Abstract
Multiple myeloma is the most common indication for high-dose chemotherapy and autologous stem cell transplantation (ASCT), and lenalidomide maintenance post-transplant is now standard. Although lenalidomide doubles progression-free survival, almost all patients eventually relapse. Post-transplant immunotherapy to improve outcomes after ASCT therefore has great merit but first requires delineation of the dynamics of immune reconstitution. We evaluated lymphocyte composition and function after ASCT to guide optimal timing of immunotherapy and to identify potential markers of relapse. Regulatory T cells (Tregs) decline as CD8+ T cells expand during early lymphocyte recovery after ASCT, markedly reducing the Treg:CD8+ effector T-cell ratio. These CD8+ T cells can respond to autologous dendritic cells presenting tumor antigen in vitro as early as day +12 post-transplant, becoming antigen-specific cytolytic T-lymphocyte effectors and thereby demonstrating preservation of cellular reactivity. CD4+ and CD8+ T cells express the negative regulatory molecules, CTLA-4, PD-1, LAG-3, and TIM-3, before and after ASCT. A subpopulation of exhausted/senescent CD8+ T cells, however, down-regulates CD28 and up-regulates CD57 and PD-1, characterizing immune impairment and relapse after ASCT. Relapsing patients have higher numbers of these cells at +3 months after transplant, but before detection of clinical disease, indicating their applicability in identifying patients at higher risk of relapse. PD-1 blockade also revives the proliferation and cytokine secretion of the hyporesponsive, exhausted/senescent CD8+ T cells in vitro. Collectively, these results identify T cell exhaustion/senescence as a distinguishing feature of relapse and support early introduction of immunotherapy to stimulate antitumor immunity after ASCT.
Keywords: multiple myeloma, autologous stem cell transplantation, T cell exhaustion, relapse, immunotherapy
INTRODUCTION
High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) can produce complete responses (CR) in up to one-third of patients with multiple myeloma (MM) (1). Nevertheless, most patients achieving a CR after ASCT eventually relapse; and in patients who fail to attain a CR, progression of disease is inevitable (2). This has remained true even with lenalidomide maintenance therapy, which is now standard after ASCT and extends progression-free survival from approximately two to four years (3-5).
The immune system participates in the control of MM, whereas compromised immunity contributes to its evolution. Myeloma-reactive T cells are present in active disease (6-9) and correlate with disease burden (9). The expansion of T cell clones after ASCT (10) and the emergence of antigen-specific T cells after allogeneic stem cell transplantation (11, 12) are associated with improved clinical outcomes. The loss of tumor-specific T cells characterizes progression from the benign precursor condition, monoclonal gammopathy of undetermined significance (MGUS), to MM (13, 14). Malignant plasma cells themselves also evade immune surveillance by various mechanisms (15-19), including the upregulation of PD-L1 (17-19).
Incorporating immune-based therapies into post-ASCT treatment regimens to induce or restore antitumor immunity offers a promising approach to target residual MM. The minimal residual disease state and lymphopenia after ASCT afford a unique platform for promoting antitumor immune responses by limiting tumor-driven immunosuppression (20), eliminating cytokine sinks (21), and transiently depleting regulatory T cells (22). Post-transplant reconstitution of immune cell subsets, however, occurs with disparate kinetics that can affect the outcome of immunotherapy. The immunomodulatory effect of lenalidomide maintenance therapy on the dynamics of immune recovery also remains undefined.
The rational development of immunotherapeutic interventions after ASCT, where relapse remains the primary cause of treatment failure, requires a comprehensive understanding of the immunologic milieu. We therefore performed a prospective analysis of immune reconstitution in 55 MM patients undergoing ASCT and lenalidomide maintenance therapy to define patterns of lymphocyte recovery and to identify immunologic markers of relapse, which could elucidate potential targets for converting durable responses to long-term cures.
MATERIALS AND METHODS
Patients
Fifty-five patients were evaluated (Table 1). All patients received pre-ASCT induction regimens that included bortezomib and/or lenalidomide, with 40 (72%) patients receiving both agents. All patients were conditioned for ASCT with high-dose melphalan (140 mg/m2 or 200 mg/m2, depending on age and comorbidity risk) and received post-ASCT lenalidomide maintenance therapy. Disease status was assessed for response to induction therapy within 30 days before ASCT, at 3 and 12 months after ASCT, and at the time of relapse where applicable. Myeloma response was assessed using International Myeloma Working Group (IMWG) criteria (23).
Table 1.
Patient characteristics
| All | Male | Female | ||
|---|---|---|---|---|
| Total patients | 55 | 35 | 20 | |
| Age | 57 (29-72) | 57.5 (29-72) | 56.8 (38-70) | |
| ISS stage | I | 27 | 18 | 9 |
| II | 10 | 6 | 4 | |
| III | 18 | 11 | 7 | |
| Induction therapy | Bortezomib-containing | 9 | 7 | 2 |
| Lenalidomide-containing | 6 | 4 | 2 | |
| Bortezomib & lenalidomide-containing | 40 | 24 | 16 | |
| Response to induction therapy (i.e., disease status immediately pre-ASCT) | CR | 10 | 6 | 4 |
| VGPR | 20 | 14 | 6 | |
| PR | 25 | 15 | 10 | |
| Stem cell dose | 6.24 (1.9-13.58) | 6.47 (1.9-13.49) | 5.84 (2.9-13.58) | |
| Response at 3mo post-ASCT | CR | 29 | 18 | 11 |
| VGPR | 13 | 8 | 5 | |
| PR | 13 | 9 | 4 | |
| Maintenance | Lenalidomide | 55 | 35 | 20 |
| Status at 12mo post-ASCT | CR | 28 | 17 | 11 |
| VGPR | 6 | 4 | 2 | |
| PR | 9 | 6 | 3 | |
| POD/relapse | 12 | 8 | 4 |
ISS, International Staging System; CR, complete response; VGPR, very good partial response; PR, partial response; SD, stable disease; ASCT, autologous stem cell transplantation; POD, progression of disease.
Blood samples
Peripheral blood mononuclear cells (PBMCs) were obtained by centrifugation over Ficoll-Paque PLUS (GE Healthcare) from peripheral blood or leukocyte concentrates from patients and healthy volunteers. Biospecimen sample collection and use adhered to protocols approved by the Institutional Review and Privacy Board of Memorial Hospital, Memorial Sloan Kettering Cancer Center (MSKCC). Leukocyte concentrates (buffy coats) purchased from the Greater New York Blood Center, American Red Cross, were also used as a source of healthy donor cells.
Flow cytometric analysis
PBMCs were incubated with fluorochrome-conjugated mAbs and analyzed on either an FC 500 (Beckman Coulter) or an LSRFortessa (Becton Dickinson) flow cytometer. FITC-, PE-, PE-Texas Red-, ECD-, APC-, PE-Cy5–, PE-Cy7–, PerCP-Cy5.5–, Pacific Blue-, and AF700-conjugated mouse anti-human mAbs included anti-CD3, anti-CD4, anti-CD8, anti-CD11c, anti-CD14, anti-CD16 (clone 3G8), anti-CD19, anti-CD25, ant-CD28, anti-CD45RA, anti-CD45RO, anti-CD80, anti-CD86, anti-CD123, anti-CTLA-4, anti–HLA-DR, anti-IL2, anti-Ki-67 (BD Pharmingen), anti-CD56, anti-CD83 (Beckman Coulter), anti-CD127, anti- IFNγ, anti-LAG-3, anti-PD-1, anti- TNFα (eBioscience), anti-CCR7, anti-TIM-3 (R&D Systems), and anti-CD57 (BioLegend). Nonreactive isotype-matched antibodies (Becton Dickinson, eBioscience, R&D Systems) were used as controls. LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Life Technologies) facilitated exclusion of dead cells. Gates were set for collection and analysis of at least 20,000 live events. Data were analyzed with FlowJo 9.5 software (TreeStar).
Generation of monocyte-derived dendritic cells (moDCs) and isolation of T lymphocytes
MoDCs were generated from PBMCs using media, media supplements, and cytokines, exactly as published (24). Xenogeneic plasma or serum (e.g., fetal calf serum) was never used in any cultures. T cells were obtained from tissue culture plastic-nonadherent PBMCs, further purified by elution from nylon wool columns (Polysciences), achieving >90% purity.
Allogeneic mixed leukocyte reactions (MLRs) and PD-1 blockade experiments
MoDCs were added in serial doses (1:30 to 1:3000, moDC:T) to triplicate wells of 1 × 105 allogeneic T cells in 96 round-bottomed well plates (Corning Life Sciences). Final volume was 100 μL/well in RPMI with 10% heat-inactivated, pooled normal human serum (NHS). For PD-1 blockade, cells were cultured at a 1:30 moDC:T cell ratio with nivolumab (10 μg/mL final; Bristol-Myers Squibb) or IgG4 isotype control added at culture initiation. Responder allogeneic T cell proliferation was measured after 5 days by a colorimetric assay according to manufacturer's instructions (CellTiter96 Aqueous One Solution Cell Proliferation Assay MTS; Promega) or by intracellular staining with Ki-67 (BD Pharmingen).
mRNA electroporation of moDCs
WT1 mRNA transcription was performed exactly as published (25, 26). Immature moDCs were electroporated with WT1 mRNA on day 5-6. After electroporation, cells were immediately transferred to culture and terminally matured by exposure to inflammatory cytokines for 48 hours (24).
Cytolytic T lymphocyte (CTL) assays
Mature WT1 mRNA-electroporated autologous moDCs were added in serial doses to triplicate wells containing 1 × 105 T cells in a 96 round-bottomed well plate (Corning Life Sciences). Final volume was 100 μL/well of RPMI-10% heat-inactivated, autologous serum, supplemented with recombinant human IL15 (10 ng/mL; R&D Systems). After 7 days of moDC-T cell culture, 5×103 target cells were added directly to each well, and cytolytic activity exerted by responder T lymphocytes was assessed after 4 to 6 hours with a colorimetric CTL assay (27). These data represented the total cytolytic activity generated in each culture according to the primary stimulation conditions, rather than per number of effector T cells irrespective of their frequency in the primary cultures. Target cells were 697 cells (HLA-A*0201+, WT1+ cell line). SKLY-16 cells (HLA-A*0201+, WT1neg cell line) served as a negative control.
Statistics
Descriptive and graphical measures were used to characterize leukocyte subpopulation patterns both longitudinally and by disease status following ASCT. Unpaired t-tests were used to explore mean differences in expression across disease categories, while paired t-tests were used to compare across time points and treatment conditions. A P value less than 0.05 was considered statistically significant. All statistical analyses were calculated using Prism 6 software (GraphPad).
RESULTS
Kinetics of lymphocyte reconstitution in MM patients after ASCT
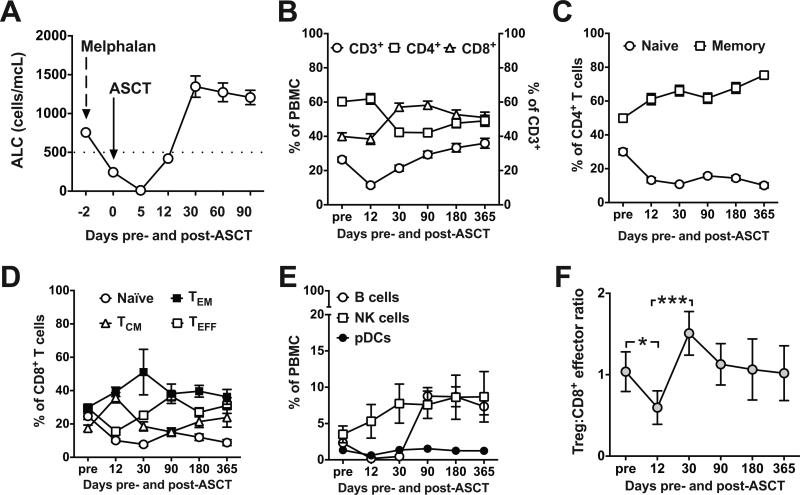
We evaluated absolute lymphocyte count (ALC) after ASCT to determine the kinetics of lymphocyte reconstitution. ALC nadir occurred at day +5, followed by early recovery at day +12 (Fig. 1A) and complete recovery by day +30 (Fig. 1B). Reconstitution of CD8+ T cells, however, outpaced that of CD4+ T cells, most likely due to the homeostatic proliferation of peripheral T cells that phenotypically resemble memory cells after chemotherapy-induced lymphopenia (28). This resulted in an inverted CD4/CD8 ratio lasting up to one year (Fig. 1B). CD4+CD45RO+ memory T cells represented the majority of CD4+ T cells at day +12 (Fig. 1C; 61.11% ± 3.27%), whereas CD4+CD45RA+ naïve T cells remained low at one year (Fig. 1C; 10.13% ± 1.5%). CD8+CCR7negCD45RO+ effector memory and CD8+CCR7+CD45RO+ central memory cells comprised the majority of CD8+ T cells at day +12 (Fig. 1D; 39.26% ± 2.8% and 35.75% ± 3.15%, respectively), with low levels of CCR7+CD45ROneg naïve CD8+ T cells present at one year (Fig. 1D; 8.81% ± 1.79%). Natural killer (NK) cells (CD3negCD56+CD16neg and CD3negCD56dimCD16+) exhibited rapid and sustained recovery after ASCT (Fig. 1E). The recovery of CD19+ B cells lagged in comparison to the other lymphocyte subsets but recovered by 3 months (Fig. 1E). Plasmacytoid dendritic cells (CD123+DR+CD11cneg) were present at similar levels before and after ASCT (Fig. 1E). Subgroup analysis based on 3-month post-ASCT disease response (i.e., PR vs. VGPR vs. CR) revealed no statistically significant differences in the pattern of lymphocyte reconstitution between groups (data not shown).
Figure 1. Patterns of lymphocyte reconstitution and regulatory T cell-to-CD8+ effector ratio in MM patients after ASCT.
(A) Absolute lymphocyte count (ALC) was calculated before high-dose melphalan conditioning, on the day of stem cell infusion, and after ASCT on days +12, 30, 60, and 90. Dotted line indicates lower limit of normal ALC. (B-F) PBMCs were analyzed by flow cytometry for each lymphocyte subpopulation before (pre; 1-7 days before melphalan) and at the indicated time points after ASCT. (B) Total T cells: CD3+ (○) cells are plotted against the LEFT Y axis. CD4+ (□) and CD8+ (Δ) T cells are plotted against the RIGHT Y axis. (C) CD4+ T cells: naïve (CD45RA+; ○) and memory (CD45RO+; □). (D) CD8+ T cells: naïve (CCR7+CD45ROneg; ○), central memory (CCR7+CD45RO+; Δ), effector memory (CCR7negCD45RO+; ■), and effector (CCR7negCD45ROneg; □). (E) B cells (CD19+; ○), NK cells (CD3negCD56+CD16neg and CD3negCD56dimCD16+; □, and plasmacytoid dendritic cells (pDC; CD123+DR+CD11cneg; ●). (F) Regulatory T cell (Treg; CD3+CD4+CD25bright CD127neg) to CD3+CD8+CD25+ effector T cell ratios. For all panels (A-F), pooled data (mean ± SD) from 55 patients are shown. *P < .05 and ***P < .001.
Regulatory T cell-to-CD8+ effector ratio declines in the early post-ASCT period
The balance between regulatory T cells (Tregs) and effector T cells shapes antitumor immune responses and the efficacy of immune-based interventions (29). We compared CD3+CD4+CD25brightCD127neg Tregs with CD3+CD8+CD25+ effector T cells after ASCT. As shown in Fig. 1F, the Treg:CD8+ effector T cell ratio at day +12 (0.59 ± 0.21) was significantly lower than before transplant (1.04 ± 0.23; P < .05) or day +30 after transplant (1.51 ± 0.27; P < .001). Tregs therefore decline early post-nadir as CD8+ T cell recovery occurs, resulting in a markedly lower Treg:CD8+ effector T cell ratio and providing a critical early window for the introduction of immune-based post-transplant consolidation therapies.
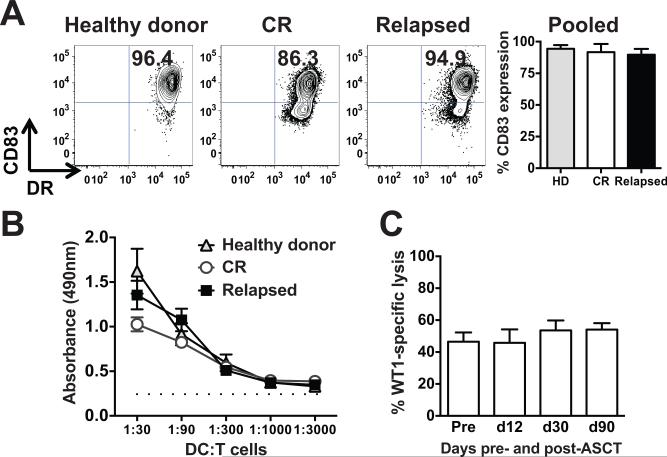
Dendritic cells from MM patients after ASCT, irrespective of disease status, induce autologous antigen-specific CTLs comparable to those stimulated by healthy donor dendritic cells
In the non-transplant setting, there are reports of defective dendritic cell (DC) function in MM (30, 31). To evaluate the integrity of DCs from patients after transplant, monocyte-derived DCs (moDCs) were generated from peripheral blood mononuclear cells (24) from patients in CR three months after transplant, patients who relapsed after transplant, and healthy donors. DCs were assessed by flow cytometry for the upregulation of the prototypical DC maturation marker, CD83 (32). Neither post-transplant status nor disease relapse impaired terminal, inflammatory cytokine-induced maturation (Fig. 2A). We then evaluated the functional capacity of these moDCs in the allogeneic mixed leukocyte reaction (alloMLR), which is a standard assay of overall DC immunogenicity (24). MoDCs from each group stimulated comparably vigorous proliferation of allogeneic T cells (Fig. 2B).
Figure 2. Dendritic cells generated from MM patients after ASCT induce autologous antigen-specific CTLs.
(A) Cytokine-matured, monocyte-derived dendritic cells (moDCs) generated from PBMCs from healthy donors, patients in CR after ASCT, and patients who relapsed after ASCT were compared for expression of the maturation marker, CD83. Representative dot plots of mature moDCs from each group are shown, along with pooled data (mean ± SD, n = 3 independent experiments). (B) Mature moDCs generated from these same three groups were added as stimulators in graded doses to a fixed number of allogeneic T cell responders from healthy volunteers (allo-MLRs). DC:T ratios ranged from 1:30 to 1:3000. T cell proliferation was measured by a flow cytometry-based colorimetric assay (triplicate means ± SEM, n = 3 independent experiments). Dotted line depicts background proliferation of T cells alone without stimulation. (C) MoDCs generated from peripheral blood obtained on day +90 after ASCT were electroporated with WT1 mRNA, terminally matured and activated by a combination of inflammatory cytokines (24), and then added in serial doses to triplicate microwells each containing 1 × 105 autologous T cells obtained pre- and post- ASCT (days +12, 30, and 90), in the presence of exogenous recombinant human IL-15 for 7 days. Antigen-specific target cell lysis by CTLs stimulated by these WT1 mRNA-electroporated moDCs was evaluated using a flow cytometry-based assay. Target cells were 697 cells (HLA-A*0201+, WT1+ cell line). SKLY-16 cells (HLA-A*0201+, WT1neg cell line) served as a negative control. Specific lysis is plotted against the Y axis, comparing the lysis activity of T cells from the indicated time points pre- and post-ASCT, after stimulation by WT1 mRNA-electroporated moDCs (triplicate means ± SEM, n = 3 independent experiments).
We also assessed the generation of antigen-specific CTLs by mRNA-electroporated moDCs from patients who had undergone ASCT for MM. MoDCs were electroporated with WT1 mRNA and then matured and activated with inflammatory cytokines for 48 hours (25, 26). The resulting mature moDCs were added in serial 3-fold dilutions to a fixed number of purified autologous T cells obtained pre- and post-ASCT (days +12, 30, and 90). Cultures were supplemented with recombinant human IL15, because unlike CD34+ HPC-derived Langerhans-type DCs, moDCs do not provide sufficient endogenous IL15 for CTL stimulation (25). Autologous WT1 mRNA-electroporated moDCs plus IL15 stimulated potent antigen-specific CTLs after only 7 days’ stimulation, demonstrating that active cellular immune responses can be elicited in vitro as early as day +12 from MM patients after ASCT (Fig. 2C). We therefore conclude that the DC compartment causes no alteration in immune responsiveness in MM patients after ASCT.
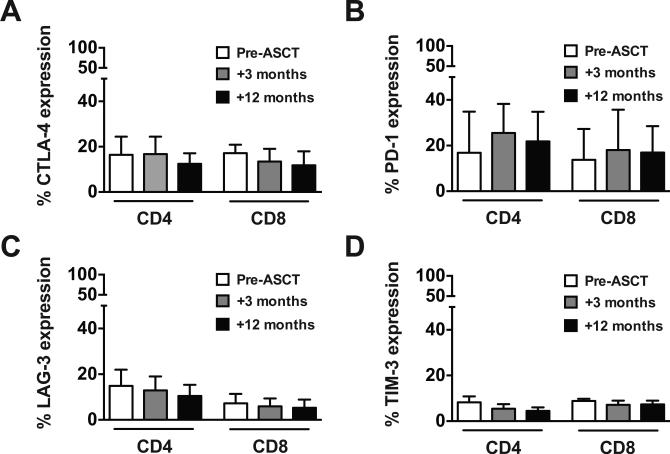
T cells retain immune checkpoint receptor expression after ASCT
Immune checkpoint pathways can impede antitumor immune responses and contribute to persistent and/or relapsed malignancy (33). To assess this key regulatory axis in the setting of ASCT, we compared T cells before and after ASCT for expression of the inhibitory receptors CTLA-4 (Fig. 3A), PD-1 (Fig. 3B), LAG-3 (Fig. 3C), and TIM-3 (Fig. 3D). CD4+ and CD8+ T cells maintained the expression of each inhibitory receptor at 3 and 12 months after transplant, with CTLA-4 and PD-1 showing higher overall expression than LAG-3 and TIM-3. These findings provide a rationale for the inclusion of checkpoint inhibition to augment T cell responses after ASCT.
Figure 3. ASCT does not alter T cell expression of inhibitory receptors.
PBMCs were analyzed by flow cytometry for expression of the inhibitory receptors CTLA-4 (A), PD-1 (B), LAG-3 (C), and TIM-3 (D) on CD4+ and CD8+ T cells before (white bar), and 3-months (gray bar) and 12-months (black bar) after ASCT. Pooled data (mean ± SD) from 10 patients are shown.
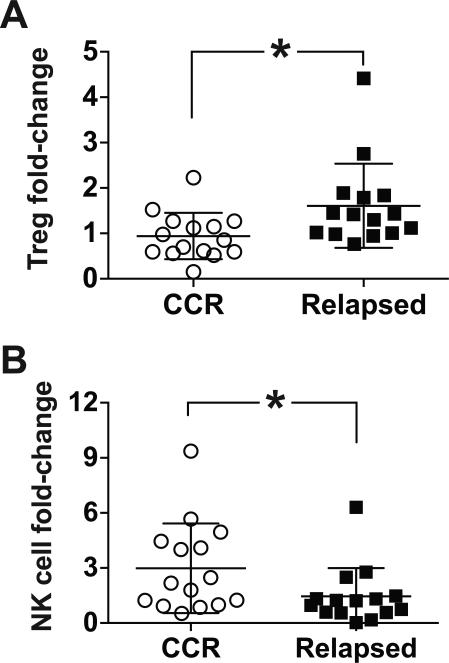
Increased regulatory T cells and decreased NK cells are associated with relapse after ASCT
Treg expansion has been implicated in MM pathogenesis (34, 35) and is associated with inferior survival (36). Early NK cell reconstitution correlates with improved progression-free survival after ASCT (37), and impaired NK cell function correlates with MM progression (38). We compared patterns of Treg and NK cell content in patients who remained in a continuous CR one year after ASCT with a subset of patients who initially achieved a CR at 3 months but subsequently relapsed after ASCT (mean time to relapse: 12.3 months; range: 5-21 months). The fold-change in Tregs was significantly greater in relapsed compared with non-relapsed patients, 1.65 ± 0.25 and 0.94 ± 0.13, respectively (Fig. 4A). Treg:CD8+ effector T cell ratio was also increased in relapsed compared with non-relapsed patients, but did not reach statistical significance (data not shown). The fold-increase in NK cells was greater in patients remaining in a continuous CR compared with relapsed patients, 2.98 ± 0.63 and 1.38 ± 0.42, respectively (Fig. 4B).
Figure 4. Regulatory T cell and NK cell trends in non-relapsed and relapsed patients after ASCT.
(A-B) PBMCs from patients who remained in a continuous CR one year after ASCT (CCR; n = 15) and patients who initially achieved a CR but relapsed beyond 3 months after ASCT (Relapsed; n = 14) were compared for Treg (CD3+CD4+CD25brightCD127neg) and NK cell (CD3negCD56+CD16neg plus CD3negCD56dimCD16+) content. The percentage of Tregs or NK cells was determined by flow cytometry at 3 and 12-months post-ASCT, or at the time of relapse. Interval fold-change was calculated by comparing Treg (A) or NK cell (B) numbers at the 12-month or relapse time point with values from the 3-month time point when all patients were in a CR. Pooled data (mean ± SD) are shown. *P < .05.
CD8+ T cell exhaustion and/or senescence distinguish relapse after ASCT
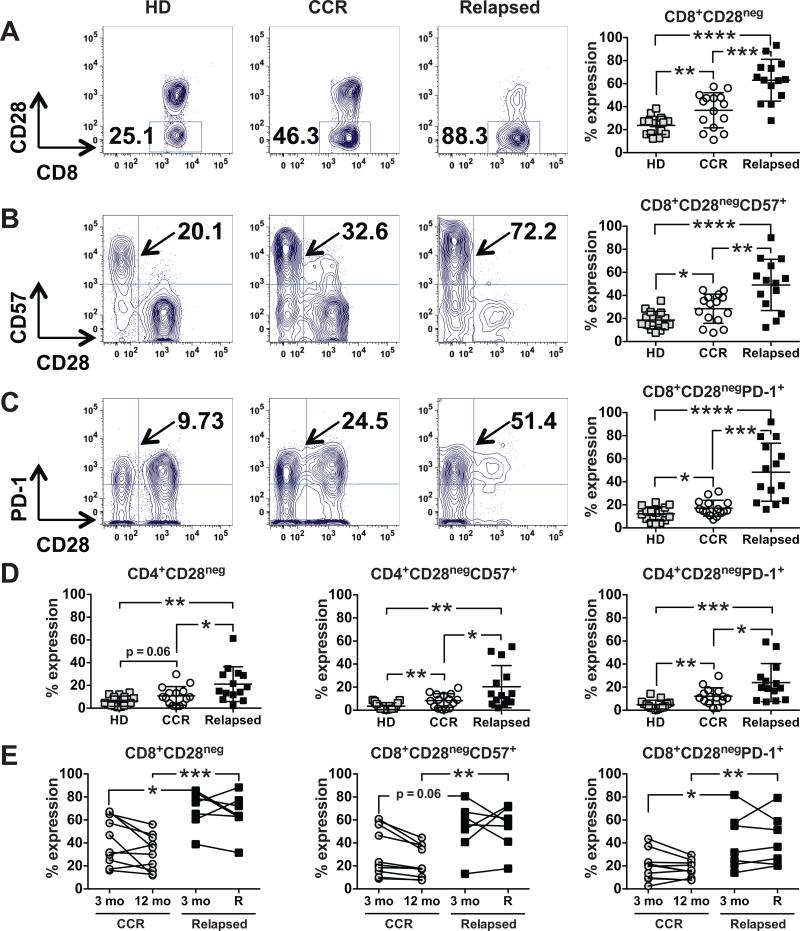
T cell exhaustion and senescence occur under conditions of chronic antigen stimulation and contribute to impaired immune responses (39, 40). Although investigators have not fully distinguished the molecular characteristics of exhaustion and senescence (41), cell surface markers can be used to segregate these T cell states phenotypically. Down-regulation of the costimulatory marker, CD28, is a feature of both exhaustion and senescence (41). Up-regulation of the glycoepitope CD57 can be used to identify T cells with low proliferative capacity (42). Senescent cells can also express PD-1, a prototypical marker of exhaustion (39, 40). Using these markers, we assessed the pattern of T cell exhaustion/senescence in the post-transplant setting. As shown in Fig. 5A, patients with relapsed disease after ASCT had a higher percentage of CD8+CD28neg cells (62.94% ± 4.86%), compared with age-matched (mean age 53.3; range 47-66) healthy donors (23.69% ± 2.03%; P < .0001) or patients in a continuous CR after ASCT (36.83% ± 3.95%; P < .001). Patients with relapsed disease after ASCT also had a similar increase in the fraction of CD8+CD28negCD57+ (Fig. 5B) and CD8+CD28negPD-1+ (Fig. 5C) T cells. CD4+ T cells showed the same trends in the frequencies of CD28neg, CD28negCD57+, and CD28negPD-1+ cells, albeit at lower levels of expression (Fig. 5D). The expression of the inhibitory receptors, CTLA-4, LAG-3, and TIM-3, was not significantly different between the groups examined (data not shown).
Figure 5. CD8+ T cell exhaustion/senescence is a prominent feature of relapse after ASCT.
(A-C) PBMCs from healthy donors (HD, □ß; n = 15), patients in a continuous CR one year after ASCT (CCR, ○; n = 15), and patients who initially achieved CR but relapsed beyond 3 months after ASCT (Relapsed, ■; n = 14) were compared by flow cytometry for (A) CD8+CD28neg, (B) CD8+CD28negCD57+, and (C) CD8+CD28negPD-1+ T cells. Representative dot plots from one patient from each group are shown, with pooled data (mean ± SD) in the far right column for each group. (D) PBMCs from the same three groups in A-C were analyzed for CD4+CD28neg, CD4+CD28negCD57+, and CD4+CD28negPD-1+ T cells. Pooled data (mean ± SD) are shown. (E) Interval changes in CD8+CD28neg, CD8+CD28negCD57+, and CD8+CD28negPD-1+ T cells were assessed by flow cytometry at 3 and 12 months post-ASCT for patients in a CCR one year after ASCT (○; n = 10) and at 3 months and at the time of relapse for patients who initially achieved a CR but relapsed beyond 3 months after ASCT (■; n = 7). *P < .05, **P < .01, ***P < .001, and ****P < .0001.
We also compared interval changes in T cell exhaustion/senescence profiles among patients who initially achieved CRs at 3 months post-ASCT, but then either relapsed thereafter or remained in a continuous CR at 12 months. As shown in Fig. 5E, patients with relapsed disease had a higher percentage of exhausted/senescent CD8+CD28neg, and CD8+CD28negPD-1+ T cells at the time of relapse than did patients remaining in a continuous CR at 12 months. Of note, patients in the relapsed group had higher baseline levels of CD8+CD28neg, CD8+CD28negCD57+, and CD8+CD28negPD-1+ T cells at the 3-month mark, preceding the onset of clinical relapse. Together these findings indicate that impaired immune function due to exhaustion and/or senescence may contribute to relapse after ASCT. The senescence/exhaustion T cell phenotype could also provide a predictive marker supporting earlier intervention to prevent relapse.
PD-1 blockade activates exhausted/senescent CD8+ T cells in vitro
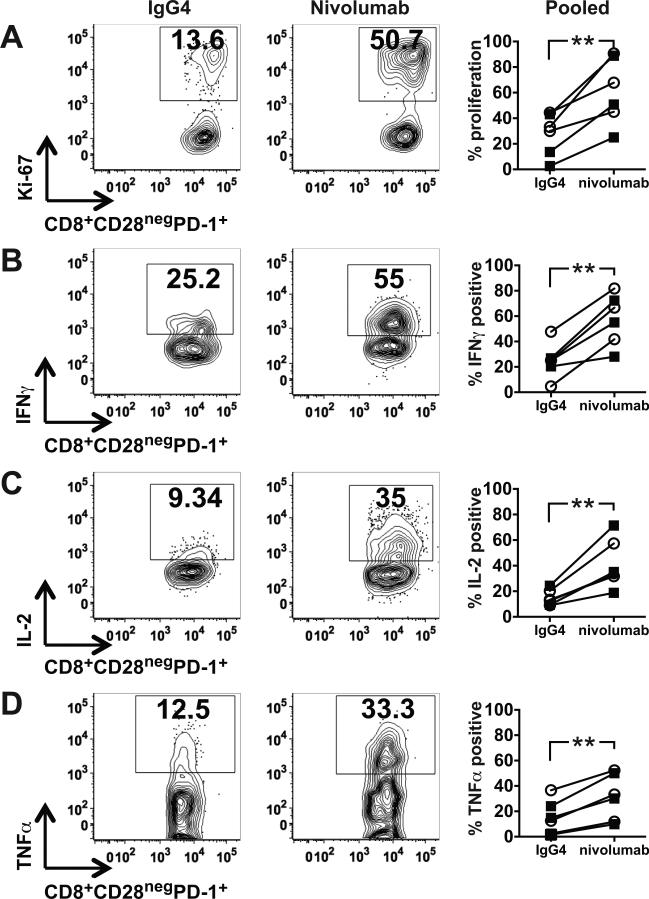
Using cells from patients in continuous CR one year after ASCT and from patients who initially achieved CR but relapsed beyond 3 months after ASCT, we tested the capacity of CD8+CD28negPD-1+ T cells to respond to stimulation by moDCs derived from healthy donors in alloMLRs as a generic response assay, with and without the PD-1 inhibitor, nivolumab. In all cases, PD-1 inhibition increased CD8+CD28negPD-1+ T cell proliferation (Fig. 6A; 61.33% ± 10.58% vs. 27.82% ± 6.78% for isotype control; P < .01). Nivolumab treatment also induced a concomitant increase in the secretion of the cytokines IFNγ, IL2, and TNFα. These findings demonstrate augmented responsiveness of a significant fraction of cells in the setting of PD-1 inhibition, thus underscoring the potential benefit of checkpoint blockade to enhance/restore T cell responses in this patient population.
Figure 6. PD-1 inhibition stimulates the proliferation and cytokine secretion of exhausted/senescent CD8+ T cells in vitro.
(A-D) Mature monocyte-derived dendritic cells generated from PBMCs from healthy donors were added as stimulators to T cells from patients in a continuous CR (CCR) one year after ASCT or T cells from patients who initially achieved CR but relapsed beyond 3 months after ASCT, at a 1:30 ratio with nivolumab or IgG4 isotype control in alloMLRs. After 5 days, cells were harvested, and CD8+CD28negPD-1+ T cells were assessed by flow cytometry for (A) proliferation by Ki-67 expression, and secretion of (B) IFNγ, (C) IL2, and (D) TNFα. Representative dot plots from one patient are shown, with pooled data (mean ± SD) from six patients, three in CCR (○) and three relapsed (■), in the far right column for each parameter. **P < .01.
DISCUSSION
This study identifies several key immunologic parameters of immune reconstitution and relapse in MM after ASCT. Lymphocyte recovery from high-dose chemotherapy-induced lymphopenia relies primarily on the peripheral expansion of memory cells with only a minor contribution from their thymic-derived naïve counterparts. The early post-transplant period is marked by a transient decline in the Treg-to-effector T cell ratio, which reverts to pre-transplant levels by one month. DCs are functionally intact, comparable to those from healthy donors, and induce autologous antigen-specific T cells with robust lytic activity in vitro. CD4+ and CD8+ T cells retain immune inhibitory receptor expression after transplant, however. A significantly increased subset of these T cells with an exhausted/senescent phenotype and reversible hyporesponsiveness to PD-1 inhibition characterize patients who relapse after transplant. Increased Tregs and decreased NK cells further characterize immune dysfunction in these patients.
Consistent with previous reports (28), disruption of the normal balance of immune cells with a bias toward T cell memory phenotypes, protracted diminution of naïve T cell output from the thymus, and inversion of CD4+/CD8+ T cell ratios characterize the immune reconstitution in our study population. Other groups have shown that T cell repertoire diversity of expanding lymphocytes after lymphopenia is limited in the absence of adequate thymopoiesis (43, 44). These changes contribute to a state of prolonged post-transplant immune deficiency and predispose patients to infections and compromised antitumor immune surveillance (28), but the specific dynamics of T-cell recovery in the context of our current understanding of exhausted/senescent T cells and checkpoint inhibitors have not been defined.
During initial lymphocyte recovery, the Treg:CD8+ effector T cell ratio is significantly reduced. This shift provides an opportunity to stimulate maximal antitumor responses in the absence of Treg-mediated suppression. DC-based vaccination in this setting offers one approach to redirect recovering T cells toward specific MHC-restricted antigen. Our data show the induction of potent autologous antigen-specific CTLs by monocyte-derived DCs (moDCs) supplemented with IL15 at day +12 after transplant, thus confirming preservation of DC and T cell function in vitro. Non-DC-based pre-ASCT vaccination supplemented with the adoptive transfer of ex vivo expanded T cells promotes accelerated reconstitution of immune responses against both microbial and MM tumor antigens in vivo (45, 46), supporting the concept of immunotherapy in the setting of post-transplant lymphopenia. We are testing the feasibility of early post-transplant vaccination using autologous Langerhans-type DCs (LCs) electroporated with mRNA encoding three MM-associated antigens in a phase I clinical trial (NCT01995708). LCs provide sufficient endogenous IL15, obviating the need for IL15 supplementation that moDCs require to induce CTLs (25). Adoptive T cell infusions should not be necessary in the setting of DC-based vaccines, unless perhaps used in a prime-boost sequence.
Inhibitory receptor blockade augments vaccine-induced T cell responses (19, 47), reviving antigen-reactive T cells from their exhausted state (39). Our data demonstrate the persistent expression of CTLA-4, PD-1, LAG-3, and TIM-3 by T cells after ASCT, providing a rationale for early inhibition of checkpoint blockade in post-transplant treatment strategies. Such intervention should promote the recognition of MM-associated antigens, boost antigen-specific T cell activation and proliferation, enhance immune reconstitution, expand anti-MM immune responses, and counteract relapse from minimal residual disease. Using the alloMLR as a proxy assay for robust immune reactivity, our data in fact demonstrate a role for PD-1 blockade in reversing the hyporesponsiveness of exhausted/senescent CD8+ T cells in vitro.
Patients who initially achieved a CR at 3 months but subsequently relapsed after ASCT were assessed for immune markers of relapse. Analysis of Tregs showed a relative increase among the relapsed cohort, a finding that is consistent with recent reports showing greater risk for disease progression with increased Tregs (35) and an association of long-term disease control with decreased Tregs (36). NK cells exhibit rapid recovery after ASCT but are less abundant in patients who relapse, thus supporting their role in conferring an anti-MM benefit after ASCT as well (37, 38).
T cell dysfunction, including exhaustion and/or senescence, contributes to tumor persistence and progression (39, 40). Our studies define a T cell exhaustion/senescence phenotype associated with post-ASCT relapse, which is most pronounced in the CD8+ T cell compartment. These cells down-regulate CD28, an essential co-stimulatory receptor involved in the activation and modulation of key cellular functions (48), up-regulate CD57, a marker of low proliferative capacity (42), and display increased PD-1 expression. Patients with relapsed MM had the highest levels of CD8+CD28neg, CD8+CD28negCD57+, and CD8+CD28negPD-1+ T cells. Patients in the continuous CR group, however, also had higher levels of exhausted/senescent T cells compared with healthy controls, possibly due to chronic malignancy and related treatments.
An intriguing finding is that patients in the relapsed group had higher baseline levels of exhausted/senescent T cells at the 3-month mark before the detection of clinical relapse, suggesting the utility of these immune biomarkers for identifying patients at higher risk of relapse who are candidates for early immunotherapy. Because T cells become exhausted/senescent from chronic antigen stimulation, this begs the question as to whether patients who eventually relapse have enough minimal residual disease persistence, despite high-dose chemotherapy, to provide sufficient chronic antigen stimulation post-ASCT to cause T cell exhaustion/senescence. Alternatively, while it is not standard practice to characterize the cell composition of autografts, apart from their CD34+ content, our findings lend sufficient rationale to evaluating whether exhausted/senescent T cells, as well as Tregs, are over-represented in the autografts of patients more likely to relapse.
Many studies suggest that exhaustion and senescence are mechanistically distinct, but the underlying molecular pathways differentiating the two have yet to be fully elucidated (41). Distinguishing T cell exhaustion from senescence and delineating the plasticity and function of these cells remain important unknowns. Exhausted T cells can be reactivated by blocking the PD-1 pathway (49). Senescent T cells can transiently up-regulate telomerase activity and proliferate (40), and blockade of p38 MAPK reverses senescence via an mTOR-independent mechanism (50). In this study, PD-1 inhibition significantly enhanced the proliferation and cytokine secretion of CD8+CD28negPD-1+ T cells, demonstrating the potential to revive these hyporesponsive cells with checkpoint blockade. Additional studies of this T cell subpopulation, including the contribution of other inhibitory receptors and immunosuppressive factors, are warranted to gain a more complete understanding of the biology of these cells with regard to post-ASCT disease status and immunotherapy.
These results provide rationale for the early introduction of immunotherapeutic modalities like vaccines and checkpoint blockade agents to induce antitumor immunity after ASCT. Our findings also underscore the contribution of immune dysregulation to disease relapse, with T cell exhaustion/senescence as an immune biomarker that deserves validation by prospective testing because of its potential value in identifying candidates for early therapeutic intervention. The reservoir of dendritic cell immunostimulatory function and the potential reactivity of T and NK cells comprise an untapped resource to alter the natural history of MM in the setting of ASCT.
ACKNOWLEDGMENTS
We gratefully acknowledge the various contributions of members of the Laboratory of Cellular Immunobiology to the development of this work. We thank the nurses, advanced practice providers, and physicians of the Adult Bone Marrow Transplant and Myeloma Services at MSKCC for assistance with sample procurement. We also thank the patients and healthy volunteers who provided samples for research.
Financial support: This work was supported by Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research and The Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center (DJC), The Society of Memorial Sloan Kettering (DJC), Cycle for Survival (DJC and AML), P30 CA008748 from the National Cancer Institute, NIH (SMD), Thomas Israel Myeloma Research Fund (SAG), U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute, NIH (SAG), Swim Across America (JWY), and P01 CA23766 from the National Cancer Institute, NIH (JWY). This research was also funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
REFERENCES
- 1.Kapoor P, Kumar SK, Dispenzieri A, Lacy MQ, Buadi F, Dingli D, et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J Clin Oncol. 2013;31(36):4529–35. doi: 10.1200/JCO.2013.49.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lonial S, Anderson KC. Association of response endpoints with survival outcomes in multiple myeloma. Leukemia. 2014;28(2):258–68. doi: 10.1038/leu.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770–81. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782–91. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 6.Pellat-Deceunynck C, Jego G, Harousseau JL, Vie H, Bataille R. Isolation of human lymphocyte antigens class I-restricted cytotoxic T lymphocytes against autologous myeloma cells. Clin Cancer Res. 1999;5(3):705–9. [PubMed] [Google Scholar]
- 7.Dhodapkar MV, Krasovsky J, Olson K. T cells from the tumor microenvironment of patients with progressive myeloma can generate strong, tumor-specific cytolytic responses to autologous, tumor-loaded dendritic cells. PNAS. 2002;99(20):13009–13. doi: 10.1073/pnas.202491499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noonan K, Matsui W, Serafini P, Carbley R, Tan G, Khalili J, et al. Activated marrow-infiltrating lymphocytes effectively target plasma cells and their clonogenic precursors. Cancer Res. 2005;65(5):2026–34. doi: 10.1158/0008-5472.CAN-04-3337. [DOI] [PubMed] [Google Scholar]
- 9.Goodyear O, Piper K, Khan N, Starczynski J, Mahendra P, Pratt G, et al. CD8+ T cells specific for cancer germline gene antigens are found in many patients with multiple myeloma, and their frequency correlates with disease burden. Blood. 2005;106(13):4217–24. doi: 10.1182/blood-2005-02-0563. [DOI] [PubMed] [Google Scholar]
- 10.Brown RD, Spencer A, Ho PJ, Kennedy N, Kabani K, Yang S, et al. Prognostically significant cytotoxic T cell clones are stimulated after thalidomide therapy in patients with multiple myeloma. Leuk Lymphoma. 2009;50(11):1860–4. doi: 10.3109/10428190903216804. [DOI] [PubMed] [Google Scholar]
- 11.Atanackovic D, Arfsten J, Cao Y, Gnjatic S, Schnieders F, Bartels K, et al. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109(3):1103–12. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]
- 12.Tyler EM, Jungbluth AA, O'Reilly RJ, Koehne G. Wilms' tumor 1 protein-specific T-cell responses in high-risk multiple myeloma patients undergoing T-cell depleted allogeneic hematopoietic stem cell transplantation and donor lymphocyte infusion. Blood. 2012;121(2):308–17. doi: 10.1182/blood-2012-06-435040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhodapkar MV, Krasovsky J, Osman K, Geller MD. Vigorous Premalignancy-specific Effector T Cell Response in the Bone Marrow of Patients with Monoclonal Gammopathy. J Exp Med. 2003;198(11):1753–7. doi: 10.1084/jem.20031030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spisek R, Kukreja A, Chen L-C, Matthews P, Mazumder A, Vesole D, et al. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204(4):831–40. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2006;103(24):9190–5. doi: 10.1073/pnas.0603503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witzens-Harig M, Hose D, Junger S, Pfirschke C, Khandelwal N, Umansky L, et al. Tumor cells in multiple myeloma patients inhibit myeloma-reactive T cells through carcinoembryonic antigen-related cell adhesion molecule-6. Blood. 2013;121(22):4493–503. doi: 10.1182/blood-2012-05-429415. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110(1):296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 18.Benson DM, Jr., Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–94. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34(5):409–18. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66(11):5527–36. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 21.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11(11):1238–43. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 23.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 24.Ratzinger G, Baggers J, de Cos MA, Yuan J, Dao T, Reagan JL, et al. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173:2780–91. doi: 10.4049/jimmunol.173.4.2780. Erratum in J Immunol. 005; 174:3818. [DOI] [PubMed] [Google Scholar]
- 25.Romano E, Cotari JW, Barreira da Silva R, Betts BC, Chung DJ, Avogadri F, et al. Human Langerhans cells use an IL-15R-alpha/IL-15/pSTAT5-dependent mechanism to break T-cell tolerance against the self-differentiation tumor antigen WT1. Blood. 2012;119(22):5182–90. doi: 10.1182/blood-2011-09-382200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung DJ, Romano E, Pronschinske KB, Shyer JA, Mennecozzi M, St Angelo ET, et al. Langerhans-type and monocyte-derived human dendritic cells have different susceptibilities to mRNA electroporation with distinct effects on maturation and activation: implications for immunogenicity in dendritic cell-based immunotherapy. Journal of translational medicine. 2013;11:166. doi: 10.1186/1479-5876-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer K, Andreesen R, Mackensen A. An improved flow cytometric assay for the determination of cytotoxic T lymphocyte activity. J Immunol Methods. 2002;259(1-2):159–69. doi: 10.1016/s0022-1759(01)00507-5. [DOI] [PubMed] [Google Scholar]
- 28.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19(5):318–30. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev. 2011;241(1):104–18. doi: 10.1111/j.1600-065X.2011.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown RD, Pope B, Murray A, Esdale W, Sze DM, Gibson J, et al. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood. 2001;98(10):2992–8. doi: 10.1182/blood.v98.10.2992. [DOI] [PubMed] [Google Scholar]
- 31.Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100(1):230–7. doi: 10.1182/blood.v100.1.230. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L-J, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. JImmunol. 1995;154:3821–35. [PubMed] [Google Scholar]
- 33.Kim HJ, Cantor H. The Path to Reactivation of Antitumor Immunity and Checkpoint Immunotherapy. Cancer immunology research. 2014;2(10):926–36. doi: 10.1158/2326-6066.CIR-14-0153. [DOI] [PubMed] [Google Scholar]
- 34.Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107(10):3940–9. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 35.Muthu Raja KR, Rihova L, Zahradova L, Klincova M, Penka M, Hajek R. Increased T regulatory cells are associated with adverse clinical features and predict progression in multiple myeloma. PLoS One. 2012;7(10):e47077. doi: 10.1371/journal.pone.0047077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pessoa de Magalhaes RJ, Vidriales MB, Paiva B, Fernandez-Gimenez C, Garcia-Sanz R, Mateos MV, et al. Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematologica. 2013;98(1):79–86. doi: 10.3324/haematol.2012.067272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rueff J, Medinger M, Heim D, Passweg J, Stern M. Lymphocyte subset recovery and outcome after autologous hematopoietic stem cell transplantation for plasma cell myeloma. Biol Blood Marrow Transplant. 2014;20(6):896–9. doi: 10.1016/j.bbmt.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Godfrey J, Benson DM., Jr. The role of natural killer cells in immunity against multiple myeloma. Leuk Lymphoma. 2012;53(9):1666–76. doi: 10.3109/10428194.2012.676175. [DOI] [PubMed] [Google Scholar]
- 39.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 40.Chou JP, Effros RB. T cell replicative senescence in human aging. Current pharmaceutical design. 2013;19(9):1680–98. doi: 10.2174/138161213805219711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11(4):289–95. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 42.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101(7):2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 43.Sarzotti M, Patel DD, Li X, Ozaki DA, Cao S, Langdon S, et al. T cell repertoire development in humans with SCID after nonablative allogeneic marrow transplantation. J Immunol. 2003;170(5):2711–8. doi: 10.4049/jimmunol.170.5.2711. [DOI] [PubMed] [Google Scholar]
- 44.Dumont-Girard F, Roux E, van Lier RA, Hale G, Helg C, Chapuis B, et al. Reconstitution of the T-cell compartment after bone marrow transplantation: restoration of the repertoire by thymic emigrants. Blood. 1998;92(11):4464–71. [PubMed] [Google Scholar]
- 45.Rapoport AP, Stadtmauer EA, Aqui N, Badros A, Cotte J, Chrisley L, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11(11):1230–7. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 46.Rapoport AP, Aqui NA, Stadtmauer EA, Vogl DT, Xu YY, Kalos M, et al. Combination immunotherapy after ASCT for multiple myeloma using MAGE-A3/Poly-ICLC immunizations followed by adoptive transfer of vaccine-primed and costimulated autologous T cells. Clin Cancer Res. 2014;20(5):1355–65. doi: 10.1158/1078-0432.CCR-13-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Binder DC, Engels B, Arina A, Yu P, Slauch JM, Fu YX, et al. Antigen-specific bacterial vaccine combined with anti-PD-L1 rescues dysfunctional endogenous T cells to reject long-established cancer. Cancer immunology research. 2013;1(2):123–33. doi: 10.1158/2326-6066.CIR-13-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229(1):12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 50.Henson SM, Lanna A, Riddell NE, Franzese O, Macaulay R, Griffiths SJ, et al. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8(+) T cells. J Clin Invest. 2014;124(9):4004–16. doi: 10.1172/JCI75051. [DOI] [PMC free article] [PubMed] [Google Scholar]