Abstract
Background
Examining trends in cardiovascular events (CVE) and mortality in U.S. health systems can guide the design of targeted clinical and public health strategies to reduce CVE and mortality rates.
Methods and Results
We conducted an observational cohort study from 2005–2011 among 1.25 million diabetic subjects and 1.25 million nondiabetic subjects from 11 health systems that participate in the SUrveillance, PREvention and ManagEment of Diabetes Mellitus (SUPREME-DM) DataLink. Annual rates (per 1000 person-years) of myocardial infarction/acute coronary syndrome (MI/ACS; ICD-9 410.0–410.91, 411.1–411.8), stroke (ICD-9 430–432.9, 433–434.9), heart failure (HF; ICD-9 428–428.9), and all-cause mortality were monitored by diabetes status, age, sex, race/ethnicity, and a prior CV history.
We observed significant declines in CVE and mortality rates in subjects with and without diabetes. However, there was substantial variation by age, sex, race/ethnicity, and prior CV history. Mortality declined from 44.7 to 27.1 (p<.0001) for those with DM and CVD, from 11.2 to 10.9 (p=.03) for those with DM only, and from 18.9 to 13.0 (p<.0001) for those with CVD only. Yet, in the approximately 85% of subjects with neither DM nor CVD, overall mortality (7.0 to 6.8; p=.10) and stroke rates (1.6 to 1.6; p=.77) did not decline and HF rates increased (0.9 to 1.15; p=.0005).
Conclusions
To sustain improvements in MI, stroke, HF, and mortality, health systems that have successfully focused on care improvement in high risk adults with diabetes and/or CVD must broaden their improvement strategies to target lower risk adults who have not yet developed diabetes or CVD.
Keywords: diabetes mellitus, myocardial infarction, stroke, heart failure, public health surveillance
Myocardial infarction (MI), stroke, and heart failure (HF) continue to be the leading causes of excess morbidity and mortality in the United States and among the 21 million Americans with diagnosed diabetes.1–3 Cardiovascular disease (CVD) rates are persistently higher in people with diabetes compared to those without diabetes and among people with prior CVD compared to people with no known CVD history.4 Several studies in the U.S. and other developed countries have documented overall declines in MI, stroke, HF and all-cause mortality within the overall adult population over the last 20 years, although with substantial race-ethnic and geographic variation and, in some instances, stable or even increasing CVD rates depending on diabetes status, type of CVD, and other demographic factors.1,5–11 Despite the overall declines in CVD rates, recent projections indicate that rates and costs of CVD will increase over the next few decades.12 Rates of diabetes are also projected to increase, potentially undermining the observed improvements in overall CVD rates.13 To limit future CVD burden through targeted clinical and public health interventions, it is important to better understand trends in CV events and mortality among specific population segments where improvement may be lagging or CV rates remain high.
While vital statistics provide reasonably accurate tracking of mortality trends in those with diabetes, surveillance of trends in CVD that relies entirely on hospital-based data does not have a defined population-based denominator.14–16 Increased use of electronic health data (EHD), particularly electronic medical record data linked with administrative claims data, can provide a powerful system for ongoing, timely, systematic monitoring of trends in CVD and mortality in those with and without diabetes within a defined population.16–20 Large health care systems with EHD are particularly well-suited to longitudinal surveillance of MI, stroke, and HF and where CVD rates in subpopulations are unavailable.
In this study, we use the SUrveillance, PREvention, and ManagEment of Diabetes Mellitus (SUPREME-DM) Datalink to examine MI, stroke, HF, and all-cause mortality rates from 2005 through 2011 among subjects enrolled in 11 large integrated health systems providing care to 16 million insured members in 10 states.19 To better understand opportunities for CVD intervention, we examine trends among those with and without diabetes by demographic subgroups (i.e., age, gender, race/ethnicity) and prior CVD history.
METHODS
Study Setting, Design, and Data Sources
SUPREME-DM is a consortium of 11 organizations of the HMO Research Network (HMORN): Geisinger Health System (Pennsylvania), Group Health Cooperative (Washington), HealthPartners (Minnesota), Henry Ford Health System (Michigan), Marshfield Clinic (Wisconsin), and Kaiser Permanente regions in Colorado (KPCO), Northern California (KPNC), Southern California (KPSC), Hawaii (KPHI), Southeast (Georgia, KPGA), and Northwest (Oregon and Washington, KPNW). These organizations chose to join the SUPREME-DM network in 2009. Research institutions embedded in these organizations have developed a distributed virtual data warehouse that extracts and standardizes patient information across health systems on demographics, pharmacy dispensing and claims, laboratory tests and results, and coding from outpatient and inpatient care encounters.21 Members of these health systems receive insurance through group plans, self-pay, Medicare, and Medicaid or other publicly-supported programs. These data were used to construct the SUPREME-DM DataLink, the largest clinically detailed private-sector diabetes population in the United States.19 Using this database, we examined trends in annual CVD and death rates in adults with and without diabetes.
Study Population
Diabetic and matched non-diabetic subjects were identified beginning January 1, 2003 and had to be enrolled within a participating health system for at least 6 months before entering the DataLink. The study period was January 1, 2005 through December 31, 2011. A subject had to be at least 20 years of age to enter the study. Thus, a subject could be enrollment eligible but not enter the study until turning 20 years of age.
Ascertainment of Diabetes and Matched Controls
We used inpatient and outpatient primary and secondary diagnosis codes, laboratory results, and pharmacy data within a 24 month time period to classify a person as having diabetes if they had: (a) one or more inpatient diabetes diagnosis based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 250.x, 357.2, 366.41, or 362.01–362.07. or (b) any combination of two or more of: 1) HbA1c ≥ 6.5%; 2) fasting plasma glucose ≥ 126mg/dl; 3) random plasma glucose ≥ 200mg/dl; 4) outpatient visit diabetes diagnosis codes (same codes as for inpatient); or 5) any filled prescription for a glucose-lowering medication. When the two criteria used for identification were of the same source (e.g. two outpatient diagnoses or two elevated laboratory values), we required they occur on separate days but no more than two years apart. Patients with two dispenses of metformin or thiazolidinediones with no other indication of diabetes were not included because these agents could be treatments for pre-diabetes, polycystic ovarian syndrome, or HIV lipodystrophy. Women during pregnancy were also excluded from diabetes identification. If two qualifying criteria were used to identify the diabetic subject, the date of the second event was used as the diabetes identification date. Similar methods accurately identify diabetes in various settings including several of our study sites.17,20 Our method may identify individuals with undiagnosed diabetes but it does not distinguish between Type 1 and Type 2 diabetes.
We frequency matched diabetic subjects with nondiabetic subjects by health care system, year of initial diabetes identification, sex, and within a 5-year age range. This was done annually to account for new diabetes cases originating from entering the source population and from new diabetes cases originating from the existing source population. If a subject in the control group developed diabetes, he/she was reclassified as a case.
Study Exposure Variables
Age, sex, and self-reported race/ethnicity (Asian, Black, Hispanic, and White) were based on EHD records. Subjects self-reporting Hispanic origin were categorized as Hispanic and, therefore, the other race/ethnic categories were non-Hispanic. American Indian/Alaskan Native and Native Hawaiian/Pacific Islander were also examined but the sample size was too small for stable estimates over time. Approximately 17% of the sample had unknown race/ethnicity and were excluded from the race/ethnicity analysis. While there is variability in unknown race/ethnicity by site, 76% of subjects are from sites with less than 15% unknown race/ethnicity information. Prior CVD was defined by at least two outpatient or one inpatient encounter with primary or secondary diagnosis for congestive heart failure, coronary artery disease, cerebrovascular accident, dysrhythmia, or other cardiac event (ICD-9 404.x1, 410–414, 420–421, 423–424, 426–427, 429, or 430–438) before January 1 of each calendar year of analyses. A subject could have up to 8 years of prior health information but almost all cases of prior CV history were ascertained with 18 months of data.
Study Outcome Variables
Major cardiovascular events (CVE) were ascertained using principal inpatient diagnoses codes for myocardial infarction/acute coronary syndrome (MI) ICD-9 codes 410.0–410.9 and 411.1–411.8, stroke ICD-9 codes 430–432.9 and 433–434.9 and heart failure (HF) ICD-9 codes 428–428.9. Similar methods have shown good positive predictive value.5,22,23 A participant could have multiple CVEs in a calendar year if such events occurred at least 7 days apart. All-cause mortality from 2005 to 2010 was determined using State death registries supplemented with internal EHR and claims data. One site used the National Death Index. Cause specific mortality was not uniformly available and thus is not included in these analyses.
Analyses
Major CVE and all-cause mortality rates per 1,000 person-years were calculated annually for the diabetic and non-diabetic subjects from each study site using total person-years as the denominator and the number of events as the numerator. For each calendar year, person-time was calculated by adding days of enrollment, stratified by diabetes status, age groups, sex, and race/ethnicity. Person-time for subjects with enrollment breaks of more than 90 days is excluded. Person-time was truncated for subjects on the date of disenrollment, death, or conversion to diabetes. Event rates were standardized to the 2010 census age and gender population distribution to facilitate comparison with other national surveillance studies. To examine the annual absolute and relative change of major CVE and all-cause mortality rates, a Poisson regression model with logarithm link was fitted. The model included fixed effects of the covariate of interest, site, time as a continuous variable, and the ‘covariate x time’ interaction with number of events as dependent variable and natural log of person-year as offset. Generalized estimation equations with a compound symmetry covariance structure were used to account for the non-independence of observations by site.24 Presented rate changes and reductions over time are based on the regression analyses results. Analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC).
Protection of Human Subjects
This study was approved by the KPCO institutional review board (IRB), and each participating site either ceded oversight to the KPCO IRB or received approval from their local site IRB.
RESULTS
The study included almost 10 million person years of observation contributed by 1,256,537 unique diabetic subjects and 1,243,276 non-diabetic subjects, frequency matched on age and sex (Table 1; Table S1). At study entry, mean age was 59 ± 14 years. Compared to those without diabetes, those with diabetes included more Hispanics (19.1 % vs 12.3%), fewer Whites (43.9% vs 53.2%), and a greater proportion with baseline CVD (16.7% vs 11.0%). Unknown race/ethnicity information declined over time as did the prevalence of prior CVD history (Table S1).
Table 1.
Demographic characteristics of study subjects at the time of study entry.
| Characteristic | Diabetes | No Diabetes | ||
|---|---|---|---|---|
| N | % | N | % | |
| Total Population | 1,256,537 | 100 | 1,243,276 | 100 |
| Age Group | ||||
| 20–44 years | 212,377 | 16.9 | 219,837 | 17.7 |
| 45–64 years | 642,204 | 51.1 | 639,589 | 51.4 |
| ≥ 65 years | 401,956 | 32.0 | 383,850 | 30.9 |
| Sex | ||||
| Men | 659,794 | 52.5 | 650,905 | 52.4 |
| Women | 596,743 | 47.5 | 592,371 | 47.6 |
| Race/Ethnicitya,b | ||||
| White | 551,420 | 43.9 | 661,209 | 53.2 |
| Hispanic | 239,702 | 19.1 | 152,737 | 12.3 |
| Black | 123,924 | 9.9 | 87,118 | 7.0 |
| Asian | 125,851 | 10.0 | 95,288 | 7.7 |
| Native Hawaiian/Pacific Islander | 10,363 | 0.8 | 5,016 | 0.4 |
| American Indian/Alaskan Native | 5,810 | 0.5 | 3,855 | 0.3 |
| More than One Race | 10,286 | 0.8 | 6,547 | 0.5 |
| Unknown Race | 189,181 | 15.1 | 231,506 | 18.6 |
| Medicaid Coverage | 39,416 | 3.1 | 19,269 | 1.5 |
| Prior Cardiovascular History | 209,953 | 16.7 | 136,675 | 11.0 |
All persons indicating Hispanic origin are classified as Hispanic thus the remaining race/ethnic categories are non-Hispanic.
Only White, Hispanic, Black, and Asian results are presented in the race/ethnicity analyses due to small sample sizes for other race/ethnic categories and difficulty in interpreting the ‘Unknown’ category.
Overall Temporal Trends by Diabetes Status
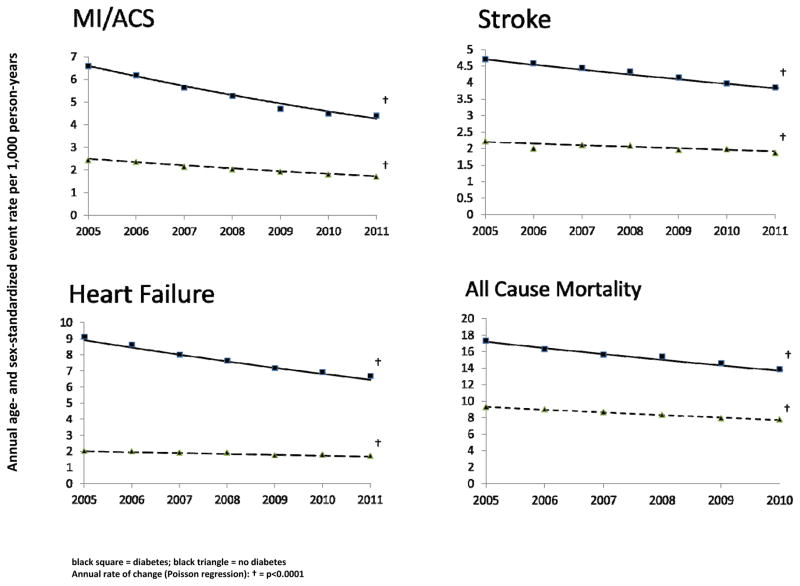
Major CVE rates and all-cause mortality declined substantially from 2005 through 2011 (Figure 1; Table S2). Myocardial infarction/acute coronary syndrome (MI) rates dropped annually by 7.0% (95% CI: 5.9–8.1%) and 6.0% (95% CI: 4.6–7.3%) in people with and without diabetes representing overall relative reductions of 35% and 31%, respectively. Stroke declined annually by 3.4% (95% CI: 2.8–3.9%) and 2.2% (95% CI: 1.5–3.0%) for overall reductions of 19% and 13%, respectively. Annual heart failure (HF) hospitalization rates fell 5.2% (95% CI: 4.7–5.7%; 28% overall reduction) in people with diabetes and 2.8% (95% CI: 1.7–4.0%; 16% overall reduction) in those without diabetes. All-cause mortality declined 20% and 17% in the diabetic and non-diabetic populations. The rates of decline were significantly greater in the diabetic population for stroke (p<0.02) and HF (p<0.001) but similar for MI and all-cause mortality. Overall rate reductions of MI, stroke, HF, and all-cause mortality were 3.0, 2.4, 7.8, and 2.3 times greater for people with diabetes than for people without diabetes. Despite this MI, stroke, HF, and all-cause mortality remained 1.8 – 3.9 fold higher in diabetic subjects.
Figure 1. Rates of myocardial infarction/acute coronary syndrome, stroke, heart failure, and all-cause mortality from 2005 – 200 by diabetes among 2.5 million insured persons across 11 health care systems.
Black square = diabetes; black triangle = no diabetes
Annual rate of change (Poisson regression): † = p<0.0001
Temporal Trends by Diabetes Status and Prior History of Cardiovascular Disease
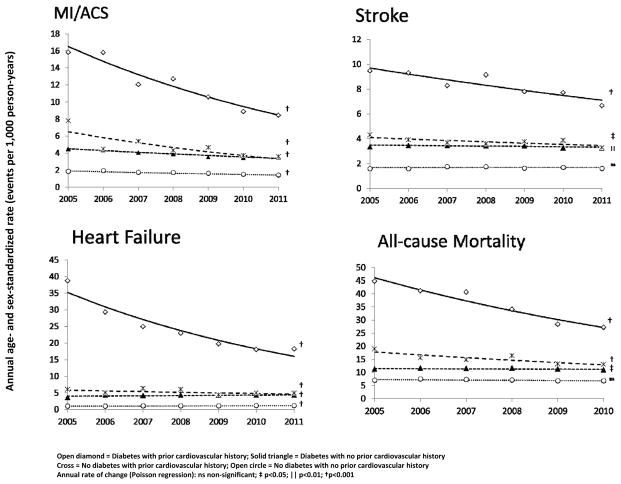
Regardless of diabetes status, subjects with a prior history of CVD had significant decreases in rates of MI, stroke, and HF from 2005 to 2011. Among subjects without a prior history of CVD, annual rate reductions were less than half as rapid for MI, while there was no improvement in stroke rates and even slight rate increases in HF (Figure 2, Table S3). In people with diabetes and a prior CV history, MI rates declined from 15.8 to 8.4 per 1,000 person-years, stroke declined from 9.5 to 6.7 per 1,000 person-years and HF declined from 38.7 to 18.2 per 1,000 person-years. These represent overall relative declines of 49%, 27%, and 55%, respectively. All-cause mortality rates in subjects with a prior history of CVD declined an overall 41% in people with diabetes and 28% in those without diabetes. There was little all-cause mortality improvement among subjects with no prior CV history (Figure 2; Table S3). When stratifying by age group (20–44 years, 45–64 years, 65 years and older), trends of greater improvement in subjects with a prior CVD history remained, especially for stroke, HF, and all-cause mortality (Table S4) and often with little or no improvement in subjects with no prior CVD history. Improvement trends in MI rates by prior CVD status were similar among subjects 65 years of age and older and there was variability among the younger adults likely due to reduced sample sizes. Despite improvements among subjects with a prior CV history, 2011 event rates remained 2–4 times higher compared to subjects without a prior CV history. Interestingly, 2011 rates were similar among subjects with diabetes and no prior CV history and subjects without diabetes and a prior CV history for MI (3.51 vs 3.56 per 1,000 person-years), stroke (3.24 vs 3.29 per 1,000 person-years), and HF (4.31 vs 4.83 per 1,000 person-years) (Figure 2, Table S3).
Figure 2. Rates of myocardial infarction/acute coronary syndrome, stroke, heart failure, and all-cause mortality from 2005 – 2011 among 2.5 million insured persons across 11 health care systems by diabetes status and prior cardiovascular history.
Open diamond = Diabetes with prior cardiovascular history; Solid triangle = Diabetes with no prior cardiovascular history; Open circle = No diabetes with no prior cardiovascular history Annual rate of change (Poisson regression): ns non-significant; ‡ p<0.05; || p<0.01; † p<0.001
Temporal Trends by Diabetes Status and by Age and Sex
Men and women, with and without diabetes, experienced relative rate reductions of 29–38% for MI, 10–20% for stroke, 12–28% for HF, and 17–20% for all-cause mortality (Table S2). However, compared to women, men experienced slightly higher rates of MI, stroke, and HF although this attenuated from 2005 to 2011 (Table S2). Cardiovascular event rates in men and women with diabetes remained 2–4 times higher compared to those without diabetes.
As expected, rates of CVE and all-cause mortality increased with age and were persistently higher in people with diabetes, especially for HF (Table S5). The greatest absolute CVE declines were in subjects 65 years of age and older. In 20–44 year old diabetic subjects, small but significant annual rate changes were observed from 2005 to 2011 for MI (−4.8%, p=0.0027), stroke (−3.7%, p=0.0037), and HF (−4.9%, p=0.0002). There were no significant improvements in MI, stroke, and HF among 20–44 year old non-diabetic subjects.
Temporal Trends by Diabetes Status and by Race and Ethnicity
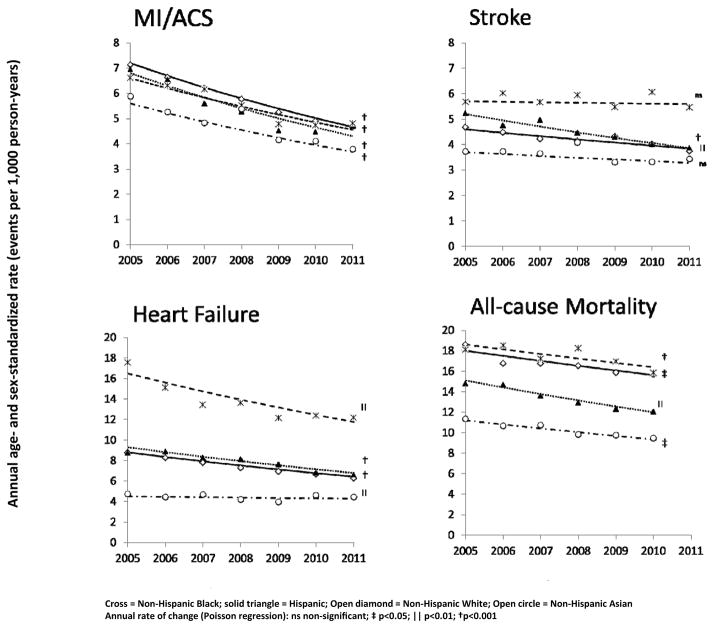
Among the diabetic population, MI rates were similar for Whites, Blacks, and Hispanics but lower for Asians (Figure 3; Table S6). However, all racial and ethnic groups experienced similar overall relative rates of decline in MI ranging from 31 – 37% and absolute rate decreases from 1.8 – 2.5 per 1,000 person-years. Significant MI rate reductions were also observed in the non-diabetic population ranging from 26–30% (Table S6).
Figure 3. Racial and ethnic rates of myocardial infarction/acute coronary syndrome, stroke, heart failure, and all-cause mortality from 2005 – 2011 among 1.25 million insured persons with diabetes across 11 health care systems.
Cross = Non-hispanic Black; solid triangle = Hispanic; Open diamond = Non-Hispanic White; Open circle = Non-Hispanic Asian
Annual rate of change (Poisson regression): ns non-significant; ‡ p<0.05; || p<0.01; † p<0.001
Reduction in stroke rates from 2005 through 2011 was variable by race/ethnicity. Stroke was highest among Blacks with essentially no change over time for the Black diabetic population and a 9% decline in the Black non-diabetic population (Figure 3; Table S6). In subjects with and without diabetes, stroke rates fell 16% and 14%, respectively, in Whites and 26% and 10% in Hispanics. Among Asians, stroke rates fell 11% in those with diabetes but increased 9% in those without diabetes, although neither of these changes was significant (Figure 3; Table S6).
In people with diabetes, HF rates in 2011 were twice as high for Blacks (12.1–17.6 per 1,000 person-years) as Whites (6.2–8.8 per 1,000 person-years) and Hispanics (6.6–8.9 per 1,000 person-years) while Asians had the lowest rates (4.0–4.7 per 1,000 person-years). Whites, Blacks, and Hispanics with diabetes saw overall reductions of 27–29% in HF hospitalization rates, but only a 5% decline was observed in Asians (Figure 3). In the non-diabetic populations, rate reductions were 15% for Whites, 9% for Hispanics, and 7% for Asians. There was a non-significant relative rate increase of 12% for non-diabetic Blacks. All-cause mortality rates decreased overall by 12–21% for diabetic Whites, Blacks, Hispanics, and Asians. Among non-diabetes, only Hispanics experience a significant decrease in all-cause mortality (Table S6).
DISCUSSION
Summary of Results
We observed substantial and sustained declines in the rates of myocardial infarction/acute coronary syndrome (MI), stroke, heart failure (HF), and all-cause mortality among insured adults with and without diabetes receiving care at 11 integrated U.S. health care delivery systems from 2005 to 2011. Stroke and HF rates declined significantly more rapidly in people with diabetes than in people without diabetes, while rates of decline were similar for MI and all-cause mortality.
We found substantial variability in CVE rates and rate changes by gender, age, race/ethnicity, and prior CV history. While rate reductions occurred in both men and women, CVE rates remained higher in men than in women irrespective of diabetes status. We also observed CVE rate decreases across all age groups for diabetic subjects. This was not the case for non-diabetic subjects, where CVE rates remained stable among the 20–44 year olds as did stroke and HF rates among those 65 years of age and older. Racial and ethnic variation was evident with Blacks having the highest CVE and mortality rates and Asians having the lowest among those with diabetes. These disparities remained over time, despite rate reductions in MI and HF. In fact, stroke rates for diabetic Blacks and diabetic Asians did not change from 2005–2011. Although CVE rates and mortality were lower among non-diabetic subjects, similar racial and ethnic disparities persisted over time.
Of particular interest is the sharp decline in CVE rates among patients with a prior CV history. In our study, CVE rate reductions among people with a prior CV history comprise the majority of the total CVE and all-cause mortality rate decline in subjects with and without diabetes. In fact, in persons with and without diabetes but with no prior CV history, minimal rate decreases and even slight rate increases were observed for stroke, HF, and all-cause mortality. Our findings are consistent within age strata further illuminating the role of secondary prevention in driving overall observed improvements in CVE and all-cause mortality.
Studies of Temporal Trends in Cardiovascular Disease
Surveillance of CVE within subgroups of individuals with diabetes in the U.S. has been limited.1,5,11,16,25 Significant declines in MI and stroke from 1990 to 2010 have been reported by diabetes status, age groups, and for Whites and Blacks.1,11 Our findings are similar, showing declines in overall MI, stroke, and HF rates and among older diabetic adults but they differ in that we found significant declines in stroke and HF in younger diabetic subjects and stable stroke rates among diabetic Blacks.1,26 We also observed significant MI rate reductions among the younger and middle-age diabetic subjects, in contrast to a recent study by Gupta and colleagues that found no decline in MI rates among the general 30–54 year old U.S. population from 2001 to 2010.6 We found similar declines for stroke and HF indicating that among younger diabetic patients CV event rates are improving. We also see this in the 45–64 year old non-diabetic subjects. It is possible that our insured patient population may have greater access to care or self-care behaviors leading to reductions in CV events. We found no CV event trend studies among Asians. Recently, declines in ischemic stroke rates among Mexican-Americans living in Texas were reported but did not distinguish by diabetes status.27 We report for the first time significant improvements in MI, stroke, heart failure, and all-cause mortality among diabetic Hispanics and Asians. Our findings highlight the value of examining trends in specific high risk or demographic subgroups to more effectively reduce disparities in cardiovascular care.28
Implications
Diabetes has been described as a coronary disease risk equivalent, but a recent meta-analysis indicates that the risk of MI is lower for patients with diabetes compared to those without diabetes but with a previous MI.4 We found a similar pattern in MI and also with stroke and HF initially, yet by 2011 these differences diminished to the point where event rates were similar between our diabetic subjects with no prior CV history and our non-diabetic subjects with a prior CV history. This may reflect the increased attention and care these insured patients with prior CV events received compared to patients with no prior CV history. Improved blood pressure and cholesterol medication adherence and control have been observed nationally, particularly among insured patients and patients with diabetes and CVD.29–32 These improvements may be in response to well-established secondary prevention and risk reduction guidelines.33,34 National and regional quality and accountability programs also emphasize improved risk factor control and care in diabetes and high CV risk patients. Given the continued high residual CV event rates in persons with prior CVD, especially those that also have diabetes, ongoing diligence in reducing subsequent CV events in these patients using statins, anti-hypertensive agents, aspirin, smoking cessation, and lifestyle changes remains an important focus.
However, our study also highlights that diabetic and non-diabetic patients who are not yet at the highest CV risk have not seen comparable reductions in CVE, despite improvements in blood pressure and cholesterol levels. These people represent roughly 85% of our study population and account for the majority of CV events. Furthermore, diabetes and obesity prevalence remain high and could erode recent decreases in CV event rates.12,13,35 Primary prevention of diabetes and key CV risk factors will be required for long-term sustained reductions in CV event rates, including greater coordination among primary care, hospital, community, and public health efforts.36–39 More effective tailoring of interventions and recommendations based on age, sex, race, and ethnicity, regardless of their current CV risk, are also necessary.28,40,41
Strengths and Limitations
Our study provides a novel approach to enhance existing national surveillance systems.16 An American Heart Association Strategic Impact Goal through 2020 is to reduce CVD and stroke mortality by 20% from 2010 rates. However, because of insufficient data, no nonfatal CVD goals were established.42 Furthermore, a number of recent large federally funded clinical trials substantially overestimated the anticipated CVE and mortality rates in diabetes and/or non-diabetes groups when planning the trials.43 This overestimation reduces the power of such trials to adequately test important hypotheses. We combined several years of routinely collected EHD across 11 large, independent, integrated health care organizations representing 16 million people, used a harmonized virtual data warehouse to facilitate monitoring across sites, identified condition-specific populations (i.e. diabetes) and longitudinally followed individuals. This enables broad-based reporting of results in a systematic and timely way and has the infrastructure to integrate other health systems and more clinical, therapeutic, and patient-reported information. In the era of learning health care systems, comparative effectiveness research, and quality assessment, data systems like the SUPREME-DM DataLink provide a powerful, flexible, cost-efficient, and timely system to monitor CV event and mortality trends in large health care systems, and thus assist in the assessment of current care strategies, and the development of new strategies to meet the evolving health care needs of the overall population and particular clinically defined or demographically defined subgroups. The longitudinal integration of clinical, demographic, and patient-reported information is also likely to improve our understanding of various health-related behaviors, risk factors, and treatment preferences and enable better tailoring of care management strategies and interventions to individuals as well as groups of individuals.44,45
Our study also has limitations that must be considered. First, our results are derived from insured adults at 11 U.S. health systems. However, these health systems encompass urban and rural regions of 10 states, include many Medicaid and Medicare-insured individuals, have a diverse racial and ethnic composition, and provide greater longitudinal power to examine important subpopulations than most nationally representative samples. The use of health system members provides a well-defined denominator for rate calculations and monitoring of trends over time within important patient subgroups. This approach avoids selection effects due to consent or survey non-response that affect NHANES and NHIS data. Future efforts to include other care delivery systems such as federally-qualified health centers or the Veterans Administration would broaden population representation. Second, diabetes or CVD status, CV events or deaths across sites and over time may be misclassified. However, our diabetes and CV event classification methods were similar to previously reported and well-validated methods and the influence of undetected diabetes or unrecognized CV events is likely to attenuate our results.17 Furthermore, given the stable incidence of diabetes in this population during the study period, the interpretation of observed trends is unlikely to differ.46 For major CV events we only included those listed as the principal discharge diagnosis from hospitalizations, a method that has had high positive predictive value in other studies.5,22,23 While coding of CV events may have varied across sites, using a consistent method over time and across sites limits potential bias in monitoring of changes over time. Nonetheless, our CV event rates are likely underestimates since secondary hospital diagnoses or events occurring outside the hospital are not captured. Misclassification of prior CVD history is also possible although this likely affects less than 1% of the study cohort and its effect would be slightly inflated CV event rates among subjects with no prior CVD history resulting in attenuation of our findings. Third, while recognizing potentially different etiologies and trends of CVD among Type 1 compared to Type 2 diabetes, we are currently unable to distinguish diabetes type. Finally, we had limited power to detect changes over time because we had only seven years of data. We did, however, observe clinically meaningful rate changes in major CV event and total mortality rates overall and within many subgroups. While some rate changes appear small from a clinical perspective, they are still important from a population-based perspective.47 As more years of data are compiled we will be even better positioned to examine changes over time.
In conclusion, our findings demonstrate that from 2005 to 2011, major CV events and all-cause mortality rates declined substantially in the roughly 15% of insured adults with diabetes, a prior CV history or both. However, among the remaining insured adults without diabetes or a prior CV history, declines in CV event rates and mortality were much lower or absent. Significant age, gender, and racial-ethnic differences in CV event and mortality rates persist and attention is urgently needed to address these disparities.48,49 Our data strongly suggest that large U.S. health systems will be unable to sustain recent decreases in CV event and overall mortality rates unless they can implement successful targeted primary prevention strategies to reduce CV risk in patients without a prior CV event history.38–41,50
Supplementary Material
Acknowledgments
Sources of Funding
This project was supported by grant number R01HS019859 from the Agency for Healthcare Research and Quality. The study sponsor had no role in the study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. E.B.S. was additionally supported by grant 1K23DK099237-01 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosures
The authors declare that they have no other relevant financial interests.
References
- 1.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–23. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med. 2009;26:142–8. doi: 10.1111/j.1464-5491.2008.02640.x. [DOI] [PubMed] [Google Scholar]
- 5.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–65. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, D’Onofrio G, Lichtman JH, Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–45. doi: 10.1016/j.jacc.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012;125:1848–57. doi: 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krumholz HM, Normand SL, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999–2011. Circulation. 2014;130:966–75. doi: 10.1161/CIRCULATIONAHA.113.007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preis SR, Pencina MJ, Hwang SJ, D’Agostino RB, Sr, Savage PJ, Levy D, Fox CS. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation. 2009;120:212–20. doi: 10.1161/CIRCULATIONAHA.108.846519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, Khatri P, Adeoye O, Ferioli S, Broderick JP, Kissela BM. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky. Stroke Study. Stroke. 2010;41:1326–31. doi: 10.1161/STROKEAHA.109.575043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koton S, Schneider AL, Rosamond WD, Shahar E, Sang Y, Gottesman RF, Coresh J. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312:259–68. doi: 10.1001/jama.2014.7692. [DOI] [PubMed] [Google Scholar]
- 12.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 13.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A Nationwide Framework for Surveillance of Cardiovascular and Chronic Lung Diseases. Washington DC: National Academy of Sciences; 2011. [PubMed] [Google Scholar]
- 15.Desai J, Geiss L, Mukhtar Q, Harwell T, Benjamin S, Bell R, Tierney E. Public health surveillance of diabetes in the United States. J Public Health Manag Pract. 2003;(Suppl):S44–51. doi: 10.1097/00124784-200311001-00008. [DOI] [PubMed] [Google Scholar]
- 16.Sidney S, Rosamond WD, Howard VJ, Luepker RV. The “heart disease and stroke statistics--2013 update” and the need for a national cardiovascular surveillance system. Circulation. 2013;127:21–3. doi: 10.1161/CIRCULATIONAHA.112.155911. [DOI] [PubMed] [Google Scholar]
- 17.Leong A, Dasgupta K, Bernatsky S, Lacaille D, Avina-Zubieta A, Rahme E. Systematic review and meta-analysis of validation studies on a diabetes case definition from health administrative records. PloS One. 2013;8:e75256. doi: 10.1371/journal.pone.0075256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai JR, Wu P, Nichols GA, Lieu TA, O’Connor PJ. Diabetes and asthma case identification, validation, and representativeness when using electronic health data to construct registries for comparative effectiveness and epidemiologic research. Med. Care. 2012;50(Suppl):S30–5. doi: 10.1097/MLR.0b013e318259c011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichols GA, Desai J, Elston Lafata J, Lawrence JM, O’Connor PJ, Pathak RD, Raebel MA, Reid RJ, Selby JV, Silverman BG, Steiner JF, Stewart WF, Vupputuri S, Waitzfelder B SUPREME-DM Study Group. Construction of a multisite DataLink using electronic health records for the identification, surveillance, prevention, and management of diabetes mellitus: the SUPREME-DM project. Prev Chronic Dis. 2012;9:E110. doi: 10.5888/pcd9.110311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klompas M, Eggleston E, McVetta J, Lazarus R, Li L, Platt R. Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care. 2012;36:914–21. doi: 10.2337/dc12-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross TR, Ng D, Brown JS, Pardee R, Hornbrook MC, Hart G, Steiner JF. The HMO Research Network Virtual Data Warehouse: A Public Data Model to Support Collaboration. EGEMS (Wash DC) 2014;2:1049. doi: 10.13063/2327-9214.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21:100–28. doi: 10.1002/pds.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saczynski JS, Andrade SE, Harrold LR, Tjia J, Cutrona SL, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21:129–40. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colin Cameron Adrian, Trivedi PK. Regression analysis of count data. Cambridge, UK; New York, NY, USA: Cambridge University Press; 1998. [Google Scholar]
- 25.Fang J, Alderman MH, Keenan NL, Ayala C. Acute myocardial infarction hospitalization in the United States, 1979 to 2005. Am J Med. 2010;123:259–66. doi: 10.1016/j.amjmed.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 26. [Accessed September, 2013];Diabetes Data & Trends. www.cdc.gov/diabetes/statistics.
- 27.Morgenstern LB, Smith MA, Sanchez BN, Brown DL, Zahuranec DB, Garcia N, Kerber KA, Skolarus LE, Meurer WJ, Burke JF, Adelman EE, Baek J, Lisabeth LD. Persistent ischemic stroke disparities despite declining incidence in Mexican Americans. Ann Neurol. 2013;74:778–85. doi: 10.1002/ana.23972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw LJ, Butler J. Targeting priority populations to reduce disparities in cardiovascular care: health equity for all. J Am Coll Cardiol. 2014;64:346–8. doi: 10.1016/j.jacc.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman HW, Blatt AJ, Huang X, Odeh MA, Superko HR. Blood cholesterol trends 2001–2011 in the United States: analysis of 105 million patient records. PloS One. 2013;8:e63416. doi: 10.1371/journal.pone.0063416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health And Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126:2105–14. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 31.Wilper AP, Woolhandler S, Lasser KE, McCormick D, Bor DH, Himmelstein DU. Hypertension, diabetes, and elevated cholesterol among insured and uninsured U.S. adults. Health Aff(Millwood) 2009;28:w1151–9. doi: 10.1377/hlthaff.28.6.w1151. [DOI] [PubMed] [Google Scholar]
- 32.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36:2271–9. doi: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumbhani DJ, Fonarow GC, Cannon CP, Hernandez AF, Peterson ED, Peacock WF, Laskey WK, Deedwania P, Grau-Sepulveda M, Schwamm LH, Bhatt DL Get With the Guidelines Steering Committee and Investigators. Temporal trends for secondary prevention measures among patients hospitalized with coronary artery disease. Am J Med. 2015;128:426.e1–9. doi: 10.1016/j.amjmed.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011;58:2432–46. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 35.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA. 2014;312:189–90. doi: 10.1001/jama.2014.6228. [DOI] [PubMed] [Google Scholar]
- 36.Green LW, Brancati FL, Albright A. Primary prevention of type 2 diabetes: integrative public health and primary care opportunities, challenges and strategies. Family Pract. 2012;29(Suppl 1):i13–23. doi: 10.1093/fampra/cmr126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 38.Fihn SD. Bending the curve on cardiovascular risk. JAMA Intern Med. 2014;174:48–50. doi: 10.1001/jamainternmed.2013.9498. [DOI] [PubMed] [Google Scholar]
- 39.Spring B, Ockene JK, Gidding SS, Mozaffarian D, Moore S, Rosal MC, Brown MD, Vafiadis DK, Cohen DL, Burke LE, Lloyd-Jones D. Better population health through behavior change in adults: a call to action. Circulation. 2013;12:2169–76. doi: 10.1161/01.cir.0000435173.25936.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capewell S, Graham H. Will cardiovascular disease prevention widen health inequalities? PLoS Med. 2010;7:e1000320. doi: 10.1371/journal.pmed.1000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stuart-Shor EM, Berra KA, Kamau MW, Kumanyika SK. Behavioral strategies for cardiovascular risk reduction in diverse and underserved racial/ethnic groups. Circulation. 2012;125:171–84. doi: 10.1161/CIRCULATIONAHA.110.968495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 43.Preiss D, Sattar N, McMurray JJ. A systematic review of event rates in clinical trials in diabetes mellitus: the importance of quantifying baseline cardiovascular disease history and proteinuria and implications for clinical trial design. Am Heart J. 2011;161:210–9. doi: 10.1016/j.ahj.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Panzer RJ, Gitomer RS, Greene WH, Webster PR, Landry KR, Riccobono CA. Increasing demands for quality measurement. JAMA. 2013;310:1971–80. doi: 10.1001/jama.2013.282047. [DOI] [PubMed] [Google Scholar]
- 45.Roger VL. Cardiovascular disease surveillance in the comparative effectiveness landscape. Circ Cardiovasc Qual Outcomes. 2009;2:404–6. doi: 10.1161/CIRCOUTCOMES.109.901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nichols GA, Schroeder EB, Karter AJ, Gregg EW, Desai J, Lawrence JM, O’Connor PJ, Xu S, Newton KM, Raebel MA, Pathak RD, Waitzfelder B, Segal J, Lafata JE, Butler MG, Kirchner HL, Thomas A, Steiner JF SUPREME-DM Study Group. Trends in diabetes incidence among 7 million insured adults, 2006–2011: the SUPREME-DM project. Am J Epidemiol. 2015;181:32–9. doi: 10.1093/aje/kwu255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427–32. doi: 10.1093/ije/30.3.427. discussion 433–4. [DOI] [PubMed] [Google Scholar]
- 48.Chatterji P, Joo H, Lahiri K. Racial/ethnic- and education-related disparities in the control of risk factors for cardiovascular disease among individuals with diabetes. Diabetes Care. 2012;35:305–12. doi: 10.2337/dc11-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd-Jones DM. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2012;125:45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ford ES, Capewell S. Proportion of the decline in cardiovascular mortality disease due to prevention versus treatment: public health versus clinical care. Annu Rev Public Health. 2011;32:5–22. doi: 10.1146/annurev-publhealth-031210-101211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.