Abstract
Background
Smoking cessation after acute myocardial infarction (AMI) decreases the risk of recurrent AMI and mortality by 30–40%, but many patients continue to smoke. The association of smoking with angina and health-related quality of life (HRQOL) after AMI is unclear.
Methods and Results
Patients in 2 U.S. multicenter AMI registries (n=4003) were assessed for smoking and HRQOL at admission and 1, 6 and 12 months after AMI. Angina and HRQOL were measured with the Seattle Angina Questionnaire (SAQ) and Short Form-12 (SF-12) physical and mental component scales (PCS and MCS). At admission, 29% never had smoked, 34% were former smokers (quit before AMI) and 37% were active smokers, of whom 46% quit by 1 year (recent quitters). In hierarchical, multivariable, regression models that adjusted for sociodemographic, clinical and treatment factors, never and former smokers had similar and the best HRQOL in all domains. Recent quitters had intermediate HRQOL levels, with angina and SF-12 MCS scores similar to never smokers. Persistent smokers had worse HRQOL in all domains compared to never smokers and worse SF-12 MCS scores than recent quitters.
Conclusion
Smoking after AMI is associated with more angina and worse HRQOL in all domains, while smokers who quit after AMI have similar angina levels and mental health as never smokers. These observations may help encourage patients to stop smoking after AMI.
Keywords: smoking, angina, quality of life, myocardial infarction
Smoking is common among patients presenting with acute myocardial infarction (AMI) and represents an important modifiable risk factor for recurrent events. Smoking cessation after AMI decreases the risk of recurrent MI and mortality by 30–50%.1–4 Although efforts to improve smoking cessation after AMI have become important performance measures for both hospitals5 and outpatient clinics,6 a large percentage of smokers do not quit after their AMI.3, 4, 7 While current educational strategies focus upon the risks of continued smoking, patients may be concerned that smoking cessation will lead to worse quality of life (e.g., increased negative affect). Such concerns may lower patients’ motivation and success with quitting.8
Patients recovering from an AMI currently have no information about how smoking cessation might alter their health status (their symptoms, function and quality of life). Although it is well established that smoking after AMI is associated with a significantly greater risk of recurrent MI and mortality,1–4 there are few studies describing how smoking relates to health-related quality of life (HRQOL) in cardiac patients,9–15 despite HRQOL often being equally or more important to patients than longevity.16, 17 Better illuminating the health status impact of continued smoking after AMI might support improved counseling of patients to quit.
To address this current gap in knowledge, we sought to describe the association between smoking status and patient HRQOL in a cohort of 4003 post-AMI subjects using data from 2 large, prospective, observational registries. Understanding the association of smoking cessation with HRQOL could have important implications for smoking prevention and the treatment of patients who are actively smoking at the time of AMI.
METHODS
Study Population and Protocol
Our analytic cohort was derived from two consecutive multicenter, prospective cohort studies of patients hospitalized with AMI in the U.S. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER) study enrolled 2498 patients with AMI from 19 US hospitals between January 2003 and June 2004.18 Similarly, 4340 patients with AMI from 24 US hospitals were enrolled into the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status (TRIUMPH) study between June 2005 and December 2008 (12 hospitals participated in both studies).19 Both studies were coordinated by Saint Luke’s Mid America Heart Institute and employed identical inclusion and exclusion criteria. Eligible patients had biomarker evidence of myocardial necrosis and additional clinical evidence supporting the diagnosis of an AMI (e.g., prolonged ischemic signs/symptoms or electrocardiographic ST changes during the initial 24 hours of admission).
Baseline data, including smoking status and health status data, were obtained through chart abstraction and a structured interview by trained research staff within 24 to 72 hours of admission. Detailed follow-up interviews were attempted on all survivors at 1, 6, and 12 months after AMI. To be eligible for the current study, patients had to survive to 1 year and not be discharged against medical advice, to another acute-care facility, or to hospice. Each participating hospital obtained Institutional Research Board approval, and all patients provided written informed consent for baseline and follow-up assessments.
Smoking Status
Smoking was assessed at baseline and each follow-up time point using the following options: (1) I have never smoked, not even a puff, (2) I have smoked in the past but less than 100 cigarettes total, (3) I stopped smoking more than 1 year ago, (4) I stopped smoking between 1 month and 1 year ago, and (5) I have smoked (even a puff) in the past 30 days.” At baseline, responses (1) and (2) were categorized as “never smokers”. Responses (3) and (4) were categorized as “former smokers”, and response (5) was categorized as “current smokers.” Those who were “current smokers” at baseline were then reclassified based on their 1-year follow-up interview responses. At 1 year, those who responded that they had quit in the past year were designated “recent quitters” while those who continued to smoke were designated “persistent smokers.” Patients with inconsistent answers at baseline and 1 year (e.g., reported being a former smoker at baseline and never smoker at 1 year) and patients who started smoking during the year (e.g., reported being a former smoker at baseline and a current smoker at 1 year) were excluded from the analyses.
Health-Related Quality of Life
Disease-specific and generic HRQOL were measured using the Seattle Angina Questionnaire (SAQ)20 and the Medical Outcomes Study 12-item Short Form (SF-12).21 The SAQ is a reliable, responsive, and valid 19-item questionnaire that assesses the symptoms, function, and quality of life (QOL) of patients with coronary artery disease and has been shown to be associated with mortality, admission for acute coronary syndrome, and healthcare costs.22, 23 For this study, we focused on the SAQ angina frequency and QOL domains. Scores range from 0 to 100, with higher scores indicating less disease burden. Based on prior work, SAQ angina frequency was categorized into the clinically interpretable framework of no (score 100) vs. any (score <100) angina.24 The SF-12 is a reliable and valid measure of generic health status that provides summary component scales for overall physical (PCS) and mental (MCS) health.25 Scores are standardized using norm-based methods to a mean of 50 and a standard deviation of 10 with higher scores indicating better health status.21
Statistical Analysis
The purpose of these analyses was to describe the association between smoking status and patient HRQOL 1 year after AMI. Baseline characteristics and post- AMI treatment of the 4 smoking groups (defined at the 12-month time point as ‘never smokers’, ‘former smokers’,’ recent quitters’, and ‘persistent smokers’) were compared using the chi-square test for categorical variables and one-way ANOVA for continuous variables. To describe the HRQOL profile over the course of 1-year after MI across the full spectrum of smoking status categories, unadjusted baseline and 1-, 6-, and 12-month follow-up HRQOL scores were compared among smoking status groups using ANOVA.
We then constructed multivariable regression models to assess the independent association of 1-year smoking status with HRQOL at one year following AMI. All models were hierarchical with site entered as a random effect to account for clustering of patients within sites. Models were logistic (SAQ angina) or linear (SAQ QOL, SF-12), as appropriate. For all models, never smokers were used as the reference group and then recent quitters were also compared to persistent smokers. As smoking might be associated with several factors that may also affect HRQOL, we performed sequential multivariable adjustment to better examine the effect of adjustment on the independent association between smoking and HRQOL. We serially adjusted for factors in the order of basic demographics (age, sex, race), clinical comorbidities (hypertension, diabetes, prior MI, prior angioplasty, prior bypass graft surgery, heart failure, ST-elevations at presentation, LV systolic dysfunction [ejection fraction <40%], lung disease, hypercholesterolemia, and history of renal failure), treatment during the index hospitalization (angioplasty, bypass graft surgery, and % of quality of care measures received [e.g., timely reperfusion for ST-elevations, beta-blocker at discharge, etc.]), post-MI treatment during the year following the index hospitalization (revascularization within 1 year; whether the patient was prescribed antiplatelet, beta blocker, statin, or angiotensin converting enzyme [ACE]/angiotensin II receptor blocker [ARB] therapy and whether the patient reported taking these medications at follow-up; and patient report of participation in cardiovascular rehabilitation), sociodemographic characteristics (marital status, education, insurance status, self-reported monthly financial reserve, and self-reported avoiding medication due to costs), and psychosocial characteristics of social support (assessed with the Enhancing Recovery in Coronary Heart Disease [ENRICHD] Social Support Inventory)26 and symptoms of depression (assessed with the 9-item Patient Health Questionnaire [PHQ-9]27).
Baseline data were fairly complete, with only 22% missing 1 covariate and 13% missing more than 1 and an average of 0.68 covariates missing per patient. Missing covariate data were imputed using multiple imputation incorporating all baseline and outcome variables. To examine the possibility of selection bias, we compared the baseline characteristics, including baseline smoking status, of those included in the analysis with those excluded due to missing or inconsistent 1-year follow-up data.
All statistical analyses were conducted using SAS Version 9.3 (SAS Institute, Cary, NC), IVEware (University of Michigan, MI), and R Version 2.11.1 (Free Software Foundation, Boston, MA).28 All tests for statistical significance were 2-tailed and were evaluated at a significance level of 0.05.
RESULTS
Patient Population
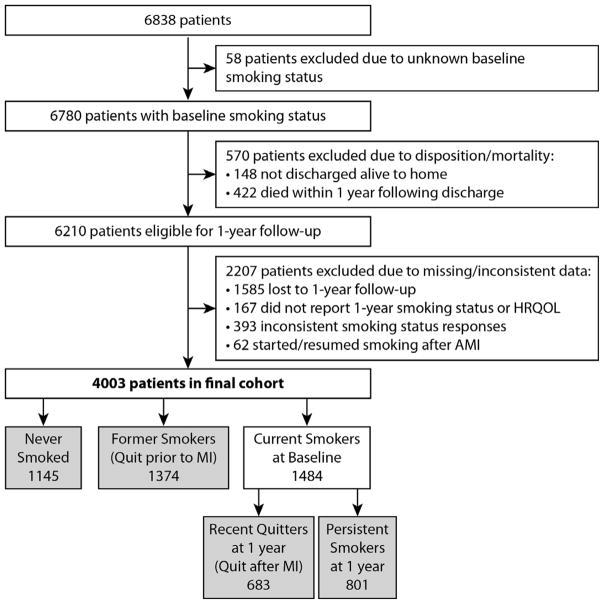
Among the 6838 patients included in TRIUMPH and PREMIER, baseline smoking status was obtained on 6780. We excluded 148 patients who were not discharged alive to home and 422 patients who did not survive to the 1-year interview. Additionally, we excluded 1585 (23%) patients who did not participate in the 1-year interview, 167 patients who participated in the interview but did not report 1-year smoking status or HRQOL, and 455 patients who gave inconsistent smoking status answers (n=393; e.g., reported being a former smoker at baseline but at 1 year reported having never smoked) or who started smoking during the year following AMI (n=62). As such, the final analytic cohort was comprised of 4003 patients (Figure 1).
Figure 1.
Flowchart of patient inclusion and exclusion in the study
Patients who were excluded due to missing or inconsistent smoking status data (n=2207), as compared with those included in the analysis (n=4003), were more likely to be younger, non-Caucasian, have poorer socioeconomic status, have cardiac and non-cardiac comorbidities, undergo less revascularization, and take less cardiac medications (Supplemental Table 1). However, there was no difference in baseline smoking status between those with and without missing/inconsistent data (p=0.116).
Description of Smoking Groups
At the time of admission for AMI, 29% of patients reported that they were life-long non-smokers, 34% were former smokers (i.e., quit prior to AMI), and 37% had smoked within 30 days of the AMI. Of those currently smoking at the time of their AMI, 46% quit smoking during the following year and were classified as recent quitters, while 54% were persistent smokers 1 year after their AMI. The baseline characteristics and post-AMI treatment of never smokers, former smokers, recent quitters, and persistent smokers are presented in Table 1. There were numerous differences among the 4 smoking status groups. For example, those who were smoking at the time of their MI, compared to those who were not, tended to be about 10 years younger, unmarried, less educated, of lower socioeconomic status, have higher depression scores, and have less comordities. They were also more likely to present with an ST-elevation MI and be treated with percutaneous coronary intervention during their index hospitalization, but less likely to participate in cardiac rehabilitation after their AMI.
Table 1.
Comparison of Baseline Characteristics and Post-AMI Treatment by 1-Year Smoking Status
| Never Smoked n = 1145 | Former Smokers n = 1374 | Recent Quitters n = 683 | Persistent Smokers n = 801 | P-Value | |
|---|---|---|---|---|---|
| Basic Demographics | |||||
| Age | 62.8 ± 13.3 | 64.1 ± 10.7 | 54.6 ± 9.8 | 54.6 ± 9.8 | < 0.001 |
| Male | 55.4% | 74.9% | 71.2% | 66.7% | < 0.001 |
| Caucasian | 75.7% | 84.0% | 72.7% | 69.8% | < 0.001 |
| Sociodemographics | |||||
| Marriage | 63.1% | 69.5% | 59.7% | 45.9% | < 0.001 |
| Less than high school education | 41.3% | 44.9% | 50.4% | 57.5% | < 0.001 |
| Insurance coverage for medications | 81.0% | 82.8% | 72.6% | 66.5% | < 0.001 |
| Monthly financial situation Not enough to make ends meet | 12.6% | 10.3% | 18.6% | 27.7% | < 0.001 |
| Avoided health care due to cost | 14.7% | 13.8% | 25.7% | 34.6% | < 0.001 |
| Psychosocial factors | |||||
| ENRICHD Social Support Score | 22.7 ± 3.7 | 22.7 ± 3.7 | 22.1 ± 4.1 | 21.2 ± 5.0 | < 0.001 |
| PHQ Depression Score | 4.5 ± 4.9 | 4.7 ± 4.9 | 5.2 ± 5.3 | 6.4 ± 5.8 | < 0.001 |
| Clinical Co-morbidities | |||||
| Hypertension | 65.5% | 68.2% | 52.4% | 58.8% | < 0.001 |
| Diabetes | 30.9% | 30.9% | 20.5% | 20.2% | < 0.001 |
| Prior MI | 17.4% | 22.6% | 12.3% | 20.3% | < 0.001 |
| Prior PCI | 15.5% | 23.3% | 11.7% | 17.4% | < 0.001 |
| Prior CABG | 12.1% | 17.8% | 5.4% | 6.9% | < 0.001 |
| Congestive Heart Failure | 7.8% | 8.9% | 2.9% | 6.5% | < 0.001 |
| STEMI | 41.5% | 42.3% | 58.7% | 49.7% | < 0.001 |
| LV systolic dysfunction | 18.8% | 20.4% | 20.1% | 17.8% | 0.425 |
| Hypercholesterolemia | 51.9% | 57.0% | 41.0% | 44.6% | < 0.001 |
| Chronic Renal Failure | 7.7% | 8.2% | 2.5% | 4.1% | < 0.001 |
| Chronic Lung Disease | 5.7% | 10.1% | 7.0% | 10.4% | < 0.001 |
| Treatment during index hospitalization | |||||
| In-hospital PCI | 63.2% | 66.7% | 75.8% | 72.7% | < 0.001 |
| In-hospital CABG | 10.7% | 13.0% | 12.2% | 6.2% | < 0.001 |
| <100% of Quality of Care Measures Received | 27.3% | 29.3% | 41.3% | 40.1% | < 0.001 |
| Post-MI treatment within 1 year of index hospitalization | |||||
| Revascularization | 7.2% | 8.2% | 7.9% | 6.4% | 0.459 |
| Antiplatelet use | 83.1% | 87.8% | 90.7% | 85.3% | < 0.001 |
| Beta blocker use | 71.0% | 73.8% | 75.4% | 68.8% | 0.022 |
| Statin use | 64.8% | 69.0% | 71.5% | 63.7% | 0.003 |
| ACE/ARB use | 50.5% | 51.0% | 56.7% | 53.1% | 0.061 |
| Cardiac Rehabilitation | 54.3% | 54.2% | 50.8% | 35.0% | < 0.001 |
Continuous variables compared using one-way analysis of variance. Categorical variables compared using chi-square or Fisher's exact test.
Unadjusted Analyses at Baseline, 1-, 6-, and 12-months
Overall, all groups showed improvement from baseline to 1 year in SAQ angina, SAQ QOL, and SF-12 MCS, but little or no improvement in SF-12 PCS. In unadjusted comparisons, there were statistically significant differences (p < 0.001) across the 4 smoking status groups for each HRQOL domain at baseline and 1, 6, and 12 months after AMI. At each separate time-point, there was a gradation of more angina and worse disease-specific and generic HRQOL across the 4 smoking status groups from never smokers having the best health status and persistent smokers the worst (Supplemental Table 2 and Supplemental Figure 1).
Multivariable Analyses of 1-year Smoking Status and 1-year HRQOL Domains
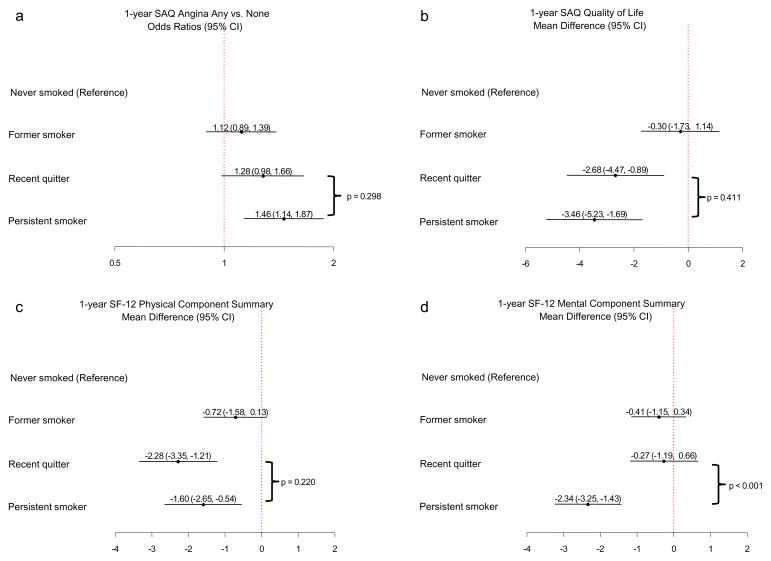
The associations between smoking status and each domain of 1-year HRQOL were somewhat attenuated at each step of sequential adjustment, but no particular step drastically changed the step-wise associations between smoking status and 1-year HRQOL, indicating that no particular factor category accounted for a greater portion of variance in the sequential analysis than other categories (Supplemental Figure 2). Thus, the final fully-adjusted models are shown as the primary results (Figure 2a–d). In the fully-adjusted models with never smokers as the reference group, former smokers were statistically similar to never smokers for all HRQOL domains. Recent quitters varied in the degree they were similar to and different from never smokers, depending upon the HRQOL domain. There were no statistically significant differences between recent quitters and never smokers in the odds of having angina at 1 year (Figure 2a) and in SF-12 MCS scores (Figure 2d). Recent quitters had worse SAQ QOL and SF-12 PCS scores compared with never smokers, having a 2.7-points lower score on the SAQ QOL scale (95% CI -4.5 to −0.9, p = 0.003) and a 2.3-points lower score on the SF-12 PCS (95% CI −3.4 to −1.2, p < 0.001) (Figures 2b and c). Persistent smokers had worse HRQOL than never smokers for all domains. Persistent smokers had a 1.5-fold increased odds of having angina at 1 year as compared with never smokers (95% CI 1.1 to 1.9, p = 0.003), 3.5-points lower scores on the SAQ QOL scale (95% CI -5.2 to −1.7, p < 0.001), 1.6 points less on the SF-12 PCS (95% CI −2.7 to -0.5, p = 0.003), and 2.3 points less on the SF-12 MCS (95% CI −3.3 to −1.4, p < 0.001) (Figures 2a–d).
Figure 2.
a-d. Multivariable analyses of the association of 1-year smoking status with HRQOL domains at 1 year following AMI.
In fully-adjusted models comparing recent quitters with persistent smokers, recent quitters were statistically similar to persistent smokers for angina (p = 0.298), SAQ QOL (p = 0.411), and SF-12 PCS (p = 0.220) (Figures 2a–c). However, recent quitters had better SF-12 MCS scores compared with persistent smokers (p < 0.0001) (Figure 2d).
DISCUSSION
In a large, multicenter cohort of AMI patients, we found a gradation in the association of smoking status with angina and HRQOL. Those who had never smoked had the least angina and the best disease-specific and general HRQOL. Based on adjusted analyses, those who quit prior to their AMI (former smokers) were very similar to those who had never smoked in all HRQOL domains. In contrast, those who continued to smoke after their AMI (persistent smokers) were significantly worse than those who had never smoked in all HRQOL domains. Those who quit smoking after their AMI (recent quitters) had intermediate levels of HRQOL, with their experience of angina and mental health status being similar to those who never smoked. Furthermore, recent quitters had markedly better general mental health than persistent smokers.
Our findings support and extend prior studies that have reported smoking to be associated with poorer HRQOL;9, 10, 12 while other studies have reported that smoking is not associated with HRQOL13 and that smoking cessation may, in fact, be associated with poorer HRQOL for some patients.14 In terms of disease-specific HRQOL, our findings complement those from studies of patients undergoing PCI, which demonstrated that never and former smokers have similar levels of angina and angina-related quality of life that are significantly better than that of those who persist in smoking.10, 11 Jang et al. further found that patients who quit smoking within 1 year after PCI had less angina than those who continued to smoke.11 In our study, although recent quitters and persistent smokers still had similar levels of angina, recent quitters did not have more angina compared to never smokers whereas persistent smokers had more angina than never smokers.
There are several potential mechanisms underlying the association between smoking and angina. Exposure to toxic compounds contained in cigarette smoke causes free-radical mediated oxidative stress and decreased nitric oxide bioavailability resulting in decreased endothelium-dependent vasodilatation.29–32 Studies have shown that smoking increases mean platelet volume, platelet activation, platelet aggregation and non-responsiveness to clopidogrel.33–36 Smoking also causes endothelial damage and activation of inflammation pathways resulting in increased expression of pro-inflammatory cytokines, adhesion molecules and other prothrombotic factors.37–39 Damage to the endothelium allows the subendothelium to be exposed to these factors as well as platelets with increased activity and the overall result can promote thrombus formation. Moreover, in patients with coronary artery disease, smoking has been associated with increased adrenergic tone and increased coronary vasospasm.37, 40
In terms of general HRQOL, our results are also consistent with those of Stafford et al.15 who looked at this association in a broad group of patients with coronary artery disease and showed a stronger inverse association between smoking status and general mental health than with general physical health. In our study, even after adjusting for social support and depressive symptoms, the general mental health status of both former and recent quitters was very similar to that of never smokers. Furthermore, recent quitters had substantially better general mental health status than persistent smokers. Our findings are also consistent with a recent systematic review and meta-analysis that concluded that smoking cessation is associated with improved mental health compared with continued smoking in both general and clinical populations . 41 There are likely to be complex mechanisms underlying the association of smoking and poor mental health that involve biological, behavioral, and environmental factors. For example, there is evidence of shared biological pathways of both smoking and depression with coronary artery disease as smoking has been associated with oxidative stress42 and increases in C-reactive protein induced inflammation43 which have been depicted in the pathophysiology of both depression and cardiovascular disorders.44–47
Similar to the approach of Smedt et al.12 and Jang et al.,11 our study extends the insights from prior studies on smoking status and HRQOL in coronary heart disease patients by comparing HRQOL across 4 smoking status categories that address the full smoking status spectrum: never smokers, former smokers (quit before AMI), recent quitters (quit after AMI), and persistent smokers (continued to smoke after AMI). However, in contrast to previous studies, our study included only subjects with AMI and is therefore directly relevant to post-AMI patients. Furthermore, our study used HRQOL measures specific to coronary artery disease, which greatly strengthens our findings and conclusions as they are specific to AMI patients who smoke. In fact, our study is the largest known study to examine the disease-specific manifestations of continued smoking after AMI.
It is important to interpret our findings through a clinical framework. In our attempt to isolate the effect of smoking with HRQOL, we knowingly adjusted for multiple covariates that are associated with both smoking and HRQOL (e.g., depression, socioeconomic status). As such, the small group differences in the multivariable analyses are potentially due to overadjustment and the complex inter-relationships between smoking and other variables, such as depressive symptoms, may underestimate the HRQOL benefits of quitting smoking (e.g., patients who quit may also become less depressed). In addition, it should be noted that the HRQOL mean differences are reported at the population level rather than at a patient level. As such, our findings likely indicate that smoking cessation is associated with a large HRQOL improvement in some patients while others may have no benefit. Finally, noting that former smokers were statistically similar to never smokers indicates that there may be a time-dependent effect of smoking on health status. Patients categorized as recent quitters could have quit just over 30 days prior to their 1-year follow-up interview after their AMI and yet it could be that longer durations of smoking cessation are associated with better HRQOL. Understanding the long-term effect of smoking cessation on HRQOL and the time-dependence of this effect will require longer study. However, even in the absence of large improvements in HRQOL with smoking cessation, we have, at a minimum, shown that smoking cessation is not associated with worse HRQOL. In addition, our results—showing better mental health for those who quit smoking—may provide helpful information for those concerned that smoking cessation may have a detrimental effect on mental health.
Our results must be interpreted in light of several study limitations. First, patient self-report was used to determine smoking status and these reports were not accompanied with biochemical measures for confirmation. However, research shows that self-report is a valid indicator of current smoking, especially when there are no strong incentives to deceive.48, 49 Second, some studies have shown that decreasing intensity (pack-years and/or number of cigarettes per day) can improve HRQOL.3 As we did not assess intensity of smoking we cannot comment on whether there were differences in angina and HRQOL within the ‘current smoker’ group associated with different smoking intensity subgroups. Third, we excluded a large number of subjects due to missing or inconsistent data. This could limit study generalizabilty and introduce possible selection bias as those excluded for missing/inconsistent data did differ from those included on several baseline characteristics and treatment factors that could be associated with smoking cessation and HRQOL. However, those excluded for missing/inconsistent data did not differ in baseline smoking status as compared with those we included. Finally, while we adjusted for many clinical and sociodemographic factors that may confound the association between HRQOL and smoking, there is always the potential for unmeasured confounding in any observational study.
In conclusion, we observed, in a large multicenter cohort of post-AMI patients, that persistent smoking after the AMI is associated with significantly more angina and worse disease-specific and general HRQOL. Those who quit smoking prior to their AMI were similar to those who had never smoked in all HRQOL domains. Within one year, the odds of having angina and the general mental health status of those who quit smoking after their AMI was also similar to those who had never smoked. Further study of the time-dependent effect of longer-term smoking cessation on HRQOL might determine if those who quit smoking after their AMI might see benefits in additional areas of HRQOL with increased time of abstinence. Our findings provide unique information to post-AMI patients and should provide strong support for counseling patients about how smoking cessation not only reduces the risk of MI and death, but is also associated with better health status over time. As such, these observations may offer current smokers increased incentive and motivation for quitting.
Supplementary Material
Acknowledgments
FUNDING SOURCES
Funding support was received for the PREMIER Registry from CV Therapeutics, Inc. and for the TRIUMPH Registry from the National Heart Lung and Blood Institute (P50 HL077113). S. Cresci’s effort is supported in part by the National Institutes of Health (Cresci R01 NR013396).
Footnotes
DISCLOSURES
J.A. Spertus owns the copyright for the Seattle Angina Questionnaire.
References
- 1.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290:86–97. doi: 10.1001/jama.290.1.86. [DOI] [PubMed] [Google Scholar]
- 2.Wilson K, Gibson N, Willan A, Cook D. Effect of smoking cessation on mortality after myocardial infarction: meta-analysis of cohort studies. Arch Intern Med. 2000;160:939–44. doi: 10.1001/archinte.160.7.939. [DOI] [PubMed] [Google Scholar]
- 3.Gerber Y, Rosen LJ, Goldbourt U, Benyamini Y, Drory Y. Smoking status and long-term survival after first acute myocardial infarction a population-based cohort study. J Am Coll Cardiol. 2009;54:2382–7. doi: 10.1016/j.jacc.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Rea TD, Heckbert SR, Kaplan RC, Smith NL, Lemaitre RN, Psaty BM. Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med. 2002;137:494–500. doi: 10.7326/0003-4819-137-6-200209170-00009. [DOI] [PubMed] [Google Scholar]
- 5.Krumholz HM, Anderson JL, Bachelder BL, Fesmire FM, Fihn SD, Foody JM, Ho PM, Kosiborod MN, Masoudi FA, Nallamothu BK. ACC/AHA 2008 performance measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to develop performance measures for ST-elevation and non-ST-elevation myocardial infarction): developed in collaboration with the American Academy of Family Physicians and the American College of Emergency Physicians: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Cardiovascular Angiography and Interventions, and Society of Hospital Medicine. Circulation. 2008;118:2596–648. doi: 10.1161/CIRCULATIONAHA.108.191099. [DOI] [PubMed] [Google Scholar]
- 6.Drozda J, Jr, Messer JV, Spertus J, Abramowitz B, Alexander K, Beam CT, Bonow RO, Burkiewicz JS, Crouch M, Goff DC, Jr, Hellman R, James T, 3rd, King ML, Machado EA, Jr, Ortiz E, O'Toole M, Persell SD, Pines JM, Rybicki FJ, Sadwin LB, Sikkema JD, Smith PK, Torcson PJ, Wong JB. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with coronary artery disease and hypertension: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement. Circulation. 2011;124:248–70. doi: 10.1161/CIR.0b013e31821d9ef2. [DOI] [PubMed] [Google Scholar]
- 7.Dawood N, Vaccarino V, Reid KJ, Spertus JA, Hamid N, Parashar S. Predictors of smoking cessation after a myocardial infarction: the role of institutional smoking cessation programs in improving success. Arch Intern Med. 2008;168:1961–7. doi: 10.1001/archinte.168.18.1961. [DOI] [PubMed] [Google Scholar]
- 8.McKee SA, O'Malley SS, Salovey P, Krishnan-Sarin S, Mazure CM. Perceived risks and benefits of smoking cessation: gender-specific predictors of motivation and treatment outcome. Addict Behav. 2005;30:423–35. doi: 10.1016/j.addbeh.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Taira DA, Seto TB, Ho KK, Krumholz HM, Cutlip DE, Berezin R, Kuntz RE, Cohen DJ. Impact of smoking on health-related quality of life after percutaneous coronary revascularization. Circulation. 2000;102:1369–74. doi: 10.1161/01.cir.102.12.1369. [DOI] [PubMed] [Google Scholar]
- 10.Haddock CK, Poston WS, Taylor JE, Conard M, Spertus J. Smoking and health outcomes after percutaneous coronary intervention. Am Heart J. 2003;145:652–7. doi: 10.1067/mhj.2003.67. [DOI] [PubMed] [Google Scholar]
- 11.Jang JS, Buchanan DM, Gosch KL, Jones PG, Sharma PK, Shafiq A, Grodzinsky A, Fendler TJ, Graham G, Spertus JA. Association of Smoking Status with Health-Related Outcomes after Percutaneous Coronary Interventions. Circ Cardiovasc Interv. 2015;8:e002226. doi: 10.1161/CIRCINTERVENTIONS.114.002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smedt DD, Clays E, Annemans L, Boudrez H, Sutter JD, Doyle F, Jennings C, Kotseva K, Pajak A, Pardaens S, Prugger C, Wood D, Bacquer DD. The association between self-reported lifestyle changes and health-related quality of life in coronary patients: the EUROASPIRE III survey. Eur J Prev Cardiol. 2013;21:796–805. doi: 10.1177/2047487312473846. [DOI] [PubMed] [Google Scholar]
- 13.Quist-Paulsen P, Bakke PS, Gallefoss F. Does smoking cessation improve quality of life in patients with coronary heart disease? Scand Cardiovasc J. 2006;40:11–6. doi: 10.1080/14017430500384855. [DOI] [PubMed] [Google Scholar]
- 14.Wiggers LC, Oort FJ, Peters RJ, Legemate DA, de Haes HC, Smets EM. Smoking cessation may not improve quality of life in atherosclerotic patients. Nicotine Tob Res. 2006;8:581–9. doi: 10.1080/14622200600790005. [DOI] [PubMed] [Google Scholar]
- 15.Stafford L, Berk M, Jackson HJ. Tobacco smoking predicts depression and poorer quality of life in heart disease. BMC Cardiovasc Disord. 2013;13:35. doi: 10.1186/1471-2261-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–24. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 17.Tsevat J, Dawson NV, Wu AW, Lynn J, Soukup JR, Cook EF, Vidaillet H, Phillips RS. Health values of hospitalized patients 80 years or older. HELP Investigators. Hospitalized Elderly Longitudinal Project. JAMA. 1998;279:371–5. doi: 10.1001/jama.279.5.371. [DOI] [PubMed] [Google Scholar]
- 18.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)- -evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–97. doi: 10.1016/j.ahj.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–76. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–41. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 21.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43–9. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 23.Arnold SV, Morrow DA, Lei Y, Cohen DJ, Mahoney EM, Braunwald E, Chan PS. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN-TIMI 36 trial. Circ Cardiovasc Qual Outcomes. 2009;2:344–53. doi: 10.1161/CIRCOUTCOMES.108.829523. [DOI] [PubMed] [Google Scholar]
- 24.Spertus JA, Salisbury AC, Jones PG, Conaway DG, Thompson RC. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110:3789–94. doi: 10.1161/01.CIR.0000150392.70749.C7. [DOI] [PubMed] [Google Scholar]
- 25.Muller-Nordhorn J, Roll S, Willich SN. Comparison of the short form (SF)-12 health status instrument with the SF-36 in patients with coronary heart disease. Heart. 2004;90:523–7. doi: 10.1136/hrt.2003.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell PH, Powell L, Blumenthal J, Norten J, Ironson G, Pitula CR, Froelicher ES, Czajkowski S, Youngblood M, Huber M, Berkman LF. A short social support measure for patients recovering from myocardial infarction: the ENRICHD Social Support Inventory. J Cardiopulm Rehabil. 2003;23:398–403. doi: 10.1097/00008483-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 28.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A Multivariate Technique for Multiply Imputing Missing Values Using a Sequence of Regression Models. Survey Methodology. 2001;27 [Google Scholar]
- 29.Smith CJ, Fischer TH. Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis. 2001;158:257–67. doi: 10.1016/s0021-9150(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 30.Barua RS, Ambrose JA, Srivastava S, DeVoe MC, Eales-Reynolds LJ. Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: an in vitro demonstration in human coronary artery endothelial cells. Circulation. 2003;107:2342–7. doi: 10.1161/01.CIR.0000066691.52789.BE. [DOI] [PubMed] [Google Scholar]
- 31.Iwata K, Iida H, Iida M, Takenaka M, Tanabe K, Fukuoka N, Uchida M. Nicorandil protects pial arterioles from endothelial dysfunction induced by smoking in rats. J Neurosurg Anesthesiol. 2013;25:392–8. doi: 10.1097/ANA.0b013e318295aa93. [DOI] [PubMed] [Google Scholar]
- 32.Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. 2013;10:219–30. doi: 10.1038/nrcardio.2013.8. [DOI] [PubMed] [Google Scholar]
- 33.Asher E, Fefer P, Shechter M, Beigel R, Varon D, Shenkman B, Savion N, Hod H, Matetzky S. Increased mean platelet volume is associated with non-responsiveness to clopidogrel. Thromb Haemost. 2014;112:137–41. doi: 10.1160/TH13-10-0845. [DOI] [PubMed] [Google Scholar]
- 34.Fusegawa Y, Goto S, Handa S, Kawada T, Ando Y. Platelet spontaneous aggregation in platelet-rich plasma is increased in habitual smokers. Thromb Res. 1999;93:271–8. doi: 10.1016/s0049-3848(98)00184-4. [DOI] [PubMed] [Google Scholar]
- 35.Imaizumi T, Satoh K, Yoshida H, Kawamura Y, Hiramoto M, Takamatsu S. Effect of cigarette smoking on the levels of platelet-activating factor-like lipid(s) in plasma lipoproteins. Atherosclerosis. 1991;87:47–55. doi: 10.1016/0021-9150(91)90231-q. [DOI] [PubMed] [Google Scholar]
- 36.Cho SY, You E, Lee HJ, Lee WI, Park TS. Smoking cession decreases mean platelet volume in healthy Korean populations. Clin Lab. 2014;60:1413–6. doi: 10.7754/clin.lab.2013.130901. [DOI] [PubMed] [Google Scholar]
- 37.Barua RS, Ambrose JA. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler Thromb Vasc Biol. 2013;33:1460–7. doi: 10.1161/ATVBAHA.112.300154. [DOI] [PubMed] [Google Scholar]
- 38.Collins T. Endothelial nuclear factor-kappa B and the initiation of the atherosclerotic lesion. Lab Invest. 1993;68:499–508. [PubMed] [Google Scholar]
- 39.Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009;102:248–57. doi: 10.1160/TH09-03-0192. [DOI] [PubMed] [Google Scholar]
- 40.Zhu BQ, Parmley WW. Hemodynamic and vascular effects of active and passive smoking. Am Heart J. 1995;130:1270–5. doi: 10.1016/0002-8703(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 41.Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. Bmj. 2014;348:g1151. doi: 10.1136/bmj.g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eiserich JP, van der Vliet A, Handelman GJ, Halliwell B, Cross CE. Dietary antioxidants and cigarette smoke-induced biomolecular damage: a complex interaction. Am J Clin Nutr. 1995;62:1490S–1500S. doi: 10.1093/ajcn/62.6.1490S. [DOI] [PubMed] [Google Scholar]
- 43.Das I. Raised C-reactive protein levels in serum from smokers. Clin Chim Acta. 1985;153:9–13. doi: 10.1016/0009-8981(85)90133-0. [DOI] [PubMed] [Google Scholar]
- 44.Berk M, Ng F, Dean O, Dodd S, Bush AI. Glutathione: a novel treatment target in psychiatry. Trends Pharmacol Sci. 2008;29:346–51. doi: 10.1016/j.tips.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Berk BC. Novel approaches to treat oxidative stress and cardiovascular diseases. Trans Am Clin Climatol Assoc. 2007;118:209–14. [PMC free article] [PubMed] [Google Scholar]
- 46.Pasco JA, Nicholson GC, Ng F, Henry MJ, Williams LJ, Kotowicz MA, Hodge JP, Dodd S, Kapczinski F, Gama CS, Berk M. Oxidative stress may be a common mechanism linking major depression and osteoporosis. Acta Neuroopsychiatrica. 2008;20:112–116. doi: 10.1111/j.1601-5215.2008.00283.x. [DOI] [PubMed] [Google Scholar]
- 47.Berk M, Wadee AA, Kuschke RH, O'Neill-Kerr A. Acute phase proteins in major depression. J Psychosom Res. 1997;43:529–34. doi: 10.1016/s0022-3999(97)00139-6. [DOI] [PubMed] [Google Scholar]
- 48.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 49.Morabia A, Bernstein MS, Curtin F, Berode M. Validation of self-reported smoking status by simultaneous measurement of carbon monoxide and salivary thiocyanate. Prev Med. 2001;32:82–8. doi: 10.1006/pmed.2000.0779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.