Abstract
The mammalian female reproductive tract interacts with sperm in various ways in order to facilitate sperm migration to the egg while impeding migrations of pathogens into the tract, to keep sperm alive during the time between mating and ovulation, and to select the fittest sperm for fertilization. The two main types of interactions are physical and molecular. Physical interactions include the swimming responses of sperm to the microarchitecture of walls, to fluid flows, and to fluid viscoelasticity. When sperm encounter walls, they have a strong tendency to remain swimming along them. Sperm will also orient their swimming into gentle fluid flows. The female tract seems to use these tendencies of sperm to guide them to the site of fertilization. When sperm hyperactivate, they are better able to penetrate highly viscoelastic media, such as the cumulus matrix surrounding eggs. Molecular interactions include communications of sperm surface molecules with receptors on the epithelial lining of the tract. There is evidence that specific sperm surface molecules are required to enable sperm to pass through the uterotubal junction into the oviduct. When sperm reach the oviduct, most bind to the oviductal epithelium. This interaction holds sperm in a storage reservoir until ovulation and serves to maintain fertilization competence of stored sperm. When sperm are released from the reservoir, they detach from and re-attach to the epithelium repeatedly while ascending to the site of fertilization. We are only beginning to understand the communications that may pass between sperm and epithelium during these interactions.
Keywords: spermatozoa, cervix, oviduct, microfluidics, fallopian tubes
Introduction
All mammals, even those that lay eggs (Nixon et al. 2011), utilize internal fertilization. That is, the male transfers sperm into the female reproductive tract and then the sperm must migrate through a portion of the tract in order to reach eggs and fertilize them.
Evolution of the female reproductive tract seems to be driven by a number of factors. One is the need to facilitate sperm migration to the egg while impeding migration of pathogens into the tract. Another is that the female tract must be able to keep sperm alive for hours, days, or even months, depending on the period between the optimal time to mate and the optimal time to initiate development of an embryo. In addition, it is believed that the female employs a strategy to attempt to select the fittest sperm for fertilization. Given all of these factors that operate on the process of reproduction, it should not be surprising that the female reproductive tract interacts in various ways with sperm in order to facilitate migration to the egg, store sperm until needed, and select sperm of the best quality to fertilize.
There are two main categories of interactions of sperm with the female reproductive tract, namely, physical and molecular. The physical category includes the swimming responses of sperm to the microarchitecture of the walls of the tract, to fluid flows, and to fluid viscoelasticity. Molecular interactions include communications of sperm surface molecules with receptors in the epithelial linings of the tract. Indirect molecular interactions, such as effects of tract secretions on sperm, effects of seminal plasma on the tract, or interactions of sperm with immune cells that enter the lumen of the tract will not be discussed in this review. Recent reviews on these topics include Ghersevich et al. (2015), Martyn et al. (2014), McGraw et al. (2014), and Rodriguez-Martinez et al. (2011).
Physical Interactions
Surfaces
The architecture of cell surfaces can affect the direction of sperm movement. It has long been observed that sperm tend to accumulate at surfaces, particularly the surfaces of slides and coverslips (Rothschild 1963; Winet, et al. 1984; Woolley 2003). When sperm that are swimming along a flat horizontal surface reach a side wall, they tend to continue swimming along the corner where the two walls meet (Denissenko et al. 2012). The surfaces of the walls of the female reproductive tract are, of course, far more complex in design than the surfaces of microscope slides. Recently, Denissenko and colleagues (Denissenko et al. 2012) took advantage of advances in microtechnology to construct microchannels of various configurations to test how angles and curved surface would affect movement of sperm. These microchannels were constructed of polymethylsiloxane (PDMS), which is a somewhat soft and elastic siliconbased polymer that more closely resembles the properties of epithelial surfaces than do glass slides, but is also optically clear. They observed that when human sperm that were swimming along a surface encountered a sharp outward turn, the sperm would leave the surface until they encountered another surface. Using this information, they constructed a “one-way running track” for sperm. When human sperm were loaded into this circular channel with scalloped walls, they tended to swim in a counterclockwise direction around the circle (Fig 1). It is very interesting that a scanning electron micrograph of the inner surface of the bovine uterotubal junction (Yaniz et al. 2000) reveals shapes that resemble the architecture of the running track (Fig 2). This resemblance indicates that the microarchitectural of the junctional walls could guide sperm to swim toward the oviduct.
Fig 1.
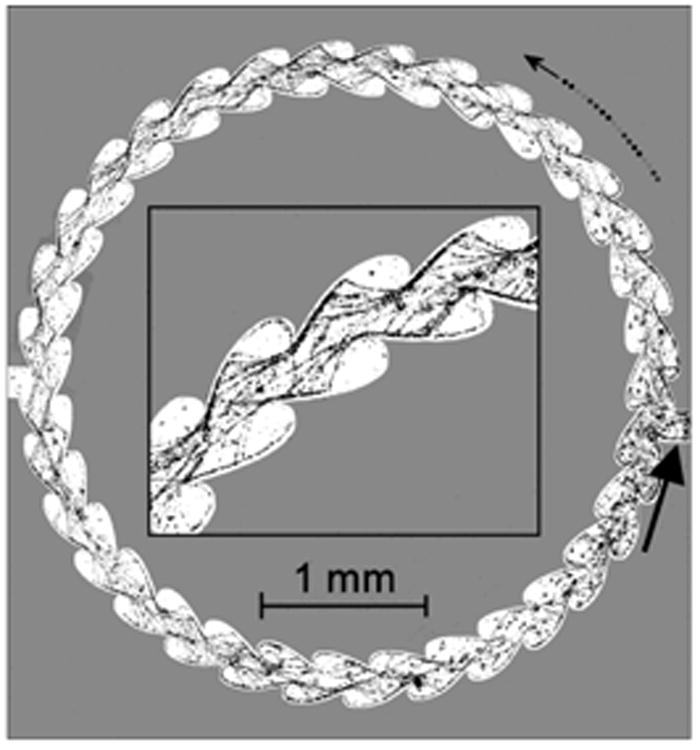
Human sperm in a microchannel designed to guide them to swim counterclockwise (curved arrow). The input site for sperm is indicated by the large, straight arrow. The insert shows a higher magnification of tracks of sperm swimming within the microchannel. Images were collected at 4 frames per second and the location of sperm heads (tiny dots) in 200 consecutive frames were summed to produce this figure (from Denissenko et al. 2012).
Fig 2.
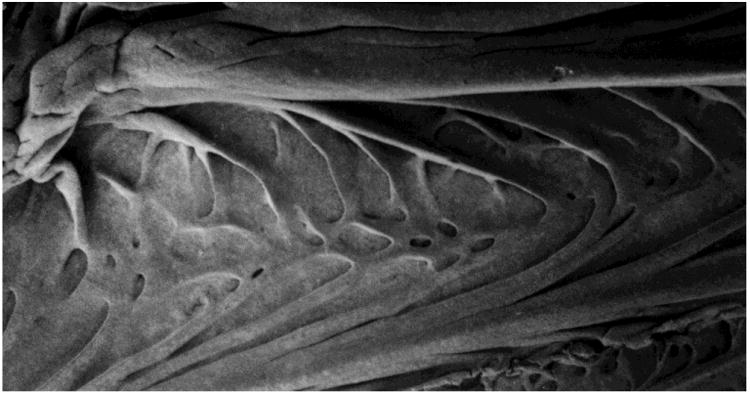
Scanning electron micrograph of the surface that lines the bovine uterotubal junction. The junction has been opened longitudinally and is oriented such that the uterus would lie to the left. Notice that mucosal folds form blunt arrows that point into the oviduct (from Yaniz et al. 2000).
Fluid flows
The fluid in the lumen of the female reproductive tract is rarely static: ciliary beating, contractions of smooth muscle in its walls, and secretion of fluids into the lumen create fluid flows (Gaddum Rosse and Blandau 1976; Miki and Clapham 2013).
At a certain low range of fluid flow velocity, sperm orient into a flow and swim against it (Miki and Clapham 2013; Kantsler et al. 2014; Tung et al. 2014, 2015). Below this range, the direction of swimming is unaffected by the flow; above this range, sperm are swept downstream (Tung et al., 2015). There is little information about the velocity of flows that exist within the female reproductive tract. Fluid flow in the mouse oviduct at a time when sperm were moving up the oviduct was measured to be 18+/-1.6 μm/sec (Miki and Clapham 2013). Bull sperm begin orienting into a flow when it reaches about 15 μm/sec (Tung et al. 2015).
According to experimental evidence, fluid flow and surfaces can act together to guide sperm through parts of the female tract. The best example is of microgrooves in the cervix. A careful study by Mullins and Saacke (1989) of serial histological sections of the bovine cervix revealed that there are microgrooves in the wall of the cervix that run its entire length from the vagina to the uterus. These microgrooves are not much wider than sperm heads and were found to be filled with sperm when the cervices were taken from naturally mated cows. The sperm were oriented toward the uterus. Mullins and Saacke proposed that the microgrooves provide a priviledged pathway toward the uterus for sperm, because the grooves would protect sperm from the strong outflow of fluids through the center of the cervical canal.
Recent developments in microfluidic technologies enabled us to test the proposal of Mullins and Saacke. A microfluidics device was created that contained longitudinally-oriented microgrooves in the ceilings of larger channels (Fig 3). When bull sperm were added to the devices and a fluid flow was applied through the channels, the sperm showed a strong tendency to enter the microgrooves and continue swimming through them against the direction of the fluid flow. In contrast, the flagellated, sexually-transmitted pathogen, Tritrichomonas foetus, did not enter the microgrooves and were swept away by the flow (Tung et al. 2015). Thus the microgrooves provided pathways for sperm, but not for T. foetus.
Fig 3.

A microfluidic device designed to model fluid flows and microgrooves within the cervix. a. Diagrams of bull sperm and Tritrichomonas foetus. b. Illustration of a bovine female reproductive tract (from Roberts et al. 1986). The pink arrow points in the direction of fluid flow through the cervix. c. Microgrooves are seen in PAS/hematoxylin-stained frozen sections of the bovine cervix (detailed methods in Suarez et al. 1997). d. Diagram of the microfluidic device that re-creates the microgrooves and fluid flows of the bovine cervix. The sperm seeding port is on the left side and the flow inlet on the right; they are connected by channels with and without microgrooves. e. Details of the channel design in the middle of the device. There are six channels for parallel experimentation: G denotes a channel with microgrooves in the upper surface and F denotes a control channel lacking microgrooves. f. A 3D drawing illustrates the details of a grooved channel. Here the main channel is 120 [μ]m in height and the microgrooves have a sectional area of 20 [μ]m × 20 [μ]m… Drawing not to scale.
Viscoelasticity
Sperm encounter viscous fluids in the female tract, some of which contain significant elastic properties. These include estrous cervical mucus (Tung et al. 2015), oviduct fluid in some species (Jansen 1978; Jansen and Bajpai 1982; Suarez et al. 1997), and the matrix of the cumulus oophorus (Dunn and Picologlou 1976), which is considered to be a viscoelastic network immersed in a viscous fluid (Wrobel et al. 2014). Viscoelastic fluids can reduce the swimming velocity of sperm (Tung et al. 2015), but can also modify the bending pattern and subsequent swimming trajectories of sperm. For example, artificial viscoelastic fluids can straighten the trajectories of hyperactivated hamster and mouse sperm (Suarez et al. 1991; Suarez and Dai 1992). Although it has not yet been tested experimentally, the viscoelasticity of the cumulus matrix could assist hyperactivated sperm in penetrating the cumulus to reach the zona pellucida. The zona pellucida itself has also been characterized as viscoelastic (Kim and Kim 2013; Wrobel et al. 2014).
Molecular Interactions
In this review, I will highlight some well-established examples of molecular interactions of mammalian sperm with cells of the female reproductive tract that are mediated by cell-surface molecules. We may have only discovered the tip of the iceberg in this regard, for sperm pass through various compartments in the female tract, and interactions can serve a number of functions.
The primary sites of known, direct molecular interactions of sperm with the tract that bear most directly on fertilization are interactions with the epithelia lining the uterotubal junction and the oviduct.
Sperm interaction with epithelium lining the uterotubal junction
The anatomy of the uterotubal junction varies considerably among mammalian species. Nevertheless, in most species, the passageway for sperm is narrow and the mucosa that forms the inner surface is thrown into folds that create a complex, branched passageway (Hafez and Black 1969; Wrobel et al. 1993; Suarez 2015). The passageway is known to be quite narrow in some species, such as the cow Bos taurus (Suarez et al. 1997). In the mouse, the passageway is also known to be more widely open during the time of mating, although still not much wider than sperm; however, it is tightly closed at some time after mating (Zamboni 1972; Suarez 1987).
Within one minute of mating, rabbit sperm have been recovered from the oviduct, proximal to the ovary (Overstreet and Cooper 1978). For these sperm, transport through the uterotubal junction is too swift to involve sperm swimming and interactions with the epithelium lining the junction. When these rapidly transported sperm were collected and evaluated, most were found to be damaged and immotile—presumably dead or dying (Overstreet and Cooper, 1978). It has been proposed that these sperm are byproducts of contractions of the tract that were meant to draw sperm up into the uterus (Overstreet and Cooper 1978; Hawk 1983). In the rabbit, the oviduct appears to be cleared of these sperm before ovulation and therefore they are unlikely to participate in fertilization. Instead, sperm that move more gradually into the oviduct, mostly by self propulsion, and then interact with its epithelium are more likely to fertilize (Overstreet et al. 1978).
Evidence gathered from a number of species indicates that most sperm swim through the uterotubal junction rather than being carried through by contractions or currents (Baker and Degen 1972; Overstreet et al. 1978; Gaddum-Rosse 1982; Smith et al. 1987). Nevertheless, recent investigations of mice in which one of several genes were disrupted using classic knockout techniques (homologous recombination using embryonic stem cells) indicate that motility alone does not enable sperm to pass through the uterotubal junction, at least in mice (reviewed by Okabe 2015). Male mice with any one of the disrupted genes produced sperm that appeared to be normal morphologically and showed normal motility, but were unable to pass through the uterotubal junction into the oviduct.
Most of the genes that are required for mouse sperm to pass through the uterotubal junction encode proteins that interact with ADAM3 (a disintegrin and metalloprotease 3) to ensure that it is correctly placed in the sperm plasma membrane. Sperm lacking a normal distribution of ADAM3 in the plasma membrane cannot pass through the junction (Yamaguchi et al. 2009). However, in Ly6k knockout mice, ADAM3 is normally distributed on the plasma membrane, but sperm are unable to pass through the junction (Fujihara et al. 2014). Yet, the normal Ly6k gene product is not present in mature sperm; therefore, the molecule ultimately required for passage of sperm through the junction remains a mystery at this time (Okabe 2015). Still another mystery arises from the observation that the sperm from none of the knockout male mice are capable of binding to the oocyte zona pellucida to fertilize oocytes in vitro, in the absence of cumulus. These sperm can fertilize when cumulus is present and can also fertilize if they are injected directly into oviducts (Okabe 2015).
Altogether these studies of knockout lines of mice indicate that a protein on the sperm plasma membrane somehow interacts with the lining of the uterotubal junction. There is strong evidence that each sperm must interact directly with the junction: genetically chimeric male mice were engineered to produce a mixture of fluorescently labeled knockout sperm that could not pass through the junction and wild type sperm that could. When these chimeras were mated to wild-type females, only the wild type sperm passed through the uterotubal junction (Nakanishi et al. 2004), indicating that the wild type sperm did not stimulate the junction to open and let through the knockout sperm. The actual mechanism of how the uterotubal junction responds to a cell surface protein by enabling sperm to pass into the oviduct remains unknown.
Sperm interactions with epithelium in the oviductal reservoir
Many of the sperm that pass into the oviduct soon bind to the oviductal epithelium (Fig 4). The buildup of bound sperm has been found to create a storage reservoir in a number of species (Hunter and Nichol 1983, Hunter and Wilmut 1984, Suarez 1987). As the time of ovulation draws near, there is some evidence that sperm begin to detach from the epithelium, and may then reattach and detach several times before they move out of the storage region (Chang and Suarez 2012).
Fig 4.

Scanning electron micrograph of bull sperm bound to cilia on the epithelium of the oviductal isthmus. The sperm are located in grooves between secondary folds of oviductal mucosa (bar = 10 μm; from Lefebvre et al. 1995).
The fertilizing capacity of sperm may be maintained by their interaction with oviductal epithelium. For example, bull sperm fertility and motility are maintained longer in vitro by incubation with oviductal epithelium (Pollard et al. 1991; Chian and Sirard 1995). Holding sperm in the lower oviduct may also serve to prevent polyspermic fertilization by allowing only a few sperm at a time to reach oocytes in the ampulla. Sperm numbers were experimentally increased at the site of fertilization in the pig by surgically inseminating sperm directly into the oviduct (Polge et al. 1970; Hunter 1973), by surgically removing the region of the reservoir and reconnecting the remaining tube (Hunter and Leglise 1971), or by injecting progesterone into the oviduct wall to inhibit smooth muscle constriction of the tube (Day and Polge 1968; Hunter 1972). Each of these treatments raised the incidence of polyspermic fertilization. Such a thorough set of investigations into increasing sperm numbers in the upper oviduct at fertilization has not yet been undertaken in any other species, however.
There is evidence that carbohydrate moieties on the surface of oviductal epithelium play a role in the interactions with sperm. Fetuin and its terminal sugar, sialic acid, competitively inhibit binding of hamster sperm, whereas desialylated fetuin does not; furthermore, colloidal gold-tagged fetuin labels the region of sperm heads that bind to epithelium (DeMott et al. 1995). Other carbohydrates have been identified as inhibitors of binding of other species of sperm to conspecific oviductal epithelium. Stallion sperm binding to explants of oviductal epithelium was inhibited by asialofetuin and its terminal sugar, galactose (Dobrinski et al. 1996). Bull sperm binding was blocked by fucose and reduced by pretreating epithelium with fucosidase, but not galactosidase (Lefebvre et al. 1997). Fucose in an alpha 1-4 linkage to N-acetylglucosamine, found in the Lewisa trisaccharide, inhibited bull sperm binding more efficiently than the monosaccharide fucose and than fucose in linkages to other sugars. Furthermore, Lewisa tagged by conjugation to fluorescein-labeled polyacrylamide bound to the heads of live bull sperm (Suarez et al. 1998). Similarly, boar sperm bind to Lewisx trisaccharide, which contains fucose in an alpha 1-3 linkage to N-acetylglucosamine and has been localized to the surfaces of epithelium in the porcine oviductal isthmus (Machado et al. 2014). Altogether, these characterizations of oviductal receptors for sperm indicate that carbohydrates are part of the sperm binding site, although the particular carbohydrates vary among species.
Little is known about the receptor molecules that contain these carbohydrates. Annexin proteins A1, A2, A4, and A5 have been tentatively identified as oviductal receptors for bull sperm, because they have been shown to interact with sperm surface proteins and to be immunolocalized to the surface of oviductal epithelium, and because antibodies to annexins reduced sperm binding in vitro (Ignotz et al. 2007).
Lewisa, the key part of the carbohydrate moiety of the bovine sperm receptor, was used to affinity purify the ligand on sperm. A 16.5 kDa sperm protein was identified by amino acid sequencing as Binder of SPerm 1(BSP1, formerly known as PDC-109; Ignotz et al. 2001). BSP1 is synthesized and secreted by bovine seminal vesicles (Manjunath et al. 1987). It is adsorbed onto sperm when sperm from the epididymis come into contact with seminal plasma secretions during or shortly after ejaculation (Ignotz et al. 2001). BSP1 purified from seminal plasma competetively inhibited bull sperm binding to oviductal epithelium. Bovine epididymal sperm lack BSP1 on their membranes and, indeed, show only minimal binding to oviductal epithelium in vitro. However, when epididymal sperm were incubated with purified BSP1, then washed to remove unadsorbed protein, and then added to explants of oviductal epithelium, incidence of sperm binding was raised to the level of ejaculated sperm (Gwathmey et al. 2003).
BSP1 is the most abundant protein in bovine seminal plasma, present at concentrations of roughly 15-50 mg/ml (Nauc and Manjunath, 2000). It is produced primarily by the seminal vesicles (Salois et al. 1999). BSP1 binds to choline phospholipids via short hydrophobic sequences (Ramakrishnan et al. 2001). This is thought to be the mechanism by which it adsorbs onto epididymal sperm when they are exposed to secretions of the seminal vesicles during and after ejaculation (Desnoyers and Manjunath, 1992; Muller et al. 1998).
Two other BSP proteins, BSP3 and BSP5, were each found to enhance sperm binding to oviductal epithelium (Gwathmey et al. 2006). BSP1, BSP3, and BSP5 all consist of a unique N terminal domain followed by two FN2 domains with heparin and phospholipid binding sites (Fan et al. 2006). All three BSP proteins are produced by the seminal vesicles, although the concentrations of BSP3 and BSP5, at 2-6 mg/ml, are only about one-tenth that of BSP1 (Nauc et al. 2000). Because each BSP alone has been shown to enhance sperm binding to oviductal epithelium, it is not required that they form heteromeric complexes in order to do so. Furthermore, since each BSP can act alone, each may play a different, if overlapping, role in mediating sperm interactions with oviductal epithelium.
Homologs of the bovine BSP proteins have been identified in a various mammalian species, including mice and humans. The murine and human homologs are expressed in the epididymis rather than in the seminal vesicles and would therefore coat testicular sperm as they pass through the epididymis (Fan et al. 2006).
Another small protein, beta-defensin (DEFB126), enhances the binding of cynomolgus macaque sperm to oviductal epithelium. DEFB126 is synthesized and secreted in the epididymis and coats the entire surface of sperm (Yudin et al. 2003). Sperm retain the coating after passing through the cervix and entering the uterus. In contrast to bull sperm binding, macaque sperm bind to secretory cells, rather than ciliated cells, of conspecific oviductal epithelium (Tollner et al. 2008).
As mentioned above, sperm binding to oviductal epithelium involves more than a simple holding mechanism to keep them in the reservoir. Sperm incubated with oviductal epithelium in vitro remain viable longer than when they are incubated in sperm medium alone (Ellington et al. 1993; Kawakami et al., 2001) or with tracheal epithelium, which contains ciliated cells like those in oviductal epithelium (Pollard et al. 1991). Viability can even be extended by incubating sperm with vesicles prepared from the apical membranes of oviductal epithelium (rabbit: Smith et al. 1997; horse: Dobrinski et al. 1997; human: Murray and Smith et al. 1997), indicating that the binding interaction directly affects sperm, rather than supporting sperm by inducing oviductal secretions. Equine sperm bound to oviductal membrane vesicles maintained low levels of cytoplasmic Ca2+ when compared with free-swimming sperm, sperm attached to Matrigel, or sperm incubated with vesicles made from kidney membranes (Dobrinski et al. 1997). A rise in cytoplasmic Ca2+ is associated with sperm capacitation; therefore, the protective effect of binding to oviductal membranes may occur through inhibition of capacitation. In fact, equine and human sperm incubated with oviduct membrane vesicles capacitated more slowly than sperm incubated in capacitating medium alone (Dobrinski et al. 1997; Murray and Smith 1997). The mechanism for inhibiting capacitation or for preventing increases of cytoplasmic Ca2+ in sperm are not known, but there is evidence that catalase, which is present in the oviduct, serves to protect against peroxidative damage to the sperm membranes, which could increase the permeability of membranes to Ca2+(LaPointe et al. 1998).
There is evidence that BSP1, the oviductal binding protein on bull sperm, prolongs sperm viability by acting to stabilize plasma membranes. It was demonstrated that adding BSP1 to phospholipid membranes reduces membrane fluidity and immobilizes cholesterol in artificial membranes and in membranes of epididymal sperm (Greube et al. 2001; Muller et al. 2002). BSP1 can also stabilize plasma membranes by inhibiting the activity of phospholipase A2 (Soubeyrand and Manjunath 1997). Thus, BSP1 could serve to stabilize sperm when bound to oviductal receptors.
Theoretically, sperm could break free from oviductal epithelium either by loss or alteration of receptors on the epithelium or by loss or alteration of ligands on sperm. Changes in the hormonal state of oviductal epithelium after ovulation were not found to reduce the density numbers of sperm that bound to epithelium (Lefebvre et al. 1995); therefore, it appears that epithelium does not release sperm by reducing available binding sites, at least in cattle. Instead, current evidence indicates that a change in sperm enables them to detach from epithelium.
Detachment of sperm from oviductal epithelium
Two changes that occur in sperm during the process of capacitation may play a role detachment: (1) modification of cell surface proteins and (2) hyperactivation of motility. Modification of sperm surface proteins could reduce binding affinity for oviductal receptors. Hyperactivation could provide the force necessary for sperm to pull away from the oviductal epithelium. For example, capacitated hamster sperm did not bind to epithelium when infused into hamster oviducts, while uncapacitated sperm did bind (Smith and Yanagimachi, 1991). Bull sperm bound to epithelium in lower numbers after they had been incubated under capacitating conditions until a significant percentage acrosome reacted in response to lysophosphatidyl choline, even though they were not hyperactivated (Lefebvre and Suarez,1996). In this case, the failure to bind could be attributed to decreased binding affinity of sperm for epithelium rather than to hyperactivation. In contrast, when mouse sperm in oviducts were observed by transillumination, only hyperactivated mouse sperm were seen to detach from epithelium (DeMott and Suarez, 1992). The hyperactivated sperm appeared to use a rocking motion to tear themselves off of the cilia on the epithelial surface (Chang and Suarez 2012). In conclusion, it is possible that hyperactivation plays a greater role in sperm detachment in some species, while decreased molecular binding affinity plays a geater role in other species.
While evidence is lacking for a release mechanism involving reduction in binding sites on the epithelium, the epithelium could play a role in sperm release by secreting factors that affect sperm. For example, hormonal signals that induce ovulation or signals from the preovulatory follicle could stimulate the oviductal epithelium to secrete factors that hasten sperm capacitation and trigger hyperactivation, thereby bringing about sperm release. Evidence for this possibility includes the observation that soluble oviductal factors enhance capacitation of bull sperm (Chian et al. 1995). Also, incubating macaque sperm in medium containing the levels of bicarbonate and glucose found in periovulatory oviduct fluid capacitates the sperm and releases oviduct-binding DEFB126 from the sperm surface (Tollner et al. 2009).
As sperm undergo capacitation, the carbohydrate portions of molecules responsible for binding sperm to epithelium may be lost from the sperm surface or modified. Capacitated hamster sperm were no longer labeled over the acrosomal region by fetuin, indicating that they had lost the ability to bind to oviductal epithelium via sialic acid. Furthermore, fewer proteins extracted from capacitated sperm were labeled by fetuin or sialic acid LFA lectin on western blots (DeMott et al. 1995).
Capacitated bull sperm showed reduced binding to oviductal epithelium (Lefebvre and Suarez, 1996), as well as to fucose (Revah et al. 2000; Ignotz et al. 2001). This might be attributable to loss or modification of the BSP proteins on the sperm surface. The address this possibility, fresh bull sperm were incubated under various capacitating conditions, and then the amounts of BSP proteins remaining on the sperm were measured by western blot of sperm extracts. Most BSP5 was lost from sperm even after incubation under minimally capacitating conditions. No loss of BSP1 was detected. Surprisingly, anti-BSP3 antibodies detected a new protein band of reduced molecular mass on the blots. Its identity was confirmed as BSP3 by mass spectrometry and the reduced size was attributed to proteolytic cleavage at the N terminus. BSP3 is not glycosylated; therefore, the reduction in size could not be due to deglycosylation (Hung and Suarez 2012). The proteolytic cleavage could be accounted for by metalloproteases or serine proteases that have been identified on the surface of sperm (Gottlieb and Meizel 1987; Hagaman et al. 1998; Honda et al. 2002). Altogether, the differential response of the BSP proteins to capacitation indicate that a complex process is involved in the detachment of sperm from oviductal epithelium.
Sperm interactions with oviductal epithelium in the upper oviduct
Years ago, we reported that bull sperm bound equally well to the epithelium of the isthmus and the ampulla (Lefebvre et al. 1995). At the time, we speculated that the reservoir was confined to the isthmus because it was the first region encountered by sperm. More recently, we were able to take a closer look at mouse sperm in the oviduct, because Acr-EGFP mutant mice that produce sperm with fluorescent acrosomes (Nakanishi et al. 1999) facilitated the study of sperm movement into the ampulla of the oviduct. Periovulatory wild type female mice were mated with males who produced the fluorescent sperm and sperm in the oviducts were examined three hours later (Chang and Suarez 2012). Unexpectedly, most of the few sperm found in the ampulla were attached to epithelium and often remained attached for several minutes. These observations indicate that sperm may continue to bind to oviductal epithelium as they move up the oviduct. This would not only explain why bull sperm could bind to ampullar epithelium but also why annexin proteins, the putative oviductal sperm receptors, could be detected in the ampulla and well as in the isthmus (Ignotz et al. 2007).
Conclusions
The physical and molecular interactions discussed in this review reveal only a superficial understanding of the interactions of sperm with the female tract. Questions that remain include (1) how sperm interactions with the uterotubal junction allow them to pass into the oviduct, (2) exactly how sperm are released from oviductal epithelium, (3) how binding and release of sperm facilitate movement to the oocyte, and (4) how sperm interact with immune cells that enter the lumen of the tract. It is also very likely that sperm interact with the female reproductive tract in other ways that have yet to be discovered.
Fig 5.
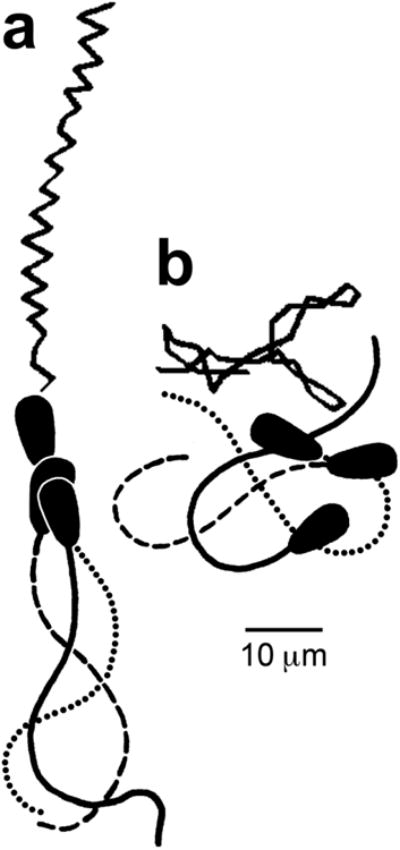
Tracings of video images and swimming tracks of activated (a) and hyperactivated (b) bull sperm. The tracings of sperm (below) represent three successive video images taken at 30 images/sec. The swimming tracks (above) are shown as lines connecting the positions of the head/tail junctions on successive video frames (30/sec) over a 1 sec period. (modified from Ho et al. 2002).
Acknowledgments
Work completed by the author in the last five years has been supported by NIH grants 1 R01 HD070038 and 1 R03 HD062471, as well as USDA-NRI grant 2008-35203-19031.
References
- Baker RD, Degen AA. Transport of live and dead boar spermatozoa within the reproductive tract of gilts. J Reprod Fertil. 1972;28(3):369–377. doi: 10.1530/jrf.0.0280369. [DOI] [PubMed] [Google Scholar]
- Chang H, Suarez SS. Unexpected flagellar movement patterns and epithelial binding behavior of mouse sperm in the oviduct. Biol Reprod. 2012;86(5):140, 1–8. doi: 10.1095/biolreprod.111.096578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chian RC, Sirard MA. Fertilizing ability of bovine spermatozoa cocultured with oviduct epithelial cells. Biol Reprod. 1995;52(1):156–162. doi: 10.1095/biolreprod52.1.156. [DOI] [PubMed] [Google Scholar]
- Day BN, Polge C. Effects of progesterone on fertilization and egg transport in the pig. J Reprod Fertil. 1968;17(1):227–230. doi: 10.1530/jrf.0.0170227. [DOI] [PubMed] [Google Scholar]
- DeMott RP, Suarez SS. Hyperactivated sperm progress in the mouse oviduct. Biol Reprod. 1992;46(5):779–785. doi: 10.1095/biolreprod46.5.779. [DOI] [PubMed] [Google Scholar]
- DeMott RP, Lefebvre R, Suarez SS. Carbohydrates mediate the adherence of hamster sperm to oviductal epithelium. Biol Reprod. 1995;52(6):1395–1403. doi: 10.1095/biolreprod52.6.1395. [DOI] [PubMed] [Google Scholar]
- Denissenko P, Kantsler V, Smith DJ, Kirkman-Brown J. Human spermatozoa migration in microchannels reveals boundary-following navigation. Proc Natl Acad Sci USA. 2012;109(21):8007–8010. doi: 10.1073/pnas.1202934109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnoyers L, Manjunath P. Major proteins of bovine seminal plasma exhibit novel interactions with phospholipid. J Biol Chem. 1992;267(14):10149–10155. [PubMed] [Google Scholar]
- Dobrinski I, Ignotz GG, Thomas PG, Ball BA. Role of carbohydrates in the attachment of equine spermatozoa to uterine tubal (oviductal) epithelial cells in vitro. Am J Vet Res. 1996;57(11):1635–1639. [PubMed] [Google Scholar]
- Dobrinski I, Smith TT, Suarez SS, Ball BA. Membrane contact with oviductal epithelium modulates the intracellular calcium concentration of equine spermatozoa in vitro. Biol Reprod. 1997;56(4):861–869. doi: 10.1095/biolreprod56.4.861. [DOI] [PubMed] [Google Scholar]
- Dunn PF, Picologlou BF. Viscoelastic properties of cumulus oöphorus. Biorheology. 1976;13(6):379–384. doi: 10.3233/bir-1976-13605. [DOI] [PubMed] [Google Scholar]
- Ellington JE, Ignotz GG, Varner DD, Marcucio RS, Mathison P, Ball BA. In vitro interaction between oviduct epithelial and equine sperm. Arch Androl. 1993;31(2):79–86. doi: 10.3109/01485019308988384. [DOI] [PubMed] [Google Scholar]
- Fan J, Lefebvre J, Manjunath P. Bovine seminal plasma proteins and their relatives: A new expanding superfamily in mammals. Gene. 2006;375:63–74. doi: 10.1016/j.gene.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Fujihara Y, Okabe M, Ikawa M. GPI-anchored protein complex, LY6K/TEX101, is required for sperm migration into the oviduct and male fertility in mice. Biol Reprod. 2014;90:60. doi: 10.1095/biolreprod.113.112888. [DOI] [PubMed] [Google Scholar]
- Gaddum-Rosse P, Blandau RJ. Comparative observations on ciliary currents in mammalian oviducts. Biol Reprod. 1976;14(5):605–609. doi: 10.1095/biolreprod14.5.605. [DOI] [PubMed] [Google Scholar]
- Gaddum-Rosse P. Some observations on sperm transport through the uterotubal junction of the rat. Am J Anat. 1981;160(3):333–341. doi: 10.1002/aja.1001600309. [DOI] [PubMed] [Google Scholar]
- Ghersevich S, Massa E, Zumoffen C. Oviductal secretion and gamete interaction. Reproduction. 2015;149(1):R1–R14. doi: 10.1530/REP-14-0145. [DOI] [PubMed] [Google Scholar]
- Gottlieb W, Meizel S. Biochemical studies of metalloendoprotease activity in the spermatozoa of three mammalian species. J Androl. 1987;8(1):14–24. doi: 10.1002/j.1939-4640.1987.tb02411.x. [DOI] [PubMed] [Google Scholar]
- Greube A, Muller K, Topfer-Petersen E, Herrmann A, Muller P. Influence of the bovine seminal plasma protein PDC-109 on the physical state of membranes. Biochemistry. 2001;40(28):8326–8334. doi: 10.1021/bi010552+. [DOI] [PubMed] [Google Scholar]
- Gwathmey TM, Ignotz GG, Suarez SS. PDC-109 (BSP-A1/A2) promotes bull sperm binding to oviductal epithelium in vitro and may be involved in forming the oviductal sperm reservoir. Biol Reprod. 2003;69(3):809–815. doi: 10.1095/biolreprod.102.010827. [DOI] [PubMed] [Google Scholar]
- Gwathmey TM, Ignotz GG, Mueller JL, Manjunath P, Suarez SS. Bovine seminal plasma proteins PDC-109, BSP-A3, and BSP-30-kDa share functional roles in storing sperm in the oviduct. Biol Reprod. 2006;75(4):501–507. doi: 10.1095/biolreprod.106.053306. [DOI] [PubMed] [Google Scholar]
- Hafez ESE, Black DL. Washington State University. The mammalian uterotubal junction. In: Hafez ESE, Blandau RJ, editors. The Mammalian oviduct; comparative biology and methodology. University of Chicago Press; Chicago: 1969. pp. 85–128. [Google Scholar]
- Hagaman JR, Moyer JS, Bachman ES, et al. Angiotensin-converting enzyme and male fertility. Proc Natl Acad Sci USA. 1998;95(5):2552–2557. doi: 10.1073/pnas.95.5.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk HW. Sperm survival and transport in the female reproductive tract. J Dairy Sci. 1983;66(12):2645–2660. doi: 10.3168/jds.S0022-0302(83)82138-9. [DOI] [PubMed] [Google Scholar]
- Ho HC, Suarez SS. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev Biol. 2002;250(1):208–217. doi: 10.1006/dbio.2002.0797. [DOI] [PubMed] [Google Scholar]
- Honda A, Yamagata K, Sugiura S, Watanabe K, Baba T. A mouse serine protease TESP5 is selectively included into lipid rafts of sperm membrane presumably as a glycosylphosphatidylinositol-anchored protein. J Biol Chem. 2002;277(19):16976–16984. doi: 10.1074/jbc.M112470200. [DOI] [PubMed] [Google Scholar]
- Hung PH, Suarez SS. Alterations to the bull sperm surface proteins that bind sperm to oviductal epithelium. Biol Reprod. 2012;87(4):88. doi: 10.1095/biolreprod.112.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RH, Leglise PC. Polyspermic fertilization following tubal surgery in pigs, with particular reference to the role of the isthmus. J Reprod Fertil. 1971;24(2):233–246. doi: 10.1530/jrf.0.0240233. [DOI] [PubMed] [Google Scholar]
- Hunter RH. Local action of progesterone leading to polyspermic fertilization in pigs. J Reprod Fertil. 1972;31(3):433–444. doi: 10.1530/jrf.0.0310433. [DOI] [PubMed] [Google Scholar]
- Hunter RH. Polyspermic fertilization in pigs after tubal deposition of excessive numbers of spermatozoa. J Exp Zool. 1973;183(1):57–63. doi: 10.1002/jez.1401830107. [DOI] [PubMed] [Google Scholar]
- Hunter RH, Nichol R. Transport of spermatozoa in the sheep oviduct: preovulatory sequestering of cells in the caudal isthmus. J Exp Zool. 1983;228(1):121–128. doi: 10.1002/jez.1402280113. [DOI] [PubMed] [Google Scholar]
- Hunter RH, Wilmut I. Sperm transport in the cow: peri-ovulatory redistribution of viable cells within the oviduct. Reprod Nutr Dev. 1984;24(5A):597–608. doi: 10.1051/rnd:19840508. [DOI] [PubMed] [Google Scholar]
- Ignotz GG, Lo MC, Perez CL, Gwathmey TM, Suarez SS. Characterization of a fucose-binding protein from bull sperm and seminal plasma that may be responsible for formation of the oviductal sperm reservoir. Biol Reprod. 2001;64(6):1806–1811. doi: 10.1095/biolreprod64.6.1806. [DOI] [PubMed] [Google Scholar]
- Ignotz GG, Cho MY, Suarez SS. Annexins are candidate oviductal receptors for bovine sperm surface proteins and thus may serve to hold bovine sperm in the oviductal reservoir. Biol Reprod. 2007;77(6):906–913. doi: 10.1095/biolreprod.107.062505. [DOI] [PubMed] [Google Scholar]
- Jansen RP. Fallopian tube isthmic mucus and ovum transport. Science. 1978;201(4353):349–351. doi: 10.1126/science.580814. [DOI] [PubMed] [Google Scholar]
- Jansen RP, Bajpai VK. Oviduct acid mucus glycoproteins in the estrous rabbit: ultrastructure and histochemistry. Biol Reprod. 1982;26(1):155–168. doi: 10.1095/biolreprod26.1.155. [DOI] [PubMed] [Google Scholar]
- Kantsler V, Dunkel J, Blayney M, Goldstein RE. Rheotaxis facilitates upstream navigation of mammalian sperm cells. Elife. 2014 May 27;3:e02403. doi: 10.7554/eLife.02403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami E, Kashiwagi C, Hori T, Tsutsui T. Effects of canine oviduct epithelial cells on movement and capacitation of homologous spermatozoa in vitro. Anim Reprod Sci. 2001;68(1-2):121–131. doi: 10.1016/s0378-4320(01)00135-x. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim J. Viscoelastic characterization of mouse zona pellucida. IEEE Trans Biomed Eng. 2013;60(2):569–575. doi: 10.1109/TBME.2012.2230444. [DOI] [PubMed] [Google Scholar]
- Lapointe S, Sullivan R, Sirard MA. Binding of a bovine oviductal fluid catalase to mammalian spermatozoa. Biol Reprod. 1998;58(3):747–753. doi: 10.1095/biolreprod58.3.747. [DOI] [PubMed] [Google Scholar]
- Lefebvre R, Chenoweth PJ, Drost M, LeClear CT, MacCubbin M, Dutton JT, Suarez SS. Characterization of the oviductal sperm reservoir in cattle. Biol Reprod. 1995;53(5):1066–1074. doi: 10.1095/biolreprod53.5.1066. [DOI] [PubMed] [Google Scholar]
- Lefebvre R, Suarez SS. Effect of capacitation on bull sperm binding to homologous oviductal epithelium. Biol Reprod. 1996;54(3):575–582. doi: 10.1095/biolreprod54.3.575. [DOI] [PubMed] [Google Scholar]
- Lefebvre R, Lo MC, Suarez SS. Bovine sperm binding to oviductal epithelium involves fucose recognition. Biol Reprod. 1997;56(5):1198–1204. doi: 10.1095/biolreprod56.5.1198. [DOI] [PubMed] [Google Scholar]
- Machado SA, Kadirvel G, Daigneault BW, Korneli C, Miller P, Bovin N, Miller DJ. LewisX-containing glycans on the porcine oviductal epithelium contribute to formation of the sperm reservoir. Biol Reprod. 2014;91(6):140. doi: 10.1095/biolreprod.114.119503. [DOI] [PubMed] [Google Scholar]
- Manjunath P, Sairam MR, Uma J. Purification of four gelatin-binding proteins from bovine seminal plasma by affinity chromatography. Biosci Rep. 1987;7(3):231–238. doi: 10.1007/BF01124794. [DOI] [PubMed] [Google Scholar]
- Martyn F, McAuliffe FM, Wingfield M. The role of the cervix in fertility: is it time for a reappraisal? Hum Reprod. 2014;29(10):2092–2098. doi: 10.1093/humrep/deu195. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Suarez SS, Wolfner MF. On a matter of seminal importance. Bioessays. 2015;37(2):142–147. doi: 10.1002/bies.201400117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K, Clapham DE. Rheotaxis guides mammalian sperm. Curr Biol. 2013;23(6):443–452. doi: 10.1016/j.cub.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Erlemann KR, Muller K, Calvete JJ, Topfer-Petersen E, Marienfeld K, Herrmann A. Biophysical characterization of the interaction of bovine seminal plasma protein PDC-109 with phospholipid vesicles. Eur Biophys J. 1998;27(1):33–41. doi: 10.1007/s002490050108. [DOI] [PubMed] [Google Scholar]
- Muller P, Greube A, Topfer-Petersen E, Herrmann A. Influence of the bovine seminal plasma protein PDC-109 on cholesterol in the presence of phospholipids. Eur Biophys J. 2002;31(6):438–447. doi: 10.1007/s00249-002-0234-2. [DOI] [PubMed] [Google Scholar]
- Murray SC, Smith TT. Sperm interaction with fallopian tube apical membrane enhances sperm motility and delays capacitation. Fertil Steril. 1997;68(2):351–357. doi: 10.1016/s0015-0282(97)81528-2. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Ikawa M, Yamada S, Parvinen M, Baba T, Nishimune Y, Okabe M. Realtime observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett. 1999;449(2-3):277–283. doi: 10.1016/s0014-5793(99)00433-0. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Isotani A, Yamaguchi R, Ikawa M, Baba T, Suarez SS, Okabe M. Selective passage through the uterotubal junction of sperm from a mixed population produced by chimeras of calmegin-knockout and wild-type male mice. Biol Reprod. 2004;71(3):959–965. doi: 10.1095/biolreprod.104.028647. [DOI] [PubMed] [Google Scholar]
- Nauc V, Manjunath P. Radioimmunoassays for bull seminal plasma proteins (BSP-A1/-A2, BSP-A3, and BSP-30-Kilodaltons), and their quantification in seminal plasma and sperm. Biol Reprod. 2000;63(4):1058–1066. doi: 10.1095/biolreprod63.4.1058. [DOI] [PubMed] [Google Scholar]
- Nixon B, Ecroyd HW, Dacheux JL, Jones RC. Monotremes provide a key to understanding the evolutionary significance of epididymal sperm maturation. J Androl. 2011;32(6):665–671. doi: 10.2164/jandrol.110.012716. [DOI] [PubMed] [Google Scholar]
- Okabe M. Mechanisms of fertilization elucidated by gene-manipulated animals. Asian J Androl. 2015 Apr 7; doi: 10.4103/1008-682X.153299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet JW, Cooper GW. Sperm transport in the reproductive tract of the female rabbit: II. The sustained phase of transport. Biol Reprod. 1978;19(1):115–132. doi: 10.1095/biolreprod19.1.115. [DOI] [PubMed] [Google Scholar]
- Polge C, Salamon S, Wilmut I. Fertilizing capacity of frozen boar semen following surgical insemination. Vet Rec. 1970;87(15):424–429. doi: 10.1136/vr.87.15.424. [DOI] [PubMed] [Google Scholar]
- Pollard JW, Plante C, King WA, Hansen PJ, Betteridge KJ, Suarez SS. Fertilizing capacity of bovine sperm may be maintained by binding of oviductal epithelial cells. Biol Reprod. 1991;44(1):102–107. doi: 10.1095/biolreprod44.1.102. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan M, Anbazhagan V, Pratap TV, Marsh D, Swamy MJ. Membrane insertion and lipid-protein interactions of bovine seminal plasma protein PDC-109 investigated by spin-label electron spin resonance spectroscopy. Biophys J. 2001;81(4):2215–2225. doi: 10.1016/S0006-3495(01)75869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revah I, Gadella BM, Flesch FM, Colenbrander B, Suarez SS. Physiological state of bull sperm affects fucose- and mannose-binding properties. Biol Reprod. 2000;62(4):1010–1015. doi: 10.1095/biolreprod62.4.1010. [DOI] [PubMed] [Google Scholar]
- Roberts SJ, Lein DH, Foote RH, Drost M. Veterinary obstetrics and genital diseases (Theriogenology) 3rd. published by the authors, Woodstock, Vt.; Distributed by David and Charles Inc.; North Pomfret, Vt: 1986. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Martínez H, Kvist U, Ernerudh J, Sanz L, Calvete JJ. Seminal plasma proteins: what role do they play? Am J Reprod Immunol. 2011;66(Suppl 1):11–22. doi: 10.1111/j.1600-0897.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- Rothschild Non-random distribution of bull spermatozoa in a drop of spermsuspension. Nature. 1963;198(488):1221. doi: 10.1038/200381a0. [DOI] [PubMed] [Google Scholar]
- Salois D, Ménard M, Paquette Y, Manjunath P. Complementary deoxyribonucleic acid cloning and tissue expression of BSP-A3 and BSP-30-kDa: phosphatidylcholine and heparin-binding proteins of bovine seminal plasma. Biol Reprod. 1999;61(1):288–297. doi: 10.1095/biolreprod61.1.288. [DOI] [PubMed] [Google Scholar]
- Smith TT, Koyanagi F, Yanagimachi R. Distribution and number of spermatozoa in the oviduct of the golden hamster after natural mating and artificial insemination. Biol Reprod. 1987;37(1):225–234. doi: 10.1095/biolreprod37.1.225. [DOI] [PubMed] [Google Scholar]
- Smith TT, Yanagimachi R. Attachment and release of spermatozoa from the caudal isthmus of the hamster oviduct. J Reprod Fertil. 1991;91(2):567–573. doi: 10.1530/jrf.0.0910567. [DOI] [PubMed] [Google Scholar]
- Smith TT, Nothnick WB. Role of direct contact between spermatozoa and oviductal epithelial cells in maintaining rabbit sperm viability. Biol Reprod. 1997;56(1):83–89. doi: 10.1095/biolreprod56.1.83. [DOI] [PubMed] [Google Scholar]
- Soubeyrand S, Manjunath P. Novel seminal phospholipase A2 is inhibited by the major proteins of bovine seminal plasma. Biochim Biophys Acta. 1997;1341(2):183–188. doi: 10.1016/s0167-4838(97)00070-8. [DOI] [PubMed] [Google Scholar]
- Suarez SS. Sperm transport and motility in the mouse oviduct: observations in situ. Biol Reprod. 1987;36(1):203–210. doi: 10.1095/biolreprod36.1.203. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Katz DF, Owen DH, Andrew JB, Powell RL. Evidence for the function of hyperactivated motility in sperm. Biol Reprod. 1991;44:375–381. doi: 10.1095/biolreprod44.2.375. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Dai X. Hyperactivation enhances mouse sperm capacity for penetrating viscoelastic media. Biol Reprod. 1992;46(4):686–69. doi: 10.1095/biolreprod46.4.686. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Brockman K, Lefebvre R. Distribution of mucus and sperm in bovine oviducts after artificial insemination: the physical environment of the oviductal sperm reservoir. Biol Reprod. 1997;56(2):447–453. doi: 10.1095/biolreprod56.2.447. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Revah I, Lo M, Kolle S. Bull sperm binding to oviductal epithelium is mediated by a Ca2+-dependent lectin on sperm that recognizes Lewis-a trisaccharide. Biol Reprod. 1998;59(1):39–44. doi: 10.1095/biolreprod59.1.39. [DOI] [PubMed] [Google Scholar]
- Suarez SS. Gamete and zygote transport. In: Plant TM, Zeleznich AJ, editors. Knobil and Neill's Physiology of Reproduction. 4th. Vol. 1. Elsevier; Oxford: 2015. pp. 197–232. [Google Scholar]
- Tollner TL, Yudin AI, Tarantal AF, Treece CA, Overstreet JW, Cherr GN. Beta-defensin 126 on the surface of macaque sperm mediates attachment of sperm to oviductal epithelia. Biol Reprod. 2008;78(3):400–412. doi: 10.1095/biolreprod.107.064071. [DOI] [PubMed] [Google Scholar]
- Tollner TL, Vandevoort CA, Yudin AI, Treece CA, Overstreet JW, Cherr GN. Release of DEFB126 from macaque sperm and completion of capacitation are triggered by conditions that simulate periovulatory oviduct fluid. Mol Reprod Dev. 2009;76(5):431–443. doi: 10.1002/mrd.20964. [DOI] [PubMed] [Google Scholar]
- Tung CK, Ardon F, Fiore AG, Suarez SS, Wu M. Cooperative roles of biological flow and surface topography in guiding sperm migration revealed by a microfluidic model. Lab Chip. 2014;14(7):1348–1356. doi: 10.1039/c3lc51297e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung CK, Hu L, Fiore AG, Ardon F, Hickman DG, Gilbert RO, Suarez SS, Wu M. Microgrooves and fluid flows provide preferential passageways for sperm over pathogen Tritrichomonas foetus. Proc Natl Acad Sci USA. 2015;112(17):5431–5436. doi: 10.1073/pnas.1500541112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winet H, Bernstein GS, Head J. Observations on the response of human spermatozoa to gravity, boundaries and fluid shear. J Reprod Fertil. 1984;70(2):511–523. doi: 10.1530/jrf.0.0700511. [DOI] [PubMed] [Google Scholar]
- Woolley DM. Motility of spermatozoa at surfaces. Reproduction. 2003;126(2):259–270. doi: 10.1530/rep.0.1260259. [DOI] [PubMed] [Google Scholar]
- Wrobel KH, Kujat R, Fehle G. The bovine tubouterine junction: general organization and surface morphology. Cell Tissue Res. 1993;271(2):227–239. doi: 10.1007/BF00318609. [DOI] [PubMed] [Google Scholar]
- Wrobel KH, Cortez R, Fauci L. Modeling viscoelastic networks in Stokes flow. Physics of Fluids. 2014;26:113102. [Google Scholar]
- Yamaguchi R, Muro Y, Isotani A, Tokuhiro K, Takumi K, Adham I, Ikawa M, Okabe M. Disruption of ADAM3 impairs the migration of sperm into oviduct in mouse. Biol Reprod. 2009;81(1):142–146. doi: 10.1095/biolreprod.108.074021. [DOI] [PubMed] [Google Scholar]
- Yaniz JL, Lopez-Gatius F, Santolaria P, Mullins KJ. Study of the functional anatomy of bovine oviductal mucosa. Anat Rec. 2000;260(3):268–278. doi: 10.1002/1097-0185(20001101)260:3<268::AID-AR60>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Yudin AI, Tollner TL, Li MW, Treece CA, Overstreet JW, Cherr GN. ESP13.2, a member of the beta-defensin family, is a macaque sperm surface-coating protein involved in the capacitation process. Biol Reprod. 2003;69(4):1118–1128. doi: 10.1095/biolreprod.103.016105. [DOI] [PubMed] [Google Scholar]
- Zamboni L. Fertilization in the mouse. In: Moghissi KS, Hafez ESE, editors. Biology of mammalian fertilization and implantation. C. C. Thomas; Springfield, Ill: 1972. pp. 213–262. [Google Scholar]


