storage modulus values (elastic-state) were used as indicator for high mechanical stability of crosslinked hybrid hydrogels.
In vitro release study
The released curcumin from curcumin-loaded SCS–DAS hybrid hydrogels was collected in 0.1 M phosphate buffer solution (pH 5.6 at 37 °C, containing 10% fetal calf serum to maintain the curcumin stability during the release process) [25]. A 250 mg of curcumin was added to the dissolved SCS in phosphate buffer solution, and then DAS was mixed with the latter solution to allow the crosslinking process occurring. After crosslinking, the curcumin-loaded hybrid hydrogel disc was transferred into 50 mL of phosphate buffer solution (pH 5.6) in falcon tube for measuring the release profile. The release medium was kept under incubation with a gentle agitation ∼150 rpm at 37 °C. At calculated time intervals of (15 min, 30 min, 45 min, 1 h, 3 h, 6 h, 12 h, 24 h, 48 h, 72 h, 96 h, 120 h, and 132 h), the released curcumin was determined. A 1.5 mL of release medium was taken to measure the released curcumin, followed by the same volume of phosphate buffer that was substituted in the release medium. The released curcumin was determined at different times up to one week with the help of curcumin calibration standard curve using the ultraviolet spectrophotometer at 425 nm. The calibration curve of curcumin was plotted between absorbance and concentration of standard solution. The released curcumin was first extracted in methanol and quantified spectrophotometrically [25], [26]. The cumulative release (%) of curcumin was given as follows:
| (4) |
Cell adhesion test
The adhesion of human gingival fibroblast cells (HGF, obtained from Provitro, GmbH, Germany) on SCS–DAS hybrid hydrogel surface was assessed. The separated solutions of both SCS and DAS in phosphate buffers were first sterilized under UV for 30 min [27]. The last solutions were mixed together according to the aforementioned studied ratios of SCS and DAS into the 48-well culture plate, and then followed with incubation at 37 °C for 2 h, to ensure formation of all hybrid hydrogels. 1 mL of DMEM contains 20,000 HGF cells that were added to each sample. After the incubation for 12 h, the attached HFG cell numbers onto SCS–DAS hydrogels were quantitatively assessed by CyQuant cell proliferation assay kit. Briefly, the fluorescence measurements were done using micro-plate wells-readers. The cultured media were removed for freezing, and then it was allowed to thaw. The kit dye was added in lysis buffer and measures the fluorescence with an excitation at 485 nm, where the emission detection is at 530 nm. The linear range of the assay under these conditions is from 50 to 50,000 cells per 200 μL sample [28].
Results and discussion
Structure of SCS–DAS hybrid hydrogels
FTIR
FTIR spectra of SCS, DAS, and SCS–DAS hybrid xerogels are presented in Fig. 1. As seen, FTIR spectrum of N-succinyl chitosan (SCS) exhibited stretching vibration peaks of —OH and —NH2 at 3416 cm−1, the weak band of —CH2 stretching at 2918 cm−1, and the C O stretching of amide groups at 1585 cm−1. The peak at 1402 cm−1 belongs to —COOH symmetric stretching vibration, and the peak at 1057 cm−1 for —OH groups of characteristic peak of —CH2OH of alcohol and C—O stretching [17], [18], [19]. FTIR spectrum of dialdehyde starch (DAS) exhibited dialdehyde stretching peaks at 2924 cm−1 and 1384 cm−1, while the peak at 3435 cm−1 is due to —OH groups of unconverted soluble starch and the peak at 1055 cm−1 belongs to —OH groups (characteristic peak of —CH2OH in primary alcohol of glucose ring). However, the peak at 1649 cm−1 might refer to periodate residues [23]. The gelation mechanism of hybrid hydrogel is ascribed to the Schiff-base reaction between amine and dialdehyde groups of SCS and DAS respectively, as shown in Scheme 2. FTIR spectrum of dried SCS–DAS xerogels was very similar with that of SCS with difficulties to notice the vibration peak belongs to the aldehyde functionalities. This perhaps is owing to the formation of hemiacetals, as were previously verified by Tan et al. [17] and Kato et al. [20]. The characteristic peak of hemiacetal was detected at 842 cm−1, suggesting that the coupling reaction between —CHO groups of DAS and —NH2 group of SCS was followed, Fig. 1.
Fig. 1.
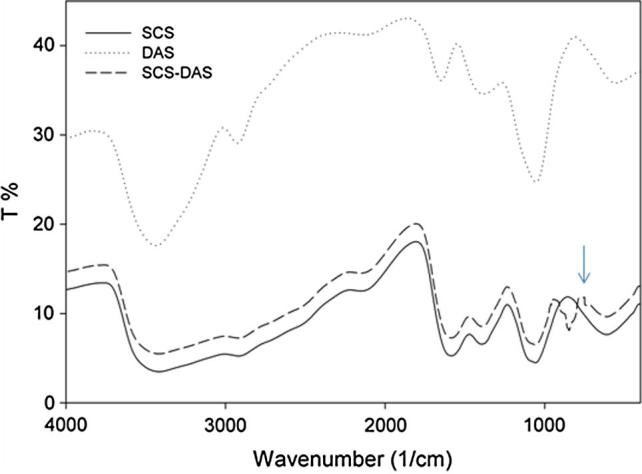
FTIR spectra of SCS, DAS, and SCS–DAS hybrid hydrogel.
SEM
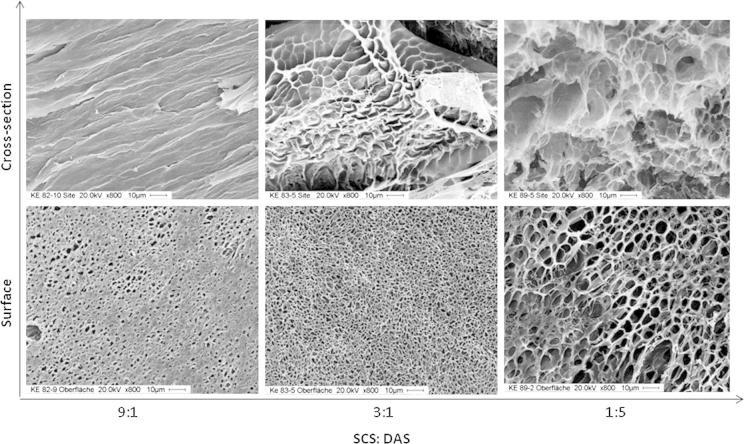
SEM images are investigated to show the microstructure morphologies of the freeze-dried SCS–DAS hybrid hydrogels due to SCS:DAS distinction ratios, Fig. 2. The surface images of SCS–DAS hybrid hydrogels are presented in the down rows Fig. 2. A honeycomb-like structure of thin polymer layer and loosely-like structured can be observed as DAS content increases in SCS–DAS hybrid hydrogel composition. It can imbibe a high water-sorption capacity as further confirmed in swelling results in Fig. 3. Meanwhile, the uniformity, tighter structure, and the homogeneity of the surface structure seem to be better and tiny-pores density declines when the SCS content increases in hybrid hydrogels. Hence, the ratio 1:5 of SCS:DAS revealed a higher content of DAS results in the creation of wider pore diameters, caves, and fluffy network structure in hybrid hydrogels. As the cross-section images which displayed in Fig. 2 (upper rows), the internal morphology structure of SCS–DAS hybrid hydrogels was fully depended on the SCS content, indicating the high volume ratio of SCS in hydrogel composition resulted in small and tiny pore sizes in the hydrogel structure. These results are completely consistent with displayed SEM images of chitosan-hyaluronic acid hydrogels by Tan et al. [17].
Fig. 2.
SEM photographs of freeze-dried SCS–DAS hybrid xerogels depending on the ration of SCS:DAS, surface images (down rows), and cross-section images (upper rows), (magnifications at 20 kV, 800×, 10 μm).
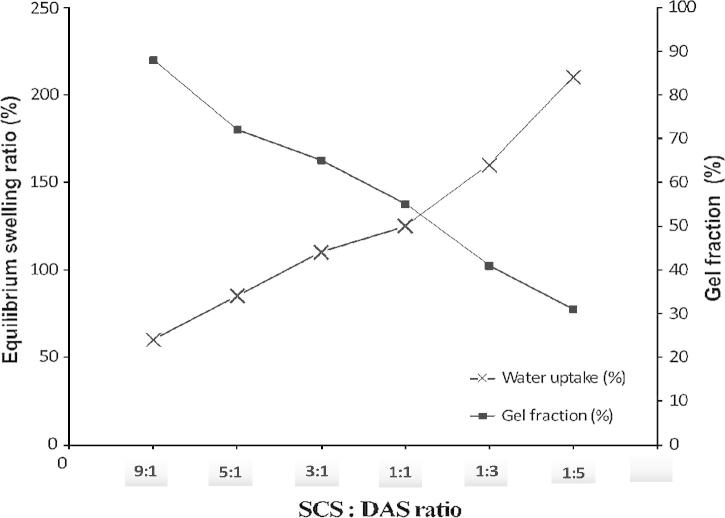
Fig. 3.
Equilibrium swelling ratio and gel volume fraction (%) of SCS–DAS hybrid hydrogels as a function of volume ratio between SCS and DAS moieties.
Physicochemical properties of SCS–DAS hybrid hydrogels
Gelation time
The gelation rate of SCS–DAS hybrid hydrogel was detected at room temperature, where 50 mg mL−1 SCS–DAS was mixed in different six ratios of SCS:DAS, where the gelation occurred within 10–80 min, Table 1. It was found that the gelation time sharply influenced ratios of two component polymers of hydrogel (i.e. SCS and DAS). Interestingly, increasing the ratios of SCS in the hybrid hydrogels, the gelation time comes faster. In addition, the gelation rate of SCS:DAS = 9:1 was the fastest, which exhibited significant faster gelation rate and tighter crosslinked density hydrogel network, than SCS–DAS hydrogel with high DAS e.g. (SCS:DAS 1:5). The result can be attributed to the high molecular weight of SCS compared to DAS; in addition the high crosslinking degree occurred accompanied by compacted hydrogel structure formation, Table 1. However, the gelation time was prolonged when the content of DAS in hydrogels was between 1:1 and 1:5.
Table 1.
Effect of hybrid hydrogel composition (SCS:DAS) on gelation time and cell adhesion (%) on hydrogel surface.
| SCS:DAS | Time of gelation (min) | Adhered HGF cells (%) on hydrogel surface |
|---|---|---|
| 9:1 | 10 | 93 |
| 5:1 | 22 | 84 |
| 3:1 | 28 | 72 |
| 1:1 | 45 | 58 |
| 1:3 | 65 | 48 |
| 1:5 | 80 | 36 |
Equilibrium swelling ratio and gel fraction of SCS–DAS hybrid hydrogels
Fig. 3 shows the equilibrium swelling ratio and gel fraction (%) of obtained crosslinked SCS–DAS hybrid hydrogels. The gel fraction (GF %) increased significantly along with increasing SCS portions in hybrid hydrogel. GF % reached to 89% for SCS:DAS = 9:1, while it decreased to 30%, when SCS portion decreased to 1:5 in SCS:DAS hybrid hydrogels, Fig. 3. Likewise, the equilibrium swelling ratio values rapidly increased with the increase of DAS content in SCS–DAS hybrid hydrogels, where swelling ratios increased dramatically from 52% to 215%, when DAS ratio contents increased in hybrid hydrogel from one to five parts. This behavior was attributed to the incorporation of high hydrophilic polymer in hydrogel composition; i.e. the soluble starch derivative compared to SCS polymer in hydrogel composition [11], [13]. Moreover, both SCS and DAS polymers possess abundant hydrophilic groups, such as —OH and —NH2 groups in DAS and SCS polymers respectively, which can easily form hydration with the water molecules. Thus, included amounts between SCS and DAS in hybrid hydrogel synthesis apparently affect the swelling behavior of obtained hybrid hydrogels. This implies that crosslinking density influences the most of hydrogel properties; that is an increase in the crosslinking density with increasing SCS contents leads to a significant water content reduction, and an increase in the gel fraction %. Thus, the ratio 9:1 of SCS:DAS hydrogels revealed the highest crosslinking density and the highest gel fraction %, consequently decreasing the hydrophilicity and direct sorption of polymer chains to water molecules resulting in polymer networks collapsing.
Weight loss (%) of SCS–DAS hybrid hydrogels
The hydrogels that are targeted for biomedical applications particularly for tissue engineering should be biodegradable. The degradation behavior or weight loss rate of in situ hybrid hydrogel scaffold has a critical influence on the biological long-term performance e.g. cell-adhesion construct of the injected polymer [4], [19]. This interest stems from the importance of understanding the in vitro weight loss profile of the synthesized hybrid hydrogels. The hydrolytic degradation or weight loss (%) of SCS–DAS hybrid hydrogel was conducted in vitro with respect to the incubation time in PBS at 37 °C and different SCS:DAS ratios, as displayed in Fig. 4. The ratio of SCS:DAS showed a considerable influence on the weight loss profile. SCS–DAS hybrid hydrogels with the ratio of 1:1–1:5 showed notable weight losing rate owing to weak crosslinking density and dissociated polymer chains in PBS, where 50% weights loss was monitored between 2 and 5 days. However, hybrid hydrogels with high SCS contents such as, 3:1–9:1 lost their weight ranged 20–45% steadily on more than 10 days, due to high crosslinked hydrogel networks and compacted structure. It is worth mentioned that, the SCS–DAS hybrid hydrogels were stable in PBS during the first two weeks of incubation referring that the hydrolysis speed of SCS–DAS via Schiff’s base is very low under the physiological conditions, particularly hydrogels with high SCS contents. The degradation results of high DAS content in hybrid hydrogel were ascribed thoroughly to the starch chains that were enzymatic-degraded quickly caused by α-amylase which is mostly present in physiological conditions [4].
Fig. 4.
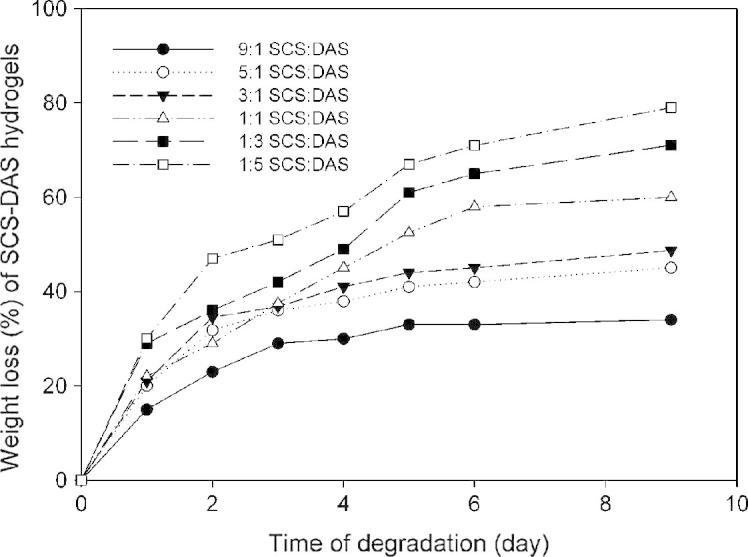
In vitro hydrolytic degradation (weight loss %) of SCS–DAS hydrogels depending on the time of degradation as function of different volume ratios between SCS and DAS polymers.
Dynamic mechanical stability of SCS–DAS hybrid hydrogels
The mechanical properties of SCS–DAS hybrid hydrogels were carried out by a dynamic oscillation rheometer as a function of different ratios between SCS and DAS, Fig. 5. This characterization allows the evaluation of the elastic (G′: storage modulus) of the hybrid hydrogel. Theoretically, the increase of crosslinking density improves the mechanical properties of hybrid hydrogels, as has been already obtained. It can be seen that, the storage modulus values for hybrid hydrogels having high SCS content are much higher compared to that measured samples with high DAS contents. For example, with increasing SCS ratios (SCS:DAS) from 1:3 to 9:1 (v/v), G′ values of hybrid hydrogels were improved correspondingly, from 2 kPa to 12 kPa. However, the high DAS content in hybrid hydrogels e.g. ratio of 1:5, exhibited deteriorated mechanical properties of hybrid hydrogels (∼1 kPa). Similarly, these results are fully dependable with reports of Tan et al. [17] and Sun et al. [19]. They demonstrated that increasing SCS contents increased the compressive modulus values of SCS–hyaluronic acid hybrid hydrogels.
Fig. 5.
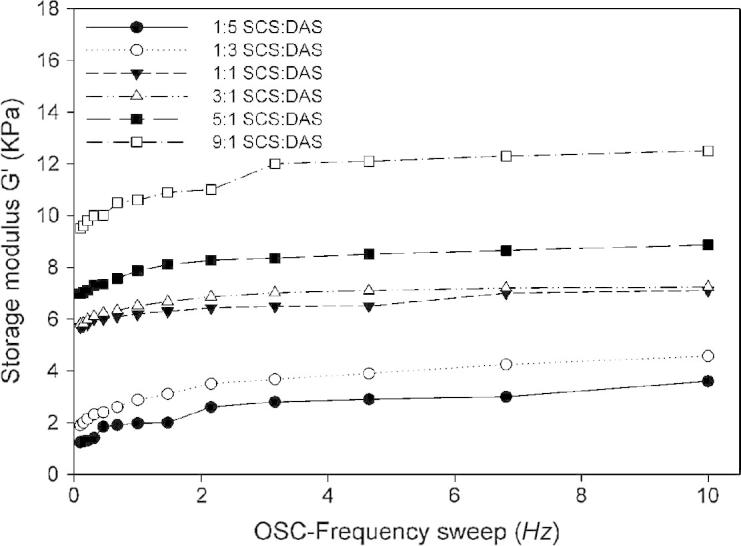
Plot of the dynamic storage modulus of SCS–DAS hybrid hydrogels versus the applied oscillation frequency at room temperature as a function of different volume ratios between SCS and DAS polymers.
In vitro release study
The in vitro release profile study which is an essential test for being the resultant materials will be used in the biomedical application. The release profiles of curcumin-loaded SCS–DAS hybrid hydrogels are presented in Fig. 6. All release behaviors showed a big burst release particularly with the high DAS content in hydrogel compositions. However, the burst release significantly was reduced with increasing the SCS content in hybrid hydrogel composition. The big burst release for most hydrogels at the beginning of release time is owing to the presence of the soluble and stuck curcumin on hydrogel surfaces and the structural permeability of hydrogels as well. Apparently, the moderate SCS contents (i.e. SCS:DAS ratios of 3:1, 5:1) revealed gradual and sustainable release behavior after 5 days, which is a proper behavior for scaffolds medical materials. As expected, the highest released curcumin (83%) was detected by the highest DAS content in hydrogels, which can be interpreted by the hydrogel wettability and pores on surface structure, (Fig. 2, Fig. 3). Conversely, the lowest released curcumin (33%) was obtained by the highest SCS content hydrogel, which tends to release plateau (zero-order release) after 2 days. The presented release results showed that the hydrogel structure affected directly the release profile of curcumin. This implies that the weak hydrogel structure due to the high DAS content showed high burst release of curcumin. However, the high crosslinked or compressed hydrogel structure due to high SCS content showed very limited and sustainable released curcumin.
Fig. 6.
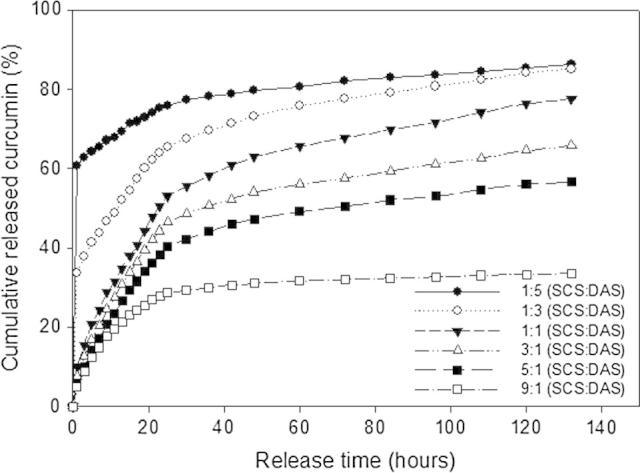
Representative cumulative release profile of curcumin-loaded SCS–DAS hydrogels with respect to different hybrid hydrogel compositions (SCS:DAS).
Cell adhesion on SCS–DAS hydrogels
Cell adhesion results on the surface of SCS–DAS hydrogels as function of different hydrogels composition were provided in Table 1. After 12 h of incubation, the percentage number of adhered HGF cells on hydrogel surface was significantly enhanced with increasing SCS moiety in hydrogel composition. However, the number of adhered cells on hydrogel surface was clearly reduced with increasing the DAS portion in hydrogel composition. This study indicates that the hydrogel composition influences sharply the behavior of surface cell adhesion, which is predominantly owing to the wettability and hydrophilicity of the host hydrogel surface. Thus, the surface of SCS–DAS hydrogels was more convenient for HGF cell adhesion, particularly with high SCS hydrogel composition due to its higher bioactivity and biocompatibility than the hydrogel surface with high DAS composition. This adhesion behavior is depending on the fact that, the cell adhesion on hydrophilic hydrogel surfaces can be improved by either reducing the degree of wettability of surface, or functionalizing the hydrogel surface with RGD peptides [29]. As a result, this assumption is coincided with the swelling degree results in Fig. 3, which summarize that presence of SCS portion in hybrid hydrogel could limit the swelling ability due to its high viscosity and molecular weight; however it enhanced drastically the cell adhesion behavior.
Conclusions
The in situ forming SCS–DAS hybrid hydrogels were successfully synthesized via Schiff’s base crosslinking reaction. The ratio between two main polymers composition of hybrid hydrogel (i.e. SCS and DAS) has pointedly influenced the physicochemical properties of resultant hybrid hydrogels. High SCS content of hybrid hydrogels showed a slightly shorter gelation time, limited water uptake, little weight loss (%), and considerable tighter hydrogel structure than those of the hydrogel possessing a high DAS content. Furthermore, the hybrid hydrogels with a high SCS content showed slower degradation rate and steady limited weight loss (%) than hydrogels with a low SCS composition. This implies that SCS–DAS hybrid hydrogels are relatively stable and have limited hydrolysis rate with a high SCS hydrogel composition in physiological conditions without any unwanted by-products, which make them good candidate hydrogel scaffolds for tissue engineering. These preliminary results indicate that, Schiff’s base crosslinking reaction has been employed to form SCS–DAS hybrid hydrogels under mild conditions without using any extraneous toxic chemical crosslinker or reagent. In addition, the increase of SCS in hybrid hydrogel composition reduced drastically the curcumin burst release and the curcumin sustained releases were monitored. In the same context, the adhered HGF cells number on hydrogel surface was greatly improved by SCS content in hybrid hydrogel composition. Thus, SCS–DAS hybrid hydrogels will have potential attraction to be used as injectable and biodegradable scaffold hydrogels for clinical purposes e.g. tissue engineering and cartilage repair.
Conflicts of interest
The author reports no financial or nonfinancial conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgments
This study is financially supported by Higher Education Ministry of Egypt, Sector of Cultural Affairs and Missions. The author greatly acknowledges Prof. Henning Menzel, Institute for Technical Chemistry, Braunschweig University of Technology (TU-BS) Germany, for the help of IR, SEM, and mechanical measurements. Also, he gratefully thanks Prof. Xin Chen, Department of Macromolecular Science, Laboratory of Advanced Materials, Fudan University, Shanghai, China, for providing some chemicals and reagents that have been employed to achieve this work.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Lao L., Tan H., Wang Y., Gao C. Chitosan modified poly(l-lactide) microspheres as cell micro carriers for cartilage tissue engineering. Colloids Surf B Biointerfaces. 2008;66:218–225. doi: 10.1016/j.colsurfb.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 2.McGlohorn J.B., Grimes L.W., Webster S.S., Burg K.J.L. Characterization of cellular carriers for use in injectable tissue-engineering composites. J Biomed Mater Res. 2003;66A:441–449. doi: 10.1002/jbm.a.10546. [DOI] [PubMed] [Google Scholar]
- 3.Tememoff J.S., Mikos A.G. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials. 2000;21:2405–2412. doi: 10.1016/s0142-9612(00)00108-3. [DOI] [PubMed] [Google Scholar]
- 4.Kamoun E.A., Menzel H. Crosslinking behavior of dextran modified with hydroxyethyl methacrylate upon irradiation with visible light-effect of concentration, coinitiator type, and solvent. J Appl Polym Sci. 2010;117:3128–3138. [Google Scholar]
- 5.Kamoun E.A., Menzel H. HES-HEMA nanocomposite polymer hydrogel: swelling behavior and characterization. J Polym Res. 2012;19:9851–9865. [Google Scholar]
- 6.Dai S., Barbari T.A. Hydrogel membranes with mesh size asymmetry based on the gradient crosslinking of poly(vinyl alcohol) J Membr Sci. 1999;156:67–79. [Google Scholar]
- 7.Ferretti M., Marra K.G., Kobayashi K., Defail A.J., Chu C.R. Controlled in vivo degradation of genipin crosslinked polyethylene glycol hydrogels within osteochondral defects. Tissue Eng. 2006;12(9):2657–2663. doi: 10.1089/ten.2006.12.2657. [DOI] [PubMed] [Google Scholar]
- 8.Ito T., Fraser I.P., Yeo Y., Highley C.B., Bellas E., Kohane D.S. Anti-inflammatory function of an in situ cross-linkable conjugate hydrogel of hyaluronic acid and dexamethasone. Biomaterials. 2007;28(10):1778–1786. doi: 10.1016/j.biomaterials.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Ruhela D., Riviere K., Szoka F.C.J. Efficient synthesis of an aldehyde functionalized hyaluronic acid and its application in the preparation of hyaluronan–lipid conjugates. Bioconjugate Chem. 2006;17(5):1360–1363. doi: 10.1021/bc0600721. [DOI] [PubMed] [Google Scholar]
- 10.Fathi E., Atyabi N., Imani M., Alinejad Z. Physically crosslinked polyvinyl alcohol–dextran blend xerogels: morphology and thermal behavior. Carbohydr Polym. 2011;84:145–152. [Google Scholar]
- 11.Kenawy E., Kamoun E.A., Eldin Mohy M.S., El-Meligy M.A. Physically crosslinked poly(vinyl alcohol)-hydroxyethyl starch blend hydrogel membranes: synthesis and characterization for biomedical applications. Arab J Chem. 2014;7(3):372–380. [Google Scholar]
- 12.Nishi K.K., Jayakrishnan A. Preparation and in vitro evaluation of primaquine-conjugated gum Arabic microspheres. Biomacromolecules. 2004;5:1489–1495. doi: 10.1021/bm0499435. [DOI] [PubMed] [Google Scholar]
- 13.Kamoun E.A., Kenawy E.S., Tamer T.M., El-Meligy M.A., Mohy Eldin M.S. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: characterization and bio-evaluation. Arab J Chem. 2015;8(1):38–47. [Google Scholar]
- 14.Lu W., Shen Y., Xie A., Zhang W. Preparation and protein immobilization of magnetic dialdehyde starch nanoparticles. Am Chem Soc J Phys Chem B. 2013;117:3720–3725. doi: 10.1021/jp3110908. [DOI] [PubMed] [Google Scholar]
- 15.Zhao L., Mitomo H., Zhai M., Yoshii F., Nagasawa N., Kume T. Synthesis of antibacterial PVA/CM-chitosan blend hydrogels with electron beam irradiation. Carbohydr Polym. 2003;53:439–446. [Google Scholar]
- 16.Yang X., Liu Q., Chen X., Yu F., Zhu Z. Investigation of PVA/ws-chitosan hydrogels prepared by combined gama-irradiation and freeze-thawing. Carbohydr Polym. 2008;73:401–408. [Google Scholar]
- 17.Tan H., Chu C.R., Payne K.A., Marra K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30:2499–2506. doi: 10.1016/j.biomaterials.2008.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dia Y.N., Li P., Zhang J.P., Wang A.Q., Wei Q. A novel pH sensitive N-succinyl chitosan/alginate hydrogel bead for nifedipine delivery. Biopharm Drug Dispos. 2008;29:173–184. doi: 10.1002/bdd.590. [DOI] [PubMed] [Google Scholar]
- 19.Sun J., Xiao C., Tan H., Hu X. Covalently crosslinked hyaluronic acid-chitosan hydrogel containing dexamethasone as an injectable for soft tissue engineering. J Appl Polym Sci. 2013;129(2):682–688. [Google Scholar]
- 20.Kato Y., Onishi H., Machida Y. Lactosaminated and intact N-succinyl-chitosans as drug carriers in liver metastasis. Int J Pharm. 2001;226(1–2):93–106. doi: 10.1016/s0378-5173(01)00777-3. [DOI] [PubMed] [Google Scholar]
- 21.Onishi H., Nagai T. Characterization and evaluation of dialdehyde starch as an erodible medical polymer and a drug carrier. Int J Pharm. 1986;30:133–141. [Google Scholar]
- 22.SuYao X., XuanMing L., ChunYi T., LiChao Z., XiaoJuan L., AiMei Z., et al. Dialdehyde starch nanoparticles as antitumor drug delivery system: an in vitro, in vivo, and immuno histological evaluation. Chin Sci Bull. 2012;57:3226–3232. [Google Scholar]
- 23.Lu W., Shen Y., Xie A., Zhang W. Preparation and protein immobilization of magnetic dialdehyde starch nanoparticles. J Phys Chem B. 2013;117:3720–3725. doi: 10.1021/jp3110908. [DOI] [PubMed] [Google Scholar]
- 24.Sun S., Wang A. Adsorption properties of N-succinyl-chitosan and cross-linked N-succinyl-chitosan resin with Pb(II) as template ions. Sep Purif Technol. 2006;51:409–415. [Google Scholar]
- 25.Wang Y.J., Pan M.H., Cheng A.L., Lin L.I., Ho Y.S., Hsieh C.Y., et al. Stability of curcumin in buffer solution and characterization of its degradation products. J Pharm Biomed Anal. 1997;15(12):1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 26.Das R.K., Kasoju N., Bora U. Encapsulation of curcumin in alginate-chitosan-pluronic composites nanoparticles for delivery to cancer cells. Nanomed Nanotechnol Biol Med. 2010;6:153–160. doi: 10.1016/j.nano.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Baman N.K., Schneider G.B., Terry T.L., Zaharian R., Salem A.K. Spatial control over cell attachment by partial solvent entrapment of poly lysine in microfluidic channels. Int J Nanomed. 2006;1(2):213–217. doi: 10.2147/nano.2006.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones L.J., Gray M., Yue S.T., Haugland R.P., Singer V.L. Sensitive determination of cell number using the CyQUANT cell proliferation assay. J Immunol Methods. 2001;254(1–2):85–98. doi: 10.1016/s0022-1759(01)00404-5. [DOI] [PubMed] [Google Scholar]
- 29.Burdick J.A., Anseth K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]