Abstract
The mammalian homeostatic oxygen sensing system (HOSS) initiates changes in vascular tone, respiration, and neurosecretion that optimize oxygen uptake and tissue oxygen delivery within seconds of detecting altered environmental or arterial PO2. The HOSS includes carotid body type 1 cells, adrenomedullary cells, neuroepithelial bodies, and smooth muscle cells (SMC) in pulmonary arteries (PA), ductus arteriosus (DA) and fetoplacental arteries. Hypoxic pulmonary vasoconstriction (HPV) optimises ventilation-perfusion matching. In utero, HPV diverts placentally-oxygenated blood from the non-ventilated lung through the DA. At birth, increased alveolar and arterial oxygen tension dilate the pulmonary vasculature and, constrict the DA, respectively, thereby transitioning the newborn to an air-breathing organism. Though modulated by endothelial-derived relaxing and constricting factors, O2-sensing is intrinsic to PA- and DASMCs. Within the SMC’s dynamic mitochondrial network, changes in PO2 alter the reduction-oxidation state of redox couples (NAD+/NADH, NADP+/NADPH) and the production of reactive oxygen species, ROS (e.g. H2O2) by Complexes I and III of the electron transport chain (ETC). ROS and redox couples regulate ion channels, transporters, and enzymes, changing intracellular calcium [Ca2+]i and calcium sensitivity and eliciting homeostatic responses to hypoxia. In PASMC, hypoxia inhibits ROS production and reduces redox couples, thereby inhibiting O2-sensitive voltage-gated potassium (Kv) channels, depolarizing the plasma membrane, activating voltage-gated calcium channels (CaL), increasing [Ca2+]i and causing vasoconstriction. In DASMC, elevated PO2 causes mitochondrial fission, increasing ETC Complex I activity and ROS production. The DASMC’s downstream response to elevated PO2 (Kv channel inhibition, CaL activation, increased [Ca2+]i and rho kinase activation) is similar to the PASMC’s hypoxic response. Impaired O2-sensing contributes to human diseases, including pulmonary arterial hypertension and patent DA.
Keywords: Hypoxic pulmonary vasoconstriction, ductus arteriosus, mitochondria, oxygen-sensitive potassium channels, pulmonary arterial hypertension, patent ductus arteriosus
Introduction
Although human life requires oxygen, all humans begin life in the hypoxic intrauterine environment. The foetus receives oxygen from the mother through the placenta. With the first breath, at the moment of birth, we are exposed to elevated oxygen concentrations in the environment. Thereafter, we live in an environment in which oxygen is usually abundant. However, as we proceed through life we may be exposed to hypoxia both as a consequence of changes in our environment, such as exposure to high altitude, or through disease (e.g. pneumonia, atelectasis). In the modern era of artificial ventilation we may also experience hyperoxia.
Oxygen is required for mammalian life because mammals have high energy demand. Oxygen’s ability to receive electrons permits it to participate in an elegant cascade within the mitochondria that ultimately produces energy-rich phosphates (ATP). Situated at the distal end of a redox cascade (the mitochondrial electron transport chain, ETC), oxygen receives electrons and in doing so, is reduced, forming water. The reductive journey of electrons down a redox gradient begins with electron donors nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2), which are produced by mitochondrial metabolic cycles, notably, the Krebs cycle. These donors pass their electrons down a redox potential gradient that is spread over 4 mega-complexes (ETC Complexes I-IV) spanning the inner mitochondrial membrane. This electron transport powers the pumping of hydrogen ions across the inner mitochondrial membrane and in so doing, generates the potential energy to power ATP synthesis.
The range of physiologically optimal oxygen tension is narrow; too much or too little oxygen can result in disease. Evolution has endowed mammals with a network of specialized tissues that sense oxygen in their local environment. These tissues couple an upstream mitochondrial O2-sensor mechanism with downstream redox-responsive effectors (ion channels and enzymes). Upon sensing small changes in PO2, these effectors initiate compensatory responses that optimize the uptake and delivery of oxygen to our tissues. This network is called the Homeostatic Oxygen Sensing System (HOSS) [95]. The components of the HOSS are strategically distributed in the body to rapidly optimize oxygen uptake and distribution in response to small changes in airway oxygen levels (FiO2) or blood oxygen levels (PO2).
The HOSS includes type 1 cells in the carotid body, and smooth muscle cells (SMC) in small pulmonary arteries (PA), fetoplacental arteries in the placenta, and the ductus arteriosus (DA). HOSS components can increase ventilation to enhance oxygen uptake (the type 1 cells in the carotid body), match ventilation to perfusion in the lung to avoid perfusing hypoxic lung segments (PASMC in small resistance-level pulmonary arteries), and redistribute blood to hypoxic organs by causing vasodilatation (SMC in the DA or systemic arteries). Additional components of this homeostatic system include the adrenomedullary chromaffin cells in the adrenal glands, which can release catecholamines to counteract hypoxic stress at birth, and neuroepithelial bodies, neuroendocrine cells in the airways, the function of which is less well understood. The HOSS should arguably be considered one of the body’s major physiologic systems, on equal footing with the cardiovascular, nervous, or endocrine systems.
Attributes of HPV
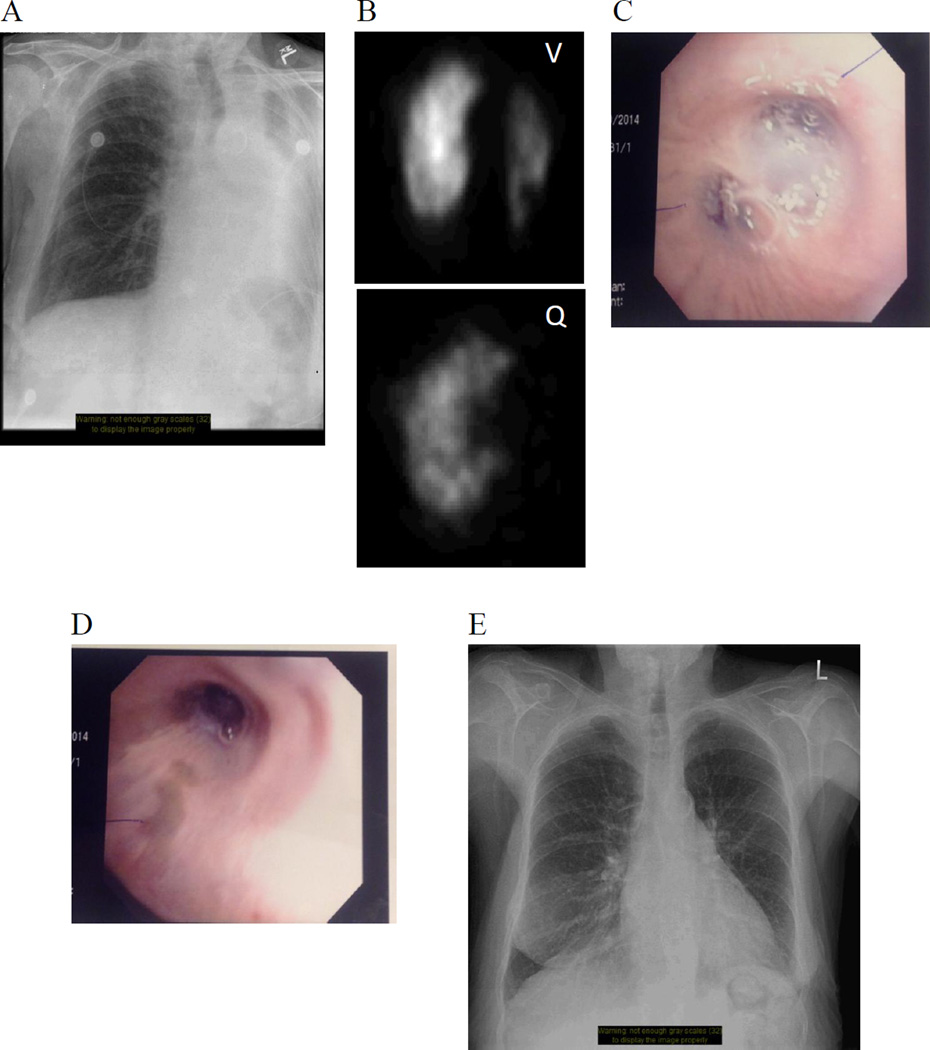
Since its first description 120 years ago by Bradford and Dean [16], and subsequent more extensive description by von Euler and Liljestrand [24], the search for the mechanism of HPV has been the subject of much research [10, 11, 64, 66–68, 93–95, 97]. The field has also seen substantial debate [88, 96]. To help clarify the study of HPV, it is essential to understand its physiologic phenotype. The basic attributes of HPV include its rapid onset. HPV onsets within seconds of exposure to hypoxia [42] and reaches a maximum within minutes [13]. HPV is also completely reversible upon restoration of normoxia [82]. In addition, HPV is primarily elicited by alveolar hypoxia, rather than pulmonary arterial hypoxemia. Consistent with this responsiveness to alveolar hypoxia, HPV is strongest in the resistance pulmonary arteries (PA), which are exposed to alveolar oxygen tensions (FiO2). The fact that HPV is reversible and localized to the region of the lung that is hypoxic is illustrated in Figure 1. In this patient, airway hypoxia caused by a mucus plug in the left lung’s main bronchus led to corresponding left lung HPV, thereby reducing left lung perfusion and optimizing systemic PO2. HPV was sustained until the mucous plug was removed by means of bronchoscopic aspiration.
Fig. 1. Hypoxic vasoconstriction by ventilation/perfusion scintigraphy.
Image series of a 77-year-old woman with chronic obstructive pulmonary disease and peripheral vascular disease who experienced transient, acute hypoxia on the hospital ward while awaiting removal of an ischemic toe. A) Plain anteroposterior film displaying left upper lung zone volume loss suggestive of bronchial obstruction. B) Ventilation (upper) and perfusion (lower) (V/Q) scintigraphy scans confirming diminished ventilation to the left lung, absent perfusion to the left upper lobe (LUL), and decreased perfusion to the left lower lobe (LLL), demonstrating appropriate hypoxic vasoconstriction of the LUL vasculature. The relatively preserved perfusion to the LLL despite absent ventilation is known as “reverse ventilation-perfusion mismatch,” and is indicative of inadequate hypoxic vasoconstriction reflex to that area. C) Bronchoscopy revealing mucous plugging of the left superior and inferior lobar bronchi. D) Post-bronchoscopic aspiration of mucous plug. E) Plain anteroposterior film displaying complete re-expansion of left lung post-aspiration
Hypoxic constriction is unique to the PA; systemic arteries, such as the mesenteric arteries [101] and renal arteries [62], relax in response to hypoxia. Likewise, at the cellular level, PASMC from PA constrict to hypoxia while cerebral artery SMC relax [51]. There is a longitudinal heterogeneity in the magnitude of HPV in cats, with HPV being greater in small, resistance PA (diameter < 300µm) and less in large, conduit PA (diameter > 500µm) arteries. Indeed, larger PA behave like systemic arteries, exhibiting neither significant hypoxic constriction nor hypoxic PASMC depolarization [50]. Similar localization of HPV to resistance PA is noted in the rat [7, 9]. On a molecular level, the downstream constrictor mechanism in HPV is well accepted to result, in large part, from a rise in intracellular calcium in PASMC [57].
Role of potassium and calcium channels in HPV
The effector mechanism of HPV involves the coordinated activity of redox- and voltage-sensitive K+ [74] and Ca2+ channels [93]. McMurtry et al showed that verapamil, an inhibitor of large-conductance, voltage-gated calcium channels (CaL) virtually eliminated HPV in an isolated perfused rat lung. This indicates that the calcium required for HPV is largely derived from the extracellular pool and enters the PA via CaL channels [57], a finding reinforced by Harder in studies conducted in isolated PA rings [35]. Conversely, HPV is enhanced by CaL channel agonists, such as BAY K8644 [59, 86]. The sensitivity of CaL channels to changes in oxygen tension varies longitudinally along the pulmonary vasculature. Resistance PA have increased CaL channel current density and greater ICa hypoxia-sensitivity, as compared to conduit PA [27].
CaL channels are voltage-dependent and cell membrane depolarization favours their activation (although the CaL can be intrinsically O2-sensitive [27]). Potassium (K+) channels are known to regulate membrane potential (Em) in multiple cell types, including SMC. In pharmacology experiments using isolated perfused rodent lungs, Hasunuma et al showed that the K+ channel blockers, tetraethylammonium (TEA) and 4-aminopyridine (4-AP), cause pulmonary vasoconstriction [36]. Post et al used the whole-cell patch clamp technique to directly demonstrate that outward potassium current (IK) creates the PASMC’s relatively negative Em (< than −50mV). This electronegativity prevents opening of the CaL channels, which have their own a voltage sensor that favours channel opening at more positive, depolarized, membrane potentials (i.e. > −20mV) [74]. The PASMC’s IK is inhibited by hypoxia, leading to membrane depolarization and vasoconstriction. Post also demonstrated that K+ channel antagonists, notably the voltage-gated K+ channel (Kv) inhibitor 4-AP, mimicked the hypoxic response in isolated rat lung as well as PA rings, increasing pulmonary artery pressure (PAP). 4-AP inhibits the hypoxia-sensitive portion of IK in isolated rat PASMC [74]. Although blockers of the CaL eliminate the majority of HPV [35, 57], hypoxia also increases intracellular calcium by enhancing release from the sarcoplasmic reticulum (SR) [79]. Hypoxia can also directly enhance calcium entry through the CaL [28].
Initially, pharmacology and electrophysiology were used to suggest the identity of the oxygen-sensitive K+ channels involved in HPV. These studies showed that the PASMC’s hypoxia-sensitive IK current was sensitive to 4-AP, but not TEA or charybdotoxin (CTX), inhibitors of large conductance, Ca2+-sensitive K+ channels (BKCa). The hypoxia-sensitive IK was predominant in PASMC isolated from resistance PA, whereas a BKCa-predominant IK was evident in PASMC derived from conduit PA (which dilate in response to hypoxia) [7]. Subsequently, correolide, an inhibitor of Kv1.x channels, provided further specificity showing that much of the hypoxia-sensitive IK in PASMC was conducted by member of the Kv1.x channel family.
Molecular studies ultimately identified specific voltage-gated, O2-sensitive K+ channels that elicit HPV, including Kv1.5 and Kv2.1 [9]. Kv1.5 mRNA and protein levels are increased in resistance PA (relative to conduit PA), and the majority of IK in these arteries is inhibited by correolide. Inhibition of Kv1.5 and Kv2.1, achieved by administering antibodies against these channels to the intracellular space using a patch pipette, results in additive inhibition of cell depolarization. Superimposition of hypoxia after addition of the anti-Kv antibodies does not result in further membrane depolarization. This suggests that these two channels contribute a majority of the hypoxia-sensitive IK. Consistent with this, Platoshyn et al demonstrated that expression of Kv1.5 is proportional to sensitivity to hypoxia, and that heterogeneity in the expression of Kv1.5 channels amongst PA segments accounts in part for regional heterogeneity in the magnitude of HPV [73]. Finally, HPV is impaired in mice lacking Kv1.5, even though expression of other putative oxygen-sensitive Kv channels (Kv 1.1, 1.2, 2.1, 3.1, and 4.3) is unaltered [8].
It is noteworthy that ion channels may be “redox-sensitive” due to their intrinsic amino acid sequence (i.e. the presence of redox-sensitive sulfhydryl groups) [21]. Certain amino acids, such as histidine, cysteine, methionine, and tryptophan are particularly sensitive to modification by ROS. Thus, ROS may cause redox changes in key amino acids, thereby altering channel conformation and function. In addition, Kv channels are tetramers of α-subunits and may assemble as heterotetramers, often with associated β-subunits. The heterotetrameric composition of a channel or its association with β-subunits may alter its O2 sensitivity. For example, when expressed in PASMC, heterotetrameric channels comprised of Kv1.5 plus Kv1.2 or Kv2.1 plus Kv9.3 α-subunits, display enhanced oxygen sensitivity compared to similar expression of homomeric channels [41].
Another line of evidence for the role of O2-sensitive K+ channels in the mechanism of HPV comes from studies of rats exposed to chronic hypoxia. Chronic hypoxia attenuates acute HPV while leaving other forms of vasoconstriction unaltered or enhanced [58], and ultimately causes a form of pulmonary hypertension that is associated with decreases in the expression of both Kv1.5 and Kv2.1 [77, 102]. This loss of acute HPV in chronic hypoxic pulmonary hypertension can be reversed by infection of the lung with Kv1.5, delivered by a serotype 5 adenovirus. Kv1.5 gene therapy also reduces pulmonary hypertension and regresses both right ventricular and PA hypertrophy [75].
Other voltage-gated channels, including Kv3.1b, have been implicated in the mechanism of HPV. Osipenko et al used patch clamp techniques to investigate the effect of hypoxia on Kv3.1b. They reported that hypoxia inhibited Kv3.1b, but not Kv1.x channels, and that addition of TEA inhibited the electrophysiological effects of hypoxia in PASMC [69] – a finding discordant to observations reported by both Hasunuma [36], Post [74], and Archer [8].
Some debate exists surrounding the role of Kv channels versus other types of K+ channels in HPV. This debate revolves around consideration of the PASMC’s resting Em. Two-pore K+ channels (K2P channels) have greater open-state probability at more negative Em than do Kv channels. Unlike Kv channels, they do not show desensitization with prolonged or repeated depolarization. Thus, it has been argued that they may be more relevant to HPV since they would be expected to be active at the PASMC’s resting Em. However, it should be noted that, under proper experimental conditions (i.e. studying PASMC at physiological temperature and PO2) using a patch clamp protocol that does not include prolonged exposure of the cell to depolarization or extreme depolarization steps, Kv channels are also active at resting Em. Candidate K2P channels include TASK channels (TWIK-related, acid-sensitive K+ channel). TASK channels conduct a background current that is voltage-independent [22]. Additional 2-pore K+ channels, including TASK-2, THIK-1 (tandem pore domain halothane-inhibited K+ channel 1), TREK-2 (TWIK-related K+ channel 2), and TWIK-2 (tandem pore domain weak inward rectifier K+ channel 2) have been detected in the PASMC [29]. However, when evaluating the potential role of K+ channels in HPV, one would expect observations to be concordant with established attributes of HPV. While it is expected that inhibition of a putative candidate channel should elicit vasoconstriction (mimicking hypoxia), blockade of K2P channels, with anandamide or bupivacaine, did not significantly increase pulmonary vascular tone [29].
Although there is no clear explanation for these divergent findings, the majority of research surrounding TASK channels occurred using proximal rather than resistance PA [32]. Moreover, one can envision heterogeneity in the channels involved in HPV amongst individuals and between species. In addition, each channel activates and inactivates at specific voltages, and therefore, there may be a rolling progression of channels inhibited by hypoxia. Those channels active at resting membrane potential would be first to be inhibited by hypoxia, but the resulting depolarization would bring into play other channels that function at more positive potentials. Other factors that result in heterogeneous reports include the failure to define PO2 and pH in many studies. Anoxia and hypoxia are distinct, and it is important to be precise when defining study conditions and ensure they replicate conditions relevant to HPV. Finally, there is diversity in the quality of the cell used when examining the mechanism of HPV. Some laboratories use conduit PAs (which are easier to harvest but lack HPV), while others use PASMC that have been passaged in culture. Cell culture down-regulates the expression of many Kv channels and attenuates hypoxia-sensitive IK. The healthier the PASMC preparation, the more negative the resting membrane potential (usually ∼ −60mV). In evaluating the quality of the evidence for a channel’s role in HPV one should consider whether the cells being used are primary isolates from resistance PAs, whether they manifest a robust response to hypoxia, whether the have a demonstrable hypoxic response (cellular constriction or a rise in [Ca2+]i) and whether the studies were performed at optimal temperature and pH.
The downstream portion of the HPV mechanism is shared with other tissues in HOSS, albeit the specific ion channels involved and the consequence of channel inhibition varies amongst tissues. For example, hypoxic inhibition of IK and depolarization of the plasma membrane activates CaL channels leading to calcium influx in the type 1 cell of the carotid body [47, 72], the adrenomedullary cells [65], and the neuroepithelial body [99]. However, in each of these tissues, the rise in calcium triggers secretion of mediators, rather than vasoconstriction. In the DASMC, the downstream electrical cascade is similar to that in the PASMC, but is triggered by increased PO2 [87].
Mitochondria – the O2 sensor mediating HPV
While the identity of HPV’s effector mechanism is reasonably well established, the sensor and mediator components of the O2-sensing system are the subject of ongoing investigation (and some controversy [88, 96]). Although there is certainly controversy, our worthy opponents in a debate on the mechanisms of HPV noted:
“Currently, the best documented hypothesis for HPV proposes that Ca2+ entry is mediated primarily via voltage-dependent L-type channels, as a result of hypoxia-induced inhibition of voltage-gated K+ channels (Kv channels) and consequent depolarization. According to this scheme, Kv channel inhibition is caused by a decrease in the ambient intracellular concentration of H2O2 which results when mitochondrial electron transport, and consequently the production of superoxide ion, falls due to the lack of O2. There is an enormous body of evidence supporting this hypothesis, which has been presented in a number of authoritative reviews” [1].
Although the O2-sensitive ion channels respond to changes in PO2, they are not themselves sensors. In fact, systemic and pulmonary arteries have a similar complement of K+ and Ca2+ channels, and respond similarly to Kv channel blockers such as 4-AP with vasoconstriction, and to CaL inhibitors, such as verapamil and nifedipine, with vasodilation. Rather, it is the vascular oxygen sensor and the sensor’s susceptibility to initiate PO2-dependent changes in production of redox mediators that differs between these vascular beds.
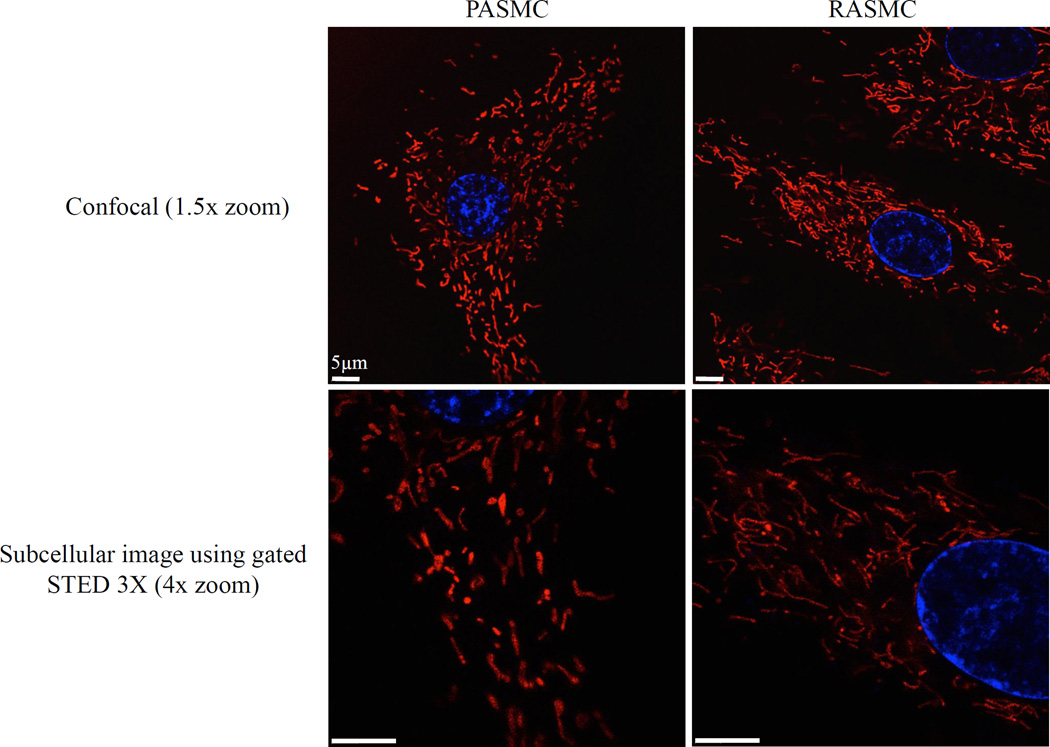
Mitochondria are leading candidates for the role of PA O2 sensors. Although mitochondria rely on molecular oxygen to produce ATP the PASMC’s oxygen sensor does not rely on ATP depletion, which would be a late and extreme signal of hypoxia. Instead, the sensor monitors mitochondrial electron transport and the related production of ROS and changes in redox couples, which are perturbed by hypoxia before ATP is depleted. Recent advances in live cell imaging using mitochondrial-targeted fluorophores reveal that the PASMC’s mitochondria are arrayed in an extensive network [15, 52] (Figure 2). Recognition of the structure and mobility of mitochondria offers biologic plausibility that these organelles are positioned to rapidly convey redox signals to ion channels in the plasma membrane as well as cytosolic enzymes within vascular SMC [63].
Fig. 2. Smooth muscle cell mitochondria are arranged in an extensive network.
Standard confocal (top) and super resolution (bottom) images of rat arterial smooth muscle cells. Cells stained with 50nM MitoTracker® Red FM and Hoechst 33342 (Molecular Probes, Life Technologies; Waltham, MA) for 20 min at 37°C and imaged using a Leica TCS STED 3X confocal microscope with and without stimulated emission depletion (STED); all images 100X oil with 1.5x or 4.0x zoom. A) Confocal image (1.5x) and B) STED confocal image (4.0x) of pulmonary artery smooth muscle cells. C) Confocal image (1.5x) and D) STED confocal image (4.0x) of renal artery smooth muscle cells
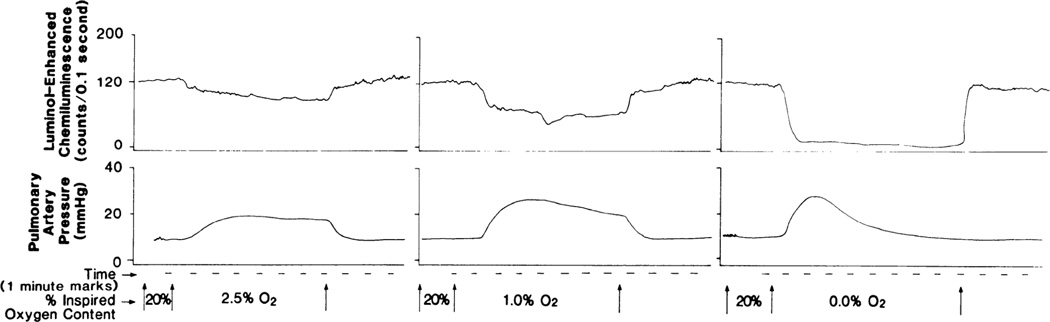
Rounds and McMurtry demonstrated that various ETC inhibitors increased pulmonary vascular resistance, mimicking the effects of hypoxia in a crystalloid-perfused isolated rat lung model [80]. They postulated that the constrictor effect related to energy depletion. However, further investigation showed that within the rapid time frame of the vasoconstriction, there was no energy depletion or change in PO2. Rather, these ETC inhibitors were changing cytosolic redox chemistry and mitochondrial ROS production. Moreover, while rotenone and antimycin A (specific inhibitors of complex I and complex III, respectively) recapitulated the effects of hypoxia, cyanide, an inhibitor of complex IV, did not [62]. These observations are consistent with the Redox Theory of HPV, which has gradually been refined since its initial publication in 1986 [3], reviewed in [96, 97]. The Redox Theory (Figure 3) postulates that the mitochondrial ETC is an important oxygen sensor that varies production of ROS in direct proportion to physiologic changes in PO2 [64]. This is plausible, since a small percentage of electron transport involves unpaired electrons that yield superoxide anion. Superoxide anion is rapidly converted by mitochondrial superoxide dismutase 2 to H2O2, a less toxic redox mediator with a wider diffusion radius. Thus, the more electron flux, the more uncoupled electrons and ROS production [5]. Conversely, reduced electron flux results in fewer radicals and less H2O2 production. This applies not only to the PA and ductus, but also the heart (where ischemia-reperfusion is well established to reduce and increase ROS production, respectively) [38]. Evidence for diffusible mitochondrial-derived ROS being the mediator of HPV has been demonstrated through many experimental strategies. First, there are concordant effects of hypoxia and inhibitors of the proximal ETC on the pulmonary vs. systemic vasculature (Table 1). Perfusion of isolated rabbit lungs with lucigenin (a detector of superoxide, O2) demonstrated endogenous production of O2.− in the lung that was inhibited by superoxide dismutase (SOD, an enzyme responsible for detoxification of O2.− to H2O2), but not catalase (an H2O2 scavenger) or dimethylthiourea (DMTU, an hydroxyl radical scavenger) [70]. These experiments have also been conducted using isolated, saline-perfused rat lungs. In this preparation, hypoxia also decreases the chemiluminsecent signal. This decrease in ROS production (whether measured with luminol or lucigenin) precedes the increase in PAP, as would be expected if it were the signal connecting the senor to the downstream effectors [4] (Figure 4). Likewise, proximal ETC inhibitors (antimycin A and rotenone) also cause pulmonary vasoconstriction and decrease ROS formation in isolated perfused rat lungs [6]. In addition, PA from rats exposed to chronic hypoxia not only have reduced HPV but display a concordant decrease in constriction to ETC inhibitors (whereas their constrictor responses to phenylephrine is enhanced). This selective impairment of oxygen sensing in response to chronic hypoxia is recapitulated in the fawn hooded rat, a model of spontaneous PAH in which there is mitochondrial fragmentation and decreased expression of ETC complex I [15] (Figure 5). H2O2 is known to be a pulmonary vasodilator, as demonstrated by Wolin and Burke. Although, in bovine pulmonary arteries, at least some of this vasodilatation relates to activation of guanylate cyclase [98]. Apart from inhibitors of ETC complexes I and III, the only other class of chemicals which is known to mimic hypoxia (without changing PO2) are redox agents - agents that can reduce (donate an electron) or oxidize (remove an electron). Treatment of rat PA and aortic rings with diamide (a sulfhydryl oxidant) or oxidized glutathione (GSSG) causes relaxation of PA rings and (in parallel patch clamp experiments) opens Kv channels, as measured by increases in whole-cell IK. Conversely, antioxidants such as co-enzyme Q10, duroquinone, or reduced glutathione (GSH) elicit dose-dependent, endothelium-independent vasoconstriction [76] and inhibit IK in PASMC (mimicking hypoxia) [76, 93] (Figure 6). Based on the concordant effects of reducing agents and ETC inhibitors in the pulmonary vasculature, it appears that states of reduction (like authentic hypoxia itself) cause pulmonary vasoconstriction, while oxidants (like normoxia itself) cause vasodilatation.
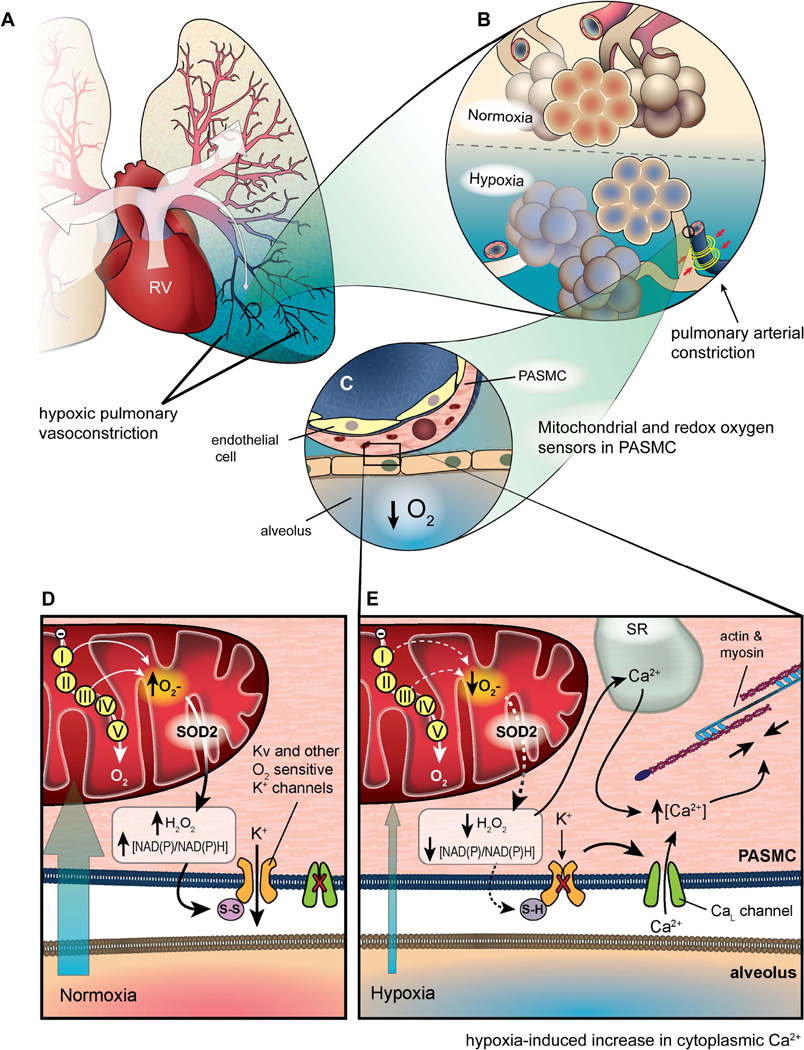
Fig. 3. The Redox Theory of HPV.
A) Illustration of a left lung experiencing regional hypoxia in the left lower lobe. Well-ventilated portions of the lung have pink blood vessels and increased blood flow (indicated by thick arrows), whereas the hypoxic region of the lung is blue with small, constricted, blue blood vessels and diminished blood flow (indicated by a thin arrow). B) Inset of lung illustrating oxygenated alveoli and dilated blood vessels in a normoxic region of the lung versus hypoxic alveoli and constricted pulmonary arteries in a hypoxic region of the lung. C) Inset of pulmonary artery illustrating the proximity of PASMC mitochondria to a hypoxic alveolus. D) Under normoxic conditions, mitochondrial ROS production and elevated NAD(P)/NAD(P)H ratio contribute to an oxidative environment, resulting in oxidized sulfhydryl groups on K+ channels, keeping them open, while the CaL channel remains closed. E) During hypoxia, diminished ROS production and reduced NAD(P)/NAD(P)H ratio result in depolarization of the cell, closing of K+ channels and subsequent opening of CaL channels. Influx of Ca2+ triggers Ca2+ release from the sarcoplasmic reticulum (SR), stimulating actin and myosin and triggering vasoconstriction
Table 1.
Similarities between hypoxia, proximal, and distal electron transport chain (ETC) inhibitors on pulmonary artery smooth muscle cells (PASMC)
| Hypoxia | Proximal ETC Inhibitors | Distal ETC Inhibitors | |
|---|---|---|---|
| Redox couples (e.g. NAD+/NADH) |
reduced | reduced | |
| Voltage-gated K+ current | ↓ | ↓ | ←→ |
| ROS levels in PASMC |
(controversial) ↓ Per Archer lab |
↓ | ↑ |
| Cytosolic Ca2+ | ↑ | ↑ | ↑* |
-the distal ETC inhibitor cyanide induces increased cytosolic Ca2+ via a mechanism discrete of that elicited by hypoxia
Fig. 4. Simultaneous measurement of ROS production via chemiluminescence and mean pulmonary artery pressure (PAP) during hypoxia in isolated rat lung.
Hypoxia-induced change in luminol-enhanced chemiluminescence begins earlier and peaks sooner than simultaneously measured change in mean pulmonary artery pressure during hypoxia ventilation. Magnitude of change in chemiluminescence is proportional to inspired O2 content. This is a representative recording from single lung. From [4], reproduced with permission.
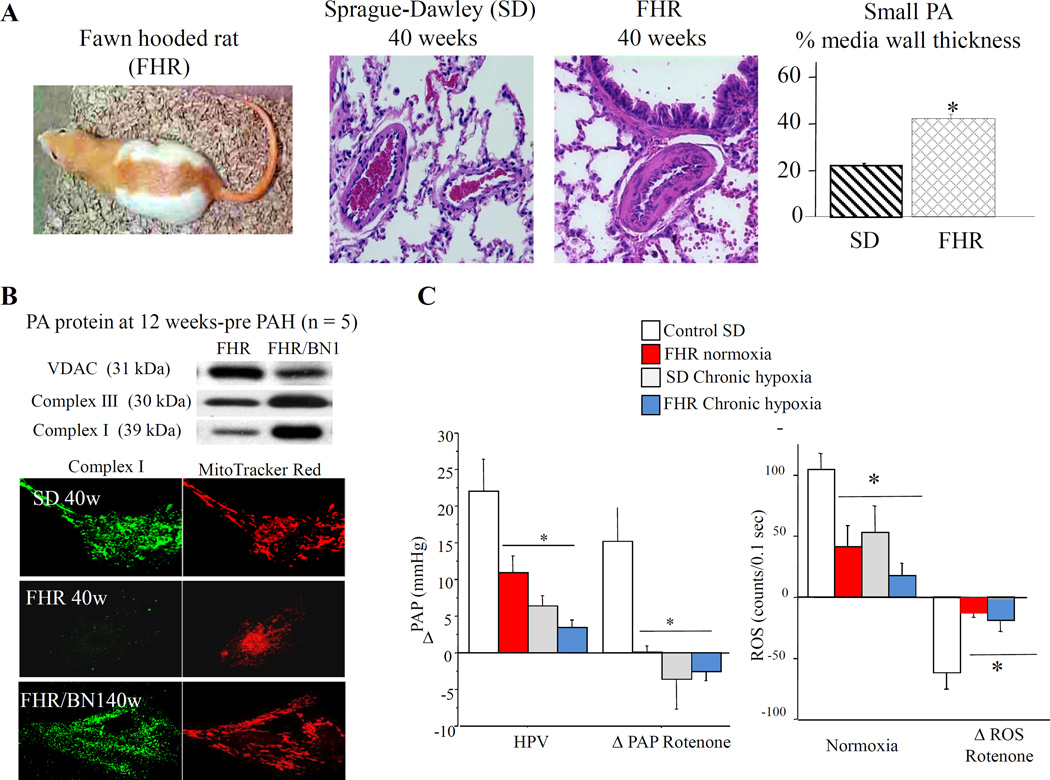
Fig. 5. Similarities between Sprague-Dawley rats exposed to chronic hypoxia and the fawn hooded rat that exhibits spontaneous PAH.
Early loss of ROS precedes spontaneous PAH in FHR. A) Lung histology reveals that 40-week-old FHRs have increased medial hypertrophy of small pulmonary arteries vs 40-week-old Sprague-Dawley rats (*P<0.05). B) Expression of electron transport chain complexes is decreased in FHR pulmonary arteries before PAH (voltage-dependant anion channel expression is increased). FHR PASMCs express less electron transport chain complex I and have fewer mitochondria arrayed in a less organized reticulum than FHR/BN1 PASMCs. C) Hypoxic and rotenone vasoconstriction and lung ROS levels (luminol) are similarly impaired in normoxic FHRs and chronically hypoxic Sprague-Dawley rats vs normoxic Sprague-Dawley rats (*P<0.05). From [15], reproduced with permission
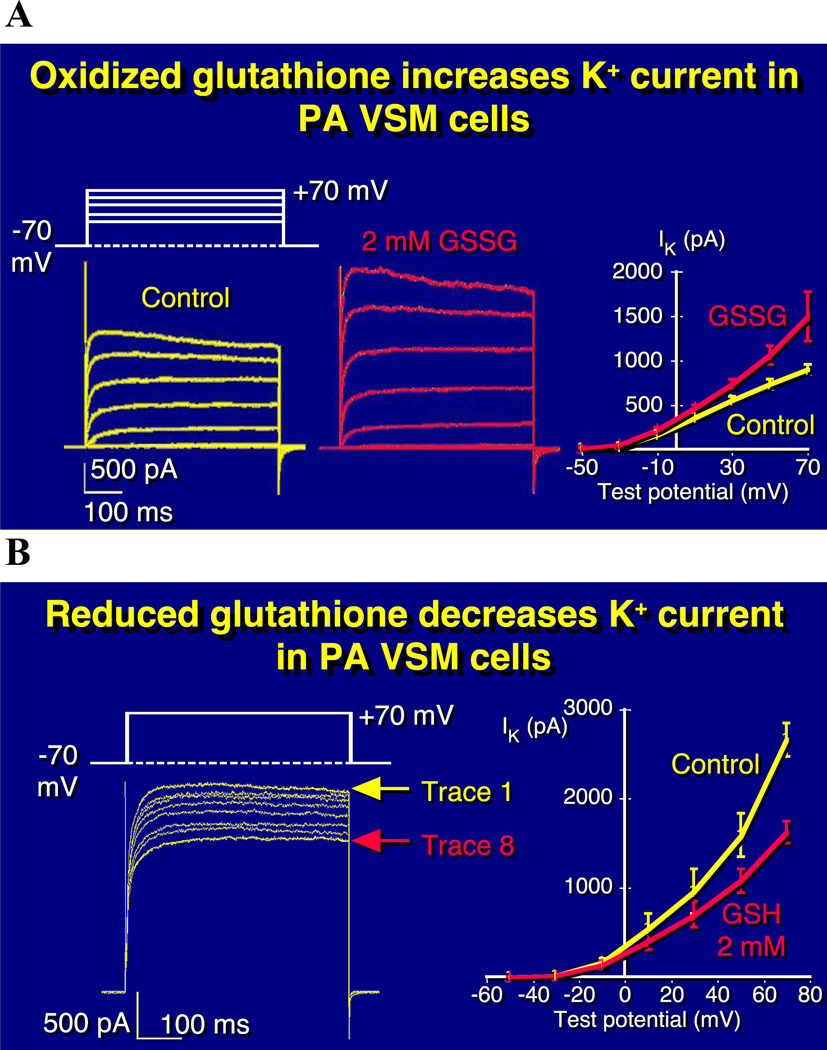
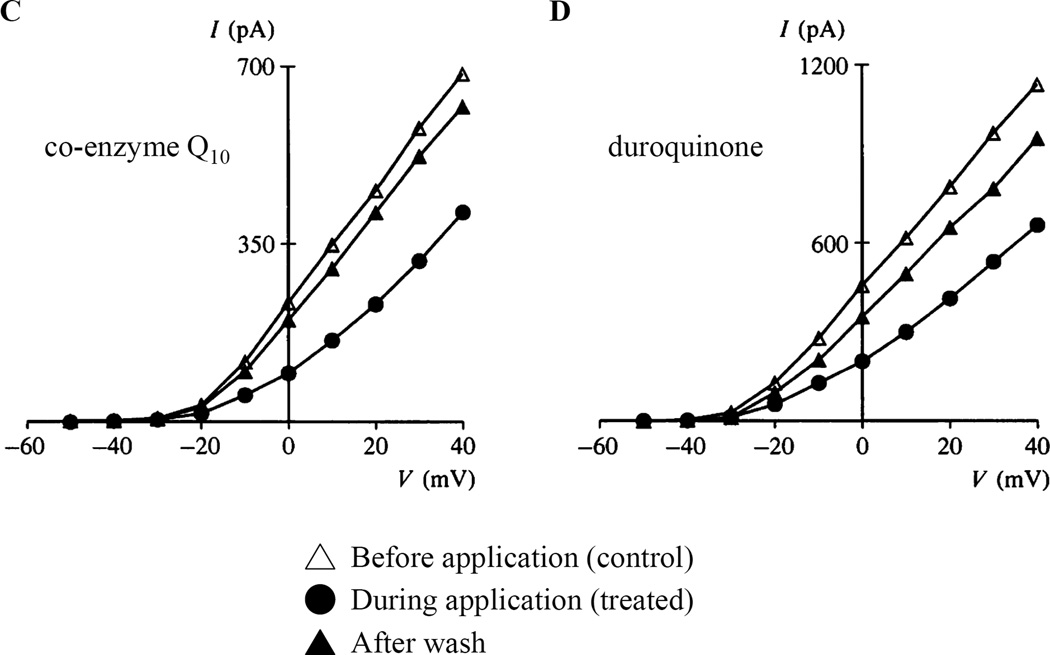
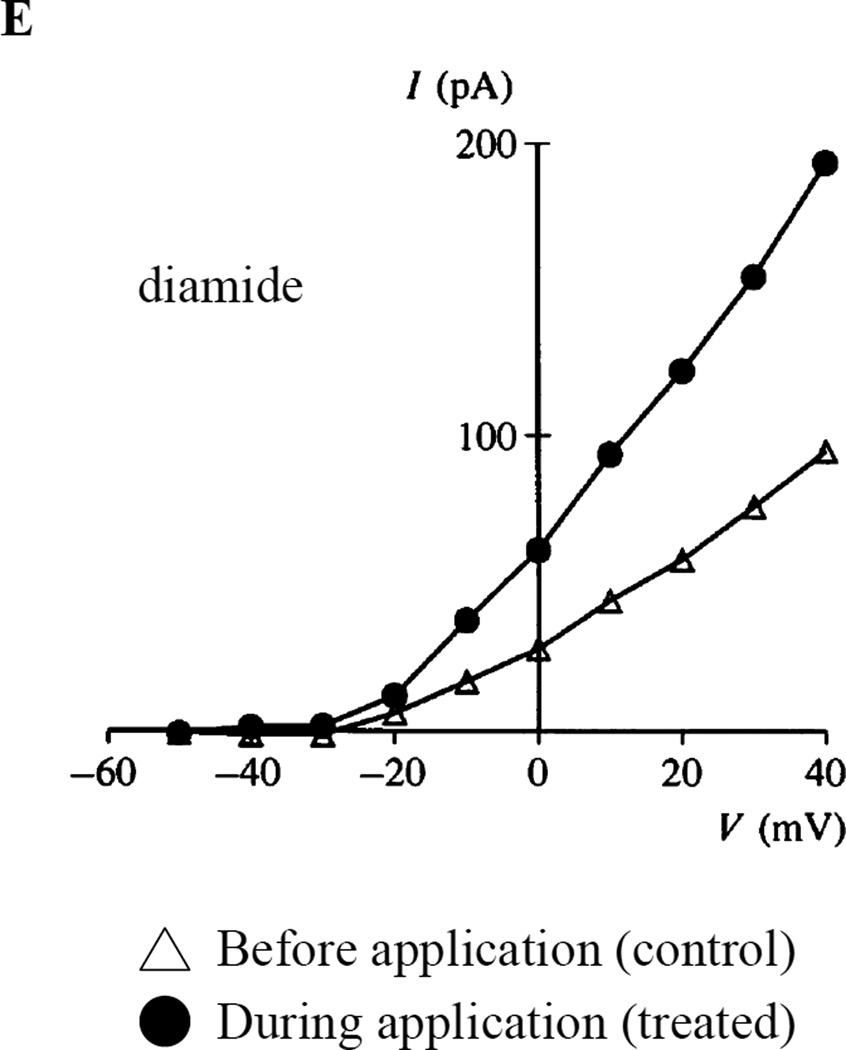
Fig. 6. Reducing agents inhibit and oxidants enhance PASMC K+ current.
Effect of intracellular oxidants and reducing agents on whole-cell potassium currents in rat pulmonary artery smooth muscle cells. A) Family of K+ currents elicited b voltage steps before (left) and after (middle) 5 min dialysis of oxidized glutathione (GSSG) and current-voltage relationship for mean (± SEM) potassium current in the presence or absence of 2mM GSSG (right). B) Series of K+ currents elicited by a repetitive voltage step from −70 to +70 mV, every 20s after start of dialysis of reduced glutathione (GSH) (left) and current-voltage relationship for mean (± SEM) potassium current in the presence or absence of 2mM GSH (right). Graphical representation of current-voltage relationships for mean (± SEM) potassium current in the presence or absence of C) 100µM co-enzyme Q10, D) 100µM duroquinone, E) 100µM diamide. 5A and 5B from [93], 5C-5E from [76], reproduced with permission
The use of comparative physiology, contrasting the pulmonary to systemic vasculature, has also been helpful in identifying the role of the mitochondrial redox O2 sensor. Compared to isolated RA rings, PA rings have greater normoxic production of H2O2 and, unlike RA rings, decrease ROS production upon exposure to both hypoxia and inhibitors of the proximal ETC (e.g. rotenone or antimycin A). As a result, whereas hypoxia decreases IK in the PASMC and causes vasoconstriction, it increases IK and elicits vasodilation in the RASMC [62].
While there is agreement in the literature that the mitochondrion is likely the oxygen sensor responsible for producing mediator ROS, whether hypoxia lowers or elevates ROS production is a source of lingering controversy [88, 96]. The hypothesis that ROS production decreases with hypoxia is based on the notion that, with decreased O2, there is reduced electron flux. Since ROS are produced by the 3% of electron flux involving uncoupled electrons, hypoxia would be predicted to decrease ROS. Conversely, the hypothesis that hypoxia increases ROS is based on the supposition that in the absence of O2, there is distal obstruction of the ETC and a retrograde accumulation of electrons leading to autoxidation of the chain and ROS production [18, 33, 89, 90, 92]. Interestingly, there is no disagreement that ROS increase with elevation of PO2 in the ductus [78], nor is there disagreement that ROS production is decreased during oxygen deprivation that accompanies cardiac ischemia [37], as recently reviewed [43].
Whether physiologic hypoxia increases or decreases mitochondrial ROS depends in part on whether there is input block concomitant with hypoxia. A reduction in electron influx into the ETC during hypoxia (resulting from simultaneous impairment of the Krebs cycle) would promote a decrease in ROS production (as we hypothesize). Conversely, ongoing input of electrons during hypoxia might favour increased ROS if the terminal electron receptor (O2) were absent. Differences in the quality of the tissue preparation in which HPV is assessed, the use of different techniques for measuring ROS, and the use of anoxia (as opposed to hypoxia by some groups) may account for divergent results. Suggestions for overcoming these differences in experimentation have been previously published [66]. It is noteworthy, that over time there has been some movement towards consensus, with the group that reports hypoxic increases in ROS conceding that mitochondrial ROS production is decreased with hypoxia, as measured with mitochondria-targeted RoGFP [91]. Interestingly, the main proponent of ROS increasing during hypoxia in the lung also noted that ROS increased during ischemia (prior to reperfusion) in the heart, noting: “Superoxide generation occurs during ischemia before reperfusion from the ubisemiquinone site of the mitochondrial electron transport chain” [14]. Interestingly, they performed their studies in isolated cardiac myocytes. We have performed similar studies of cardiac ischemia/reperfusion, but did so measuring ROS in the Langendorff perfused heart model. We found, as do most groups, that the ischemic period is one of low, not high ROS and that high ROS occurred with reperfusion [38]. Consequently, these groups, whether studying the left ventricle or the pulmonary vasculature, seem to disagree about the effect of oxygen deprivation on ROS production. One suspects the difference relates to the severity of hypoxia (versus anoxia), the use of cells versus organs and the duration/severity of oxygen deprivation.
The distal half of the pathway proposed to mediate redox regulation of pulmonary vascular tone during normoxia (normoxic vasodilatation caused by H2O2–induced Kv channel opening) is operant in systemic arteries [71].
The ductus arteriosus (DA)
The DA is a large conduit artery that connects the left pulmonary artery to the descending aorta. In utero, where the foetus’ systemic PO2 is < 40mmHg, the DA is maintained in a state of hypoxic vasodilatation. This ductal patency diverts placentally-oxygenated blood ejected from the right ventricle away from the unventilated foetal lungs, and instead directs it to the systemic vasculature. Upon birth, as the infant breathes oxygen-rich air, the FiO2 rises and systemic PO2 increases, leading to DA constriction. DA constriction is regulated by endothelial-derived vasodilator and vasoconstrictor mediators, including various prostaglandins [45, 56] and endothelin [20]. Anatomical patency of the ductus arteriosus is considered physiological during the first three days of life [83], but persistence of ductal patency (PDA) subsequent to this can lead to pulmonary hypertension and congestive heart failure [34]. The prevalence of PDA is estimated to be 57 per 100 000 live births, with considerably higher incidence in preterm infants (birth weight 501–1500g) [39]. Epidemiological findings highlight the importance of oxygen sensing in promoting PDA, including the increased incidence of PDA in infants born into environmental hypoxia at high altitude (4000+ m) compared to babies born at sea level [2].
Oxygen sensing in the DA
An early theory for oxygen-induced DA constriction is summarized in the endothelin hypothesis [19]. In this theory, a rise in PO2 elicited increased synthesis of this potent peptide vasoconstrictor, resulting in DA constriction. Invoking a circulating vasoconstrictor mediator to explain DA constriction is similar to early theories of HPV, where the proposed mechanism involved oxygen-sensitive release of vasoconstrictive circulating mediators, such as endothelin [23, 46] or leukotrienes [54]. However, like HPV, the core of oxygen-induced DA constriction is intrinsic to the vasculature and requires neither the endothelium nor endothelin itself to occur. Like HPV, O2-induced DA constriction is mediated by a mitochondrial O2 sensor within the SMC that regulates K+ and Ca2+ channels, as well as rho kinase, to initiate and sustain DA constriction.
In 1996, Tristani-Firouzi et al, using foetal rabbits and employing a similar strategy to Post et al in studies of HPV [74], demonstrated that the Kv channel inhibitor 4-AP induced DA constriction and that O2 caused little additional constriction, suggesting a Kv-channel-dependent mechanism of constriction [87]. They showed that the O2-sensitive IK in DASMC is a Kv current, which, when inhibited, causes membrane depolarization and activation of the CaL channel, thereby elevating intracellular calcium and causing vasoconstriction. Roughly two thirds of the net rise in DASMC [Ca2+]i originated from the extracellular compartment and entered the DASMC via the CaL channel.
O2 elicits constriction of the DA even in absence of endothelium [25], suggesting that, similar to the PA, the O2-sensing mechanism is intrinsic to the SMC. Indeed, in human DA rings, simultaneous inhibition of prostaglandin synthase, endothelin receptors, endothelin converting enzyme and nitric oxide synthase failed to attenuate O2-inudced DA constriction. However, this cocktail did inhibit constriction to exogenous endothelin and lower endothelin synthesis by the ductus. Targeted inhibition of L-type Ca2+ channels in human DA blocks normoxic constriction of the vessel [61]. Preterm DA rings exhibit reduced O2-mediated constriction and, as would be predicted, manifest reduced O2-sensitive IK density. Thus, the preterm DA is an interesting model, as both the sensor and constrictor mechanisms are immature and can be targeted individually. The decrease in O2-sensitive IK in preterm DASMC is associated with decreased expression of Kv1.5 and Kv2.1 (with preserved expression of the L-type Ca2+ channel). Gene transfer of Kv1.5 or Kv2.1 channels improves O2-constriction in preterm rabbit DA rings. When this therapy is made more precise and targeted solely to the SMC, gene transfer of Kv1.5 also enhances O2-mediated constriction in isolated human DA rings [85].
The mitochondrion is also the upstream O2 sensor in the DA, and similar to resistance PA, DASMC mitochondria alter ROS production (specifically, H2O2) in proportion to PO2 (Table 1). Quantification of DA ROS production (measured using luminol and lucigenin chemiluminescence) revealed increased ROS levels in normoxic versus hypoxic DA. This increase in ROS was inhibited by catalase, confirming that it reflected H2O2. Likewise, removal of H2O2 by inclusion of catalase in the patch clamp pipette solution increased outward IK in isolated, hypoxic, DASMC, studied in the whole cell configuration. Conversely, H2O2 supplementation using tert-butyl hydroperoxide decreased IK, suggesting that increased ROS levels inhibit K+ channels within the DA. Additionally, oxidizing agents can initiate constriction of dilated hypoxic DA rings, and dilation of constricted normoxic rings can be triggered by antioxidants [78]. Regulation of K+ channels by O2 occurs in human DA in a manner opposing that seen in adult pulmonary arteries [61]. Exposure of human DA rings to chronic normoxia (ex vivo) decreases expression of Kv channels (including Kv1.5 and Kv2.1), and dampens DA sensitivity to 4-AP or O2-induced vasoconstriction. Adenoviral transfer of O2-sensitive Kv channel genes restores 4-AP and O2 sensitivity. In a final example of the reverse response of the DA versus the PA to changes in redox state, proximal ETC inhibitors mimic hypoxia in the DA, increasing outward IK, decreasing ROS production and reversing O2-mediated vasoconstriction [63].
Recently we have explored how changes in mitochondrial form controls mitochondrial function. Mitochondria exist in dynamic networks that are rapidly joining through a process called fusion, and dividing through a process called fission. The movement and division of mitochondria are collectively referred to as mitochondrial dynamics. Mitochondrial dynamics are regulated by a relatively small number of conserved, large GTPases that promote fission (dynamin related protein 1, Drp1) or fusion, (mitofusin 1 and 2, Mfn1 and Mfn2; and optic atrophy 1, OPA1), reviewed in [12]. Hong et al demonstrated that mitochondrial fission is required for O2-induced DA constriction and subsequent closure [40]. Using DA from both human infants and full term rabbits, expression of the mitochondrial fission gene Drp1 was measured under hypoxic and normoxic conditions. Upon exposure to normoxia, both Drp1 activation and mitochondrial fission increase in term DASMC. Fission increases mitochondrial complex I activity and ROS production [40]. Inhibition of mitochondrial fission using mdivi-1 selectively inhibits O2-induced ROS production and DA constriction, without altering constriction to other agonists. The changes in the structure of the mitochondrial network occurred in seconds, prior to changes in ROS production or tone, suggesting that fission is an obligatory initial step in oxygen sensing in the DA [40].
Future Directions
Numerous aspects of the oxygen-sensing mechanism require further elucidation. While the opposing response of ion channels to ROS in PASMC (constriction in response to decreased ROS) and DASMC (constriction in response to increased ROS) is well documented, the basis for this opposing response requires further study.
Also, the relative importance of the various O2-sensitive K+ channels remains somewhat controversial and warrants additional investigation. Certainly, mutation of TASK channels, one of the channel types implicated in oxygen sensing, is one newly recognized cause of heritable PAH [48].
Although it is well established that the calcium driving DA and PA constriction originates in large part from the extracellular compartment and enters the cells via CaL channels, the relative significance of oxygen-sensitive calcium release from either the SR or store operated channels remains unclear.
Finally, the recognition that disorders of oxygen sensing can result in human diseases such as PAH and PDA offers new insight into disease pathogenesis [10, 31]. In chronic hypoxic pulmonary hypertension (a model for WHO Group 3 pulmonary hypertension in humans), the suppression of both mitochondrial-derived H2O2 production and expression of Kv1.5 is evident, and can be ameliorated with Kv1.5 gene therapy [75]. Likewise, the preterm ductus arteriosus does not constrict well to oxygen and is deficient in the expression of Kv1.5 and other O2-sensitive Kv channels. Augmentation of Kv channel expression is therapeutic in models of patent preterm DA [85]. It is noted that Kv1.5 is present in numerous tissues other than the pulmonary vasculature, including atrial myocytes [26], the ventricle [53], pancreatic islets [49], and the brain and spinal cord [81]. There is great variation in the electophysiologic properties of Kv1.5 amongst tissues. This relates tissue-specific expression of different splice variants of Kv1.5. There is also heterogeneity in the composition of Kv1.5 channels based on variable α-subunit composition of the channel tetramer and variation in the associated channel β-subunits.
Systemic induction of Kv1.5 as a form of therapy would certainly elicit unwanted effects. For example, Kv1.5 over-expression has anti-apoptotic effects in the brain [100], but pro-apoptotic effects in pancreatic beta cells [44]. Thus, site-specific manipulation of Kv1.5 is likely required for any potential therapeutic application. This approach has been used successfully in preclinical studies of diseases of the pulmonary vasculature [75], ductus arteriosus [85], and the heart [17]. Indeed, downregulation of Kv1.5 is a hallmark of PAH, seen in the PASMC in all animal models of PAH, as well as human PAH.
Targeting the mitochondria to reduce ROS production has been investigated as a potential therapy for numerous pathologies; specifically, MitoQ, an antioxidant quinone moiety cross-linked to triphenylphosphonium cation, can pass through both the plasma membrane and mitochondrial membrane, where it accumulates within the mitochondrial matrix and is adsorbed and is continually recycled by Complex II of the ETC [84]. MitoQ has been tested as a potential therapy for Alzheimer’s disease [55], hypertension and associated cardiovascular disease [30], and metabolic syndrome [60]. It should be noted, however, that increased ROS is pathological in these cases, whereas diminished ROS production is observed in chronic hypoxic pulmonary hypertension and PDA, at least by some investigators. Further investigation into targeting the mitochondria to produce physiological levels of H2O2 without stimulating potentially pathological over-production might be warranted to restore oxygen sensing. As an alternative to targeting the mitochondria to alter ROS production, the proteins associated with mitochondrial dynamics, specifically, Drp1 may hold therapeutic promise. Inhibiting Drp1 in preclinical studies has resulted in decreased fission and cell proliferation in human PASMC from PAH patients as well as in rodent PASMC [52]; suggesting regulators of the mitochondrial network are viable therapeutic targets for diseases of impaired oxygen sensing.
Conclusions
The ability of specialized tissues to sense changes in oxygen and initiate homeostatic responses that optimize oxygen uptake and delivery is integral to normal development and environmental adaptation. In all tissues within the HOSS, a redox O2 sensor (likely within the mitochondrion) alters production of diffusible mediators (including ROS, such as H2O2). This redox signal alters the function of downstream effector ion channels (i.e. voltage-gated K+ and Ca2+ channels) and enzymes (e.g. rho kinase), which mediates vascular tone in the PA and DA. The clinical significance of oxygen sensing is three-fold: physiological, as the optimization of oxygen uptake is crucial to both development in utero as well as adaptation to changing environmental oxygen tension; medical, as HPV can be exploited in patients undergoing single-lung anaesthesia to maintain systemic oxygenation [68]; and pathological, as subversion of the oxygen-sensing pathways is relevant to disease pathogenesis in conditions such as PAH and cancer.
Acknowledgments
Funding Sources: This work is supported by the National Institutes of Health RO1-HL071115 and 1RC1HL099462, Canadian Institutes for Health Research Foundation Award 333058, the Canada Foundation for Innovation (CFI) 33518 and 229252, and a Canada Research Chair 229252 (SLA).
Footnotes
This article is published as part of the Special Issue on Ion Channels and Sensors in Oxygen Adaptation
References
- 1.Aaronson PI, Robertson TP, Knock GA, Becker S, Lewis TH, Snetkov V, Ward JP. Hypoxic pulmonary vasoconstriction: mechanisms and controversies. J Physiol. 2006;570:53–58. doi: 10.1113/jphysiol.2005.098855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzamora-Castro V, Battilana G, Abugattas R, Sialer S. Patent ductus arteriosus and high altitude. Am J Cardiol. 1960;5:761–763. doi: 10.1016/0002-9149(60)90052-7. [DOI] [PubMed] [Google Scholar]
- 3.Archer SL, Will JA, Weir EK. Redox status in the control of pulmonary vascular tone. Herz. 1986;11:127–141. [PubMed] [Google Scholar]
- 4.Archer SL, Nelson DP, Weir EK. Simultaneous measurement of O2 radicals and pulmonary vascular reactivity in rat lung. J Appl Physiol (1985) 1989;67:1903–1911. doi: 10.1152/jappl.1989.67.5.1903. [DOI] [PubMed] [Google Scholar]
- 5.Archer SL, Nelson DP, Weir EK. Detection of activated O2 species in vitro and in rat lungs by chemiluminescence. J Appl Physiol (1985) 1989;67:1912–1921. doi: 10.1152/jappl.1989.67.5.1912. [DOI] [PubMed] [Google Scholar]
- 6.Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 7.Archer SL, Huang JM, Reeve HL, Hampl V, Tolarova S, Michelakis E, Weir EK. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ Res. 1996;78:431–442. doi: 10.1161/01.res.78.3.431. [DOI] [PubMed] [Google Scholar]
- 8.Archer SL, London B, Hampl V, Wu X, Nsair A, Puttagunta L, Hashimoto K, Waite RE, Michelakis ED. Impairment of hypoxic pulmonary vasoconstriction in mice lacking the voltage-gated potassium channel Kv1.5. FASEB J. 2001;15:1801–1803. doi: 10.1096/fj.00-0649fje. [DOI] [PubMed] [Google Scholar]
- 9.Archer SL, Wu XC, Thebaud B, Nsair A, Bonnet S, Tyrrell B, McMurtry MS, Hashimoto K, Harry G, Michelakis ED. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res. 2004;95:308–318. doi: 10.1161/01.RES.0000137173.42723.fb. [DOI] [PubMed] [Google Scholar]
- 10.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 11.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archer SL. Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369:2236–2251. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 13.Baraka AS, Taha SK, Yaacoub CI. Alarming hypoxemia during one-lung ventilation in a patient with respiratory bronchiolitis-associated interstitial lung disease. Can J Anaesth. 2003;50:411–414. doi: 10.1007/BF03021041. [DOI] [PubMed] [Google Scholar]
- 14.Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 15.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 16.Bradford JR, Dean HP. The Pulmonary Circulation1. J Physiol. 1894;16:34–158. doi: 10.1113/jphysiol.1894.sp000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunner M, Kodirov SA, Mitchell GF, Buckett PD, Shibata K, Folco EJ, Baker L, Salama G, Chan DP, Zhou J, Koren G. In vivo gene transfer of Kv1.5 normalizes action potential duration and shortens QT interval in mice with long QT phenotype. Am J Physiol Heart Circ Physiol. 2003;285:H194–H203. doi: 10.1152/ajpheart.00971.2002. [DOI] [PubMed] [Google Scholar]
- 18.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 19.Coceani F, Armstrong C, Kelsey L. Endothelin is a potent constrictor of the lamb ductus arteriosus. Can J Physiol Pharmacol. 1989;67:902–904. doi: 10.1139/y89-141. [DOI] [PubMed] [Google Scholar]
- 20.Coceani F, Kelsey L, Seidlitz E. Evidence for an effector role of endothelin in closure of the ductus arteriosus at birth. Can J Physiol Pharmacol. 1992;70:1061–1064. doi: 10.1139/y92-146. [DOI] [PubMed] [Google Scholar]
- 21.Duprat F, Guillemare E, Romey G, Fink M, Lesage F, Lazdunski M, Honore E. Susceptibility of cloned K+ channels to reactive oxygen species. Proc Natl Acad Sci U S A. 1995;92:11796–1800. doi: 10.1073/pnas.92.25.11796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elton TS, Oparil S, Taylor GR, Hicks PH, Yang R, Jin H, Chen Y. Normobaric hypoxia stimulates endothelin-1 gene expression in the rat. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1992;263:R1260–R1264. doi: 10.1152/ajpregu.1992.263.6.R1260. [DOI] [PubMed] [Google Scholar]
- 24.Euler USv, Liljestrand G. Observations on the Pulmonary Arterial Blood Pressure in the Cat. Acta Physiologica Scandinavica. 1946;12:301–320. [Google Scholar]
- 25.Fay FS. Guinea pig ductus arteriosus. I. Cellular and metabolic basis for oxygen sensitivity. Am J Physiol. 1971;221:470–479. doi: 10.1152/ajplegacy.1971.221.2.470. [DOI] [PubMed] [Google Scholar]
- 26.Feng J, Wible B, Li GR, Wang Z, Nattel S. Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circ Res. 1997;80:572–579. doi: 10.1161/01.res.80.4.572. [DOI] [PubMed] [Google Scholar]
- 27.Franco-Obregon A, Lopez-Barneo J. Differential oxygen sensitivity of calcium channels in rabbit smooth muscle cells of conduit and resistance pulmonary arteries. J Physiol. 1996;491(Pt 2):511–518. doi: 10.1113/jphysiol.1996.sp021235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco-Obregón A, Urena J, López-Barneo J. Oxygen-sensitive calcium channels in vascular smooth muscle and their possible role in hypoxic arterial relaxation. Proceedings of the National Academy of Sciences. 1995;92:4715–4719. doi: 10.1073/pnas.92.10.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardener MJ, Johnson IT, Burnham MP, Edwards G, Heagerty AM, Weston AH. Functional evidence of a role for two-pore domain potassium channels in rat mesenteric and pulmonary arteries. Br J Pharmacol. 2004;142:192–202. doi: 10.1038/sj.bjp.0705691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 31.Gupte SA, Wolin MS. Oxidant and redox signaling in vascular oxygen sensing: implications for systemic and pulmonary hypertension. Antioxid Redox Signal. 2008;10:1137–1152. doi: 10.1089/ars.2007.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurney AM, Osipenko ON, MacMillan D, McFarlane KM, Tate RJ, Kempsill FE. Two-pore domain K channel, TASK-1, in pulmonary artery smooth muscle cells. Circ Res. 2003;93:957–964. doi: 10.1161/01.RES.0000099883.68414.61. [DOI] [PubMed] [Google Scholar]
- 33.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Hamrick SE, Hansmann G. Patent ductus arteriosus of the preterm infant. Pediatrics. 2010;125:1020–1030. doi: 10.1542/peds.2009-3506. [DOI] [PubMed] [Google Scholar]
- 35.Harder DR, Madden JA, Dawson C. Hypoxic induction of Ca2+-dependent action potentials in small pulmonary arteries of the cat. J Appl Physiol (1985) 1985;59:1389–1393. doi: 10.1152/jappl.1985.59.5.1389. [DOI] [PubMed] [Google Scholar]
- 36.Hasunuma K, Rodman DM, McMurtry IF. Effects of K+ channel blockers on vascular tone in the perfused rat lung. Am Rev Respir Dis. 1991;144:884–887. doi: 10.1164/ajrccm/144.4.884. [DOI] [PubMed] [Google Scholar]
- 37.Henry TD, Archer SL, Nelson D, Weir EK, From A. Enhanced chemiluminescence as a measure of oxygen-derived free radical generation during ischemia and reperfusion. Circ Res. 1990;67:1453–1461. doi: 10.1161/01.res.67.6.1453. [DOI] [PubMed] [Google Scholar]
- 38.Henry TD, Archer SL, Nelson D, Weir EK, From AH. Postischemic oxygen radical production varies with duration of ischemia. Am J Physiol. 1993;264:H1478–H1484. doi: 10.1152/ajpheart.1993.264.5.H1478. [DOI] [PubMed] [Google Scholar]
- 39.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 40.Hong Z, Kutty S, Toth PT, Marsboom G, Hammel JM, Chamberlain C, Ryan JJ, Zhang HJ, Sharp WW, Morrow E, Trivedi K, Weir EK, Archer SL. Role of dynamin-related protein 1 (Drp1)-mediated mitochondrial fission in oxygen sensing and constriction of the ductus arteriosus. Circ Res. 2013;112:802–815. doi: 10.1161/CIRCRESAHA.111.300285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hulme JT, Coppock EA, Felipe A, Martens JR, Tamkun MM. Oxygen sensitivity of cloned voltage-gated K(+) channels expressed in the pulmonary vasculature. Circ Res. 1999;85:489–497. doi: 10.1161/01.res.85.6.489. [DOI] [PubMed] [Google Scholar]
- 42.Jensen KS, Micco AJ, Czartolomna J, Latham L, Voelkel NF. Rapid onset of hypoxic vasoconstriction in isolated lungs. J Appl Physiol (1985) 1992;72:2018–2023. doi: 10.1152/jappl.1992.72.5.2018. [DOI] [PubMed] [Google Scholar]
- 43.Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SJ, Widenmaier SB, Choi WS, Nian C, Ao Z, Warnock G, McIntosh CH. Pancreatic beta-cell prosurvival effects of the incretin hormones involve post-translational modification of Kv2.1 delayed rectifier channels. Cell Death Differ. 2012;19:333–344. doi: 10.1038/cdd.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitterman JA, Edmunds LH, Jr, Gregory GA, Heymann MA, Tooley WH, Rudolph AM. Patent ducts arteriosus in premature infants. Incidence, relation to pulmonary disease and management. N Engl J Med. 1972;287:473–477. doi: 10.1056/NEJM197209072871001. [DOI] [PubMed] [Google Scholar]
- 46.Li H, Elton T, Chen Y, Oparil S. Increased endothelin receptor gene expression in hypoxic rat lung. American Journal of Physiology-Lung Cellular and Molecular Physiology. 1994;266:L553–L560. doi: 10.1152/ajplung.1994.266.5.L553. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- 48.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, Germain M, Tregouet DA, Borczuk A, Rosenzweig EB, Girerd B, Montani D, Humbert M, Loyd JE, Kass RS, Chung WK. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 2013;369:351–361. doi: 10.1056/NEJMoa1211097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacDonald PE, Wheeler MB. Voltage-dependent K(+) channels in pancreatic beta cells: role, regulation and potential as therapeutic targets. Diabetologia. 2003;46:1046–1062. doi: 10.1007/s00125-003-1159-8. [DOI] [PubMed] [Google Scholar]
- 50.Madden JA, Dawson CA, Harder DR. Hypoxia-induced activation in small isolated pulmonary arteries from the cat. J Appl Physiol (1985) 1985;59:113–118. doi: 10.1152/jappl.1985.59.1.113. [DOI] [PubMed] [Google Scholar]
- 51.Madden JA, Vadula MS, Kurup VP. Effects of hypoxia and other vasoactive agents on pulmonary and cerebral artery smooth muscle cells. Am J Physiol. 1992;263:L384–L393. doi: 10.1152/ajplung.1992.263.3.L384. [DOI] [PubMed] [Google Scholar]
- 52.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J, Archer SL. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mays DJ, Foose JM, Philipson LH, Tamkun MM. Localization of the Kv1.5 K+ channel protein in explanted cardiac tissue. J Clin Invest. 1995;96:282–292. doi: 10.1172/JCI118032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDonnell TJ, Westcott J, Czartolomna J, Voelkel NF. Role of peptidoleukotrienes in hypoxic pulmonary vasoconstriction in rats. American Journal of Physiology-Heart and Circulatory Physiology. 1990;259:H751–H758. doi: 10.1152/ajpheart.1990.259.3.H751. [DOI] [PubMed] [Google Scholar]
- 55.McManus MJ, Murphy MP, Franklin JL. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2011;31:15703–15715. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McMurphy DM, Heymann MA, Rudolph AM, Melmon KL. Developmental changes in constriction of the ductus arteriosus: responses to oxygen and vasoactive agents in the isolated ductus arteriosus of the fetal lamb. Pediatr Res. 1972;6:231–238. doi: 10.1203/00006450-197204000-00004. [DOI] [PubMed] [Google Scholar]
- 57.McMurtry IF, Davidson AB, Reeves JT, Grover RF. Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs. Circ Res. 1976;38:99–104. doi: 10.1161/01.res.38.2.99. [DOI] [PubMed] [Google Scholar]
- 58.McMurtry IF, Petrun MD, Reeves JT. Lungs from chronically hypoxic rats have decreased pressor response to acute hypoxia. Am J Physiol. 1978;235:H104–H109. doi: 10.1152/ajpheart.1978.235.1.H104. [DOI] [PubMed] [Google Scholar]
- 59.McMurtry IF. BAY K 8644 potentiates and A23187 inhibits hypoxic vasoconstriction in rat lungs. Am J Physiol. 1985;249:H741–H746. doi: 10.1152/ajpheart.1985.249.4.H741. [DOI] [PubMed] [Google Scholar]
- 60.Mercer JR, Yu E, Figg N, Cheng KK, Prime TA, Griffin JL, Masoodi M, Vidal-Puig A, Murphy MP, Bennett MR. The mitochondria-targeted antioxidant MitoQ decreases features of the metabolic syndrome in ATM+/−/ApoE−/− mice. Free Radic Biol Med. 2012;52:841–849. doi: 10.1016/j.freeradbiomed.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 61.Michelakis E, Rebeyka I, Bateson J, Olley P, Puttagunta L, Archer S. Voltage-gated potassium channels in human ductus arteriosus. Lancet. 2000;356:134–137. doi: 10.1016/S0140-6736(00)02452-1. [DOI] [PubMed] [Google Scholar]
- 62.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res. 2002;90:1307–1315. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- 63.Michelakis ED, Rebeyka I, Wu X, Nsair A, Thebaud B, Hashimoto K, Dyck JR, Haromy A, Harry G, Barr A, Archer SL. O2 sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res. 2002;91:478–486. doi: 10.1161/01.res.0000035057.63303.d1. [DOI] [PubMed] [Google Scholar]
- 64.Michelakis ED, Thebaud B, Weir EK, Archer SL. Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J Mol Cell Cardiol. 2004;37:1119–1136. doi: 10.1016/j.yjmcc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 65.Mojet MH, Mills E, Duchen MR. Hypoxia-induced catecholamine secretion in isolated newborn rat adrenal chromaffin cells is mimicked by inhibition of mitochondrial respiration. J Physiol. 1997;504:175–189. doi: 10.1111/j.1469-7793.1997.175bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol (1985) 2005;98:390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- 67.Moudgil R, Michelakis ED, Archer SL. The role of k+ channels in determining pulmonary vascular tone, oxygen sensing, cell proliferation, and apoptosis: implications in hypoxic pulmonary vasoconstriction and pulmonary arterial hypertension. Microcirculation. 2006;13:615–632. doi: 10.1080/10739680600930222. [DOI] [PubMed] [Google Scholar]
- 68.Nagendran J, Stewart K, Hoskinson M, Archer SL. An anesthesiologist's guide to hypoxic pulmonary vasoconstriction: implications for managing single-lung anesthesia and atelectasis. Curr Opin Anaesthesiol. 2006;19:34–43. doi: 10.1097/01.aco.0000192777.09527.9e. [DOI] [PubMed] [Google Scholar]
- 69.Osipenko ON, Tate RJ, Gurney AM. Potential role for kv3.1b channels as oxygen sensors. Circ Res. 2000;86:534–540. doi: 10.1161/01.res.86.5.534. [DOI] [PubMed] [Google Scholar]
- 70.Paky A, Michael JR, Burke-Wolin TM, Wolin MS, Gurtner GH. Endogenous production of superoxide by rabbit lungs: effects of hypoxia or metabolic inhibitors. J Appl Physiol (1985) 1993;74:2868–2874. doi: 10.1152/jappl.1993.74.6.2868. [DOI] [PubMed] [Google Scholar]
- 71.Park SW, Noh HJ, Sung DJ, Kim JG, Kim JM, Ryu SY, Kang K, Kim B, Bae YM, Cho H. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflugers Arch. 2015;467:285–297. doi: 10.1007/s00424-014-1513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peers C. Effects of doxapram on ionic currents recorded in isolated type I cells of the neonatal rat carotid body. Brain Res. 1991;568:116–122. doi: 10.1016/0006-8993(91)91386-f. [DOI] [PubMed] [Google Scholar]
- 73.Platoshyn O, Yu Y, Ko EA, Remillard CV, Yuan JX. Heterogeneity of hypoxia-mediated decrease in I(K(V)) and increase in [Ca2+](cyt) in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L402–L416. doi: 10.1152/ajplung.00391.2006. [DOI] [PubMed] [Google Scholar]
- 74.Post JM, Hume JR, Archer SL, Weir EK. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992;262:C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- 75.Pozeg ZI, Michelakis ED, McMurtry MS, Thebaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- 76.Reeve HL, Weir EK, Nelson DP, Peterson DA, Archer SL. Opposing effects of oxidants and antioxidants on K+ channel activity and tone in rat vascular tissue. Exp Physiol. 1995;80:825–834. doi: 10.1113/expphysiol.1995.sp003890. [DOI] [PubMed] [Google Scholar]
- 77.Reeve HL, Michelakis E, Nelson DP, Weir EK, Archer SL. Alterations in a redox oxygen sensing mechanism in chronic hypoxia. J Appl Physiol (1985) 2001;90:2249–2256. doi: 10.1152/jappl.2001.90.6.2249. [DOI] [PubMed] [Google Scholar]
- 78.Reeve HL, Tolarova S, Nelson DP, Archer S, Weir EK. Redox control of oxygen sensing in the rabbit ductus arteriosus. J Physiol. 2001;533:253–261. doi: 10.1111/j.1469-7793.2001.0253b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol 525 Pt. 2000;3:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rounds S, McMurtry IF. Inhibitors of oxidative ATP production cause transient vasoconstriction and block subsequent pressor responses in rat lungs. Circ Res. 1981;48:393–400. doi: 10.1161/01.res.48.3.393. [DOI] [PubMed] [Google Scholar]
- 81.Roy ML, Saal D, Perney T, Sontheimer H, Waxman SG, Kaczmarek LK. Manipulation of the delayed rectifier Kv1.5 potassium channel in glial cells by antisense oligodeoxynucleotides. Glia. 1996;18:177–184. doi: 10.1002/(SICI)1098-1136(199611)18:3<177::AID-GLIA2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 82.Shirai M, Ninomiya I, Sada K. Constrictor response of small pulmonary arteries to acute pulmonary hypertension during left atrial pressure elevation. Jpn J Physiol. 1991;41:129–142. doi: 10.2170/jjphysiol.41.129. [DOI] [PubMed] [Google Scholar]
- 83.Skinner J. Diagnosis of patent ductus arteriosus. Semin Neonatol. 2001;6:49–61. doi: 10.1053/siny.2000.0037. [DOI] [PubMed] [Google Scholar]
- 84.Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 85.Thebaud B, Michelakis ED, Wu XC, Moudgil R, Kuzyk M, Dyck JR, Harry G, Hashimoto K, Haromy A, Rebeyka I, Archer SL. Oxygen-sensitive Kv channel gene transfer confers oxygen responsiveness to preterm rabbit and remodeled human ductus arteriosus: implications for infants with patent ductus arteriosus. Circulation. 2004;110:1372–1379. doi: 10.1161/01.CIR.0000141292.28616.65. [DOI] [PubMed] [Google Scholar]
- 86.Tolins M, Weir EK, Chesler E, Nelson DP, From AH. Pulmonary vascular tone is increased by a voltage-dependent calcium channel potentiator. J Appl Physiol (1985) 1986;60:942–948. doi: 10.1152/jappl.1986.60.3.942. [DOI] [PubMed] [Google Scholar]
- 87.Tristani-Firouzi M, Reeve HL, Tolarova S, Weir EK, Archer SL. Oxygen-induced constriction of rabbit ductus arteriosus occurs via inhibition of a 4-aminopyridine-, voltage-sensitive potassium channel. J Clin Invest. 1996;98:1959–1965. doi: 10.1172/JCI118999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ward JP. Point: Hypoxic pulmonary vasoconstriction is mediated by increased production of reactive oxygen species. J Appl Physiol (1985) 2006;101:993–995. doi: 10.1152/japplphysiol.00480.2006. discussion 999. [DOI] [PubMed] [Google Scholar]
- 89.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 90.Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91:719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 91.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res. 2010;106:526–535. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, Schumacker PT. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med. 2013;187:424–432. doi: 10.1164/rccm.201207-1294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weir EK, Archer SL. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB J. 1995;9:183–189. doi: 10.1096/fasebj.9.2.7781921. [DOI] [PubMed] [Google Scholar]
- 94.Weir EK, Reeve HL, Peterson DA, Michelakis ED, Nelson DP, Archer SL. Pulmonary vasoconstriction, oxygen sensing, and the role of ion channels: Thomas A. Neff lecture. Chest. 1998;114:17S–22S. doi: 10.1378/chest.114.1_supplement.17s-a. [DOI] [PubMed] [Google Scholar]
- 95.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weir EK, Archer SL. Counterpoint: Hypoxic pulmonary vasoconstriction is not mediated by increased production of reactive oxygen species. J Appl Physiol (1985) 2006;101:995–998. doi: 10.1152/japplphysiol.00480a.2006. discussion 998. [DOI] [PubMed] [Google Scholar]
- 97.Weir EK, Archer SL. The role of redox changes in oxygen sensing. Respir Physiol Neurobiol. 2010;174:182–191. doi: 10.1016/j.resp.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolin MS, Burke TM. Hydrogen peroxide elicits activation of bovine pulmonary arterial soluble guanylate cyclase by a mechanism associated with its metabolism by catalase. Biochem Biophys Res Commun. 1987;143:20–25. doi: 10.1016/0006-291x(87)90623-1. [DOI] [PubMed] [Google Scholar]
- 99.Youngson C, Nurse C, Yeger H, Cutz E. Oxygen sensing in airway chemoreceptors. Nature. 1993;365:153–155. doi: 10.1038/365153a0. [DOI] [PubMed] [Google Scholar]
- 100.Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 1997;278:114–117. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]
- 101.Yuan XJ, Tod ML, Rubin LJ, Blaustein MP. Contrasting effects of hypoxia on tension in rat pulmonary and mesenteric arteries. Am J Physiol. 1990;259:H281–H289. doi: 10.1152/ajpheart.1990.259.2.H281. [DOI] [PubMed] [Google Scholar]
- 102.Yuan XJ, Wang J, Juhaszova M, Gaine SP, Rubin LJ. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet. 1998;351:726–727. doi: 10.1016/S0140-6736(05)78495-6. [DOI] [PubMed] [Google Scholar]