Abstract
Purpose of review
Dementia is a major cause of disability and institutionalization. Besides age and APOE genotype, there are currently no established, clinically relevant, non-invasive markers of dementia. We conducted a literature search of recent observational epidemiological studies evaluating the relevance of high-density lipoprotein cholesterol (HDL-C) and apolipoproteins as biomarkers of future and prevalent risk of dementia.
Recent findings
HDL-C and apolipoproteins, such as apolipoprotein E (apoE) have been suggested to play important roles in brain function and have been associated with dementia and Alzheimer’s disease (AD) in observational studies. However, findings have been inconsistent, especially across study designs. In recent years, modern proteomic approaches have enabled the investigation of further apolipoproteins involved in the deposition and clearance of beta-amyloid, a determining factor for subsequent neurodegeneration.
Summary
Associations in cross-sectional studies are not always indicative of a prospective relationship. Large studies find that plasma HDL-C and apoE are inversely associated with dementia. Higher apoJ levels might be a marker of prevalent dementia, but were not associated with risk of future dementia. The investigation of HDL-C and apolipoproteins in relation to dementia represents an area of opportunity. Additional prospective studies that account for potential confounding factors and that explore potential effect modifiers such as APOE genotype and sex are needed to fully investigate the potential of these non-invasive measures in disease prediction.
Keywords: Alzheimer’s disease, apolipoproteins, dementia, high-density lipoproteins
INTRODUCTION
Dementia is a major cause of disability and institutionalization with a prevalence worldwide that is estimated to increase from 46.8 million people to over 74.7 million by 2030 [1, 2, 3]. Current approved dementia drugs mask cognitive and psychological disease symptoms but don’t cure most forms of dementia [4]. Besides need for additional treatment options, there is an urgent need for the discovery of novel early disease markers of dementia as well as markers to monitor dementia severity which might be useful as novel treatment targets.
Cholesterol has been suggested to play an important role in brain function. Indeed, of all organs, the highest concentrations of cholesterol are found in the brain, containing 23 % of the total cholesterol pool [5]. Apolipoproteins combine with cholesterol to form soluble lipoproteins that serve as transporters in aqueous solutions, including both blood and cerebrospinal fluid (CSF) circulating within the ventricles of the brain. Several apolipoproteins including apoE, apoA-I, and apoJ [6, 7, 8] are found in CSF where they combine with cholesterol to form lipoproteins that are relatively higher in protein (concentrations), and thus have densities similar to plasma high-density lipoprotein cholesterol (HDL-C) [9]. Apolipoproteins are involved in the deposition and clearance of beta-amyloid, a determining factor for subsequent neurodegeneration [10, 11, 12]. In addition, APOE E4 is the strongest known genetic risk factor for late onset Alzheimer’s disease (AD) and recently variation in the gene encoding another apolipoprotein; apoJ (aka clusterin), has been identified as an important risk locus in genome-wide association studies of (AD) [13].
Plasma levels of HDL-C have been inversely associated with dementia [14]. As opposed to studies using CSF, the investigation of blood markers is intriguing for the potential to identify novel non-invasive biomarkers for dementia. Plasma levels of HDL are typically indicated by either the cholesterol concentration in HDL or by measurement of plasma apoA-I concentrations, the key protein component in HDL in plasma [9]. Interestingly, even apolipoproteins such as apoA-I and apoC-III that are not expressed in the brain, are also found in CSF, indicating they are able to cross the blood brain barrier [7, 8, 15]. As yet, the differences in concentrations of apolipoproteins in plasma and CSF have not been well studied and it is unclear whether and how strong plasma and CSF levels correlate [6, 8, 16, 17]. Whether plasma-derived apolipoproteins might play a biological role in brain function and neurological disease development or whether plasma levels merely reflect CSF levels is currently unknown. However, regardless of the potential causal role of apolipoproteins, plasma markers represent a promising opportunity to study non-invasive markers of dementia. Therefore, the purpose of this review is to examine recent observational epidemiological studies evaluating the relevance of HDL-C and apolipoproteins in CSF and in the bloodstream in relation to dementia.
Identification of study reports
Data Sources
A systematic literature search of the MEDLINE database (accession date September 11, 2015) was performed to identify studies that investigated the association of HDL-C and apolipoproteins with dementia. The following search terms were used: (Dementia OR dementing OR Alzheimer OR Alzheimers OR “Alzheimer’s”) AND (“high-density lipoprotein” OR “high-density lipoproteins” OR HDL OR HDL-C OR apolipoprotein OR apolipoproteins OR apoA OR apoC OR apoE OR apoE2 OR apoE3 OR apoE4 OR apoJ OR apoD OR apoH OR clusterin). The search was limited to studies performed in adult humans and published from August 2010 to July 2015 in the English language.
Study selection
Titles and abstracts of 1625 studies were screened for eligibility. Inclusion criteria were as follows: observational epidemiological studies (cross-sectional or prospective) in humans investigating levels of HDL-C, apoA-I, apoA-II, apoA-IV, apoD, apoE, apoH, apoJ/clusterin, in un-pooled blood plasma, serum or cerebrospinal fluid (CSF) as single exposure variables (not as a component of risk scores) in relation to all-cause dementia (including AD, vascular dementia and other dementia forms). We excluded studies investigating only pre-clinical AD or cognitive decline as the outcome variable and we excluded case-only studies. Furthermore, we excluded studies with fewer than 10 participants per comparison group. In addition, reference lists of included studies were screened for eligible articles and we performed an additional hand search for updates of cohorts included.
Data extraction methods
The following information was extracted from included studies: first author, publication year, name of the study, country, study characteristics, follow-up duration for prospective studies, average (baseline) exposure concentration, outcome including number of cases and diagnostic criteria, main findings, and adjustment for covariates in statistical models.
Search results
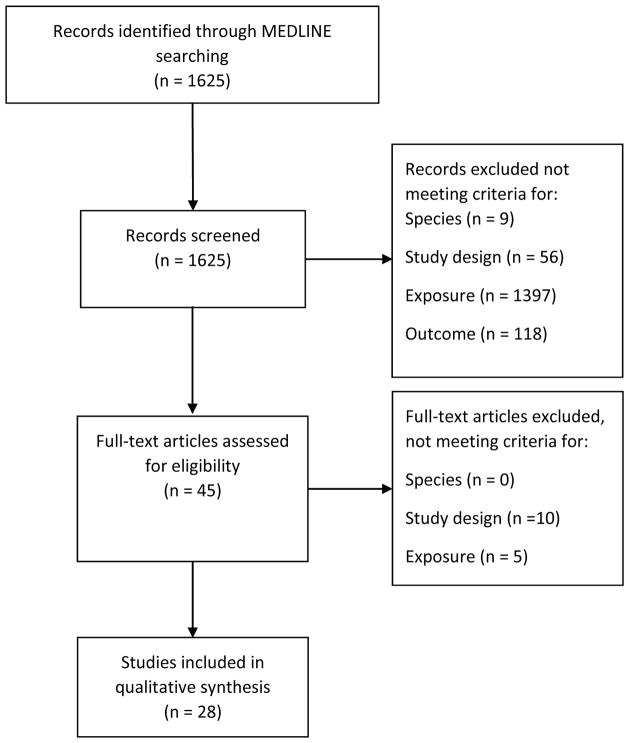
A flowchart of the study selection process is shown in Figure 1. Of the 1625 publications retrieved via the MEDLINE search, 1476 publications were excluded based on screening the title and abstract for relevance (most common reason for exclusion was that the assessed exposures were not plasma measures of HDL-C or apolipoproteins). The full texts of the remaining 40 publications were assessed for eligibility. A total of 28 studies were included in the qualitative synthesis of which 12 had a prospective study design [14, 16, 18, 19**, 20, 21, 22, 23, 24, 25**, 26, 27] and 16 had a cross-sectional design [28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43]. By hand search of reference lists and recent updates of included studies one additional publication was identified [44].
Figure 1.
Flow chart of the study selection process.
New insights into the role of HDL-C and apolipoproteins in dementia
HDL-C
Recent prospective studies (Table 1) investigating the associations of HDL-C with incident dementia have been inconsistent [14, 18, 20, 24, 25**]. In the Women’s Health and Aging Study II, where 99 women were followed-up for 9 years, HDL-C was neither related to incident dementia nor probable or possible AD, after accounting for age, body mass index (BMI) and blood glucose at baseline [24]. Similarly, baseline HDL-C levels did not differ between twin pairs in which one twin subsequently developed dementia whereas the second twin did not develop dementia. [20].
Table 1.
Characteristics of prospective studies investigating the association of HDL and apolipoproteins with dementia
| First author, year, name of the study, country | Study characteristics | Exposure, average (baseline) concentration | Outcome (no. of cases), diagnostic criteria | Main findings | Adjustments, notes |
|---|---|---|---|---|---|
| Ancelin 2013, Three-City (3C) study, France [18] | n = 7,053 participants. Multi-site cohort study of community-dwelling persons (mean age 74 years, male 39%). Follow-up: 7y. Baseline: 1999–2001. |
Fasting serum HDL-C (mmol/L): Men: overall geometric mean: 1.44 Q1: <1.19 Q2+Q3: 0.19-<1.63 Q4: ≥ 1.63 Women: overall geometric mean: 1.73. Quartile values not reported. |
All cause dementia (men: n = 184; women: n = 297): Neuropsychological and neurological examination by trained psychologists and neurologists, MRI or PET if available, consensus of neurologists committee according to DSM-IV revised criteria; AD: NINCDS-ADRDA. | A tendency for higher risk of dementia in men with lowest HDL-C. HR (95% CI) for all-cause dementia in all men: Q1: 1.45 (1.03–2.04) Q2+Q3: reference Q4: 0.97 (0.67–1.39). Women: no results shown. Reported that HDL-C was not significantly associated with all-cause dementia or AD. |
Age (y), sex (m; f), center (Bordeaux; Dijon; Montpellier), years of education (5; 9; 12; >12 y). Results were slightly attenuated with adjustment for additional covariates (HR in Q1 was 1.36 [0.97–1.93] after including hypertension, diabetes, depression and genotype). When restricted to men without vascular pathologies, the HR for all-cause dementia was 1.64 (1.02–2.66) among men in lowest HDL-C (Q1). Participants had to show up at exam year 2, 4 and 7 to be diagnosed with dementia. |
| Apostolova 2015, Alzheimer’s Disease Neuroimaging Initiative (ADNI), USA and Canada [19**]1 | n = 298 subjects with MCI (mean age 75 y, male 64%). Follow up: 36 months. Baseline: 2005–2008. |
Mean (SD) plasma apoE (log10 μg/mL): Individuals progressing to AD: 1.66 (0.18) Individuals not progressing to AD: 1.71 (0.18). Mean (SD) plasma apoJ (log10 μg/mL): individuals progressing to AD: 2.49 (0.07) Individuals not progressing to AD: 2.48 (0.07). |
AD (n = 161): NINCDS-ADRDA | Lower baseline apoE concentration (p = 0.007) but no difference in apoJ concentration (p=0.6) in individuals progressing from MCI to AD compared to individuals not progressing from MCI to AD. | None. |
| Gatz 2010, Study of Dementia in Swedish Twins (SATSA), Sweden [20] | n = 30 same-sex twin pairs enrolled in Swedish Twin Registry (mean age 71 y, male n = 7 pairs). Follow-up: 3 years. Baseline: 1987 | Serum HDL-C, apoA-I, and apoB concentration. Baseline levels not reported. |
Incident dementia (AD: n = 18, VD: n = 4, mixed AD and vascular n = 2, secondary cause dementia n = 2, unspecified dementia n = 4) in one twin whereas the other twin remains non-dement | No difference in HDL-C (p=0.3), or apoA1 (p=0.3) concentration comparing twins. Higher apoB concentration (p=0.0062) and apoB to apoA1 ratio (p=0.006) comparing the twins developing dementia to non-dement twins. |
Implicit adjustment for genetics but potentially shared familial risk factors for dementia. |
| Mielke 2012, Women’s Health and Aging Study II, USA [24] | n = 99 women free of dementia at baseline (mean age 74 y), Follow-up 9 years. Baseline: 1994–1995 | Nonfasting serum HDL-C. Baseline level not reported. |
Dementia (n = 17): DSM-IV, Probable and possible AD (n = 18): NINCDS-ADRDA. | HDL-C not significantly associated with dementia. HR (95% CI) comparing extreme tertiles was: 0.7 (0.3–1.8) for T3. In analysis restricted to probable and possible AD cases, the HR (95% CI) for T3 was T3: 1.6 (0.5–5.5). | Age (y), BMI (kg/m2), blood glucose at baseline (continuous). Non-fasting blood samples. |
| Rasmussen 2015, Copenhagen General Population Study (CGPS) and Copenhagen City Heart Study (CCHS), Denmark [25**] | n = 75,708 participants from two combined studies in Copenhagen. Baseline: CGPS=2003; CCHS=At blood sampling in 1991–1994 or 2001–2003. Follow-up: 2011 (mean of 4 years). No overall mean age at baseline, but mean age of non-cases was 57 years (45% male) and 73 years (40% male) for future cases. |
Mean (SD) plasma HDL-C (mmol/L): All dementia: 1.2 (0.02) AD: 1.7 (0.03) No dementia: 1.6 (0.002) Median plasma apoE (mg/dL) in age and sex stratified tertiles: T1: 3.1 T2: 4.1 T3: 5.8 |
Register data only, based on World Health Organization International Classification of Disease, 8th revision and 10th revision. n = 1,060 progressed to dementia (443 of which to AD). | Lower HDL-C concentration in participants who developed dementia, compared to the non-cases (p< 0.001). The lowest risk of dementia was found among those with highest apoE levels. Adjusted HR (95% CI) for all dementia comparing apoE tertiles: T1: 1.22 (1.01–1.47) T2: 1.09 (0.91–1.30) T3 (highest apoE): reference HR (95% CI) for AD: T1: 1.53 (1.13–2.08) T2: 1.31 (0.98–1.76) T3 (highest apoE): reference. |
Age (y), sex (m; f), body mass index (kg/m2), hypertension (yes; no), diabetes mellitus (yes; no), smoking (yes; no), alcohol consumption (yes; no), physical inactivity (yes; no), menopause status (yes; no), hormonal replacement therapy, lipid-lowering therapy (yes; no), education (<8; ≥8y), total cholesterol (mmol/L), low-density lipoprotein cholesterol (mmol/L), triglycerides (mmol/L), and HDL-C (mmol/L), APOE genotype (e4/-; e4/e4). APOE genotype was a strong confounder. Results were not modified by APOE genotype. No verification or validation of endpoints. |
| Reitz 2010, Northern Manhattan Study (NOMAS), USA [14] | n = 1130 elderly individuals sampled from Medicare recipients free of cognitive impairment at baseline: 1999–2001 (mean age 76 y, male: 34%). Follow-up: 4 y. |
Fasting plasma HDL-C (mgl/dL) range in HDL-C quartiles: Q1: ≤38.00 Q2: 38.01–46.00 Q3: 46.01–56.00 Q4: >56.00 |
Follow-up assessments every 18 months and medical records. Probable and possible AD (n = 101); Probable AD (n = 98), NINCDS-ADRDA. | Lowest risk of AD among those with highest HDL-C levels. Adjusted HR (95% CI) for probable and possible AD: Q1: reference Q2: 0.8 (0.4–1.5) Q3: 1.1 (0.6–1.9) Q4: 0.4 (0.2–0.9), pfor trend=0.10 HR (95% CI) for probable AD: Q1: reference Q2: 0.8 (0.4–1.6) Q3: 0.9 (0.5–1.9) Q4: 0.4 (0.2–0.9), pfor trend=0.06. |
Age (y), sex (m; f), education (y), ethnic group (White/non-Hispanic; Black/non-Hispanic; Hispanic), APOE genotype (e4/-; e4/e4), diabetes mellitus (yes;no), hypertension (yes;no), heart disease (yes;no), BMI (kg/m2), lipid lowering treatment (yes; no). |
| Schrijvers 2011, Rotterdam study, the Netherlands [26]1 | Case-cohort among non-demented participants. Random subcohort n= 926 [n = 61 developed dementia (n = 52 AD)] and additional cases of parent cohort [n = 178 developed dementia (n = 156 AD)] during follow-up. Baseline: 1997–1999. Mean follow-up: 7y. | Fasting mean (SD) plasma apoJ (μg/mL): Subcohort at risk for dementia: 115 (25) |
Incident dementia (n = 237, including n = 208 AD during follow-up: register linkage and diagnosed by a panel of neurologists, neuropsychologists at study exams every 3–4 years using NINCDS-ADRDA criteria. | No association between plasma apoJ and incident AD. All values per 1 SD increase in apoJ at baseline: HR (95% CI) per 1 SD higher apoJ: 1.00 (0.85–1.17) for AD and 0.96 (0.82–1.12) for all-cause dementia. |
Age (y), sex (m; f), education level (primary education; more than primary education), APOE genotype (e4/-; e4/e4), diabetes (yes; no), smoking (currently smoking; not currently smoking), coronary heart disease (yes; no), hypertension (yes; no). In a cross-sectional analysis comparing 60 participants with AD at baseline to the non-demented, higher plasma apoJ was significantly associated with greater odds of having AD (p trend over quartiles =0.004) as well as greater severity of AD. |
Additional reports from the ADNI study [16, 21, 22, 23, 38] and Rotterdam study [27] identified are not included in the table.
AD, Alzheimer’s disease; BMI, Body mass index; DSM-IIIR, Diagnostic and Statistical Manual of Mental Disorders, third edition, text revision; HR, Hazard Ratio; MCI, Mild cognitive impairment; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association; Q: Quintile. T, Tertile.
In contrast, a recent report using data from 75,708 participants of the Copenhagen General Population Study and the Copenhagen City Heart Study, showed that HDL-C levels were significantly lower in participants progressing to dementia or AD [25**]. This observation is further supported by evidence from a report including 1130 individuals randomly sampled from Medicare recipients in the U.S. Individuals in the highest quartile of HDL-C concentrations had a significantly lower risk of future probable or possible AD compared to individuals in the lowest quartile [14]. This association was robust to adjustment for age, sex, educational attainment, ethnic group, BMI, APOE genotype and several comorbidities and lipid-lowering medication intake [14].
The association between HDL-C and incident dementia or AD during a 7-year follow-up period was modified by sex in participants of the Three-City study, a multi-center study conducted in France that enrolled 7053 community-dwelling elderly [18]. There was no association between HDL-C and all-cause dementia or AD in the total sample, however, when the study sample was restricted to individuals without stroke, angina pectoris, myocardial infarction, arteritis, and cardiovascular surgery, participants in the lowest quartile of HDL-C had an increased risk for all-cause dementia (HR: 1.49, 95% CI: 0.99–2.23 compared to the quartiles 2 and 3) in men only. Further adjustment for baseline intima media thickness assessed at the baseline examination strengthened the association (HR: 1.64, 95% CI: 1.02–2.66). HDL-C levels were unrelated to AD in men and unrelated to incident all-cause dementia or AD in women without vascular comorbidities [18].
Six cross-sectional studies were identified that addressed the association of HDL-C in relation to dementia [28, 29, 30, 32, 33, 39], providing conflicting results (Table 2). Comparing 150 participants with AD to 197 control individuals from the Texas Alzheimer’s Disease Research and Care Consortium, Warren et al found lower HDL-C levels in participants with AD [39]. A recent study noted a significant lower HDL-C concentration in participants with late onset AD compared to participants without AD, but not early onset AD [29]. Another recent study of relatively small sample size focused on comparing HDL-C concentrations in I) participants with AD with cardiovascular comorbidities and risk factors, II) participants with AD without cardiovascular comorbidities and risk factors, and III) control subjects. Importantly, only participants with AD and cardiovascular comorbidities and risk factors had lover HDL-C levels in comparison to controls but not participants with AD without cardiovascular comorbidities and risk factors [30]. Another study of relatively small sample size including 29 participants with AD and 19 control subjects found no difference in HDL-C levels [28]. Similarly, investigations in subsamples of the Personalized Medicine Research Project sample including 455 participants [32] and the Australian Imaging, Biomarkers and Lifestyle (AIBL) study including 564 participants [33] revealed no difference in HDL-C concentrations comparing participants with AD to controls [32, 33].
Table 2.
Characteristics of cross-sectional studies investigating the association of HDL and apolipoproteins with dementia
| First author, year, name of the study, country | Study population, characteristics, | Exposure, average concentration | Outcome: diagnostic criteria | Main findings | Adjustments |
|---|---|---|---|---|---|
| Ahmed 2014, Australia [28] | n = 29 patients with AD (mean age 67 y, male 34%), n = 19 control subjects (mean age 69 y, male 53%). Study year: not reported. | Fasting mean serum HDL-C (mmol/L): AD: 1.8 Controls: 2.0 |
AD: Consensus amongst neurologist, neuropsychologist, and occupational therapist. | No difference in HDL-C concentration (p>0.05) in the AD group compared to the control group. | None. |
| Chang 2014, China [40]1 | n = 44 participants with AD (mean age 80 y), n = 62 control subjects (mean age 80 y). Study year: not reported. | Mean (SD) serum apoA-I (g/L): AD: 1.06 (0.21) Controls: 0.93 (0.20); Mean serum apoE (mg/L): AD: 37.73 (17.44) Controls: 36.69 (10.37) |
AD: Examination by neurological physicians according to ICD-10. | Higher apoA-I concentration (p = 0.006) in the AD group compared to the control group. No difference in apoE concentration in the AD group compared to the control group (p=0.8). | Controls age and sex matched. Gender distribution not specified. |
| Czapski 2012, Poland [29] | n = 68 participants with early onset AD (male 35%), n = 187 patients with late onset AD (male 28%), n = 80 control subjects recruited from the general population (mean age 71 in women, 72 in men, male 28%). Study year: not reported. | Mean (SD) serum HDL-C (mg/dL): Early onset AD: 60.6 (17.9) Late onset AD: 59.4 (16.7) Controls: 66 (17.6) |
Early and late onset AD: NINCDS-ADRDA. |
Lower HDL-C concentration (p<0.01) in the late onset AD group, but not in the early onset AD group (p<0.05) compared with the control group. | None. |
| Dias 2014, Germany [30] | n = 27 participants with AD (mean age 81 y, male 37%), n = 16 participants with AD with cardiovascular comorbidities and risk factors (mean age 79 y, male 44%), n = 33 control subjects without evidence of cognitive impairment and without vascular comorbidities and risk factors (mean age 73 y, male 33%). Study year: not reported. | Fasting plasma HDL-C (concentration not specified) | AD: NINCDS-ADRDA. | Lower HDL-C concentration (p<0.05) in participants with AD with cardiovascular comorbidities and risk factors but not AD without cardiovascular comorbidities and risk factors compared (p>0.05) to control subjects. | None. |
| Ghebranious 2011, the Personalized Medicine Research Project cohort, USA [32] | n = 455 participants (AD n = 153, mean age 78 years, male 40%: controls n = 302, mean age 87 years, male 43%). Study year: not reported. | Mean (SD) HDL-C (mg/dL): AD: 59.5 (16.8) Controls: 62.9 (17.0) |
Late onset AD: NINCDS-ADRA. | No difference in HDL-C concentration (p=0.08) comparing participants with AD to controls. | None. |
| Gupta 2011, AIBL cohort, Australia [33] | n = 199 with AD (mean age 78 years, sex not listed; n = 365 controls (cognitively normal “non-memory complainers”: mean age 70 y). Study year: not reported. |
Fasting mean (SD) plasma HDL-C (mmol/L): AD: 1.68 (0.20) Controls: 1.67 (0.22) Fasting mean (SD) plasma apoE (mg/dL) AD: 14.23 (2.63) Controls: 15.43 (2.66) |
AD: NINCDS-ADRA. | No difference in HDL-C concentration (p=0.6) comparing participants with AD to controls. Lower apoE concentration (p<0.044) in participants with AD compared to controls. |
Age (y), APOE genotype (e4/-; e4/e4). |
| Kandimalla 2013, India [34] | n= 44 participants with AD (mean age 62 y, male: 59%), n = 63 participants with non-Alzheimer dementias (NAD; mean age 57 y, male: 91%; and n = 46 age-matched control individuals (mean age 61 y, male: 70%). Study year: not reported. | Mean (SD) CFS apoE (μg/mL): AD: 22.36 (2.35) NAD: 22.10 (3.49) Controls: 23.22 (2.21). |
AD: NINCDS-ADRDA NAD: Diagnostic and Statistical Manual of Mental Disorders (4th edition; American Psychiatric Association, 1994). | No difference in apoE concentration comparing participants with AD or NAD to controls (p>0.005). | None. Bonferroni corrected p-value. |
| Lojkowska 2013, Poland [35] | n = 55 participants with AD (mean age 70 y, male 33 %), n = 30 controls (mean age 70 y, male 33%) | Serum mean (SD) apoE (mg/dL): AD: 5.7 (1.3) Controls: 6.0 (1.6) |
AD: NINCDS-ADRDA, DSM-IV. | No significant difference in apoE concentration (p>0.05) comparing participants with AD to controls. | None. |
| Marksteiner 2014, Austria [36] | n = 33 participants with AD (mean age 81 y, male 36%) and n = 23 controls (age 71 y, male 57%). Study year: not reported. | Plasma, mean (SD) apoA-I (μg/mL): AD: 176 (18) Controls: 103 (17) |
Probable AD: NINCDS-ADRDA. | Higher apoA-I concentration (p<0.001) in participants with AD compared to controls. | None. |
| Martínez-Morillo 2014, Sweden [37] | n = 43 participants with AD (median age 61 y, male: 47%) and n = 43 participants without AD (median age 78 y, male: 36%). Study year: not reported. | Plasma and CSF apoE (concentrations not reported) | AD: DSM-IIIR and NINCDS-ADRDA | No difference in CSF (p = 0.5) and plasma apoE (p = 0.8) concentrations comparing participants with AD to controls. | None. |
| Schürmann 2011, German Study on Aging Cognition and dementia in primary care patients (AgeCoDe), Germany [41] | n = 67 participants with AD (mean age 85 y, male: 30%). Study year not reported. | Mean (SD) plasma apoJ (μg/mL): AD: 158.5 (45.3) Controls: 161.5 (47.6) |
AD: DSM-IV, ICD-10 (SIDAM) and NINCDS-ADRD. Global Deterioration Scale and Blessed Dementia Scale for non-interviewed participants. | No difference in plasma apoJ concentration (p = 0.7) comparing participants with AD to controls. | None. |
| Silajdžić 2012, Sweden [43] | n = 127 participants with AD (mean age 75 y, male 29%) and n = 171 controls (mean age 74 y, male 36%). Study year not reported | Non-fasting mean (SD) plasma apoJ (μg/mL): AD: 33.3 (4.8) Controls: 33.3 (3.9) |
AD: DSM-IIIR and NINCDS-ADRADA | No difference in apoJ (p>0.05) concentrations comparing participants with AD to controls. | None. |
| Warren 2013, Texas Alzheimer’s Disease Research and Care Consortium (TARCC) Longitudinal Research Cohort, United States [39] | n = 150 participants with AD (age 80 y, male 30%), n = 197 controls (age 70 y, male 69 %). Study year: not reported. | Mean (SD) HDL-C (mg/dL): AD: 67 (138) Controls: 70.6 (211) |
Probable AD: NINCDS-ADRDA | Lower HDL-C concentration (p=0.020) in participants with AD compared to controls. | None. |
| Xing 2012, China [42] | n = 104 participants with AD (mean age 80 y, male 39%), n = 104 controls (age 79 y, male 44%). Study year 2010–2011) | Mean (SD) plasma apoJ (μg/mL): AD: 167.01 (13.4) Controls: 162.74 (10.03) |
Probable AD: NINCDS-ADRDA | Higher apoJ concentration (p=0.01) in participants with AD compared to controls. | None. |
Paper refers to apoA.
Abbreviations: AD, Alzheimer’s disease; DSM-IIIR, Diagnostic and Statistical Manual of Mental Disorders, third edition, text revision; MCI, Mild cognitive Impairment; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association.
In summary, recent prospective studies of moderate to large sample size provide evidence for an inverse association between HDL-C level and risk of all-cause dementia and AD that is robust to multivariable adjustment. However, future studies that carefully investigate the effect of comorbidities and potential effect modifiers like sex on this association are warranted.
Apolipoprotein A-I
ApoA-I is the major protein component of HDL-C in plasma [9]. Only one prospective study was identified relating apoA-I concentrations to AD [20]. The Study of Dementia in Swedish Twins compared the concentration of apoA-I in 23 discordant twin pairs (one twin developed dementia over 3 years, the other does not) and found no difference in apoA-I concentrations [20]. Cross-sectional investigations from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study [45] and another small case-control study from Austria found 1.06 and 1.7 times higher plasma apoA-I concentrations, respectively, in individuals with AD compared with controls [23, 36]. In an age- and sex-matched case-control study, higher apoA-I levels have been associated with prevalent AD [40].
Apolipoprotein E
The recognition of the APOE genotype as a major AD susceptibility variant has led to efforts to study apoE – the main protein in HDL-C in CSF – in relation to dementia. In a prospective analysis of the ADNI study, in participants with mild cognitive impairment (MCI), higher CSF apoE levels were associated with a lower risk of conversion to AD [16]. Recently, the large Copenhagen General Population Study and the Copenhagen City Heart Study confirmed an inverse association between plasma apoE and dementia [25**]. Furthermore, the latter study also addressed the question of whether apoE level is associated with dementia and AD independent of apoE genotype. While apoE4 carriers have the lowest apoE levels, the risk for AD associated with apoE4 genotype is not solely explained by the apoE plasma level as the magnitude or risk associated with the genotype is far greater than predicted as per the associated difference in apoE plasma level among carriers vs noncarriers of the E4 variant [25**]. Interestingly, in the latter study, apoE levels increased with increasing age and age is a strong predictor for dementia and AD. However, in parallel, apoE4 carriers tend to have the lowest apoE levels but the highest risk of dementia. Cross-sectional studies on apoE mainly indicated lower apoE levels in participants with dementia compared with controls, but the majority of studies did not adjust for potential confounding factors [21, 22, 33, 38, 44]. Although CSF apoE levels were correlated with plasma apoE levels (r = 0.23, p = 0.004) in the ADNI cohort [16], CSF apoE levels did not differ between participants with AD and controls in univariate analysis. However, lower CSF apoE levels were observed in participants with AD compared with controls when accounting for t-tau levels, age, gender, and APOE genotype [16]. In conclusion, emerging evidence suggests that lower apoE levels are associated with dementia prevalence and incidence [25**].
Apolipoprotein J
As one of the most prominent transport proteins in the brain and also one of the top genetic markers recently related to AD [13], apoJ is of particular interest for dementia etiology [46, 47]. Investigators of the ADNI cohort reported that plasma apoJ levels did not differ between individuals with MCI progressing to AD and individuals with MCI not progressing to AD [19**]. Although apoJ levels were significantly positively related to prevalent AD or all-cause dementia at baseline, apoJ levels did not predict incident all-cause dementia during 7 years of follow-up in the well-characterized population-based Rotterdam study [26]. The latter results highlight the importance of prospective studies in the evaluation of apolipoproteins in the pathophysiology of dementia. Smaller, cross-sectional case-control studies revealed inconclusive results regarding the association of apoJ with AD [41, 42, 43]. Given the recent identification of CLU/APOJ genetic variants as strong markers in GWAS [13], we expect that additional investigations on this marker are to come.
Other apolipoproteins
Evaluating 146 plasma biomarkers assessed in baseline blood plasma samples of the ADNI study participants, significantly lower apoA-II, apoC-III, apoA-IV, and apoH concentrations were observed in participants with AD compared to controls, whereas no difference was observed for apoC-I and apoD [23]. In the same cohort, lower plasma apoD and apoA-II concentrations were observed in individuals with MCI progressing to AD compared to controls in univariate analysis [22].
Diagnostic criteria of dementia
The comparison of study results is challenging because diagnostic criteria for dementia and AD vary and criteria might be susceptible to subjectivity and misclassification [48]. E.g. ischemic vascular disease and dementia are tightly linked, especially at old age, and most dementia at old age includes both mixed vascular and AD pathology [49]. Thus, the distinction between vascular dementia and non-vascular dementia has been questioned and HDL-C and apolipoproteins should be regarded among the important vascular contributions to cognitive impairment and dementia [49]. A recent guideline highlights the importance of reporting study results in the context of varying standards for diagnosing dementia: Standards of Reporting of Neurological Disorders (STROND) [48].
Sources of data on the role of plasma markers in relation to dementia originate from mainly two complementary study types. First, register-based epidemiological studies rely exclusively on phenotype information from register data, where only cases that present to the medical system are included [25**]. Where available, registers represent a compelling data source for epidemiological studies when caution with regard to validity of the data including change of data quality over time and completeness of data is appropriately noted [50]. It is unclear if the cases included in the large Copenhagen General Population Study and the Copenhagen City Heart Study are representative of the study base [25**, 51]. However, register-based studies can include participants at younger ages and allow for follow up over many years [50]. In contrast, studies based on data collected by researchers that closely follow participants with repeated screening tests and examinations by neuropsychologists are often limited to collection of blood samples from participants at a higher mean age when their disease incidence is highest [18, 26]. The high costs of many years of detailed cognitive tests and adjudication of neurological diseases into late life simply prohibits the longer follow-up duration required for studying younger age groups. Therefore, smaller studies with costly repeated assessments of the outcome mainly include study participants at an advanced age at baseline in order to ensure sufficient number of cases in relatively short follow up. However, it is possible that weight loss and other characteristics associated with cognitive decline [52] could lead to changes in cholesterol levels, including HDL-C levels [53]. This phenomenon could cause reverse causation and as such it is possible that these investigations underestimate the true association of HDL-C at a more vulnerable time in life. However, as with neurological disease, the pathological process of atherosclerotic plaque deposition starts many years earlier (i.e., in the third decade of life) and investigation of cholesterol measures in even older individuals (age 65 and above) also confirm an inverse association between HDL-C and CHD [54]. As yet, only few studies focusing on the lifespan course of dementia have been initiated [55, 56]. To the best of our knowledge, no large-scale studies exists with blood samples stored since early adulthood from participants followed with many years of detailed cognitive tests and adjudication of neurological diseases into late life. Nevertheless, smaller studies with researcher-collected data are feasible, cost-effective, and can provide insights that could potentially be further expanded in populations of younger adults in the future.
In summary, it is important to recognize the considerable heterogeneity across phenotypes presented in the different studies. Furthermore, the well-adjudicated, smaller studies and the larger register-based studies likely complement each other.
CONCLUSION
Studies identified were mostly cross-sectional, of small to moderate sample size and mainly unadjusted for potential confounding factors. Larger studies indicate that plasma levels of HDL-C and apoE are inversely associated with dementia risk, which was robust to multivariable adjustment. Higher apoJ might be a marker of prevalent dementia, but was not associated with future dementia. Studies on measures of CSF apolipoproteins are limited, but couldn’t confirm associations observed for apoE. The investigation of HDL-C and apolipoproteins in relation to dementia represents an area of opportunity and prospective studies are needed investigating changes of these markers in relation to incident dementia.
KEY POINTS.
Dementia is a major cause of disability and institutionalization
Larger studies indicate that plasma levels of HDL-C and apoE are inversely associated with dementia risk, which was robust to multivariable adjustment. Higher apoJ might be a marker of prevalent dementia, but was not associated with future dementia. Studies on measures of CSF apolipoproteins are limited, but couldn’t confirm associations observed for apoE
Prospective large scale epidemiological studies are needed investigating the change of HDL-C and apolipoproteins in relation to risk of dementia and AD
Acknowledgments
Funding
The authors were not sponsored to write this review. The reviewed proteins are related to work funded by the NINDS (1R01NS089638-01A1, PI: Jensen).
Footnotes
Conflicts of interest
None of the authors have any conflicts of interest.
Majken K. Jensen is listed as co-inventor on a patent US88463212B. Awarded Sept. 2014, and a patent application 61/798,575, filed May 15, 2013.
References
- 1.Alzheimer’s Disease International. World Alzheimer Report. London: Alzheimer’s Disease International; 2015. [Google Scholar]
- 2.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003 Aug;60(8):1119–22. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 3.Whiteford HA, Ferrari AJ, Degenhardt L, et al. The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS One. 2015;10(2):e0116820. doi: 10.1371/journal.pone.0116820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman R. Alzheimer’s disease and other dementias: advances in 2014. Lancet Neurol. 2015 Jan;14(1):4–6. doi: 10.1016/S1474-4422(14)70301-1. [DOI] [PubMed] [Google Scholar]
- 5.Dietschy JM. Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol Chem. 2009 Apr;390(4):287–93. doi: 10.1515/BC.2009.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roheim PS, Carey M, Forte T, Vega GL. Apolipoproteins in human cerebrospinal fluid. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4646–9. doi: 10.1073/pnas.76.9.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demeester N, Castro G, Desrumaux C, et al. Characterization and functional studies of lipoproteins, lipid transfer proteins, and lecithin:cholesterol acyltransferase in CSF of normal individuals and patients with Alzheimer’s disease. J Lipid Res. 2000 Jun;41(6):963–74. [PubMed] [Google Scholar]
- 8.Koch S, Donarski N, Goetze K, et al. Characterization of four lipoprotein classes in human cerebrospinal fluid. J Lipid Res. 2001 Jul;42(7):1143–51. [PubMed] [Google Scholar]
- 9.Kontush A, Chapman MJ. HDL: close to our memories? Arterioscler Thromb Vasc Biol. 2008 Aug;28(8):1418–20. doi: 10.1161/ATVBAHA.108.169714. [DOI] [PubMed] [Google Scholar]
- 10.Holtzman DM. Role of apoe/Abeta interactions in the pathogenesis of Alzheimer’s disease and cerebral amyloid angiopathy. J Mol Neurosci. 2001 Oct;17(2):147–55. doi: 10.1385/JMN:17:2:147. [DOI] [PubMed] [Google Scholar]
- 11.Zlokovic BV, Deane R, Sallstrom J, et al. Neurovascular pathways and Alzheimer amyloid beta-peptide. Brain Pathol. 2005 Jan;15(1):78–83. doi: 10.1111/j.1750-3639.2005.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Q, Lee CY, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008 Jun 12;58(5):681–93. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert JC, Heath S, Even G, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009 Oct;41(10):1094–9. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 14.Reitz C, Tang MX, Schupf N, et al. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch Neurol. 2010 Dec;67(12):1491–7. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitas RE, Boyles JK, Lee SH, et al. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J Biol Chem. 1987 Oct 15;262(29):14352–60. [PubMed] [Google Scholar]
- 16.Toledo JB, Da X, Weiner MW, et al. CSF Apo-E levels associate with cognitive decline and MRI changes. Acta Neuropathol. 2014 May;127(5):621–32. doi: 10.1007/s00401-013-1236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruchaga C, Kauwe JS, Nowotny P, et al. Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer’s disease. Hum Mol Genet. 2012 Oct 15;21(20):4558–71. doi: 10.1093/hmg/dds296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ancelin ML, Ripoche E, Dupuy AM, et al. Sex differences in the associations between lipid levels and incident dementia. J Alzheimers Dis. 2013;34(2):519–28. doi: 10.3233/JAD-121228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Apostolova LG, Hwang KS, Avila D, et al. Brain amyloidosis ascertainment from cognitive, imaging, and peripheral blood protein measures. Neurology. 2015 Feb 17;84(7):729–37. doi: 10.1212/WNL.0000000000001231. Recent report from the Alzheimer’s Disease Neuroimaging Initiative suggesting that apoE but not apoJ predicts progression to AD in participants with MCI over 36 months follow-up. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gatz M, Reynolds CA, Finkel D, et al. Dementia in Swedish twins: predicting incident cases. Behav Genet. 2010 Nov;40(6):768–75. doi: 10.1007/s10519-010-9407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo LH, Alexopoulos P, Wagenpfeil S, et al. Plasma proteomics for the identification of Alzheimer disease. Alzheimer Dis Assoc Disord. 2013 Oct-Dec;27(4):337–42. doi: 10.1097/WAD.0b013e31827b60d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnstone D, Milward EA, Berretta R, Moscato P. Multivariate protein signatures of pre-clinical Alzheimer’s disease in the Alzheimer’s disease neuroimaging initiative (ADNI) plasma proteome dataset. PLoS One. 2012;7(4):e34341. doi: 10.1371/journal.pone.0034341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llano DA, Devanarayan V, Simon AJ. Evaluation of plasma proteomic data for Alzheimer disease state classification and for the prediction of progression from mild cognitive impairment to Alzheimer disease. Alzheimer Dis Assoc Disord. 2013 Jul-Sep;27(3):233–43. doi: 10.1097/WAD.0b013e31826d597a. [DOI] [PubMed] [Google Scholar]
- 24.Mielke MM, Bandaru VV, Haughey NJ, et al. Serum ceramides increase the risk of Alzheimer disease: the Women’s Health and Aging Study II. Neurology. 2012 Aug 14;79(7):633–41. doi: 10.1212/WNL.0b013e318264e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Rasmussen KL, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Plasma levels of apolipoprotein E and risk of dementia in the general population. Ann Neurol. 2015 Feb;77(2):301–11. doi: 10.1002/ana.24326. Using data from 75,708 participants of the Copenhagen General Population Study and the Copenhagen City Heart Study, this large Danish register based study showed HDL-C levels were significantly lower in participants progressing to dementia or AD. [DOI] [PubMed] [Google Scholar]
- 26.Schrijvers EM, Koudstaal PJ, Hofman A, Breteler MM. Plasma clusterin and the risk of Alzheimer disease. JAMA. 2011 Apr 6;305(13):1322–6. doi: 10.1001/jama.2011.381. [DOI] [PubMed] [Google Scholar]
- 27.IJsselstijn L, Dekker LJ, Koudstaal PJ, et al. Serum clusterin levels are not increased in presymptomatic Alzheimer’s disease. J Proteome Res. 2011 Apr 1;10(4):2006–10. doi: 10.1021/pr101221h. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed RM, MacMillan M, Bartley L, et al. Systemic metabolism in frontotemporal dementia. Neurology. 2014 Nov 11;83(20):1812–8. doi: 10.1212/WNL.0000000000000993. [DOI] [PubMed] [Google Scholar]
- 29.Czapski GA, Maruszak A, Styczynska M, et al. Association between plasma biomarkers, CDK5 polymorphism and the risk of Alzheimer’s disease. Acta Neurobiol Exp (Wars) 2012;72(4):397–411. doi: 10.55782/ane-2012-1911. [DOI] [PubMed] [Google Scholar]
- 30.Dias IH, Polidori MC, Li L, et al. Plasma levels of HDL and carotenoids are lower in dementia patients with vascular comorbidities. J Alzheimers Dis. 2014;40(2):399–408. doi: 10.3233/JAD-131964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doecke JD, Laws SM, Faux NG, et al. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch Neurol. 2012 Oct;69(10):1318–25. doi: 10.1001/archneurol.2012.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghebranious N, Mukesh B, Giampietro PF, et al. A pilot study of gene/gene and gene/environment interactions in Alzheimer disease. Clin Med Res. 2011 Mar;9(1):17–25. doi: 10.3121/cmr.2010.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta VB, Laws SM, Villemagne VL, et al. Plasma apolipoprotein E and Alzheimer disease risk: the AIBL study of aging. Neurology. 2011 Mar 22;76(12):1091–8. doi: 10.1212/WNL.0b013e318211c352. [DOI] [PubMed] [Google Scholar]
- 34.Kandimalla RJ, Wani WY, Anand R, et al. Apolipoprotein E levels in the cerebrospinal fluid of north Indian patients with Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2013 May;28(3):258–62. doi: 10.1177/1533317513481097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lojkowska W, Witkowski G, Bednarska-Makaruk M, et al. Correlations between cerebellar and brain volumes, cognitive impairments, ApoE levels, and APOE genotypes in patients with AD and MCI. Curr Alzheimer Res. 2013 Nov;10(9):964–72. doi: 10.2174/15672050113106660161. [DOI] [PubMed] [Google Scholar]
- 36.Marksteiner J, Imarhiagbe D, Defrancesco M, et al. Analysis of 27 vascular-related proteins reveals that NT-proBNP is a potential biomarker for Alzheimer’s disease and mild cognitive impairment: a pilot-study. Exp Gerontol. 2014 Feb;50:114–21. doi: 10.1016/j.exger.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Morillo E, Hansson O, Atagi Y, et al. Total apolipoprotein E levels and specific isoform composition in cerebrospinal fluid and plasma from Alzheimer’s disease patients and controls. Acta Neuropathol. 2014 May;127(5):633–43. doi: 10.1007/s00401-014-1266-2. [DOI] [PubMed] [Google Scholar]
- 38.Soares HD, Potter WZ, Pickering E, et al. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch Neurol. 2012 Oct;69(10):1310–7. doi: 10.1001/archneurol.2012.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren MW, Hynan LS, Weiner MF. Lipids and adipokines as risk factors for Alzheimer’s disease. J Alzheimers Dis. 2012;29(1):151–7. doi: 10.3233/JAD-2012-111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang L, Wang Y, Ji H, et al. Elevation of peripheral BDNF promoter methylation links to the risk of Alzheimer’s disease. PLoS One. 2014;9(11):e110773. doi: 10.1371/journal.pone.0110773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schurmann B, Wiese B, Bickel H, et al. Association of the Alzheimer’s disease clusterin risk allele with plasma clusterin concentration. J Alzheimers Dis. 2011;25(3):421–4. doi: 10.3233/JAD-2011-110251. [DOI] [PubMed] [Google Scholar]
- 42.Xing YY, Yu JT, Cui WZ, et al. Blood clusterin levels, rs9331888 polymorphism, and the risk of Alzheimer’s disease. J Alzheimers Dis. 2012;29(3):515–9. doi: 10.3233/JAD-2011-111844. [DOI] [PubMed] [Google Scholar]
- 43.Silajdzic E, Minthon L, Bjorkqvist M, Hansson O. No diagnostic value of plasma clusterin in Alzheimer’s disease. PLoS One. 2012;7(11):e50237. doi: 10.1371/journal.pone.0050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta VB, Wilson AC, Burnham S, et al. Follow-up plasma apolipoprotein E levels in the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing (AIBL) cohort. Alzheimers Res Ther. 2015;7(1):16. doi: 10.1186/s13195-015-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010 Jan 19;74(3):201–9. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitali C, Wellington CL, Calabresi L. HDL and cholesterol handling in the brain. Cardiovasc Res. 2014 Aug 1;103(3):405–13. doi: 10.1093/cvr/cvu148. [DOI] [PubMed] [Google Scholar]
- 47.Thambisetty M, Simmons A, Velayudhan L, et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry. 2010 Jul;67(7):739–48. doi: 10.1001/archgenpsychiatry.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennett DA, Brayne C, Feigin VL, et al. Development of the Standards of Reporting of Neurological Disorders (STROND) checklist: A guideline for the reporting of incidence and prevalence studies in neuroepidemiology. Neurology. 2015 Jul 10; doi: 10.1212/WNL.0000000000001866. [DOI] [PubMed] [Google Scholar]
- 49.Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015 Jun;11(6):710–7. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thygesen LC, Ersboll AK. When the entire population is the sample: strengths and limitations in register-based epidemiology. Eur J Epidemiol. 2014 Aug;29(8):551–8. doi: 10.1007/s10654-013-9873-0. [DOI] [PubMed] [Google Scholar]
- 51.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011 Jul;39(7 Suppl):30–3. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 52.Soto ME, Secher M, Gillette-Guyonnet S, et al. Weight loss and rapid cognitive decline in community-dwelling patients with Alzheimer’s disease. J Alzheimers Dis. 2012;28(3):647–54. doi: 10.3233/JAD-2011-110713. [DOI] [PubMed] [Google Scholar]
- 53.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007 Aug 15;298(7):786–98. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 54.Castelli WP, Doyle JT, Gordon T, et al. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation. 1977 May;55(5):767–72. doi: 10.1161/01.cir.55.5.767. [DOI] [PubMed] [Google Scholar]
- 55.Anstey KJ, Christensen H, Butterworth P, et al. Cohort profile: the PATH through life project. Int J Epidemiol. 2012 Aug;41(4):951–60. doi: 10.1093/ije/dyr025. [DOI] [PubMed] [Google Scholar]
- 56.Cadar D, Pikhart H, Mishra G, et al. The role of lifestyle behaviors on 20-year cognitive decline. J Aging Res. 2012;2012:304014. doi: 10.1155/2012/304014. [DOI] [PMC free article] [PubMed] [Google Scholar]