Abstract
Purpose of the review
This review summarizes information pertaining to the preclinical development of new apolipoprotein (apo) E mimetic peptides that stimulate cellular cholesterol efflux.
Recent findings
Small α-helical peptides based on the C-terminal domain of apoE have been developed for therapeutic applications. These peptides stimulate cellular cholesterol efflux via the ATP-binding cassette transporter A1 (ABCA1) with high potency, like native apolipoproteins on a molar basis. This potent activity has been related to the unique ability of these peptides to maintain α-helix structure upon dilution. Recent structure-activity studies improving the safety features of these mimetic peptides have greatly improved their potential for clinical use. These studies have identified structural features of the class A α-helix motif that induce muscle toxicity and hypertriglyceridemia, which may have implications for the design other HDL mimetic peptides.
Summary
ABCA1 is an integral membrane protein that plays a central role in biology. Its principal function is to mediate the efflux of cholesterol and phospholipid from cells to extracellular apolipoprotein (apo)s, preventing a build-up of excess cholesterol in membranes. This process generates HDL particles that perform a variety of functions to protect against disease. A number of these functions can be viewed as directly or indirectly supporting ABCA1 activity, thus constituting a positive feed-back system to optimize cellular lipid efflux responses and disease prevention. Consequently, therapeutic approaches that mimic the activities of apolipoproteins may prove highly effective to combat disease. One such approach involves the use of peptides. The broad biological relevance of ABCA1 suggests these apolipoprotein mimetic peptides may be useful for the treatment of a number of diseases, such as atherosclerosis, diabetes and Alzheimer’s disease.
Keywords: ABCA1, cellular cholesterol efflux, therapeutic peptides, atherosclerosis, diabetes, Alzheimer’s disease
Introduction
High density lipoproteins (HDL) are thought to protect against arterial cholesterol deposition and risk of coronary events (1, 2**). This protection has been related, in part, to the activity of HDL and its apolipoproteins mediating cellular cholesterol efflux and transporting cholesterol to the liver for excretion in feces, a process termed reverse cholesterol transport (RCT). The ATP-binding cassette transporter A1 (ABCA1) plays an essential role in this RCT process. ABCA1 belongs to a large family of ATP-binding cassette transporters. It is present at the plasma membrane of a wide-variety of cells where it mediates the efflux of cholesterol and phospholipid to extracellular apolipoprotein (apo) acceptors, such as apoA-I, apoE and small, lipid-poor HDL particles (3, 4). In the liver and intestine, the formation of nascent HDL particles via ABCA1 is responsible for producing the bulk of plasma HDL. Locally within tissues, the lipid efflux function of ABCA1 is probably most important for preventing a build-up of excess cholesterol in cellular membranes. This cholesterol efflux function is vital to the prevention of degenerative disease, because cellular cholesterol accumulation can be cytotoxic, inflammatory, and alter the structure/function of the plasma membrane (5–7). Nascent HDL particles generated via ABCA1 subsequently mediate cholesterol efflux via other cell-surface transporters such as ABCG1 (8, 9). Thus the activity of ABCA1 is necessary for producing substrate particles that maintain cell lipid efflux responses, lipid homeostasis, and tissue integrity/function.
Therapeutic aspects - ABCA1 agonist peptides
One viable approach for enhancing ABCA1 lipid efflux therapeutically invokes the use of small peptides based on HDL apolipoproteins. To date, a number of apolipoprotein mimetic peptides have been designed toward different HDL activities and molecular targets (10–13). Consequently, each exhibits its own unique formulation requirements and pharmacology. Very few of these peptides however, target ABCA1 with high potency and selectivity (13–15). ABCA1 transfers cellular lipid to lipid-poor acceptors and, thus, is unique compared to other lipid efflux transporters. Therefore, synthetic peptides can be optimized (i.e. screened) for cholesterol efflux activity, without having to formulate the peptides with phospholipid. My laboratory has found that a stable α-helical structure represents an important factor for creating peptides with potent ABCA1 cholesterol efflux activity (14). This is also likely to be a key feature for improving the overall translatability of such peptides, as discussed below.
Peptide secondary structure and activity
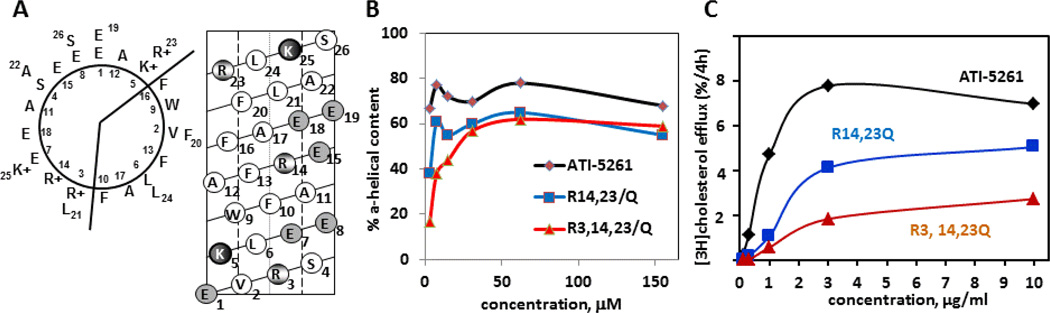
The C-terminal lipid-binding domain of apoE has proven amenable to constructing small α-helical peptides for mediating cellular lipid efflux (15). This domain represents the portion of apoE responsible for mediating ABCA1 cholesterol efflux with high affinity, and is enriched in salt-bridges that stabilize α-helix structure (3, 15). Synthetic peptides based on this domain, such as ATI-5261, stimulate cellular cholesterol efflux similar to native apolipoproteins. The potent activity of peptide ATI-5261 has been related to its unique ability to retain α-helical structure at very low concentrations, as individual peptide strands (16). Figure 1 shows that retention of α-helix structure was attributed to key positively charged amino acids (R3, R14 and R23) located within ionic pairs (i+4) distributed down the length of the peptide. Ablation of these interactions using uncharged glutamine substitutions disrupted peptide secondary structure and activity upon dilution. It is widely known that α-helicity represents an important determinant for mediating ABCA1 lipid efflux; thus the ability of mimetic peptides to retain α-helix structure is likely to be critical in vivo, when the peptide distributes to various tissues and is extensively diluted following administration.
Figure 1. Secondary structure and activity of ABCA1 agonist peptide ATI-5261.
Panel A (left diagram) shows the primary amino acid sequence of ATI-5261 on a helical wheel diagram. Position 1 corresponds to the first amino acid. The peptide forms a class A amphipathic α-helix, with cationic amino acids located at the lipid water-interface and negatively charged residues positioned in the middle of the polar surface. The helical net diagram in Panel A (right) depicts the ATI-5261 α-helix cut down the center at position 1, then flattened. Salt-bridges are located between negatively charged amino acids (shaded circles) and cationic residues (partially shaded) at positions E1-K5, R3-E7, R14-E18, and E19-R23. Panel B - ATI-5263 is able to retain a high α-helical content as the peptide is diluted from 500 - 10 µg/ml (310 - 6 µM), under conditions that favor dissociation of oligomer forms to individual peptide strands (14, 16). In contrast, the R→Q variants of ATI-5261 failed to retain α-helix structure upon dilution, indicating salt bridge interactions were required to stabilize the secondary structure. The high α-helical content observed with increasing peptide concentration is typically attributed to peptide self-association. Panel C- Peptides with R→Q substitutions are poor mediators of cholesterol efflux, as determined using J774 macrophages treated with cAMP to up-regulate ABCA1. Data were redrawn from ref 16.
Solution properties
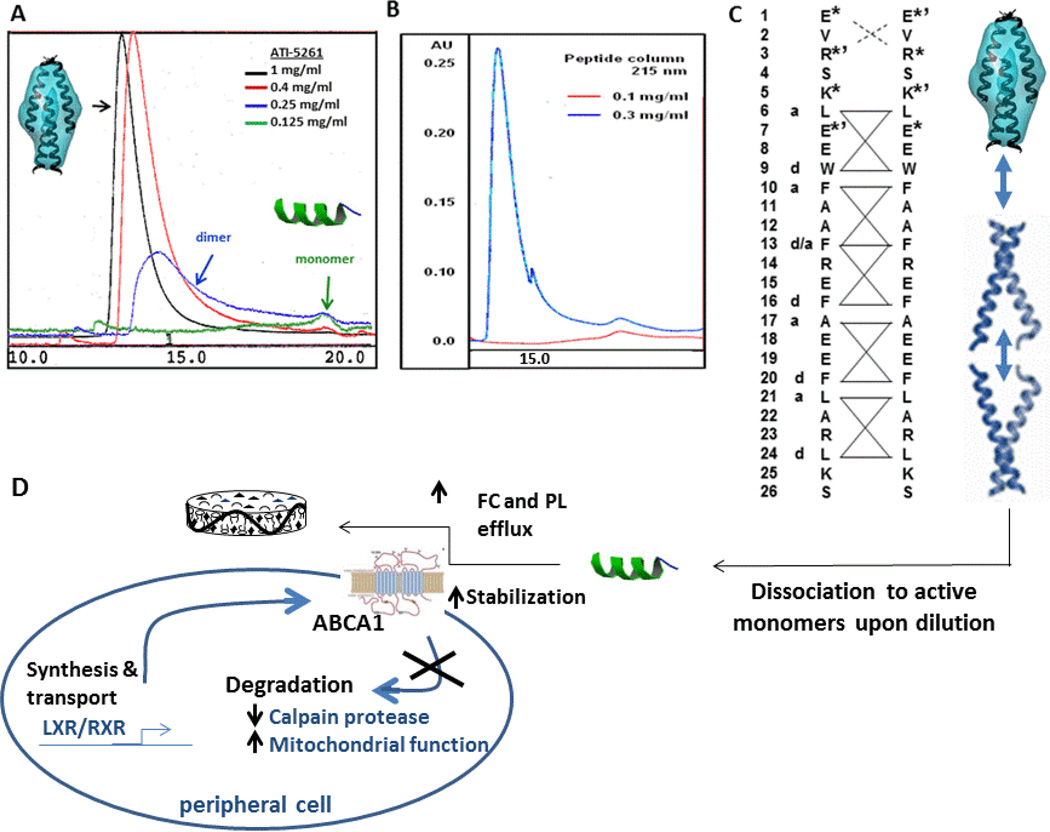
Mimetic peptides based on apolipoproteins are often amphipathic with hydrophobic surfaces. This feature generally limits peptide solubility in the absence of lipid, since amphipathic peptides self-associate to shield hydrophobic residues from the water environment. Such an effect can be particularly troublesome for peptides that are relatively unstructured, since they tend to aggregate in a non-specific manner to form precipitates. In contrast, peptides that we have engineered with high α-helix content self-associate in a specific manner to form well-defined oligomers in solution, as peptide concentration increases (14). Such behavior may be related to aspect of coiled-coil protein structure inherent to native α-helical segments of apoA-I and apoE that have been incorporated into the peptide design (17, 18). These features include sites for putative charge interactions between adjacent α-helical strands to orient helix-helix contact, as well as a regular repeating pattern of hydrophobic residues (a – d motif) to facilitate self-association (Figure 2). In this manner, protein-like structures can be designed using motifs that facilitate inter-helical interactions (19). As a result, exceedingly high stock concentrations of helical peptides can be achieved to afford convenient methods of administration, such as subcutaneous or intravenous injection (15).
Figure 2. Oligomer behavior of ATI-5261 in solution.
At peptide concentrations >0.1 mg/ml PBS (pH=7.4) ATI-5261 forms a unique tetrameric assembly in solution in the absence of lipid. Panel A- FPLC profile illustrating the assembly dissociates to low molecular weight forms (i.e. dimers and monomers) upon dilution. Panel B – ATI-5261 spontaneously adopts a tetramer form when dilute samples are concentrated, i.e. demonstration that the oligomer behavior is reversible. Panel C - Two ATI-5261 strands aligned by primary amino acid sequence (top to bottom) using a model for depicting coiled-coil arrangement of protein strands. The model predicts positive – negative ionic pairs toward the amino terminus that set the registry for self-association, with inter-helical strands aligning via a regular repeating a–d motif pattern of hydrophobic amino acids. Two dimers wedged together at the c-terminal ends would result in the formation of a tetramer with an elongated ovoid shape, as determined by SAXS (14). Panel D - With extensive dilution, the tetrameric form dissociates to dimers and monomers for mediating ABCA1 lipid efflux activity. The peptide orients on the edge of a discoidal lipid complexes.
Safety features
Previous studies demonstrated that ATI-5261 displayed efficacy reducing substantial atherosclerosis in dietary and genetic mouse models of hyperlipidemia (15). These effects were associated with a sustained increase in macrophage specific RCT. Moreover the lipid-free peptide was found to be effective, circumventing the need to formulate the peptide with phospholipid. Consequently, the lipid-free ATI-5261 peptide was considered a potential therapeutic candidate for entering into clinical development for the treatment of acute coronary syndrome and atherosclerosis.
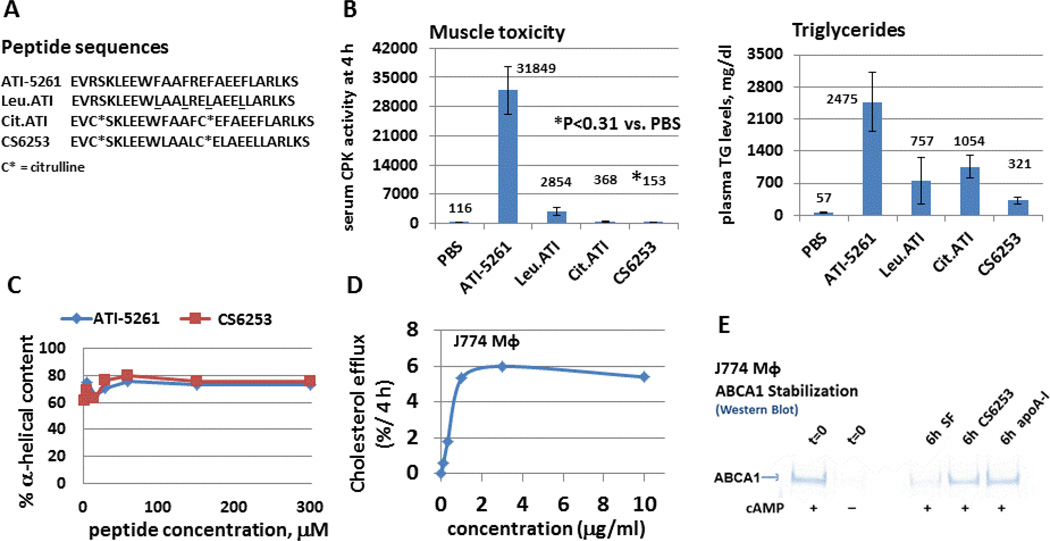
Preclinical toxicology studies revealed, however, transient increases in plasma triglycerides (TG) using pharmacological doses of ATI-5261, similar to that seen with other HDL mimetic peptides (20). In addition, injection of ATI-5261 at a dose 10-fold higher than that used to decrease atherosclerosis in mice induced muscle toxicity (Figure 3). This was accompanied by an increase in the plasma concentration of muscle enzymes including creatine phosphokinase, consistent with a cytotoxic response. Cytotoxic and TG elevating effects were observed using ATI-5261 formulated with and without phospholipid, and in several animal species, such as mice, rats, rabbits and cynomolgus monkeys (not shown).
Figure 3. Creation of safe ABCA1 agonist peptides.
Panel A - Peptide analogs of ATI-5261 are shown. The underlined phenylalanine residues in ATI-5261 were replaced with aliphatic leucine residues to create the Leu.ATI peptide, and cationic arginine residues (R3, R14) replaced with citrulline to make Cit.ATI. CS6253 contains both types of substitutions. Panel B - Safety and TG elevating responses of lipid free peptides (300 mg/kg, single ip injection) were evaluated using wild-type C57Bl/6 mice at 4 hour post-injection, i.e. when the cytotoxic response to the parent ATI-5261 peptide was maximal. The left figure shows that ATI-5261 increased serum creatine phosphokinase (CPK) activity vs injection of PBS vehicle alone. Peptides with either leucine or citrulline substitutions displayed markedly reduced cytotoxic responses; however, the simultaneous use of both types of substitutions (CS6253) was required to completely eliminate the cytotoxicity. As depicted in the right figure, ATI-5261 also increased plasma TG levels; this response was markedly reduced with CS6253. Values in Panel B (both figures) are means ±SD, n=4 mice; numbers above bars are means. Panel C- CS6253 retained α-helical structure upon dilution, similar to ATI-5261. Panel D - CS6253 mediated cellular cholesterol efflux from macrophages in a concentration-dependent manner, as judged using J774 macrophages treated with cAMP. Representative Km values for CS6253, ATI-5261, and apoA-I calculated from concentration dependence curves were 0.80±0.4, 0.86±0.25 and 3.4±0.6 µg/ml (n=3), respectively. Panel E - CS6253 and apoA-I stabilize ABCA1 protein. Treatment (18 h) of J774 macrophages with cAMP upregulated ABCA1 protein vs. no cAMP (t=0 wells). Subsequent 6 h incubation of cAMP treated cells in serum free medium resulted in ABCA1 degradation; whereas, inclusion of either CS6253 or apoA-I (10 µg/ml) in medium prevented the loss of ABCA1 protein, as determined by Western blot analysis of whole-cell lysates.
Lead optimization studies were subsequently conducted to identify features of ATI-5261 that were responsible for the adverse side-effects. This was aided by detailed structural knowledge of the peptide (Figure 1), which allowed manipulation of the amino acid composition in a targeted fashion without altering α-helicity and lipid efflux activity. Initial studies focused on amino acids thought to influence the interaction of amphipathic peptides with phospholipid bilayers, such as cationic- and/or aromatic-residues (21, 22). Peptide analogs were created with conservative amino acid substitutions to test each feature separately, and then both in combination. Replacement of phenylalanine residues with aliphatic leucine residues reduced a majority of the peptide cytotoxicity and ~50% of the TG elevations in plasma. Similar effects were noted upon replacement of interfacial arginine residues with uncharged citrulline. Use of both citrulline and leucine substitutions simultaneously in the same peptide eliminated the cytotoxic responses, as well as a majority (~90%) of the TG elevating effects. The resulting peptide (CS6253) with both the citrulline and leucine substitutions stimulated ABCA1 lipid efflux with high potency and stabilized ABCA1 protein, similar to wild-type apoA-I (Figure 3). These results were consistent with the ability of citrulline to maintain H-bonding and α-helicity, when used to replace arginine residues (R3 and R14). Collectively, the data indicated that the adverse side-effects of ATI-5261 were dependent on structural features linked to the class A α-helix motif. The presence of interfacial cationic residues together with aromatic phenylalanine residues in the same α-helix peptide were required to induce muscle toxicity and TG elevations. However, the problematic residues could be replaced with conservative substitutions without altering peptide secondary structure and activity. CS6253 was also found to reduce atherosclerotic plaque lesions in apoE knock-out mice in a manner analogous to that seen with ATI-5261 (23).
Potential for the treatment of diabetes and Alzheimer’s disease
Recent studies indicate that HDL displays several distinct activities that modulate glucose homeostasis (i.e. enhance insulin secretion and glucose tolerance), and that mimetic peptides can recapitulate some of these effects (24–32). Interestingly, a number of these reported functions involve ABCA1, and still others can be viewed as supporting ABCA1 activity. The latter includes activities of HDL to increase glucose uptake and utilization by cells, improve mitochondrial energy production, and control ABCA1 protein concentration via post-translational mechanisms.
Genetic manipulation of apoA-I in transgenic mice has revealed a novel role of HDL in stimulating glucose oxidation and mitochondrial respiration in vivo (33). Increased mitochondrial respiration and ATP production are expected to have a positive impact on ABCA1 activity and other cell surface transporters that require energy for activity. Such effects would be considered beneficial to optimize lipid efflux responses and disease prevention (34). Mitochondrial function has the potential to modulate ABCA1 protein at several levels. This includes regulation of ABCA1 gene expression via production of oxysterols (35**–37). Moreover, increased permeabilization of the mitochondrial outer membrane during disease is often associated with the release of caspases and reactive oxygen species into the cytosol. Caspases can degrade calpastatin, an endogenous inhibitor of calpain (38). As a result, calpain activity in cells is likely to increase with mitochondrial dysfunction and/or disease to facilitate ABCA1 degradation. Thus it is reasonable to speculate that HDL and its mimetic peptides may protect ABCA1 from degradation by improving mitochondrial function and modulating the calpastatin-calpain system. HDL apolipoproteins are also known to stabilize ABCA1 concentration in membranes via cell-surface interactions that protect the transporter from degradation.
Optimizing ABCA1 lipid efflux activity may also be an important factor in protecting against Alzheimer’s disease (39–41). In the brain, ABCA1 is thought to facilitate the lipidation of apoE and clearance of β-amyloid (42, 43). In this manner, ABCA1 may protect against cognitive decline, particularly in the context of the apoE4 phenotype (43). Thus targeting ABCA1 therapeutically with a small peptide may be useful for the treatment of human Alzheimer’s disease associated with apoE4.
Conclusions
HDL mimetic peptides based on the C-terminal domain of apoE have been developed that target ABCA1 for the treatment of disease. A lead compound, CS6253, has emerged from preclinical optimization studies that is highly safe, mediates ABCA1 cholesterol efflux with high potency, stabilizes ABCA1 cell-surface concentration, and reduces substantial atherosclerosis in mouse models under conditions of excess lipid burden. The importance of ABCA1 in different tissues further suggests CS6253 may be useful therapeutically to combat diabetes and Alzheimer’s disease. Future studies investigating the ability of CS6253 to improve glucose homeostasis, mitochondrial function, and inflammation are warranted. The role of specific apoE isoforms improving glucose utilization and preserving mitochondrial function in the aging brain also represent potentially interesting areas of investigation, particularly in context of dementia.
Key points.
Small peptides that stimulate ABCA1 cholesterol efflux are being developed for therapeutic applications
A lead peptide (CS6253) has emerged from optimization studies, which is highly safe and effective in mediating cellular cholesterol efflux
Studies in mice indicate CS6253 exerts ant-atherosclerosis and anti-diabetic effects
Acknowledgements
The author would like to thank Drs. Vasanthy Narayanaswami, Greg Hura, and Bo Hang for expert assistance elucidating the structure and biophysical properties of ATI-5261, CS6253 and their analogs. Appreciation is also extended to Drs. Jan Johansson and Salman Azhar for directing preclinical testing of the peptides in various animal models, including expertise related to safety and anti-diabetic actions. The excellent technical- and research-skills of Stefani Bittner and Juveria Tabassum (Azhar lab) is also greatly appreciated.
Financial support and sponsorship
The work was supported by funds from the Tobacco-Related Disease Research Program (TRDRP) of the state of California grant 17RT-0082, NIH grant R21-HL085791, and Artery Therapeutics. Work at Lawrence Berkeley National Laboratory was conducted under contract DE-AC02-05CH11231 with the United States Department of Energy, Office of Science, Office of Biological and Environmental Research.
Conflict of interest statement
JKB invented peptides ATI-5261, CS6253 and their analogs. The work produced several patent applications, which were filed at the US Patent and Trademark office on behalf of the United States Government, Department of Energy, by his employer Lawrence Berkeley National Laboratory (LBNL). LBNL has licensed these peptides to private investors to facilitate their clinical development. The author holds no position of employment, responsibility and/or decision making with Artery Therapeutics Inc. (ATI), but owns a small number of ATI shares as a result of past consulting activities with the company.
Cited Literature
- 1.Siddiqi HK, Kiss D, Rader D. HDL-cholesterol and cardiovascular disease: rethinking our approach. Curr Opin Cardiol. 2015;30:536–542. doi: 10.1097/HCO.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 2. Saleheen D, Scott R, Javad S, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabet Endochrin. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. Outstanding work revealing that serum cholesterol efflux capacity is independently associated with incident cardiovascular disease.
- 3.Vedhachalam C, Narayanaswami V, Neto N, et al. The C-terminal lipid-binding domain of apolipoproteinE is a highly efficient mediator of ABCA1-dependent cholesterol efflux that promotes the assembly of high-density lipoproteins. Biochemistry. 2007;46:2583–2593. doi: 10.1021/bi602407r. [DOI] [PubMed] [Google Scholar]
- 4.Heinecke JW. Small HDL promotes cholesterol efflux by the ABCA1 pathway in macrophages: implications for therapies targeted to HDL. Circ Res. 2015;116:1101–1103. doi: 10.1161/CIRCRESAHA.115.306052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westerterp M, Bochem AE, Yvan-Charvet L, et al. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ Res. 2014;114:157–170. doi: 10.1161/CIRCRESAHA.114.300738. [DOI] [PubMed] [Google Scholar]
- 7.Westerterp M, Murphy AJ, Wang M, et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelissen IC, Harris M, Rye KA, et al. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 9.Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res. 2006;47:2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.White CR, Garber DW, Anantharamaiah GM. Anti-inflammatory and cholesterol-reducing properties of apolipoprotein mimetics: a review. J Lipid Res. 2014;55:2007–2012. doi: 10.1194/jlr.R051367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoekenbroek RM, Stroes ES, Hovingh GK. ApoA-I mimetics - Handb. Exp Pharmacol. 2015;224:631–648. doi: 10.1007/978-3-319-09665-0_21. [DOI] [PubMed] [Google Scholar]
- 12.Getz GS, Reardon CA. The structure/function of apoprotein A-I mimetic peptides: an update. Curr Opin Endocrinol Diabetes Obes. 2014;21:129–133. doi: 10.1097/MED.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 13.Osei-Hwedieh DO, Amar M, Sviridov D, Remaley AT. Apolipoprotein mimetic peptides: mechanisms of action as anti-atherogenic agents. Pharmacol Ther. 2011;130:83–91. doi: 10.1016/j.pharmthera.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y, Patel AB, Narayanaswami V, et al. HDL mimetic peptide ATI-5261 forms an oligomeric assembly in solution that dissociates to monomers upon dilution. Biochemistry. 2011;50:4068–4076. doi: 10.1021/bi2002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bielicki JK, Zhang H, Cortez Y, et al. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J Lipid Res. 2010;51:1496–1503. doi: 10.1194/jlr.M003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Y, Patel AB, Narayanaswami V, Bielicki JK. Retention of α-helical structure by HDL mimetic peptide ATI-5261 upon extensive dilution represents an important determinant for stimulating ABCA1 cholesterol efflux with high efficiency. Biochem Biophys Res Comm. 2013;441:71–76. doi: 10.1016/j.bbrc.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Borhani DW, Rogers DP, Engler JA, Brouillette CG. Crystal structure of truncated human apolipoproteinA-I suggests a lipid-bound conformation. Proc Natl Acad Sci. 1997;94:12291–12296. doi: 10.1073/pnas.94.23.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choy N, Raussens V, Narayanaswami V. Inter-molecular coiled-coil formation in human apolipoprotein E C-terminal domain. J Mol Biol. 2003;334:527–539. doi: 10.1016/j.jmb.2003.09.059. [DOI] [PubMed] [Google Scholar]
- 19.Zhou NE, Kay CM, Hodges RS. Synthetic model proteins: positional effects of inter-chain hydrophobic interactions on stability of two-stranded α-helical coiled-coils. J Bio Chem. 1992;267:2664–2670. [PubMed] [Google Scholar]
- 20.Amar MJA, Sakurai T, Sakurai-Ikuta A, et al. A novel apolipoprotein C-II mimetic peptide that activates lipoprotein lipase and decreases serum triglycerides in apolipoprotein E-knockout mice. J. Pharmacol Exp Ther. 2015;352:227–235. doi: 10.1124/jpet.114.220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra VK, Palgunchari MN, Segrest JP, Anantharamaiah GM. Interactions of synthetic peptide analogs of the class A amphipathic helix with lipids: evidence for the snorkel hypothesis. J Biol Chem. 1994;269:7185–7191. [PubMed] [Google Scholar]
- 22.Anantharamaiah GM, Mishra VK, Garber DW, et al. Structural requirements for anti-oxidative and anti-inflammatory properties of apolipoprotein A-I mimetic peptides. J Lipid Res. 2007;48:1915–1923. doi: 10.1194/jlr.R700010-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Bielicki JK, Narayanaswami V, Hura G, et al. Mechanistic studies of HDL mimetic peptide ATI-5261 reveals aspects of class A α-helix structure that induce cytotoxicity and hypertriglyceridemia in vivo: design of safe analogs with potent anti-atherosclerosis and anti-diabetic actions. Arterio Thromb Vasc Biol. 2015;35:A28. [Google Scholar]
- 24.Drew BG, Rye KA, Duffy SJ, et al. The emerging role of HDL in glucose metabolism. Nature Reviews Endocrin. 2012;8:237–245. doi: 10.1038/nrendo.2011.235. [DOI] [PubMed] [Google Scholar]
- 25.Brunham LR, Kruit JK, Pape TD, et al. β-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nature Med. 2007;13:340–347. doi: 10.1038/nm1546. [DOI] [PubMed] [Google Scholar]
- 26.De Hann W, Karasinska JM, Ruddle P, Hayden MR. Hepatic ABCA1 expression improves β-cell function and glucose tolerance. Diabetes. 2014;63:4076–4082. doi: 10.2337/db14-0548. [DOI] [PubMed] [Google Scholar]
- 27.Umemoto T, Han CY, Mitra P, et al. Apolipoprotein AI and high-density lipoprotein have anti-inflammatory effects on adipocytes via cholesterol transporters, ATP-binding cassette A-I, ATP-binding cassette G-1, and scavenger receptor B-1. Circ Res. 2013;112:1345–1354. doi: 10.1161/CIRCRESAHA.111.300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Hann W, Bhattacharjee A, Ruddle P, et al. ABCA1 in adipocytes regulates adipose tissue lipid content, glucose tolerance, and insulin sensitivity. J Lipid Res. 2014;55:516–523. doi: 10.1194/jlr.M045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGrath KC, Li XH, Whitworth PT, et al. High density lipoproteins improve insulin sensitivity in high-fat diet-fed mice by suppressing hepatic inflammation. J Lipid Res. 2014;55:421–430. doi: 10.1194/jlr.M043281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalla-Riva J, Stenkula KG, Petriova J, Lagerstedt JO. Discoidal HDL and apoA-I-derived peptides improve glucose uptake in skeletal muscle. J Lipid Res. 2013;54:1275–1282. doi: 10.1194/jlr.M032904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson SJ, Drummond G, Kim DH, et al. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J Lipid Res. 2008;49:1658–1669. doi: 10.1194/jlr.M800046-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanella L, Li M, Kim DH, et al. ApoA1 mimetic peptide reverses adipocyte dysfunction in vivo and in vitro via an increase in heme oxygenase (HO-1) and Wnt10b. Cell Cycle. 2012;11:706–714. doi: 10.4161/cc.11.4.19125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehti M, Donelan E, Abplanalp W, et al. High-density lipoprotein maintains skeletal muscle function by modulating cellular respiration in mice. Circulation. 2013;128:2364–2371. doi: 10.1161/CIRCULATIONAHA.113.001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pahnke J, Frohlich C, Krohn M, et al. Impaired mitochondrial energy production and ABC transporter function – a crucial interconnection in dementing proteopathies of the brain. Mech Aging and Develop. 2013;134:506–515. doi: 10.1016/j.mad.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Graham A, Allen A. Mitochondrial function and regulation of macrophage sterol metabolism and inflammatory responses. World J Cardiol. 2015;7:277–286. doi: 10.4330/wjc.v7.i5.277. Interesting work describing how mitochondrial function regulates the expression of genes involved in cholesterol metabolism and efflux, via production of oxysterols.
- 36.Allen A, Graham A. Mitochondrial function is involved in regulation of cholesterol efflux to apolipoprotein (apo) A-I from murine RAW 264-7 macrophages. Lipid in Health and Disease. 2012;11:169–177. doi: 10.1186/1476-511X-11-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karunakaran D, Thrush AB, Nguyen M, et al. Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by anti-miR33 in atherosclerosis. Circ Res. 2015;117:266–278. doi: 10.1161/CIRCRESAHA.117.305624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato M, Nonaka T, Maki M, et al. Caspases cleave the amino-terminal calpain inhibitory unit of calpastatin during apoptosis in human Jurkat T cells. J Biochem. 2000;127:297–305. doi: 10.1093/oxfordjournals.jbchem.a022607. [DOI] [PubMed] [Google Scholar]
- 39.Nordestgaard LT, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Loss-of-function mutation in ABCA1 and risk of Alzheimer’s disease and cerebrovascular disease. Alzheimer’s Dement. 2015 doi: 10.1016/j.jalz.2015.04.006. pii: S1552-5260(15)00180-6. doi: 1016/j.jalz/2015.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Pahnke J, Langer O, Krohn M. Alzheimer’s and ABC transporters – new opportunities for diagnostics and treatment. Neurobiology of Disease. 2014;72:54–60. doi: 10.1016/j.nbd.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boehm-Cagan A, Michaelson DM. Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene. J Neuroscience. 2014;34:7293–7301. doi: 10.1523/JNEUROSCI.5198-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wahrle S, Jiang H, Parsadanian M, et al. ABCA1 is required for normal central nervous system apoE levels and for lipidation of astrocyte-secreted apoE. J. Biol Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- 43.Fitz NF, Cronican AA, Saleem M, et al. Abca1 deficiency affects Alzheimer’s disease-like phenotype in human apoE4 but not apoE3-targeted replacement mice. J Neuroscience. 2012;32:13125–13136. doi: 10.1523/JNEUROSCI.1937-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]