It is unknown how the immune response in early life becomes appropriately activated for protection while avoiding excessive immune activation due to diverse new antigens. T cells are integral to adaptive immunity, with mouse studies indicating that tissue localization of T cell subsets is important for protective immunity1–4 and immunoregulation5,6. In humans, however, the early development and function of T cells in tissues remain unexplored. We present here an analysis of lymphoid and mucosal tissue T cells derived from pediatric organ donors in the first two years of life (compared to adults), revealing early compartmentalization of T cell differentiation and regulation. While adult tissues contain a predominance of memory T cells7,8, naïve recent thymic emigrants are the main subset in pediatric blood and tissues with effector memory T cells (TEM) found only in the lungs and small intestine. Additionally, regulatory T (Treg) cells comprise a high proportion (30–40%) of CD4+ T cells in pediatric tissues but are present at much lower frequencies (1–10%) in adult tissues. Pediatric tissue Treg cells suppress endogenous T cell activation, with early T cell functionality confined to mucosal sites with the lowest Treg:TEM cell ratios, suggesting in situ control of immune responses in early life.
Knowledge of human immune responses during early life remains sparse, due to the difficulty and impracticality of obtaining blood and tissue samples from infants. Our current view of normal infant immune responses, including studies on T cell differentiation and function is largely based on the sampling of umbilical cord blood9–11 or fetal tissue12,13 which reflect immune responses in utero but not to diverse antigens encountered in early life. Fundamental information on the establishment of adaptive immunity during infancy, including how T cells respond, differentiate, and populate tissue sites remains undefined. Through collaboration with organ procurement agencies, we have established protocols to obtain lymphoid and mucosal tissues from individual organ donors where consent was given for use of tissues for research7,8. We previously showed that distinct subsets of T cells, including naive and previously activated effector and memory subsets, were differentially compartmentalized in lymphoid and mucosal tissues of older children and adults7,8,14,15. To investigate T cell immunity in tissues during early life, we obtained multiple lymphoid and mucosal tissues (and blood) from a cohort of infant/pediatric organ donors in the first two years of life (n=17, Supplementary Table 1), which we compared to tissues from a cohort of adult donors stratified into young adults, aged 15–25yrs (n=23) and adults >25years (n=24) (Supplementary Table 2). Tissues analyzed are representative of circulatory, lymphoid, and mucosal sites, and include blood, spleen, inguinal lymph nodes (ILN), lung-draining lymph nodes (LLN), mesenteric lymph nodes (MLN), lungs, jejunum, ileum and colon.
Pediatric donors contained reduced T cell:B cell ratios compared to adult donors in blood, spleen, MLN and intestinal sites, indicating reduced T cell numbers in these tissues in early life, while the CD4+:CD8+ T cell composition was similar in pediatric and young adult donors, showing a predominance of CD4+ T cells in lymphoid tissues (Supplementary Fig. 1a). However, major functional subsets of T cells delineated by CD45RA and CCR7 expression, including naïve (CD45RA+CCR7+) and previously activated T effector memory (CD45RA−CCR7−, TEM) cell subsets exhibited distinct frequencies and distributions in pediatric compared to young adult tissues, while central memory (CD45RA−CCR7+, TCM), and terminal effector (-CD45RA+CCR7−, TEMRA) cell subsets were similar in both age groups (Fig. 1a,b). In pediatric donors (2 months-2 years of age), naïve T cells were the predominant population (70–95%) of CD4+ and CD8+ T cells in the blood, spleen, lymph nodes and colon, whereas in young adults (15–25 years of age), naïve T cells were present only in blood and lymphoid sites at frequencies <50% (Fig. 1a,b). CD4+ T cells in pediatric tissues also exhibited a high proportion of CD31+ cells denoting recent thymic emigrants (RTE)16 (>60% in lymphoid tissues; 25–40% in mucosal sites, Fig. 1c), exceeding the proportion of CD4+ RTE in young adult lymphoid tissue (<30%) and mucosal sites (<10%) (Fig. 1c). Pediatric and young adult thymii exhibited similar percentages of CD4+CD8+ (double positive, DP) thymocytes (Fig. 1d), and naive T cells were largely RTE in both groups (Fig. 1c). Therefore, the reduction in tissue RTEs in young adults does not correlate with differences in thymopoiesis, and could derive from differential seeding, retention or activation at these sites.
Figure. 1. Naïve T cells predominate in infant tissues with TEM cells confined to mucosal sites.

(a) Flow cytometry plots of CD45RA/CCR7 expression by CD4+ and CD8+ T cells in pediatric blood and tissues from a representative infant (left, donor 118, 2 months) and older baby (right, donor 213, 2 yrs). (b) Mean frequency ± SEM of each T cell subset (naïve, TCM, TEM, TEMRA) expressed as a percent of CD4+ (top) or CD8+ (bottom) T cells in circulation, lymphoid, and mucosal tissue (ordered from circulation, red; to mucosal sites, green) separated by age group (pediatric (0–2 years) 15 donors, red; young adult (15–25 years), 9 donors, black). (c) Graph showing mean frequency ± SEM of CD31 expression by total CD4 T cells (top) and naive CD4+ T cells (bottom) from 8 pediatric and 9 young adult donors as in (b). (d) Representative staining of CD4+ and CD8+ T cell populations in thymic tissue of an infant (top, donor 107, 4 months) and young adult (bottom, donor 102, 22 yrs) and compiled frequencies of CD4+CD8+ (double positive, DP) cells in thymii of 6 pediatric and 5 young adult donors. Statistical significance represents comparisons between the indicated frequencies in pediatric and young adult donors in the same tissue sites measured by multiple t tests adjusted for multiple comparisons indicated as *P < 0.05; **P < 0.001. Individual donors used, descriptive statistics and individual p-values are shown in (Supplementary Tables 2, 3 and 4).
In pediatric tissues, memory T cells (mainly TEM cells) were found in frequencies >20% only in the lungs, jejunum and ileum, and were significantly less abundant (p<0.05) in blood, spleen, lymph nodes, and colon (Fig. 1a,b; Supplementary Fig. 1b, Table 3). By contrast, in young adults, TEM cells predominated in all mucosal sites (>90%) and comprised 30–40% of lymphoid T cells (Fig. 1b). The compartmentalization of early T cell memory to the lungs and small intestines, but not the draining LN, suggests local in situ priming to inhaled and ingested antigens, respectively. We examined whether infant tissue TEM cells expressed markers of tissue resident memory T (TRM) cells1,3,14, including expression of the activation marker CD69, and/or the integrin CD103 involved in tissue retention17,18. CD69 is expressed by the majority of TEM cells in all pediatric tissues, similar to TEM cells in adult tissues, while blood TEM cells are CD69− (Fig. 2a,b)8. However, a significantly lower frequency of CD8+ TEM cells in pediatric mucosal sites co-express CD69 and CD103, compared to >90% CD8+ TEM cells in adult mucosal sites (Fig. 2c, d, Supplementary Table 3)8, suggesting that infant mucosal memory T cells have not fully differentiated into TRM cells.
Figure. 2. Distinct properties of early tissue memory T cells in lymphoid and mucosal sites.

(a) Representative histograms of CD69 expression by naïve (red) and TEM (blue) CD4+ (top) and CD8+ (bottom) T cells from an infant donor (donor 127, 16 months). (b) Mean frequencies ± SEM of CD69 CD4+ (top) or CD8+ (bottom) TEM cells from 15 pediatric (0–2 years, red) or 9 young adults (15–25 years, black). (c) Representative flow cytometry plots of CD69 and CD103 expression on CD8+ TEM cells from a pediatric donor (top, donor 63, 26 months) and a young adult donor (bottom, donor 105, 20 years). (d) Mean frequencies ± SEM of CD69+CD103+ CD8+ (left) and CD69+CD103− (right) TEM in 9 pediatric (white) and 8 young adult (black) donors. Statistical significance for each site indicated as *P < 0.05; ** P < 0.001. (e) Cytokine content in culture supernatants (pg/ml, mean ±SEM) of infant (n=4, white) and adult (n=3, black) MLN T cells following stimulation with anti-CD2/CD3/CD28-coupled beads for four days. Unstimulated cells produced <1pg/ml cytokines (data not shown). Statistical significance indicated as *P < 0.05. (f) Average IFN-γ production from indicated tissues of 5 pediatric donors and 3 adult donors stimulated with PMA/ionomycin for 4 h. (g) IFN-γ production versus CD45RA expression from CD3+ T cells of donor 213 (2 years) stimulated with PMA/ionomycin for 4 hours. Individual donors used, descriptive statistics and individual P values are shown in (Supplementary Tables 2 and 3).
To further establish the differentiation status of T cells in pediatric tissues, we analyzed their capacity for effector cytokine production. While lymph node T cells from adult tissues produced substantial levels of IL-2, IFN-γ and IL-4 following T cell receptor (TCR)/CD3-mediated stimulation, pediatric T cells produced lower levels of IL-2 and IL-4, and negligible IFN-γ (Fig. 2e). Low level IFN-γ production by pediatric compared to adult lymphoid T cells was also observed following TCR-independent stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin (Fig. 2f), suggesting cell intrinsic differences. Pediatric lymphoid T cells also did not produce high levels of IL-8 (Supplementary Fig. 2), as observed with newborn cord blood T cells9. However, pediatric memory T cells in the small intestines produced IFN-γ rapidly in response to PMA stimulation, exceeding the low proportion of IFN-γ producers in the LN and colon (Fig. 2g). Thus, memory T cells generated during early life in mucosal sites, acquire the capacity to secrete proinflammatory cytokines.
We considered whether Treg cells have differential distribution or function in pediatric versus adult tissues. Treg cells are a subset of CD4+ T cells that express the transcription factor FOXP3 and the IL-2Rα chain (CD25), and are essential for establishment of self-tolerance and immune homeostasis (for reviews, see19–21). In humans, there are few studies of Treg cells in tissues22,23, and the role of Treg cells in early immune responses is unknown. In infant tissues, a high proportion (20–40%) of CD4+ T cells were CD25+FOXP3+, compared to a lower frequency in young adult tissues (Fig. 3a). Quantitation of Treg cells based on gating of CD25+CD127−FOXP3+ CD4+ T cells24,25 (Fig. 3b, Supplementary Fig. 3) revealed an elevated frequency of Treg cells (10–30%) in all infant/pediatric tissues, contrasting with a 6–10-fold lower frequency of Treg cells (2–5% CD4+ T cells) in adult tissues (Fig. 3c, Supplementary Fig. 4a, Table 4). Treg cell distribution in tissues also differed between pediatric and adult donors, with high frequencies of Treg cells in pediatric mucosal and lymphoid tissues, while in adults, Treg cell frequencies were highest in lymphoid tissues (often LLN) as compared to mucosal sites (Fig. 3c,d; Supplementary Fig. 4b). A heat map of tissue Treg cell frequencies in individual donors from infancy until 63 years shows the sharp reduction in Treg cell frequencies after childhood (17–20 years) (Supplementary Fig.4a).
Figure. 3. Elevated frequency and broad tissue distribution of Treg cells in pediatric as compared to adult tissues.
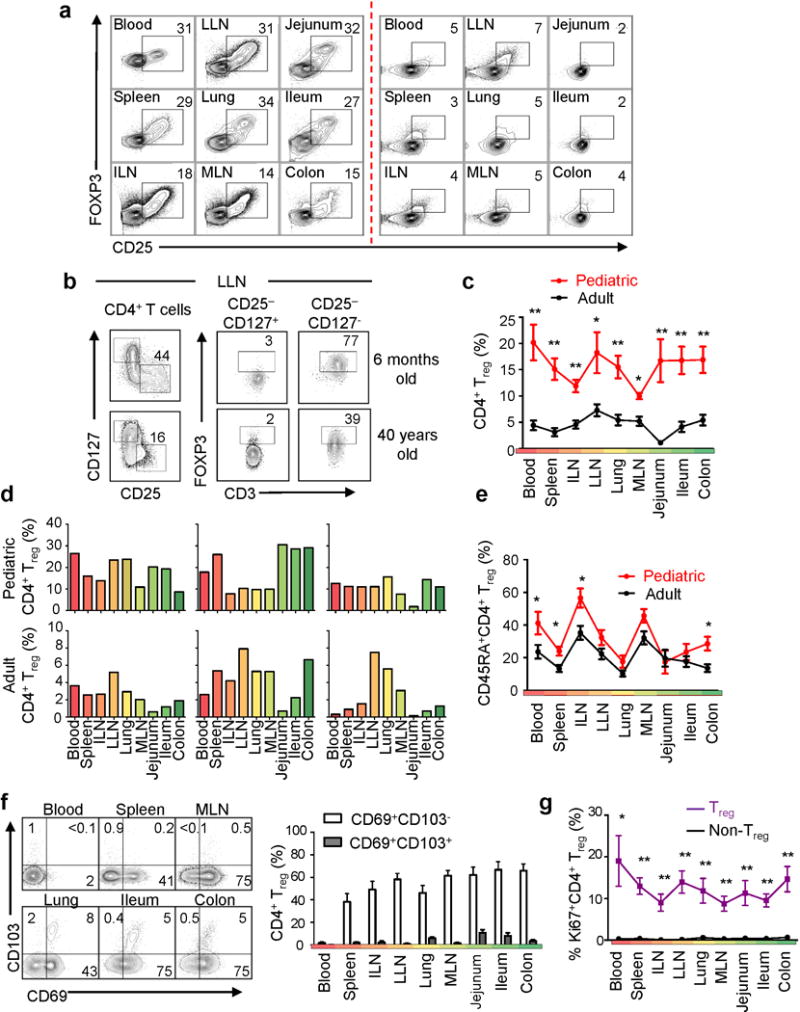
(a) Frequency of CD25+FOXP3+CD4+ T cells in tissues from an infant (donor 118, 2 months) and adult (donor 105, 20 years). (b) Gating strategy for Treg cells from pediatric (top, donor 68, 6 months) and adult (bottom, donor 110, 40 yrs) LLN showing and initial CD25+CD127− gate (left) subsequently gated on FOXP3 expression, versus non-Treg (CD25−CD127+) cells as a control. (c) Treg cell frequency (mean±SEM) in tissues from 13 pediatric (red) and 24 adult (black) donors expressed as percent CD25+CD127−FOXP3+ cells from total CD4+ T cells (left). Statistical significance indicated as *P < 0.05; **P < 0.001. (d) Treg cell tissue distribution in pediatric and adult donors. Graphs show Treg cell frequency tissues from pediatric (top row, 2 months, 4 months, 1 year) and adult (lower, 20 years, 38 years, 51 years) donors. (e) CD45RA expression by Treg cells from 13 pediatric (red) and 24 adult (black) donors, with * P < 0.05. (f) CD69 and CD103 expression on Treg cells shown as representative flow cytometry plots(left) and compiled percentages (mean±SEM) of CD69+CD103− (white) and CD69+CD103+ (grey) populations from 16 donors (right). (g) Percent Ki67 expression (mean±SEM) by Treg (purple) and non-Treg (CD4+CD25−CD127+) cells (black) in blood and tissues from 12 donors. Statistical significance indicated as *P < 0.05; **P < 0.001. Individual donors used, descriptive statistics and P values are shown in (Supplementary Tables 2, 4 and 5).
We examined potential differences in Treg cell phenotypes and turnover between tissues and the circulation. In pediatric and adult tissues, Treg cells in mucosal tissues were predominantly CD45RA−, while 40–60% Treg cells in lymphoid tissues and blood were CD45RA+ (Fig. 3e, Supplementary Fig. 5a), suggesting tissue-intrinsic differences in activation state26,27. Pediatric and adult tissue Treg cells also expressed CD69 (similar to TRM) but not CD103, while blood Treg cells were CD69−CD103− (Fig. 3f). All tissue and circulating Treg cells showed extensive proliferation compared to non-Treg cell counterparts (Fig. 3g, Supplementary Fig. 5b, Table 5). Thus, Treg cells in human tissues exhibit distinct properties independent of age, influenced by CD45RA- phenotypes28, and/or tissue-specific signals.
Pediatric Treg cells expressed higher amounts of FOXP3 (which is associated with increased suppressive capacity29) compared to adult Treg cells in lymphoid tissues, while levels of FOXP3 expression in adult and pediatric blood Treg cells were similar (Fig. 4a,b). Functionally, depletion of CD25+CD4+ Treg cells from pediatric lymph node T cell cultures resulted in increased T cell proliferation (Fig. 4c) and cytokine production (Fig. 4d, Supplementary Table 6), while adult LN T cells were similarly activated with and without CD25 depletion (Fig. 4c,d). A similar increase in T cell proliferation was observed when Treg cells were depleted from cultures derived from the lungs and colon (Supplementary Fig. 6). Together, these results suggest that early in life, Treg cells may be suppressing tissue immune responses to a greater extent than later in life, and are consistent with recent results in mice showing distinct roles for perinatal Treg cells in establishing self-tolerance30
Figure. 4. Pediatric tissue Treg cells suppress endogenous T cell proliferation and function.
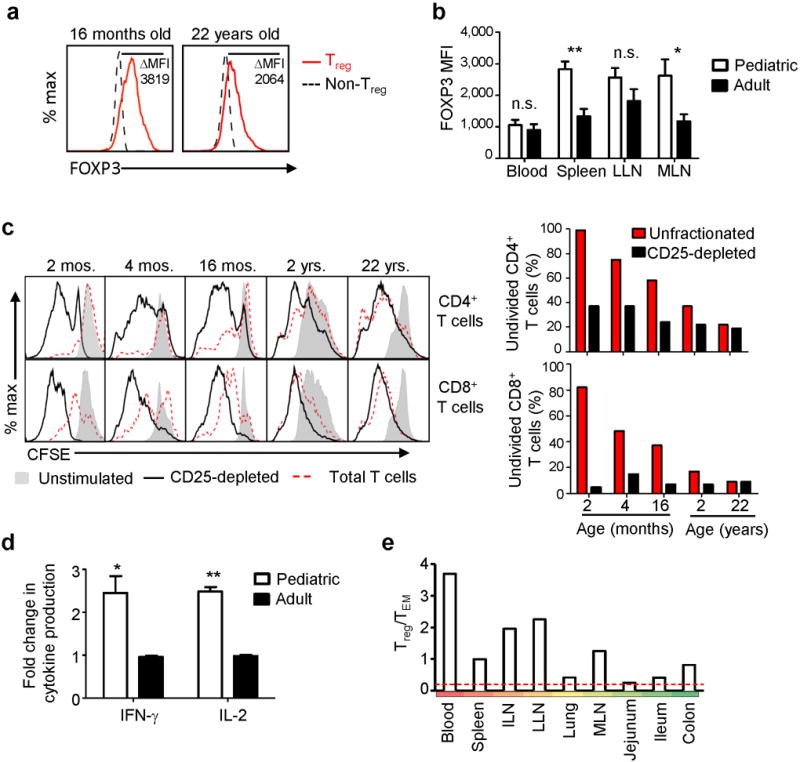
(a) FOXP3 expression by pediatric and adult Treg cells. Representative FOXP3 expression by MLN CD4+CD25+CD127− cells (red) compared to CD25−CD127+FOXP3− cells (black dashed) from a pediatric (16 months, left) and adult (22 yrs, right) donor. ΔMFI = MFI(Treg cells)–MFI(control non-Treg cells). (b) Normalized FOXP3 MFI as in (a) of Treg cells from blood and lymphoid tissues of 10 pediatric (white) and 17 adult (black) donors. *P < 0.05; **P < 0.001; n.s.: not significant. (c) Proliferation of unfractionated T cells (red dotted) or CD25-depleted T cells (black), isolated from pediatric and adult MLN of indicated ages following anti-CD3/CD28 stimulation. Left: CFSE dilution of activated compared to unstimulated (grey shaded) CD4+ (top) and CD8+ (bottom) T cells. Right: Percent of CFSE+ (undivided) CD4+ (top) or CD8+ (bottom) T cells stimulated with or without CD25-depletion for each individual infant and adult donor. (d) Cytokine production in the presence or absence of Treg cells. Spleen and MLN T cells from pediatric and adult tissues were activated as in (c) with or without CD25 depletion and IFN-γ and IL-2 in supernatants was measured after 48 hours Graph shows fold change of cytokine production by the CD25-depleted versus unfractionated pediatric (white, n=4) and adult (black, n=4) T cells, with *P < 0.05; **P < 0.001. (e) Average Treg:TEM cell ratios in tissues from pediatric donors (0–2 yrs, n=17). Red dashed line indicates the maximum Treg:TEM cell ratio in adult tissues.
Our results provide a new view of infant T cell distribution and function throughout the body, with important implications for promoting immune health and protection in early life. We show that naïve and Treg cells populate lymphoid and mucosal tissues during infancy, with differentiation of functional memory T cells occurring in certain mucosal sites. We propose that the higher Treg:TEM cell content in infants as compared to adult tissues (Fig. 4e) leads to in situ regulation of T cell differentiation, confining early T cell differentiation activation and memory formation to lungs and small intestines as sites of continuous, high level antigen exposure. Our results suggest immunization may be targeted during infancy to sites with a low ratio of Treg:TEM cells for promoting immune protection during the early years and beyond.
Online methods
Acquisition of human tissue
Human tissues were obtained from deceased (brain dead) organ donors at the time of organ acquisition for life-saving clinical transplantation. Infant donor tissue was obtained in collaboration with the Columbia University/New York Presbyterian hospital pediatric liver transplant program. Adult donor tissues were obtained through an approved protocol and material transfer agreement with LiveOnNY (formerly New York Organ Donor Network, NYODN). Organ donors were free of chronic disease and cancer, were Hepatitis B, C, and HIV negative, and were 79% male. The study does not qualify as human subjects research, as confirmed by the Columbia University IRB, as tissue samples were obtained from deceased individuals. Cord blood was obtained as a discarded sample from Dr. Kang Liu, Columbia University. Adult blood and pediatric thymus tissues was acquired through the Columbia Center for Translational Immunology (CCTI) human studies core, which operates in accordance with the Columbia University human research protection office institutional review board.
Lymphocyte isolation from human lymphoid and non-lymphoid tissues
Tissue samples were maintained in cold saline and brought to the laboratory within 2–4hrs of organ procurement. Samples were rapidly processed using enzymatic and mechanical digestion to obtain lymphocyte populations with high viability as described in detail7,8. Tissues were minced and incubated at 37°C in enzymatic digestion media: RPMI (Thermo Fisher, Waltham, MA) containing 10% FBS (Thermo Fisher), L-glutamate (Thermo Fisher), sodium pyruvate (Thermo Fisher), nonessential amino acids (Thermo Fisher), penicillin-streptomycin (Thermo Fisher), collagenase D (1mg/mL, Roche, Indianapolis, IN), trypsin inhibitor (1mg/mL, Thermo Fisher), and DNase I (0.1 mg/mL, Roche). Digested tissue was further disrupted using the gentleMACS tissue dissociator (Miltenyi Biotech, San Diego, CA); the resulting suspension was passed through a tissue sieve (10 to 150 mesh size), followed by pelleting through centrifugation. Residual red blood cells (RBC) were lysed using AKC lysis buffer (Corning Cellgro, Manassas, VA), and dead cells and debris were removed by centrifugation through 30% percoll (GE Healthcare Life Sciences, Pittsburgh, PA). Lymphocytes were isolated from blood using lymphocyte separation media (Cellgro) and AKC lysis buffer as described7.
Flow Cytometry Analysis
The following flurochrome-conjugated antibodies were used for surface staining: anti-human CD3 (Brilliant Violet 650, 1:100, OKT3, BioLegend, San Diego, CA), CD4 (PeCy7, 1:100, SK3, BioLegend), CD8 (APC-Cy7, 1:100, SK1, BD Biosciences), CD19 (PE Texas Red, 1:100, SJ25-C1, Invitrogen or APC, HIB19, Biolegend), CD25 (FITC, 1:50, 2A3, BD Biosciences, San Jose, CA), CD31 (APC, 1:100, WM59, eBioscience, San Diego, CA), CD45RA (Brilliant Violet 605, 1:100, HI100, BioLegend), CD45RO (PerCpFl710, 1:100, UCHL1, eBioscience), CD69 (Brilliant Violet 421, 1:100, FN50, BioLegend or BUV395, 1:100, FN50, BD Biosciences), CD103, (Alexa Fluor 647, 1:100, Ber-ACT8, BioLegend), CD127 (BV711, 1:100, A019D5, BioLegend), CCR7 (Alexa Fluor 488, 1:100, TG8, BioLegend). For intracellular staining, surface stained cells were resuspended and incubated in fixation buffer (eBioscience), washed, resuspended in 0.1mL permeabilization buffer (eBioscience) and stained with anti-Foxp3 antibodies (PE, 1:20, 236A/E7, eBioscience) and Ki67 (α700, 1:100, Ki-67, BioLegend) for 30 min at room temperature and washed twice with permeabilization buffer. Stained cells were acquired on a 6-laser LSRII analytical flow cytometer (BD Biosciences) in the CCTI flow cytometry core and analyzed using FlowJo software (Treestar, Ashland, OR).
T cell proliferation and Treg cell depletion
T cells isolated from tissue suspensions as above were purified using a human T cell enrichment kit (STEMCELL Technologies, Vancouver, BC). A portion of these cells was subjected to CD25 depletion using BD IMag CD25 Magnetic Particles (BD Biosciences), with the CD25-depleted population being collected in the column flow-through. Purified T cells (unfractionated and CD25-depleted) were labeled with 2.5μM CFSE for 15min. at 37 °C, washed with media+FBS to quench excess CFSE, and plated in 96-well round bottom plates (100,000 cells per well) +/− anti-CD2/CD3/CD28-coupled beads (one bead/cell; T cell activation/expansion kit, Miltenyi Biotech) for four days at 37 °C incubator with 5% CO2, after which cells were harvested and analyzed by flow cytometry.
Cytokine Analysis
Supernatants from T cell cultures treated as above were analyzed for cytokine content by Luminex using a cytokine human 25-plex panel (Life Technologies, Grand Island, NY), as performed by the Human Immunology Core (University of Pennsylvania, Philadelphia, PA). For intracellular cytokine staining, isolated T cells were cultured for 4 h with PMA/ionomycin in the presence of Brefeldin A as described7. Cells were then stained for surface markers, fixed, and permeabilized as above and stained with antibodies for IL-8 (PerCP, 1:20, BH0814, BioLegend) and IFN-γ (Alexa Fluor 700, B27, BD Biosciences). Cytokine concentrations were also measured in supernatants using cytometric bead array with the BD Biosciences Human Th1/Th2 cytokine kit II following stimulation with anti-CD2/CD3/CD28-coupled beads as indicated above for two days.
Statistical analysis and data visualization
Descriptive statistics were calculated using Microsoft Excel. Statistical significance was calculated for each subset using student’s T tests or by two-way ANOVA assuming unequal variance and adjusted for multiple comparisons by Holm-Sidak using Graphpad PRISM (Graphpad software, Inc., La Jolla, CA). We did not use statistical methods to predetermine sample size, nor were investigators blinded to sample identity or results.
Supplementary Material
Acknowledgments
This work was supported by the US National Institutes of Health (NIH) (grant no. AI100119; D.L.F., AI106697: D.L.F., F31AG047003; J.J.C.T., AI083022; K.L.B.) and a BD Bioscience Research Grant (J.J.C.T). These studies were performed in the Columbia Center for Translational Immunology (CCTI) Flow Cytometry Core funded in part through an S10 Shared Instrumentation Grant from the NIH (grant no. S10RR027050), with the excellent technical assistance of S.-H. Ho. We gratefully acknowledge the generosity of the organ donor families and the efforts of the LiveOnNY transplant coordinators and staff for making this study possible. We also wish to thank S. Mickel, and B. Kumar for assistance with tissue processing, B. Levin and N. Yudanin for assistance with statistical analyses, and K. Zens, N. Yudanin and E. Lamouse-Smith for critical reading of this manuscript.
Footnotes
Author contributions
J.J.C.T designed experiments, generated and analyzed data from infant and adult donor tissues, made figures, wrote and edited the paper; K.L.B generated phenotype and functional data from infant donors, analyzed data and made figures; Y.O, M.K, N.M, and A.G obtained tissues from organ donors for these studies; C.G and T.G assisted with processing of donor tissue; H.L coordinated tissue donation and acquisition from adult donors; T.K coordinated acquisition for all infant donors; D.L.F designed experiments, analyzed data, maintained protocols for human tissue acquisition, wrote and edited the paper.
Disclosure:
The authors declare no competing financial interests with regard to this study.
References
- 1.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 2.Schenkel JM, et al. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teijaro JR, et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 5.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;14:1007–1013. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burzyn D, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sathaliyawala T, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thome JJC, et al. Spatial Map of Human T Cell Compartmentalization and Maintenance over Decades of Life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbons D, et al. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat Med. 2014;20:1206–1210. doi: 10.1038/nm.3670. [DOI] [PubMed] [Google Scholar]
- 10.Chalmers IM, Janossy G, Contreras M, Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998;92:11–18. [PubMed] [Google Scholar]
- 11.Peoples JD, et al. Neonatal cord blood subsets and cytokine response to bacterial antigens. Am J Perinatol. 2009;26:647–657. doi: 10.1055/s-0029-1220788. [DOI] [PubMed] [Google Scholar]
- 12.Mold JE, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, et al. CD4 T cells with effector memory phenotype and function develop in the sterile environment of the fetus. Sci Transl Med. 2014;6:238ra272. doi: 10.1126/scitranslmed.3008748. [DOI] [PubMed] [Google Scholar]
- 14.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thome JJ, Farber DL. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol. 2015;36:428–435. doi: 10.1016/j.it.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimmig S, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner DL, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7:501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laidlaw BJ, et al. CD4(+) T Cell Help Guides Formation of CD103(+) Lung-Resident Memory CD8(+) T Cells during Influenza Viral Infection. Immunity. 2014;41:633–645. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Invest. 2013;123:939–944. doi: 10.1172/JCI57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr. 2013;25:708–714. doi: 10.1097/MOP.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pesenacker AM, Broady R, Levings MK. Control of tissue-localized immune responses by human regulatory T cells. Eur J Immunol. 2014 doi: 10.1002/eji.201344205. [DOI] [PubMed] [Google Scholar]
- 23.Ferraro A, et al. Expansion of th17 cells and functional defects in T regulatory cells are key features of the pancreatic lymph nodes in patients with type 1 diabetes. Diabetes. 2011;60:2903–2913. doi: 10.2337/db11-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seddiki N, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seddiki N, et al. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006;107:2830–2838. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- 27.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Booth NJ, et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184:4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 29.Allan SE, Song-Zhao GX, Abraham T, McMurchy AN, Levings MK. Inducible reprogramming of human T cells into Treg cells by a conditionally active form of FOXP3. Eur J Immunol. 2008;38:3282–3289. doi: 10.1002/eji.200838373. [DOI] [PubMed] [Google Scholar]
- 30.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348:589–594. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


