Abstract
Hereditary breast and ovarian cancer syndrome carries significant mortality for young women if effective preventive and screening measures are not taken. Preventive salpingo-oophorectomy is currently the only method known to reduce the risk of ovarian cancer-related death. Histopathological analyses of these surgical specimens indicate that a high proportion of ovarian cancers in women at high risk and in the general population arise from the fallopian tube. This paradigm shift concerning the cell of origin for the most common subtype of ovarian cancer, high-grade serous carcinoma, has sparked a major effort within the research community to develop new and robust model systems to study the fallopian tube epithelium as the cell of origin of “ovarian” cancer. In this review, evidence supporting the fallopian tube as the origin of ovarian cancer is presented as are novel experimental model systems for studying the fallopian tube epithelium in high risk women as well as in the general population. This review also addresses the clinical implications of the newly proposed cell of origin, the clinical questions that arise, and novel strategies for ovarian cancer prevention.
Hereditary pre-disposition to breast and ovarian cancer carries significant morbidity. A diagnosis of a deleterious BRCA1 or BRCA2 mutation implies an exceptionally high risk of developing breast and ovarian cancer and causes a significant psychological burden. Some of these effects can be mitigated by effective prevention methods or extensive screening. While the use of MRI, alone or alternated with mammography, is associated with a reduction in the incidence of advanced breast cancer, effective screening methods for ovarian cancer are just emerging and many limitations to these methods exist (recently reviewed in [1] and references within). The lack of effective early detection tools underscores the importance of developing prevention methods that significantly reduce ovarian cancer risk and remain acceptable to women at high risk. To date, bilateral salpingo-oophorectomy is the only effective method for ovarian cancer risk reduction in BRCA1 or BRCA2 mutation carriers. However, when preventive bilateral salpingo-oophorectomy is performed as recommended before age 40, it causes early iatrogenic menopause, that might lead to significant morbidity from menopausal symptoms, as well as cardiovascular, neurologic, and metabolic disease [1]. Although the menopausal symptoms can be partially alleviated by hormonal supplementation[2], the fear of menopause-related morbidity coupled with the irreversibility of the surgical procedure reduces compliance with this life saving approach [2, 3]. Compliance is further complicated by the partial penetrance of ovarian cancer in this population, resulting in some women having unnecessary preventive surgery. Hence, improved prevention tools for ovarian cancer, or effective screening methods are urgently needed. Here, we review the emerging data indicating that a majority of high-grade serous carcinomas (HGSC), the most common subtype of ovarian cancer in BRCA1/2 mutation carriers and in the general population, arise primarily from the fallopian tube. This new understanding of tumor pathogenesis holds promise for improved prevention and effective screening.
Ovarian cancer risk reduction in BRCA1/2 mutation carriers – the fallopian tube emerges as site of origin
The ovarian surface epithelium (OSE) was proposed as the cell of origin for ovarian cancer by Fathalla in 1971 [4] and was the only candidate cell-of-origin for over 30 years. Fathalla questioned the high frequency of ovarian cancer in humans as compared to other species and suggested that the human ovary, in contrast to that of other species, undergoes “extravagant” cycles of ovulation without conception. He hypothesized that the repeated tear and repair of the OSE leads to transformation. His hypothesis was further supported by epidemiological evidence showing a higher incidence of ovarian cancer in groups denied ovarian rest periods, such as nuns and infertile women [5]. Although this hypothesis was appealing, the exact process of OSE transformation to HGSC was never precisely defined. The search for the cell of origin of HGSC took a turn following the discovery of BRCA1 and BRCA2 as the genes predominantly responsible for high-risk (or hereditary) breast and ovarian cancer syndromes. Shortly after this discovery [6, 7], salpingo-oophorectomy became standard practice for ovarian cancer risk reduction in BRCA1/2 mutation carriers after childbearing age. Only a decade later, a prospective study showed that salpingo-oophorectomy indeed reduces ovarian cancer risk and all-cause mortality in BRCA1 mutation carriers [8]. The wide acceptance of salpingo-oophorectomy even prior to the formal establishment of its clinical utility reflects the need for ovarian cancer preventive measures in this high-risk population.
The availability of specimens from prophylactic salpingo-oophorectomies allowed for careful examination of ovaries and fallopian tubes of healthy BRCA1/2 mutation carriers [9–11]. These analyses ultimately drew attention to the fallopian tube secretory epithelial cell. The fallopian tube epithelium comprises two epithelial cell types: fallopian tube secretory and ciliated cells. Piek et al. found that fallopian tubes removed prophylactically from women with a high predisposition to ovarian cancer showed dysplastic lesions characterized by the presence of secretory, but not ciliated, cells and containing a higher than normal proliferative index [9]. These lesions were more commonly found in the fimbriated end of the fallopian tube than in its other segments [11]. Subsequently, a protocol for close examination of the fallopian tubes was developed, termed Sectioning and Extensive Examining of the FIMbria (SEE-FIM) [11]. This careful and systematic evaluation of fallopian tubes led to the reproducible identification of early serous carcinoma precursors in the fallopian tube.
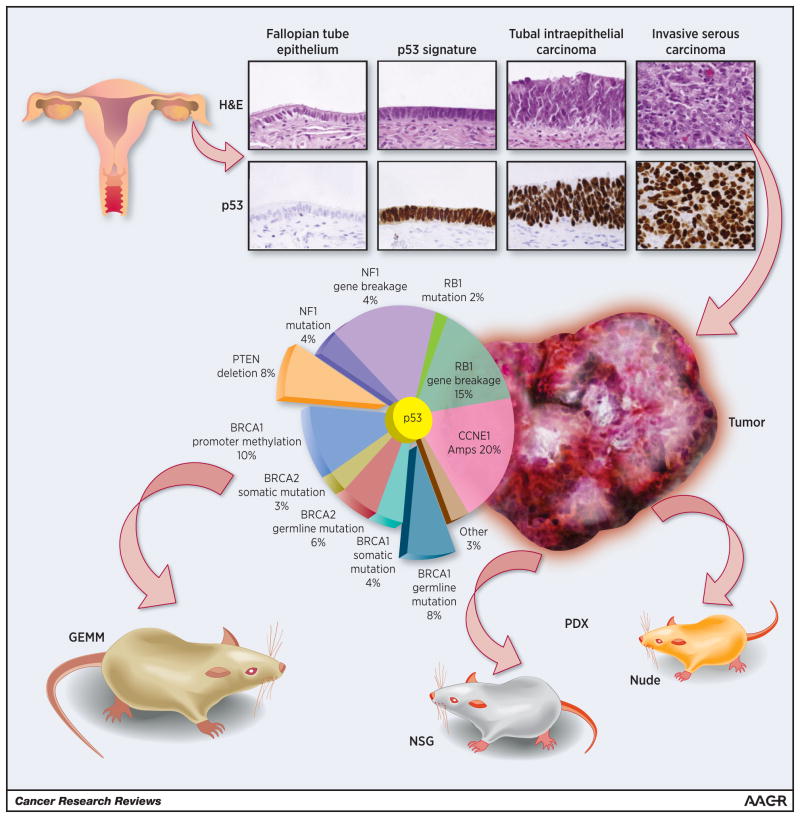
The first clearly defined step in the morphological continuum of benign tubal transformation is the “p53 signature”, characterized by stretches of benign-appearing secretory cells that exhibit evidence of DNA damage, TP53 mutations, and concomitant p53 protein stabilization[12]. The next recognizable step is the Serous Tubal Intraepithelial Carcinoma (STIC). STICs are characterized by a multilayered epithelium that lacks polarity and are composed of malignant secretory cells with evidence of DNA damage and p53 protein stabilization as well as a high proliferative index[10, 11] (Figure 1). Areas of transition between p53 signatures and STIC have been noted, suggesting continuity of both lesions [9]. Furthermore, TP53 mutational analysis showed identical TP53 mutations in the p53 signature and STIC from the same patient, further supporting clonality [13]. Interestingly, the frequency of p53 signatures is similar in BRCA1/2 mutation carriers and the general age-matched population [12, 14], while STIC is found more commonly in healthy BRCA1/2 carriers (2–8%) than in the general healthy population (0–3%) [14–17]. This suggests that while the emergence of p53 signatures is BRCA1/2-independent, the transformation into carcinoma is BRCA1/2 function-dependent. The functional role of p53 in the earlier step, and that of BRCA1/2 in the subsequent step, is an area of intense investigation.
Figure 1. Modeling high-grade serous ovarian carcinoma from the fallopian tube.
Top panel. Histological images and p53 immunostaining of normal fallopian tube epithelium and the p53 signature and STIC tubal lesions. Bottom panel. The Cancer Genome Atlas defined the genetic alterations in high-grade serous ovarian carcinoma. Animal models can be divided into two broad classes: PDX and GEM models. PDX models involve transplantation of a human tumor into an immunocompromised animal (nude or NSG mice). GEM models incorporate the relevant human genetic aberrations into an intact animal engineered to express the relevant alterations.
The emergence of the STIC lesion as a precursor to HGSC in BRCA1/2 mutation carriers has led to extensive evaluation of the fallopian tubes of women with sporadic HGSC. These studies reveal that approximately 50–60% of all HGSCs harbor a STIC lesion in the fimbriated end of the fallopian tubes if they are extensively examined [13, 18, 19]. For the remainder of STIC-negative cases there are a number of reasons why a STIC lesion may not be identified, including: (1) these HGSC may originate from other extrauterine Müllerian epithelium[20, 21], (2) sampling error – a number of studies have demonstrated that deeper levels can identify more STIC lesions [14, 17, 22], (3) Inter-observer variability – even among trained gynecological pathologists there is only a fair-to-good reproducibility for the diagnosis of STIC[23–25], (4) Inter-observer variability may be complicated by p53 negative STICs and these may necessitate additional immunohistochemical markers[26], and (5) advanced stage disease may mask the identification of a STIC lesion. The process of STIC transformation to HGSC is poorly characterized. Would all STICs eventually become invasive? What molecular changes enable invasiveness? Answering these questions requires robust model systems of HGSC pathogenesis, which have emerged in recent years.
Model systems to study the fallopian tube secretory cell as the cell of origin
The histopathological work that led to a paradigm shift in our understanding of ovarian cancer pathogenesis underscored the need to develop tractable experimental model systems to study the fallopian tube and its susceptibility to neoplastic transformation. Without experimental tools, it was almost impossible to gain mechanistic insight into the genetic and physiological factors contributing to tumor development. Several model systems for studying the fallopian tube secretory cell transformation have recently been described, mostly non-BRCA1/2-related model systems, as summarized in Table 1. The current model systems can be divided into four major types: ex vivo, cell line, genetically engineered mouse models, and patient-derived tumor xenograft models. The latter, while not useful for directly evaluating the cell of origin, constitute an invaluable clinical and research tools for addressing HGSC.
Table 1.
Fallopian tube related model systems
| Model system | Genetic alterations | BRCA related? | Precursor lesions | Reference |
|---|---|---|---|---|
| Ex-vivo | ||||
| Epithelial 2D culture system | None | No | N/A | [27] |
| 3D culture of fallopian tube secretory cells | None | No | N/A | [28] |
| Transformed cell lines | ||||
| Transformed fallopian tube secretory cells (viral oncogenes) | hTERT + SV40 Large T-Ag + SV40 Small T-Ag +H-RASV12/c-Myc | No | Immortalized cells using hTERT+SV40 Large T-Ag + SV40 Small T-Ag | [29] |
| Transformed fallopian tube secretory cells (no viral oncogenes) | hTERT + P53 shRNA+ CDK4R24C (targeting Rb)+ PP2A B56γ shRNA + c-Myc | No | Immortalized cells using hTERT + P53 shRNA+ CDK4R24C | [29] |
| Transformed fallopian tube secretory cells (viral oncogenes) | hTERT + SV40 Large T-Ag +HRAS+cMYC | No (BRCA1 accumulation was observed) | None | [30] |
| Genetically engineered mouse models | ||||
| AmhrII driven in fallopian tube mesenchymal cells | PTEN + DICER double knock out | No | None | [34] |
| OVGP-1 driven model | SV40 Large T-Ag | No | P53 signature and sTIC | [35] |
| PAX8 driven model | BRCA1 or BRCA2 deletion + P53 deletion or mutation +PTEN deletion | Yes | sTIC | [36] |
| OSE hilum model | Conditional knockout of P53 and RB in mouse OSE hilum (OSE and fallopian tube junction) | No | Cells transplanted intraperitoneally to recipient immune-deficient mice form HGSC. | [21] |
| Patient derived xenografts | ||||
| Ovarian, fallopian tube, and primary peritoneal cancer engrafted in SCID mice | Representative of patient spectrum of genetic alterations | No | No | [43] |
| HGSC engrafted in NSG mice | Representative of patient spectrum of genetic alterations. All with mutated P53. | 7 out of 10 samples | No | [42] |
An ex vivo model system for fallopian tube epithelium was developed using a polarized 2D culture system that preserves epithelial architecture, polarity, and cell differentiation [27]. Using this model system, fallopian tube secretory cells were shown to possess a limited ability to respond to DNA damage as compared to their ciliated neighbors, a finding that may explain their propensity to transform in response to genotoxic stress. In a 3D culture system recently described by Lawrenson et al., 2D primary fallopian tube secretory cell cultures are sub-cultured into spheroid structures lined by a monolayer of secretory cells and filled with a matrix resembling the in vivo extra-cellular matrix. The spheroids retained some characteristics of the fallopian tube epithelium, such as PAX8, and OVGP-1 expression and a low proliferative index. Using expression profiling and immunostaining for secretory markers, Lawrenson et al. showed that 3D cultures are phenotypically closer to the in vivo fallopian tube secretory cells than are their parental 2D cultures [28].
A number of investigators have developed immortalized secretory epithelial cell line models. These models have proven powerful in defining the contribution of a given genetic event towards neoplastic transformation [29, 30]. The fallopian tube transformation model systems were generated by in vitro targeting of human fallopian tube cells with retroviral vectors containing pre-determined oncogenic genetic alterations. One of these systems targeted fallopian tube secretory cells by over-expression of hTERT and SV-40 viral oncogenes to derive immortalized cells. Their transformation by expression of oncogenic cMYC or HRASV12 and injection into the peritoneum of immunocompromised mice yields tumors histologically and genomically consistent with HGSC [29]. In addition, these authors generated a more biologically relevant model system by omitting the use of viral oncogenes and immortalizing fallopian tube secretory cells by over-expressing hTERT, targeting p53 using shRNA, and over-expressing a mutant cyclin-dependent kinase 4 - CDK4R24C, mimicking loss of pRB function. Transformation of the non-viral immortalized cells was achieved by knocking down the B56γ subunit of PP2A and over expressing cMyc. Both viral and non-viral transformed cells formed tumors in immunocompromised mice that phenocopied human HGSC [29]. In a similar model system, Jazaeri et al. infected benign fallopian tube secretory cells with viral vectors containing a mixture of potentially oncogenic genetic alterations. The transformative potential of the different alterations was tested using a positive selection of transformed clones. The results showed that the combined over-expression of hTERT, SV40 Large T-Ag, and oncogenic c-MYC and H-RAS transforms fallopian tube secretory cells. Interestingly, shRNA for BRCA1 was not selected as a transforming alteration, suggesting that BRCA1 knock-down is not sufficient for fallopian tube secretory cell transformation [30]. These models have since been used in a number of genome-wide gain-of-function and loss-of-function screens to identify novel ovarian cancer oncogenes and tumor suppressors [31–33].
Genetically engineered mouse models as a tool to study in-vivo fallopian tube transformation, prevention and early detection
The transformation of fallopian tube cells into HGSC was recently modeled in two genetically engineered mouse (GEM) models. The first model targeted combined deletion of Dicer and Pten in the fallopian tube, using the AmhrII promoter. These mice developed carcinoma morphologically consistent with HGSC. While salpingectomy blocked tumor development, no epithelial precursors were identified in this model [34]. A more recent model described by Sherman-Baust et al. used the Müllerian specific Ovgp-1 promoter to drive SV40 large T-antigen expression specifically to the fallopian tube epithelium. The mice in this model exhibited the entire morphological spectrum of fallopian tube transformation, including “p53 signature,” STIC, and HGSC that metastasized to the ovary in 56% of mice [35].
Our group recently described another model system that mimics the inactivation of the BRCA pathway which enables tumor development in murine secretory fallopian tube cells. Using an inducible system to activate the Cre recombinase, the mouse fallopian tube epithelium was targeted for deletion of Brca1 or Brca2, Tp53, and Pten [36] (Figure 1). The model was driven by the Pax8 promoter, thus targeting specifically the aforementioned alterations to the fallopian tube, uterus, and kidney. The thyroid, another PAX8-expressing organ, was not affected in our model [37] nor were any changes in the kidneys observed. In 100% of mice, STIC developed in the fallopian tubes followed by disseminated murine HGSC. Tumors in our murine model metastasized primarily to the ovaries and to the peritoneum, similar to the human disease. Interestingly, the STIC and early invasive lesions were often not grossly visible, whereas the ovarian metastases were. This mimics the scenario in patients where the bulk of the tumor involves the ovary, even when a STIC and invasive tumor exist in the fallopian tube. Mouse tumors resembled the human disease in terms of tumor protein markers, such as Keratin, PAX8, PAX2, WT-1, and Stathmin1, and expressed the serum biomarker commonly used in the follow-up of ovarian cancer patients, CA-125.
Importantly, an assay to determine genomic copy number variations showed that the mouse tumors exhibit a high degree of copy number variation, similar to the characteristic genomic alterations that were recently described in the analysis of nearly 500 human HGSCs [38]. The copy number alterations observed in our mouse model were similar to the genes amplified and deleted in human samples [38]. It should be noted that mice with deletions of only Tp53 and Pten with intact Brca1/2 genes developed STIC, but not HGSC, suggesting that Brca1/2 alterations are critical for fallopian tube transformation in this context. Furthermore, the importance of targeting all three genes in this model underscores the role of DNA repair in HGSC carcinogenesis. BRCA1/2 and p53 play critical roles in DNA damage response pathways. Some data suggests that PTEN may also play a role in DNA repair (reviewed in [39]). Aberrations in all three genes significantly enhance tumor formation in our model. As with hereditary disease, DNA repair defects are central to the pathogenesis of sporadic HGSC. Somatic, germline and epigenetic alterations in DNA repair mechanisms are common events [38, 40]. Therefore, although the PAX8 model was designed to mimic the hereditary disease, it also serves as a compelling model for the sporadic disease.
While this model provides an invaluable tool to study the early steps of fallopian tube transformation, it has, like all models, some limitations. For instance, the universal deletion of all three genes in the Pax8-positive population might give rise to potential polyclonal populations in the invasive cancer. In addition, by simultaneously deleting the three genes we lose the ability to discern the potential importance of sequential genetic loss. Finally, the anatomy of the mouse female genital tract is inherently different than the human counterpart[36]. The mouse has a bicornuate uterus and an extensively coiled oviduct that is enveloped, along with the ovary, within a membrane sac called the ovarian bursa. In humans, the fallopian tube is not tightly coiled and is not covered by a bursa. How these anatomic structures and their associated microenvironment impact tumor development is unclear. From a technical perspective, this is a complex model that requires numerous crosses and extensive breeding. The availability of novel genome editing techniques such as CRISPR/CAS will make future models easier to obtain[41]. Specifically, these novel techniques would enable using the PAX8 promoter for efficient targeting of additional genetic alterations to the FTSEC to test their role in HGSC pathogenesis. Despite the limitations described above, this GEM can be a useful tool for testing prevention strategies and early detection methods. The high penetrance of tumors and the uniformity of time to tumor formation allow for 1) administration of preventive measures at different time points prior to tumor formation in order to test their effectivity, 2) testing early detection methods at pre-defined intervals, when the presence of either pre-invasive or early invasive cancers is known, and 3) testing of emerging novel therapeutic approaches.
A GEM model of HGSC that highlights a potential alternative cell of origin for HGSC was recently reported [21]. This model highlights the junction between the OSE and the fallopian tube epithelium. Physically located at the hilum of the ovary and characterized by ALDH1 expression, this junction serves as the stem cell niche of OSE and, in turn, as a cell of origin of HGSC. Activation of a Cre-LoxP system by adenoviral Cre infection specifically in this compartment inactivated Tp53 and Rb1 which led to neoplastic lesions and atypical cells in a higher proportion of mice. Furthermore, when transplanted intraperitoneally into immunodeficient mice, these activated cells resulted in HGSC which was positive for common tumor markers such as PAX8, CK8, and WT-1. This is the first reference to this niche as the cell of origin of HGSC in mice [21] However, the human anatomical equivalent of this specific niche is currently unknown, and while this location is in close physical proximity to the distal fallopian tube, the exact relation between the two niches is unclear.
An alternative to GEM models are patient-derived tumor xenograft (PDX) models. PDXs consist of patient tumors engrafted in immunodeficient mice which are serially expanded. PDXs maintain the histologic, genomic, and clinical characteristics of the original patient tumor (Figure 1). PDXs are an attractive option for HGSC models because samples are readily available as surgery currently remains the mainstay of treatment of most HGSC patients and tumor take in immunodeficient mice is high [42, 43]. The major advantages of using PDX as a model system for studying HGSC are: 1) the human origin of PDX includes human stromal cells and vasculature that are gradually replaced by their mouse counterparts [43]; 2) PDX obviate the need to determine the cell of origin; and 3) molecular characteristics are not predetermined, but rather represent the human spectrum of molecular changes. However, as compared to GEMs, PDX cannot be used to test either the early pathogenesis of HGSC or the impact of the tumor microenvironment, including the immune system, on tumor progression and response to treatment.
Clinical implications of the fallopian tube hypothesis
The identification of a fallopian tube origin of HGSC in BRCA1/2 mutation carriers has extensive clinical implications in terms of the pathological analysis of risk-reducing surgery specimens, treatment of early lesions found in risk reducing surgeries, and preventive strategies. The widespread use of the SEE-FIM protocol for analysis of risk-reducing salpingo-oophorectomy sections has led to identification of unsuspected tubal carcinoma and non-invasive STIC in 2–8% of patients [44, 45]. However, the natural history of these findings is unknown, as is the appropriate treatment. In a study conducted by Wethington et al., a staging surgery that included a hysterectomy, omentectomy, and peritoneal washings was recommended to all patients with STIC randomly found at risk-reducing surgery. Only 7 out of 12 patients consented to the surgery at the reporting institution, but none of the surgical staging procedures yielded additional cancer, except in one patient with positive washings. The patients did not receive adjuvant post-operative chemotherapy. At a median follow-up of 28 months, none of the 12 patients had disease recurrence [46]. In a similar study by Conner et al., 2 of 11 patients with an incidental finding of STIC, without evidence of invasive disease, had received chemotherapy. After a median of five years of follow-up, disease recurred in 1 out of 11 patients [47]. In a third study, out of 17 cases of incidental finding of STIC, 4 received chemotherapy and after a median follow up of 80 months, one patient had a recurrence of invasive disease [44]. These reports suggest a very favorable outcome for patients with an incidental finding of STIC, and yet, disease recurrence remains a risk. The characterization of the outcome of patients with pre-invasive lesions requires a much longer follow-up study and a randomized trial may be required to determine whether these patients should receive any kind of therapy. The growing use of the SEE-FIM protocol will inevitably lead to the detection of more incidental early lesions, and the need for treatment guidelines can be expected to markedly increase.
The impact of the fallopian tube hypothesis on ovarian cancer risk reduction methods
Another important aspect of the fallopian tube origin of serous carcinoma is the optimal method of risk reduction in women at high risk. Prophylactic salpingo-oophorectomy is a very effective method of ovarian cancer risk reduction [8], but leads to significant morbidity caused by iatrogenic early menopause. Presumably, the understanding that ovarian cancer in high-risk women often arises in the fallopian tube may lead to a two-step risk-reducing surgery. First, after childbearing age BRCA1/2 mutation carriers will undergo a prophylactic salpingectomy, and only after natural menopause will the women be offered a bilateral oophorectomy. This approach is more expensive than either salpingectomy alone or bilateral salpingo-oophorectomy [48], and needs to be validated in prospective studies. However, as is common with prevention strategies, testing its efficacy will require large patient cohorts and long follow-up periods. Another caveat of prophylactic salpingectomy is the loss of the well-established breast cancer protective effect of oophorectomy in high risk population [8]. Although a major disadvantage, this could be overcome by prophylactic bilateral mastectomy or rigorous breast cancer early detection efforts using breast MRI. Meanwhile, the growing acceptance of the fallopian tube hypothesis, together with the plausible logic of this strategy, is leading to an increased use of prophylactic salpingectomy in high-risk population [49–52].
In average risk women the utility of opportunistic salpingectomy as an ovarian cancer risk reducing method was recently evaluated in a large Swedish population-based cohort study by Falconer et al. This study, although limited by its retrospective nature and small numbers of ovarian cancer cases, shows ovarian cancer risk reduction in women with salpingectomy compared to non-exposed population, and a 50% ovarian cancer risk reduction by bilateral salpingectomy compared to unilateral salpingectomy[53]. An interesting prospective evaluation study of the efficacy of prophylactic salpingectomy is currently being conducted in British Columbia. Although final results have not yet been published, the study has shown that an educational initiative toward opportunistic salpingectomy at the time of hysterectomy or as sterilization is generally well accepted and does not add significantly to operation time, duration of hospital stay, or transfusion rate [54]. Therefore, although the efficacy of opportunistic salpingectomies for HGSC prevention is still not validated in prospective trials, this is another example of clinical practice change based on basic research findings and plausible models.
A new paradigm leads to new questions
The change of paradigm for the organ of origin of HGSC revolutionizes many of the most fundamental concepts of ovarian cancer pathogenesis. For instance, the role of the ovary changes from the cell of origin to a metastatic niche. However, the temporal correlation between transformation and seeding to the ovary is largely unknown. Do benign fallopian tube secretory cells shed to the ovary, which is a supportive niche for transformation? Is transformation necessary for migration as in other cancer types, or is there an as yet unrecognized intermediate step that acquires shedding ability? Fathalla’s incessant ovulation hypothesis suggested that frequent ovulatory cycles raise the risk of ovarian cancer through repeated cycles of damage and repair [4]. If the ovary is not a major site of transformation, its role in initializing transformation needs to be revised from comprising the “seed” to being the “soil” [55, 56].
Another field of research that may be significantly changed by the newly proposed cell of origin is the search for biomarkers for early detection of the disease. While historically this search focused on ovarian proteins, research now should also include fallopian tube proteins and markers. GEM models could be used to search for serum markers of HGSC in the milieu of fallopian tube secreted proteins.
In conclusion, although the identification of a new ovarian cancer cell of origin solved an enigma that lasted a quarter of a century, the number of unsolved questions continues to rise, making this a fascinating era in ovarian cancer research and treatment.
Acknowledgments
Grant support
This work was supported by the Israeli Science Foundation (RP), The European Commission FP7-PEOPLE-CIG grant (RP), Women’s health grant at Rambam (RP), Israeli Cancer Association (RP), Israeli Cancer Research Fund (RP), National Cancer Institute at the NIH P50-CA083636 (RD), NIH U01 CA-152990 (RD), NIH R21 CA-156021 (RD); the Honorable Tina Brozman ‘Tina’s Wish’ Foundation (RD), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (RD), the Robert and Debra First Fund (RD), the Gamel Family Fund (RD), and Department of Obstetrics and Gynecology at the University of Pennsylvania Perelman School of Medicine (RD).
The authors thank Drs. Frances Balkwill, Keren Levanon, Paola Vermeer and Kris Sarosiek for critical reading of the manuscript and the Drapkin and Perets labs for fruitful discussions. We thank Michael Cooper (Cooper Graphics: www.Cooper247.com) for the medical illustration. We apologize to our colleagues whose work could not be cited due to space constraints.
References
- 1.Finch A, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Women’s health. 2012;8(5):543–55. doi: 10.2217/whe.12.41. [DOI] [PubMed] [Google Scholar]
- 2.Kim SH, Frey MK, Blank SV. Occult tubal carcinoma found at risk reducing salpingectomy in a BRCA1 carrier. Gynecologic oncology case reports. 2014;9:1–2. doi: 10.1016/j.gynor.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia C, et al. Risk management options elected by women after testing positive for a BRCA mutation. Gynecologic oncology. 2014;132(2):428–33. doi: 10.1016/j.ygyno.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971;2(7716):163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 5.Fraumeni JF, Jr, et al. Cancer mortality among nuns: role of marital status in etiology of neoplastic disease in women. Journal of the National Cancer Institute. 1969;42(3):455–68. [PubMed] [Google Scholar]
- 6.Wooster R, et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994;265(5181):2088–90. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]
- 7.Miki Y, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 8.Kauff ND, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. Journal of clinical oncology. 2008;26(8):1331–7. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piek JM, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. The Journal of pathology. 2001;195(4):451–6. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 10.Carcangiu ML, et al. Atypical epithelial proliferation in fallopian tubes in prophylactic salpingo-oophorectomy specimens from BRCA1 and BRCA2 germline mutation carriers. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2004;23(1):35–40. doi: 10.1097/01.pgp.0000101082.35393.84. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros F, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. The American journal of surgical pathology. 2006;30(2):230–6. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. The Journal of pathology. 2007;211(1):26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 13.Kindelberger DW, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. The American journal of surgical pathology. 2007;31(2):161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 14.Shaw PA, et al. Candidate serous cancer precursors in fallopian tube epithelium of BRCA1/2 mutation carriers. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2009;22(9):1133–8. doi: 10.1038/modpathol.2009.89. [DOI] [PubMed] [Google Scholar]
- 15.Reitsma W, et al. Support of the ‘fallopian tube hypothesis’ in a prospective series of risk-reducing salpingo-oophorectomy specimens. European journal of cancer. 2013;49(1):132–41. doi: 10.1016/j.ejca.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Mingels MJ, et al. Tubal epithelial lesions in salpingo-oophorectomy specimens of BRCA-mutation carriers and controls. Gynecologic oncology. 2012;127(1):88–93. doi: 10.1016/j.ygyno.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Rabban JT, et al. Early detection of high-grade tubal serous carcinoma in women at low risk for hereditary breast and ovarian cancer syndrome by systematic examination of fallopian tubes incidentally removed during benign surgery. The American journal of surgical pathology. 2014;38(6):729–42. doi: 10.1097/PAS.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 18.Carlson JW, et al. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(25):4160–5. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przybycin CG, et al. Are all pelvic (nonuterine) serous carcinomas of tubal origin? The American journal of surgical pathology. 2010;34(10):1407–16. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- 20.Dubeau L, Drapkin R. Coming into focus: the nonovarian origins of ovarian cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24(Suppl 8):viii28–viii35. doi: 10.1093/annonc/mdt308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flesken-Nikitin A, et al. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495(7440):241–5. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahe E, et al. Do deeper sections increase the frequency of detection of serous tubal intraepithelial carcinoma (STIC) in the “sectioning and extensively examining the FIMbriated end” (SEE-FIM) protocol? International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2013;32(4):353–7. doi: 10.1097/PGP.0b013e318264ae09. [DOI] [PubMed] [Google Scholar]
- 23.Carlson JW, et al. Serous tubal intraepithelial carcinoma: diagnostic reproducibility and its implications. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2010;29(4):310–4. doi: 10.1097/PGP.0b013e3181c713a8. [DOI] [PubMed] [Google Scholar]
- 24.Visvanathan K, et al. Diagnosis of serous tubal intraepithelial carcinoma based on morphologic and immunohistochemical features: a reproducibility study. The American journal of surgical pathology. 2011;35(12):1766–75. doi: 10.1097/PAS.0b013e31822f58bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vang R, et al. Validation of an algorithm for the diagnosis of serous tubal intraepithelial carcinoma. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2012;31(3):243–53. doi: 10.1097/PGP.0b013e31823b8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novak M, et al. Stathmin 1 and p16 are sensitive adjunct biomarkers for serous tubal intraepithelial carcinoma. Gynecologic oncology. 2015 doi: 10.1016/j.ygyno.2015.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levanon K, et al. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29(8):1103–13. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrenson K, et al. In vitro three-dimensional modeling of fallopian tube secretory epithelial cells. BMC cell biology. 2013;14:43. doi: 10.1186/1471-2121-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(18):7547–52. doi: 10.1073/pnas.1017300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jazaeri AA, et al. Molecular requirements for transformation of fallopian tube epithelial cells into serous carcinoma. Neoplasia. 2011;13(10):899–911. doi: 10.1593/neo.11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn GP, et al. In vivo multiplexed interrogation of amplified genes identifies GAB2 as an ovarian cancer oncogene. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(3):1102–7. doi: 10.1073/pnas.1311909111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren Y, et al. Targeted tumor-penetrating siRNA nanocomplexes for credentialing the ovarian cancer oncogene ID4. Science translational medicine. 2012;4(147):147ra112. doi: 10.1126/scitranslmed.3003778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veigel D, et al. Fatty acid synthase is a metabolic marker of cell proliferation rather than malignancy in ovarian cancer and its precursor cells. International journal of cancer. Journal international du cancer. 2014 doi: 10.1002/ijc.29261. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, et al. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(10):3921–6. doi: 10.1073/pnas.1117135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherman-Baust CA, et al. A genetically engineered ovarian cancer mouse model based on fallopian tube transformation mimics human high-grade serous carcinoma development. The Journal of pathology. 2014;233(3):228–37. doi: 10.1002/path.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perets R, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer cell. 2013;24(6):751–65. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traykova-Brauch M, et al. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nature medicine. 2008;14(9):979–84. doi: 10.1038/nm.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481(7381):287–94. doi: 10.1038/nature10760. [DOI] [PubMed] [Google Scholar]
- 40.Patch AM, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–94. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 41.Yang H, et al. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154(6):1370–9. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topp MD, et al. Molecular correlates of platinum response in human high-grade serous ovarian cancer patient-derived xenografts. Molecular oncology. 2014;8(3):656–68. doi: 10.1016/j.molonc.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weroha SJ, et al. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(5):1288–97. doi: 10.1158/1078-0432.CCR-13-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powell CB, et al. Long term follow up of BRCA1 and BRCA2 mutation carriers with unsuspected neoplasia identified at risk reducing salpingo-oophorectomy. Gynecologic oncology. 2013;129(2):364–71. doi: 10.1016/j.ygyno.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Driel CM, et al. Stopping ovarian cancer screening in BRCA1/2 mutation carriers: Effects on risk management decisions & outcome of risk-reducing salpingo-oophorectomy specimens. Maturitas. 2015;80(3):318–22. doi: 10.1016/j.maturitas.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 46.Wethington SL, et al. Clinical outcome of isolated serous tubal intraepithelial carcinomas (STIC) International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2013;23(9):1603–11. doi: 10.1097/IGC.0b013e3182a80ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conner JR, et al. Outcome of unexpected adnexal neoplasia discovered during risk reduction salpingo-oophorectomy in women with germ-line BRCA1 or BRCA2 mutations. Gynecologic oncology. 2014;132(2):280–6. doi: 10.1016/j.ygyno.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon JS, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstetrics and gynecology. 2013;121(1):14–24. doi: 10.1097/aog.0b013e3182783c2f. [DOI] [PubMed] [Google Scholar]
- 49.Narod SA. Salpingectomy to prevent ovarian cancer: A Countercurrents Series. Current oncology. 2013;20(3):145–7. doi: 10.3747/co.20.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamran MW, et al. Opportunistic and interventional salpingectomy in women at risk: a strategy for preventing pelvic serous cancer (PSC) European journal of obstetrics, gynecology, and reproductive biology. 2013;170(1):251–4. doi: 10.1016/j.ejogrb.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 51.Schenberg T, Mitchell G. Prophylactic bilateral salpingectomy as a prevention strategy in women at high-risk of ovarian cancer: a mini-review. Frontiers in oncology. 2014;4:21. doi: 10.3389/fonc.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins IM, et al. The tubal hypothesis of ovarian cancer: caution needed. The Lancet Oncology. 2011;12(12):1089–91. doi: 10.1016/S1470-2045(11)70222-4. [DOI] [PubMed] [Google Scholar]
- 53.Falconer H, et al. Ovarian cancer risk after salpingectomy: a nationwide population-based study. Journal of the National Cancer Institute. 2015;107(2) doi: 10.1093/jnci/dju410. [DOI] [PubMed] [Google Scholar]
- 54.McAlpine JN, et al. Opportunistic salpingectomy: uptake, risks, and complications of a regional initiative for ovarian cancer prevention. American journal of obstetrics and gynecology. 2014;210(5):471 e1–11. doi: 10.1016/j.ajog.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Bahar-Shany K, et al. Exposure of fallopian tube epithelium to follicular fluid mimics carcinogenic changes in precursor lesions of serous papillary carcinoma. Gynecologic oncology. 2014;132(2):322–7. doi: 10.1016/j.ygyno.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 56.King SM, et al. The impact of ovulation on fallopian tube epithelial cells: evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocrine-related cancer. 2011;18(5):627–42. doi: 10.1530/ERC-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]