Abstract
The complex interactions between colorectal neoplasia and immune cells in the tumor microenvironment remain to be elucidated. Experimental evidence suggests that microRNA MIR21 (miR-21) suppresses antitumor T-cell–mediated immunity. Thus, we hypothesized that tumor MIR21 expression might be inversely associated with T-cell density in colorectal carcinoma tissue. Utilizing 538 rectal and colon cancer cases in the Nurses’ Health Study and the Health Professionals Follow-up Study, we measured tumor MIR21 expression by quantitative reverse-transcription polymerase chain reaction assay. Densities of CD3+, CD8+, CD45RO (PTPRC)+ and FOXP3+ cells in tumor tissue were determined by tissue microarray immunohistochemistry and computer-assisted image analysis. Ordinal logistic regression analysis was conducted to assess the association of MIR21 expression (ordinal quartiles as a predictor variable) with T-cell density (ordinal quartiles as an outcome variable), adjusting for tumor molecular features including microsatellite instability; CpG island methylator phenotype; KRAS, BRAF, and PIK3CA mutations; and LINE-1 methylation. We adjusted two-sided α level to 0.012 for multiple hypothesis testing. Tumor MIR21 expression was inversely associated with densities of CD3+ and CD45RO+ cells (Ptrend < 0.0005). Multivariate odds ratio of the highest vs. lowest quartile of MIR21 for a unit increase in quartile categories of CD3+ or CD45RO+ cells was 0.44 (95% confidence interval [CI], 0.28 to 0.68) or 0.41 (95% CI, 0.26 to 0.64), respectively. Our data support a possible role of tumor epigenetic deregulation by non-coding RNA in suppressing antitumor T-cell–mediated adaptive immune response, and suggest MIR21 as a potential target for immunotherapy and prevention in colorectal cancer.
Keywords: colorectum, epigenetics, immunoprevention, molecular pathological epidemiology, non-coding RNA
Introduction
Accumulating evidence indicates that innate and adaptive immunity influences tumor evolution (1). Attesting to an important role of T-cell–mediated adaptive immunity in inhibiting tumor progression, therapeutic antibodies specific for immune checkpoint molecules, including CTLA4, PDCD1 (programmed cell death 1; PD-1), and CD274 (programmed cell death 1 ligand 1; PD-L1) can effectively enhance antitumor T-cell activity in various cancers (2, 3). Emerging evidence suggests complex roles of tumor genetic alterations and tumor-host interactions in response to T-cell-based immunotherapies (4, 5). Although these immunotherapies appeared to be less effective for colorectal cancer, intense infiltrates of T cells in colorectal cancer tissue have been associated with better patient survival (6–8), and studies have suggested a potential role of immune checkpoint pathways in suppressing antitumor immune responses in a subset of colorectal cancers (9, 10). A high degree of microsatellite instability (MSI-high) in colorectal cancer is associated with intense infiltrates of T cells, as mismatch repair defects in MSI-high tumors causes numerous frameshift mutations and truncated proteins (neopeptides), which elicit antitumor T-cell–mediated adaptive immunity (11–13). However, MSI status is not the sole determinant of immune response to colorectal cancer, because the numbers of tumor-infiltrating T cells considerably overlap between MSI-high and microsatellite stable (MSS) colorectal tumors (7, 9, 13). Hence, other factors may influence the antitumor immune response to colorectal cancer.
MicroRNAs are short non-coding RNAs (18–24 nucleotides in length) that play substantial roles in epigenetic gene regulation in diverse biological and pathological processes, including immunity and carcinogenesis (14, 15). Among various microRNAs, MIR21 (miR-21) has been shown to play roles in immunity and colorectal carcinogenesis (16–18). In fact, high MIR21 expression in colorectal cancer tissue has been associated with worse clinical outcome, suggesting MIR21 as a prognostic tumor biomarker (19, 20). MIR21 is expressed in colorectal cancer cells (20, 21), and MIR21 increases amounts of IL10 (interleukin 10) and prostaglandin E2 (PGE2) in the tumor microenvironment in vivo (22–24). IL10 and PGE2 can suppress antitumor T-cell–mediated adaptive immunity through the inhibition of the antigen-presenting capacities of dendritic cells and the recruitment of myeloid-derived suppressor cells into the tumor microenvironment (25, 26). Therefore, we hypothesized that higher MIR21 expression might be associated with fewer T cells in colorectal cancer tissue. A better understanding of the relationship between microRNAs and immune cells in the tumor microenvironment may open opportunities to use microRNAs for immunotherapy and prevention of colorectal cancer.
To test our hypothesis, we analyzed two U.S. nationwide prospective cohort studies (the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS)) and examined tumor MIR21 expression in relation to densities of CD3+, CD8+, CD45RO (PTPRC)+, and FOXP3+ T cells in colorectal cancer tissue.
Methods
Study population
We used the databases of two U.S. nationwide prospective cohort studies, NHS (121,701 women who enrolled in 1976) and HPFS (51,529 men who enrolled in 1986) (27, 28). Every 2 years, participants were sent follow-up questionnaires to gather information on health and lifestyle factors, and to identify newly diagnosed cancers and other diseases. Medical records were reviewed, and the cause of death was assigned by study physicians. The National Death Index was used to ascertain deaths of study participants and identify unreported lethal colorectal cancer cases. Formalin-fixed paraffin-embedded (FFPE) tissue blocks were collected from hospitals where participants with colorectal cancer had undergone tumor resection. Hematoxylin and eosin-stained tissue sections from all colorectal cancer cases were reviewed by a pathologist (S. Ogino), who was unaware of other data. Tumor differentiation was categorized as well to moderate or poor (>50% vs. ≤50% glandular area). Based on the availability of data on tumor MIR21 expression and T-cell densities, a total of 538 colorectal cancer cases were included. Written informed consent was obtained from all study participants. Tissue collection and analyses were approved by the human subjects committee at the Harvard T.H. Chan School of Public Health and the Brigham and Women’s Hospital (Boston, MA, USA).
RNA isolation and quantitative reverse-transcription (RT) polymerase chain reaction (PCR) for MIR21
RNA was extracted from colorectal cancer tissue and adjacent non-tumor colonic mucosa in whole-tissue sections of FFPE specimens with the use of RecoverAll™ Total Nucleic Acid Isolation Kit (Ambion Inc, Austin, TX). The quantitative RT-PCR assays for MIR21 and RNU6-2 were performed according to miScript PCR System protocol (Qiagen, Valencia, CA). Briefly, complementary DNA (cDNA) was synthesized with the use of miScript II RT Kit (Qiagen, Valencia, CA). Each reaction was performed in 25 μL solution containing 1× final concentration QuantiTect SYBR Green PCR Master Mix (Qiagen, Valencia, CA) and each miScript Primer Assay (Qiagen, Valencia, CA) specific for MIR21 (catalog number, MS00009079) and RNU6-2 (catalog number, MS00033740) in a 96-well optical PCR plate. Amplification and detection of MIR21 and RNU6-2 were performed with the StepOnePlus Real-Time PCR Systems (Applied Biosystems, San Diego, CA) with the use of the following reaction conditions: 15 minutes at 95°C and 40 cycles of 15 seconds at 94°C, 30 seconds at 55°C, and 30 seconds at 70°C. The cycle threshold (Ct) values in the quantitative reverse-transcription PCR for MIR21 and RNU6-2 decreased linearly with the amount of input cDNA using 10-fold dilution series from the same specimen (r2 > 0.99; Fig. 1A). The inter-assay coefficient of variation of Ct values from the same specimen in five different batches was ≤1% for MIR21 and RNU6-2 in our validation study using five colorectal cancers (Table 1).
Figure 1.
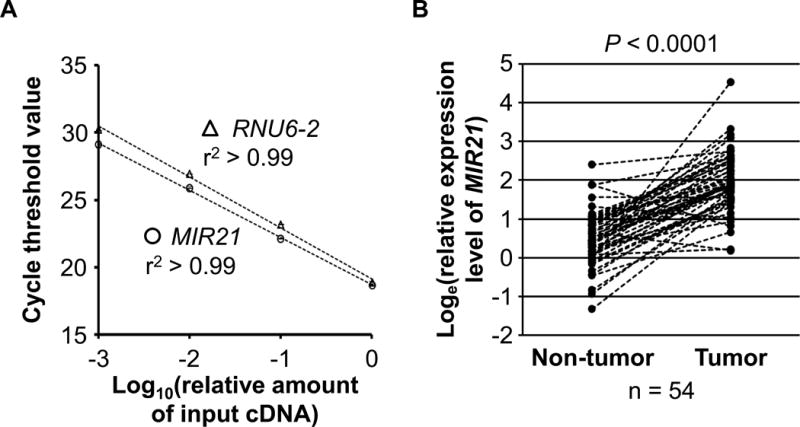
MIR21 expression in colorectal cancer.
A, Quantitative reverse-transcription PCR assays for MIR21 and RNU6-2 using 10-fold dilution series (1:1000, 1:100, 1:10, and 1:1) from the same specimen. Mean cycle threshold values (± standard deviation) of triplicate runs and the coefficient of determination (r2) in the assays for MIR21 and RNU6-2 are shown. cDNA, complementary DNA; PCR, polymerase chain reaction. B, MIR21 expression in 54 pairs of colorectal cancer and adjacent non-tumor colonic mucosa. A statistical analysis was performed using two-sided Wilcoxon signed rank test.
Table 1.
Inter-assay coefficients of variation in quantitative reverse-transcription PCR assays for MIR21 and RNU6-2
| Targets in quantitative reverse-transcription PCR assays
|
||||
|---|---|---|---|---|
|
MIR21
|
RNU6-2
|
|||
| Mean cycle threshold ± SD | Inter-assay coefficient of variation (%) | Mean cycle threshold ± SD | Inter-assay coefficient of variation (%) | |
| Specimen 1 | 19.5 ± 0.06 | 0.28 | 19.9 ± 0.13 | 0.65 |
| Specimen 2 | 19.5 ± 0.07 | 0.36 | 21.2 ± 0.15 | 0.73 |
| Specimen 3 | 19.3 ± 0.09 | 0.44 | 21.0 ± 0.18 | 0.86 |
| Specimen 4 | 20.0 ± 0.09 | 0.46 | 22.1 ± 0.09 | 0.39 |
| Specimen 5 | 18.0 ± 0.10 | 0.55 | 21.0 ± 0.12 | 0.59 |
|
| ||||
| Mean coefficient of variation (%) | 0.42 | 0.64 | ||
Abbreviations: PCR, polymerase chain reaction; SD, standard deviation. Inter-assay coefficient of variation of cycle threshold values from the same specimen was assessed by repeating assays in five different batches with the use of five colorectal cancers.
Each specimen was analyzed in duplicate for each target in a single batch, and we used the average of the two Ct values for each target. Spearman’s rank-correlation coefficient between the two Ct values (in duplicated runs) was 0.99 in quantitative PCR assays for MIR21 and RNU6-2. MIR21 expression level in each specimen was calculated as a relative unitless value normalized with RNU6-2 using the 2−ΔCt method (where ΔCt = “the average Ct value of MIR21” – “the average Ct value of RNU6-2”) as previously described (29).
Analyses of MSI, DNA methylation, and KRAS, BRAF, and PIK3CA mutations
DNA was extracted from archival colorectal cancer tissue blocks. MSI status was analyzed with use of 10 microsatellite markers (D2S123, D5S346, D17S250, BAT25, BAT26, BAT40, D18S55, D18S56, D18S67, and D18S487) as previously described (30). We defined MSI-high as the presence of instability in ≥30% of the markers, and MSI-low/microsatellite stable (MSS) as instability in <30% of the markers. Methylation analyses of long interspersed nucleotide element-1 (LINE-1) (31, 32) and eight promoter CpG islands specific for CpG island methylator phenotype (CIMP) (CACNA1G, CDKN2A, CRABP1, IGF2, MLH1, NEUROG1, RUNX3, and SOCS1) (33, 34) were performed. PCR reaction and pyrosequencing were performed for KRAS (codons 12, 13, 61, and 146) (35, 36), BRAF (codon 600) (30), and PIK3CA (exons 9 and 20) (37, 38).
Immunohistochemistry and quantification of the density of T cells
We constructed a tissue microarray, and conducted immunohistochemistry for CD3, CD8, CD45RO (one of the PTPRC protein isoforms), and FOXP3 (7). We used automated scanning microscope and the Ariol image analysis system (Genetix, San Jose, CA, USA) to measure densities (cells/mm2) of CD3+, CD8+, CD45RO+, and FOXP3+ T cells in colorectal cancer tissue as previously described (7).
Statistical analysis
All statistical analyses were conducted using SAS (version 9.3, SAS Institute, Cary, NC) and all P values were two-sided. Neither MIR21 expression, T-cell density, nor log-transformed values of MIR21 or T-cell density fit a normal distribution with the use of the Kolmogorov-Smirnov test for normality (P ≤ 0.048). Thus, we tested our primary hypothesis using a linear trend test in an ordinal logistic regression model to assess associations of tumor MIR21 expression (an ordinal quartile predictor variable as a continuous variable) with the density of CD3+, CD8+, CD45RO+, or FOXP3+ T cells in colorectal cancer tissue (an ordinal quartile outcome variable). Because we tested four primary hypotheses (for CD3+, CD8+, CD45RO+, and FOXP3+ T cells as outcome variables), we adjusted two-sided α level to 0.012 (= 0.05/4) by simple Bonferroni correction. All other analyses including evaluation of individual odds ratio (OR) estimates represented secondary analyses. In those secondary analyses, in view of multiple comparisons, we interpreted our data cautiously, in addition to the use of the adjusted α level of 0.012.
We performed multivariable ordinal logistic regression analysis to control for potential confounders. The multivariable model initially included age (continuous), sex, year of diagnosis (continuous), family history of colorectal cancer in a first-degree relative (present vs. absent), tumor location (proximal colon vs. distal colon vs. rectum), tumor differentiation (well to moderate vs. poor), MSI (high vs. MSI-low/MSS), CIMP (high vs. low/negative), KRAS (mutant vs. wild-type), BRAF (mutant vs. wild-type), and PIK3CA (mutant vs. wild-type), and LINE-1 methylation level (continuous). For cases with missing information in any of the covariates, we assigned a separate (“missing”) indicator variable. A backward stepwise elimination with a threshold of P = 0.05 was used to select variables in the final models. We assessed the proportional odds assumption in the ordinal logistic regression model, which was generally satisfied (P > 0.05).
All cross-sectional univariable analyses for clinical, pathological, and molecular associations (with variables listed in Table 2) were secondary exploratory analyses, and we adjusted two-sided α level to 0.003 (= 0.05/14) by simple Bonferroni correction for multiple hypothesis testing. To assess associations between the ordinal categories (first to fourth quartile) of tumor MIR21 expression and categorical data, the chi-square test was performed. To compare mean age and mean LINE-1 methylation levels, an analysis of variance assuming equal variances was performed.
Table 2.
Clinical, pathological, and molecular features according to tumor MIR21 expression in 538 colorectal cancer cases
| Characteristica | Total No. (n = 538) |
Tumor MIR21 expression (quartile)
|
P valueb | |||
|---|---|---|---|---|---|---|
| Q1 (lowest) (n = 135) |
Q2 (second) (n = 134) |
Q3 (third) (n = 134) |
Q4 (highest) (n = 135) |
|||
| Mean age ± SD (year) | 67.6 ± 8.3 | 66.7 ± 8.2 | 67.2 ± 8.5 | 68.8 ± 8.1 | 67.5 ± 8.3 | 0.19 |
| Sex | 0.044 | |||||
| Men | 185 (34%) | 50 (37%) | 44 (33%) | 56 (42%) | 35 (26%) | |
| Women | 353 (66%) | 85 (63%) | 90 (67%) | 78 (58%) | 100 (74%) | |
| Year of diagnosis | 0.006 | |||||
| Prior to 1995 | 201 (38%) | 63 (47%) | 55 (41%) | 42 (32%) | 41 (30%) | |
| 1996 to 2000 | 202 (38%) | 43 (32%) | 54 (40%) | 44 (34%) | 61 (45%) | |
| 2001 to 2008 | 131 (24%) | 28 (21%) | 25 (19%) | 45 (34%) | 33 (25%) | |
| Family history of colorectal cancer in a first-degree relative | 0.21 | |||||
| Absent | 422 (79%) | 105 (78%) | 111 (83%) | 96 (74%) | 110 (82%) | |
| Present | 109 (21%) | 29 (22%) | 22 (17%) | 34 (26%) | 24 (18%) | |
| Tumor location | 0.30 | |||||
| Cecum | 96 (18%) | 20 (15%) | 22 (17%) | 27 (20%) | 27 (20%) | |
| Ascending to transverse colon | 173 (32%) | 39 (29%) | 40 (30%) | 43 (32%) | 51 (38%) | |
| Splenic flexure to sigmoid | 149 (28%) | 39 (29%) | 44 (33%) | 31 (23%) | 35 (26%) | |
| Rectosigmoid and rectum | 117 (22%) | 36 (27%) | 27 (20%) | 33 (25%) | 21 (16%) | |
| Disease stage | 0.009 | |||||
| I | 110 (21%) | 37 (28%) | 29 (22%) | 23 (18%) | 21 (16%) | |
| II | 173 (34%) | 44 (34%) | 45 (35%) | 44 (35%) | 40 (31%) | |
| III | 164 (32%) | 31 (24%) | 44 (34%) | 48 (39%) | 41 (32%) | |
| IV | 67 (13%) | 18 (14%) | 11 (8.5%) | 10 (8.0%) | 28 (21%) | |
| Tumor differentiation | 0.24 | |||||
| Well to moderate | 486 (91%) | 121 (90%) | 127 (95%) | 119 (89%) | 119 (88%) | |
| Poor | 51 (9.5%) | 13 (9.7%) | 7 (5.2%) | 15 (11%) | 16 (12%) | |
| MSI status | 0.26 | |||||
| MSI-low/MSS | 440 (84%) | 114 (87%) | 115 (86%) | 107 (82%) | 104 (79%) | |
| MSI-high | 86 (16%) | 17 (13%) | 18 (14%) | 24 (18%) | 27 (21%) | |
| MLH1 hypermethylation | 0.15 | |||||
| Absent | 461 (87%) | 118 (89%) | 121 (91%) | 109 (83%) | 113 (85%) | |
| Present | 69 (13%) | 14 (11%) | 12 (9.0%) | 23 (17%) | 20 (15%) | |
| CIMP status | 0.015 | |||||
| Low/negative | 440 (83%) | 113 (86%) | 119 (89%) | 108 (82%) | 100 (75%) | |
| High | 90 (17%) | 19 (14%) | 14 (11%) | 24 (18%) | 33 (25%) | |
| BRAF mutation | 0.003 | |||||
| Wild-type | 444 (84%) | 120 (90%) | 116 (88%) | 110 (82%) | 98 (75%) | |
| Mutant | 86 (16%) | 13 (9.8%) | 16 (12%) | 24 (18%) | 33 (25%) | |
| KRAS mutation | 0.11 | |||||
| Wild-type | 311 (59%) | 76 (58%) | 67 (51%) | 88 (66%) | 80 (61%) | |
| Mutant | 216 (41%) | 55 (42%) | 64 (49%) | 46 (34%) | 51 (39%) | |
| PIK3CA mutation | 0.86 | |||||
| Wild-type | 408 (83%) | 99 (84%) | 104 (82%) | 105 (82%) | 100 (85%) | |
| Mutant | 82 (17%) | 19 (16%) | 23 (18%) | 23 (18%) | 17 (15%) | |
| Mean LINE-1 methylation level (%) ± SD | 61.6 ± 9.6 | 61.6 ± 8.4 | 59.6 ± 10.4 | 62.1 ± 10.2 | 63.0 ± 9.1 | 0.032 |
Abbreviations: CIMP, CpG island methylator phenotype; LINE-1, long interspersed nucleotide element-1; MSI, microsatellite instability; MSS, microsatellite stable; Q1 to Q4, quartile 1 to quartile 4; SD, standard deviation.
Percentage indicates the proportion of cases with a specific clinical, pathological, or molecular feature in colorectal cancer cases with each tumor MIR21 expression. There were cases that had missing values for any of the characteristics except for age and sex.
To assess associations between the ordinal categories (first to fourth quartile) of tumor MIR21 expression and categorical data, the chi-square test was performed. To compare mean age and mean LINE-1 methylation levels, an analysis of variance was performed. We adjusted two-sided α level to 0.003 (= 0.05/14) by simple Bonferroni correction for multiple hypothesis testing.
Results
MIR21 expression in colorectal cancer
To test the hypothesis of an inverse relationship between MIR21 expression and T-cell infiltration in colorectal cancer tissue, we measured MIR21 expression with RT-PCR assays on 538 colorectal cancer cases within the NHS and the HPFS databases. In 54 pairs of colorectal cancer and adjacent non-tumor colonic mucosa, MIR21 expression was generally higher in colorectal cancer than in paired adjacent nontumor colonic mucosa (Wilcoxon signed rank test, P < 0.0001; Fig. 1B).
Table 2 shows clinical, pathological, and molecular features of the 538 cases according to tumor MIR21 expression. Higher tumor MIR21 expression was associated with BRAF mutation (P = 0.003; with adjusted α level of 0.003 for multiple hypothesis testing).
Association of tumor MIR21 expression with T-cell density in colorectal cancer tissue
We measured the densities of CD3+, CD8+, CD45RO+, and FOXP3+ T cells in colorectal cancer tissue by immunohistochemistry and image analysis. Supplementary Table S1 shows pairwise correlations between the densities of CD3+, CD8+, CD45RO+, and FOXP3+ T cells. Except for between CD8+ and FOXP3+ T cells (P = 0.16), all of the other pairwise correlations were statistically significant (with Spearman’s rank-correlation coefficients ranging 0.18 to 0.48; all P < 0.0001).
Table 3 shows a distribution of colorectal cancer cases according to tumor MIR21 expression (quartiles) and the density of T cells in colorectal cancer tissue (quartiles). In our primary hypothesis testing, we conducted univariable and multivariable ordinal logistic regression analyses to assess the associations of tumor MIR21 expression (as an ordinal quartile predictor variable) with the density of CD3+, CD8+, CD45RO+, or FOXP3+ T cells in colorectal cancer tissue (an ordinal quartile outcome variable) (Table 4 and Supplementary Table S2 with all covariates). Tumor MIR21 expression was inversely associated with the densities of CD3+ T cells and CD45RO+ T cells in univariable and multivariable ordinal logistic regression analyses (all Ptrend < 0.0005; with adjusted α level of 0.012 for multiple hypothesis testing). Compared with cases in the lowest quartile of tumor MIR21 expression, those in the highest quartile were inversely associated with the densities of CD3+ T cells (multivariable OR, 0.44; 95% confidence interval [CI], 0.28 to 0.68; for a unit increase in quartile categories) and CD45RO+ T cells (multivariable OR, 0.41; 95% CI, 0.26 to 0.64; for a unit increase in quartile categories). Tumor MIR21 expression was not significantly associated with the density of CD8+ or FOXP3+ T cells (Ptrend > 0.03 in univariable analysis with adjusted α level of 0.012). We also used tumor MIR21 expression after adjusting for cellularity in colorectal cancer tissue, and observed similar associations of tumor MIR21 expression with the density of T cells (Supplementary Methods and Supplementary Table S3).
Table 3.
Distribution of colorectal cancer cases according to tumor MIR21 expression and the density of T cells
| Total No. | Tumor MIR21 expression (quartile)
|
Ptrenda | ||||
|---|---|---|---|---|---|---|
| Q1 (lowest) |
Q2 (second) |
Q3 (third) |
Q4 (highest) |
|||
| CD3+ cell density (quartile) | 0.0004 | |||||
| Q1 (0–115 cells/mm2) | 130 (25%) | 26 (20%) | 29 (22%) | 31 (25%) | 44 (33%) | |
| Q2 (116–252 cells/mm2) | 129 (25%) | 26 (20%) | 30 (23%) | 35 (28%) | 38 (29%) | |
| Q3 (253–533 cells/mm2) | 130 (25%) | 42 (32%) | 33 (25%) | 30 (24%) | 25 (19%) | |
| Q4 (≥534 cells/mm2) | 129 (25%) | 38 (28%) | 38 (30%) | 28 (23%) | 25 (19%) | |
| CD8+ cell density (quartile) | 0.27 | |||||
| Q1 (0–66 cells/mm2) | 128 (25%) | 23 (18%) | 31 (24%) | 40 (32%) | 34 (26%) | |
| Q2 (67–185 cells/mm2) | 127 (25%) | 42 (33%) | 26 (20%) | 28 (23%) | 31 (24%) | |
| Q3 (186–410 cells/mm2) | 128 (25%) | 30 (24%) | 34 (26%) | 28 (23%) | 36 (27%) | |
| Q4 (≥411 cells/mm2) | 127 (25%) | 31 (25%) | 39 (30%) | 27 (22%) | 30 (23%) | |
| CD45RO+ cell density (quartile) | 0.0002 | |||||
| Q1 (0–183 cells/mm2) | 131 (25%) | 24 (18%) | 30 (23%) | 31 (24%) | 46 (35%) | |
| Q2 (184–430 cells/mm2) | 130 (25%) | 32 (25%) | 33 (25%) | 39 (31%) | 26 (20%) | |
| Q3 (431–805 cells/mm2) | 131 (25%) | 26 (20%) | 37 (27%) | 30 (24%) | 38 (29%) | |
| Q4 (≥806 cells/mm2) | 130 (25%) | 48 (37%) | 33 (25%) | 27 (21%) | 22 (16%) | |
| FOXP3+ cell density (quartile) | 0.032 | |||||
| Q1 (0–14 cells/mm2) | 124 (25%) | 25 (20%) | 31 (26%) | 32 (27%) | 36 (28%) | |
| Q2 (15–25 cells/mm2) | 124 (25%) | 29 (23%) | 24 (20%) | 34 (28%) | 37 (28%) | |
| Q3 (26–48 cells/mm2) | 124 (25%) | 38 (31%) | 27 (22%) | 27 (22%) | 32 (25%) | |
| Q4 (≥49 cells/mm2) | 123 (25%) | 32 (26%) | 38 (32%) | 28 (23%) | 25 (19%) | |
Abbreviations: Q1 to Q4, quartile 1 to quartile 4.
Ptrend value was calculated by the linear trend test across the ordinal (first to fourth quartile) categories of tumor MIR21 expression as a continuous variable in univariable ordinal logistic regression model for the density of CD3+, CD8+, CD45RO+, or FOXP3+ T cells (an ordinal quartile outcome variable). Because we assessed four primary outcome variables, we adjusted two-sided α level to 0.012 (= 0.05/4) by simple Bonferroni correction.
Table 4.
The association of tumor MIR21 expression with the density of T cells
| Univariable OR (95% CI) | Multivariable OR (95% CI)a | ||
|---|---|---|---|
| Model for CD3+ cell density (n = 518, as an outcome variable) | |||
| MIR21 expression | Q1 (lowest) | 1 (reference) | 1 (reference) |
| Q2 (second) | 0.88 (0.57–1.36) | 0.85 (0.55–1.31) | |
| Q3 (third) | 0.67 (0.43–1.04) | 0.59 (0.37–0.92) | |
| Q4 (highest) | 0.47 (0.31–0.73) | 0.44 (0.28–0.68) | |
| Ptrendb | 0.0004 | < 0.0001 | |
| Model for CD8+ cell density (n = 510, as an outcome variable) | |||
| MIR21 expression | Q1 (lowest) | 1 (reference) | 1 (reference) |
| Q2 (second) | 1.14 (0.74–1.77) | 1.25 (0.80–1.96) | |
| Q3 (third) | 0.72 (0.46–1.12) | 0.76 (0.48–1.19) | |
| Q4 (highest) | 0.89 (0.58–1.38) | 0.99 (0.63–1.54) | |
| Ptrendb | 0.27 | 0.50 | |
| Model for CD45RO+ cell density (n = 522, as an outcome variable) | |||
| MIR21 expression | Q1 (lowest) | 1 (reference) | 1 (reference) |
| Q2 (second) | 0.70 (0.46–1.09) | 0.72 (0.46–1.12) | |
| Q3 (third) | 0.57 (0.37–0.89) | 0.54 (0.34–0.84) | |
| Q4 (highest) | 0.45 (0.29–0.70) | 0.41 (0.26–0.64) | |
| Ptrendb | 0.0002 | < 0.0001 | |
| Model for FOXP3+ cell density (n = 495, as an outcome variable) | |||
| MIR21 expression | Q1 (lowest) | 1 (reference) | 1 (reference) |
| Q2 (second) | 0.98 (0.63–1.54) | 0.93 (0.59–1.46) | |
| Q3 (third) | 0.73 (0.47–1.14) | 0.61 (0.39–0.96) | |
| Q4 (highest) | 0.66 (0.42–1.02) | 0.55 (0.35–0.86) | |
| Ptrendb | 0.032 | 0.003 | |
Abbreviations: CI, confidence interval; OR, odds ratio; Q1 to Q4, quartile 1 to quartile 4.
The multivariable ordinal logistic regression analysis model initially included age, sex, year of diagnosis, family history of colorectal cancer in parent or sibling, tumor location, tumor differentiation, microsatellite instability, CpG island methylator phenotype, KRAS, BRAF, and PIK3CA mutations, and LINE-1 methylation level. A backward stepwise elimination with a threshold of P = 0.05 was used to select variables in the final models. Variables remaining in the final multivariable ordinal logistic regression models are shown in Supplementary Table S2.
Ptrend value was calculated by the linear trend across the ordinal (first to fourth quartile) categories of MIR21 expression as a continuous variable in the ordinal logistic regression model for the density of CD3+, CD8+, CD45RO+, or FOXP3+ T cells (an ordinal quartile outcome variable). Because we assessed four primary outcome variables, we adjusted two-sided α level to 0.012 (= 0.05/4) by simple Bonferroni correction.
In our exploratory analyses, higher tumor MIR21 expression was significantly associated with higher colorectal cancer-specific mortality (Ptrend = 0.003), whereas higher CD8+ T-cell density was significantly associated with lower colorectal cancer-specific mortality (Ptrend = 0.012; Supplementary Methods and Supplementary Table S4).
Discussion
We conducted this study to test the hypothesis that tumor MIR21 expression might be inversely associated with the density of T cells in colorectal cancer tissue in a human population. We demonstrated that microRNA expression analysis, by RT-PCR assay, on FFPE tissue specimens was feasible and robust, in agreement with the previous studies (19, 20). Utilizing the database of the 538 colorectal cancer cases in the two U.S. nationwide prospective cohort studies, we found that tumor MIR21 expression was inversely associated with the densities of CD3+ and CD45RO+ T cells in human colorectal cancer tissue. Our first-line population-based data support an immunosuppressive role of MIR21 in colorectal cancer.
High densities of CD3+ pan-T cells and T-cell subpopulations (CD8+, CD45RO+, and FOXP3+ T cells) in colorectal carcinoma have been associated with better patient survival, indicating a major role of T-cell–mediated adaptive immunity in inhibiting colorectal tumor progression (39–41). Therefore, both tumor molecular and immunity analyses are increasing important in cancer research and clinical practice. MicroRNAs play substantial roles in carcinogenesis and immunity and are potential biomarkers or therapeutic targets (42). One possible mechanism of the immunosuppressive effect of MIR21 is based on its ability to suppress the expression of PDCD4, which normally inhibits the translation of IL10 mRNA. Without this suppression, more IL10 is present in the tumor microenvironment (22, 23), which inhibits the antigen-presenting capacities of dendritic cells (25). Tumor MIR21 expression has been shown to inversely correlate with tumor PDCD4 expression assessed by immunohistochemistry on human colorectal cancer tissue (43, 44). Taken together, it seems to be plausible that MIR21 may suppress antitumor immune responses through increased IL10 in colorectal cancer. In addition, emerging evidence indicates that MIR21 suppresses tumor expression of HPGD (hydroxyprostaglandin dehydrogenase 15-(NAD); or 15-PDGH), which converts PGE2 to its biologically inactive metabolite (24). Hence, MIR21 may increase PGE2 in the tumor microenvironment, which can lead to suppression of antitumor T-cell–mediated adaptive immunity (26). Our human population-based data, along with these lines of experimental evidence, support the hypothesis that MIR21 suppresses antitumor T-cell–mediated immune response to colorectal cancer, although additional studies are needed to clarify the exact mechanism. MicroRNA-targeting therapies for human disease including cancer are currently being investigated (42, 45, 46). In light of our findings, it would be intriguing for future research to explore a potential strategy of inhibiting MIR21 and thus its immunosuppressive effect in immunotherapy and prevention for colorectal cancer.
Higher tumor MIR21 expression was associated with BRAF mutation in the present study, which has not been examined in colorectal cancer before. Oncogenic mutation of BRAF activates the mitogen-activated protein kinase (MAPK) signaling pathway (47). Experimental evidence suggests that activation of the RAF-MAPK signaling pathway may increase MIR21 expression in cancer (48). Taken together, BRAF mutation might increase MIR21 expression through the activation of the MAPK signaling pathway, although additional experimental studies are needed to test this hypothesis.
One limitation of the current study is its cross-sectional nature. Hence, we cannot exclude a possibility of reverse causation. It is possible that the interaction of T cells with tumor cells might cause low expression of MIR21 in tumors. However, our specific hypothesis was based on several lines of experimental evidence indicating that MIR21 suppresses T-cell–mediated immune response to tumor (22–26). Because experimental systems cannot perfectly recapitulate the complexities of human tumors or the immune system, analyses of human population are essential in translational medicine. Another limitation is measurement of MIR21 expression in colorectal cancer tissue, which contains a mixture of neoplastic and non-neoplastic cells including immune cells. Nonetheless, a number of studies have shown that MIR21 is expressed in neoplastic cells, but not substantially in immune cells (20, 21). We also recognize the limitations in evaluating T cells in human colorectal cancer tissue. We evaluated the well-characterized T-cell markers such as CD3, CD8, CD45RO, and FOXP3 with the use of tissue microarray immunohistochemistry and computer-assisted image analysis to objectively quantify the T-cell densities in a large number of cases. The favorable prognostic associations of the densities of these T-cell populations in our cohort studies were consistent with previous studies of other populations (6, 8), suggesting that the density of T cells, as assessed by immunohistochemistry, might be considered a reliable measure of the adaptive immune response to colorectal tumors.
Strengths of this study include the use of our molecular pathological epidemiology database of more than 500 colorectal cancer cases in the two U.S. nationwide, prospective cohort studies, which integrates epidemiologic exposures, clinicopathologic features, key tumor molecular features, and immune reaction status in colorectal cancer tissue (49, 50). This population-based colorectal cancer database enabled us to rigorously examine the association of tumor MIR21 expression with the density of T cells, controlling for potential confounders. In addition, our colorectal cancer specimens were derived from a large number of hospitals in diverse settings across the U.S. (but not based on a limited number of hospitals), which increase the generalizability of our findings. As another strength, we used robust laboratory assays, including microRNA analysis and tissue image analysis that could objectively quantify specific T cells in tumor tissue.
In conclusion, tumor MIR21 expression is inversely associated with the densities of CD3+ and CD45RO+ T cells in colorectal cancer tissue. Our data support a possible role of MIR21 in down-regulating antitumor T-cell–mediated adaptive immunity, and suggest MIR21 as a potential target for immunotherapy and immunoprevention in colorectal cancer.
Supplementary Material
Acknowledgments
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Funding: This work was supported by U.S. National Institutes of Health (NIH) grants [P01 CA87969 to M.J. Stampfer; UM1 CA186107 to M.J. Stampfer; P01 CA55075 to W.C. Willett; UM1 CA167552 to W.C. Willett; P50 CA127003 to C.S.F.; R01 CA137178 to A.T.C.; R01 CA151993 to S.O.; R35 CA197735 to S.O.; and K07 CA190673 to R.N.]; and by grants from The Paula and Russell Agrusa Fund for Colorectal Cancer Research, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. K.M. is supported by a fellowship grant from Uehara Memorial Foundation and a grant from Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers from Japanese Society for the Promotion of Science. S.A.K. is supported by Early Exchange Postdoctoral Fellowship Grant from Asan Medical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- cDNA
complementary DNA
- CI
confidence interval
- CIMP
CpG island methylator phenotype
- Ct
cycle threshold
- FFPE
formalin-fixed paraffin-embedded
- HPFS
Health Professionals Follow-up Study
- LINE-1
long interspersed nucleotide element-1
- MSI
microsatellite instability
- MSS
microsatellite stable
- NHS
Nurses’ Health Study
- OR
odds ratio
- PCR
polymerase chain reaction
- PGE2
prostaglandin E2
- SD
standard deviation
Footnotes
Conflict of interest: No conflict of interest exists related to this manuscript.
Use of standardized official symbols: We use HUGO (Human Genome Organisation)-approved official symbols for genes and gene products, including BRAF, CACNA1G, CD3, CD8, CD274, CDKN2A, CRABP1, CTLA4, FOXP3, HPGD, IGF2, IL10, KRAS, MIR21, MLH1, NEUROG1, PDCD1, PDCD4, PIK3CA, PTPRC, RNU6-2, RUNX3, and SOCS1; all of which are described at www.genenames.org. Gene names are italicized, and gene product names are non-italicized.
References
- 1.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 2.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 7.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–66. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–92. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 9.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158:527–35. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tougeron D, Fauquembergue E, Rouquette A, Le Pessot F, Sesboue R, Laurent M, et al. Tumor-infiltrating lymphocytes in colorectal cancers with microsatellite instability are correlated with the number and spectrum of frameshift mutations. Mod Pathol. 2009;22:1186–95. doi: 10.1038/modpathol.2009.80. [DOI] [PubMed] [Google Scholar]
- 13.Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–20. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–24. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 15.Tili E, Michaille JJ, Croce CM. MicroRNAs play a central role in molecular dysfunctions linking inflammation with cancer. Immunol Rev. 2013;253:167–84. doi: 10.1111/imr.12050. [DOI] [PubMed] [Google Scholar]
- 16.Pencheva N, Tavazoie SF. Control of metastatic progression by microRNA regulatory networks. Nat Cell Biol. 2013;15:546–54. doi: 10.1038/ncb2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–36. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 18.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oue N, Anami K, Schetter AJ, Moehler M, Okayama H, Khan MA, et al. High miR-21 expression from FFPE tissues is associated with poor survival and response to adjuvant chemotherapy in colon cancer. Int J Cancer. 2014;134:1926–34. doi: 10.1002/ijc.28522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–36. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sempere LF, Preis M, Yezefski T, Ouyang H, Suriawinata AA, Silahtaroglu A, et al. Fluorescence-based codetection with protein markers reveals distinct cellular compartments for altered MicroRNA expression in solid tumors. Clin Cancer Res. 2010;16:4246–55. doi: 10.1158/1078-0432.CCR-10-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–7. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 23.Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhao JG, et al. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal. 2011;4:ra75. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu L, Byrnes K, Han C, Wang Y, Wu T. miR-21 targets 15-PGDH and promotes cholangiocarcinoma growth. Mol Cancer Res. 2014;12:890–900. doi: 10.1158/1541-7786.MCR-13-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–28. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishihara R, Lochhead P, Kuchiba A, Jung S, Yamauchi M, Liao X, et al. Aspirin use and risk of colorectal cancer according to BRAF mutation status. JAMA. 2013;309:2563–71. doi: 10.1001/jama.2013.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 30.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–6. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogino S, Kawasaki T, Nosho K, Ohnishi M, Suemoto Y, Kirkner GJ, et al. LINE-1 hypomethylation is inversely associated with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Int J Cancer. 2008;122:2767–73. doi: 10.1002/ijc.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irahara N, Nosho K, Baba Y, Shima K, Lindeman NI, Hazra A, et al. Precision of pyrosequencing assay to measure LINE-1 methylation in colon cancer, normal colonic mucosa, and peripheral blood cells. J Mol Diagn. 2010;12:177–83. doi: 10.2353/jmoldx.2010.090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogino S, Kawasaki T, Brahmandam M, Cantor M, Kirkner GJ, Spiegelman D, et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn. 2006;8:209–17. doi: 10.2353/jmoldx.2006.050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogino S, Kawasaki T, Kirkner GJ, Kraft P, Loda M, Fuchs CS. Evaluation of markers for CpG island methylator phenotype (CIMP) in colorectal cancer by a large population-based sample. J Mol Diagn. 2007;9:305–14. doi: 10.2353/jmoldx.2007.060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogino S, Kawasaki T, Brahmandam M, Yan L, Cantor M, Namgyal C, et al. Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diagn. 2005;7:413–21. doi: 10.1016/S1525-1578(10)60571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imamura Y, Lochhead P, Yamauchi M, Kuchiba A, Qian ZR, Liao X, et al. Analyses of clinicopathological, molecular, and prognostic associations of KRAS codon 61 and codon 146 mutations in colorectal cancer: cohort study and literature review. Mol Cancer. 2014;13:135. doi: 10.1186/1476-4598-13-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nosho K, Kawasaki T, Ohnishi M, Suemoto Y, Kirkner GJ, Zepf D, et al. PIK3CA mutation in colorectal cancer: relationship with genetic and epigenetic alterations. Neoplasia. 2008;10:534–41. doi: 10.1593/neo.08336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao X, Morikawa T, Lochhead P, Imamura Y, Kuchiba A, Yamauchi M, et al. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin Cancer Res. 2012;18:2257–68. doi: 10.1158/1078-0432.CCR-11-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maker AV, Ito H, Mo Q, Weisenberg E, Qin LX, Turcotte S, et al. Genetic evidence that intratumoral T-cell proliferation and activation are associated with recurrence and survival in patients with resected colorectal liver metastases. Cancer Immunol Res. 2015;3:380–8. doi: 10.1158/2326-6066.CIR-14-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turcotte S, Katz SC, Shia J, Jarnagin WR, Kingham TP, Allen PJ, et al. Tumor MHC class I expression improves the prognostic value of T-cell density in resected colorectal liver metastases. Cancer Immunol Res. 2014;2:530–7. doi: 10.1158/2326-6066.CIR-13-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kibe S, Yutani S, Motoyama S, Nomura T, Tanaka N, Kawahara A, et al. Phase II study of personalized peptide vaccination for previously treated advanced colorectal cancer. Cancer Immunol Res. 2014;2:1154–62. doi: 10.1158/2326-6066.CIR-14-0035. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. 2014;13:622–38. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 43.Fassan M, Pizzi M, Giacomelli L, Mescoli C, Ludwig K, Pucciarelli S, et al. PDCD4 nuclear loss inversely correlates with miR-21 levels in colon carcinogenesis. Virchows Arch. 2011;458:413–9. doi: 10.1007/s00428-011-1046-5. [DOI] [PubMed] [Google Scholar]
- 44.Chang KH, Miller N, Kheirelseid EA, Ingoldsby H, Hennessy E, Curran CE, et al. MicroRNA-21 and PDCD4 expression in colorectal cancer. Eur J Surg Oncol. 2011;37:597–603. doi: 10.1016/j.ejso.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 46.Guinea-Viniegra J, Jimenez M, Schonthaler HB, Navarro R, Delgado Y, Concha-Garzon MJ, et al. Targeting miR-21 to treat psoriasis. Sci Transl Med. 2014;6:225re1. doi: 10.1126/scitranslmed.3008089. [DOI] [PubMed] [Google Scholar]
- 47.Ogino S, Lochhead P, Giovannucci E, Meyerhardt JA, Fuchs CS, Chan AT. Discovery of colorectal cancer PIK3CA mutation as potential predictive biomarker: power and promise of molecular pathological epidemiology. Oncogene. 2014;33:2949–55. doi: 10.1038/onc.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang TH, Wu F, Loeb GB, Hsu R, Heidersbach A, Brincat A, et al. Up-regulation of miR-21 by HER2/neu signaling promotes cell invasion. J Biol Chem. 2009;284:18515–24. doi: 10.1074/jbc.M109.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song M, Nishihara R, Wang M, Chan AT, Qian ZR, Inamura K, et al. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut. 2015 doi: 10.1136/gutjnl-2014-308852. in press (published online) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


