Abstract
Aims
Glucocorticoids, such as dexamethasone, are widely used anti-inflammatory drugs. Their use is frequently associated with the development of steroid- associated diabetes. Pancreatic β-cell dysfunction has been suggested to be one of the main causes of steroid-associated diabetes. However, the mechanism is not fully understood. Glycogen synthase kinase-3β (GSK-3β) is a multifunctional serine/threonine kinase and plays an important role in energy metabolism, cell growth and apoptosis. Therefore, the contribution of GSK-3β in dexamethasone-induced pancreatic β-cell apoptosis was determined in the present study.
Main Methods
The effect of dexamethasone treatment on rat pancreatic β-cell line (INS-1) apoptosis (determined by TUNEL and Flow Cytometry), generation of reactive oxidative stress (ROS), and the phosphorylation status of GSK-3β was determined. The inhibitory effect of GSK-3β inhibitor-lithium chloride (LiCl) on dexamethasone-induced β-cell apoptosis was also evaluated.
Key Findings
Dexamethasone (0.1 μM) treatment induced INS-1 apoptosis, which was associated with increased GSK-3β activation and increased NOX4-derived ROS generation. Pretreatment of INS-1 with LiCl inhibited dexamethasone induced ROS generation and INS-1 apoptosis.
Significance
This study provides a new mechanism of Dex induced pancreatic β cell apoptosis and may serve as a new therapeutic option for treating GCs induced diabetes.
Keywords: Dexamethasone, Apoptosis, GSK-3β, ROS
INTRODUCTION
Glucocorticoids (GCs), such as dexamethasone, are widely used anti-inflammatory drug. They represent the standard therapy for asthma, rheumatoid arthritis, inflammatory bowel disease and other systemic diseases. While the GCs have well known therapeutic effects, they also induce a series of complex side effects involving in multiple organs and systems such as skin, bone, muscle, central nervous system, and endocrine system (Schacke et al., 2002). One of the major side effects of GCs therapy is that prolonged exposure to GCs induced hyperglycemia and the development of diabetes. The mechanisms of GCs associated diabetes are complex. Studies have shown that GCs can stimulate gene transcription of enzymes involved in gluconeogenesis and lead to increased glucose synthesis. Excess GCs also cause insulin resistance, which reduces the effectiveness of insulin in suppressing hepatic glucose production and in increasing glucose uptake and usage in skeletal muscle (Andrews and Walker, 1999). In addition, GCs usage induces pancreatic β-cell dysfunction including apoptosis, leading to reduced production of insulin (1993, Avram et al., 2008, Ranta et al., 2006, Ullrich et al., 2007). All of these effects result in hyperglycemia and induction of diabetes. Studies have shown that prolonged exposure to high glucocorticoids levels may lead to an increase in reactive oxygen species (ROS) production, which might be one of the mechanisms of dexamethasone- induced β cell apoptosis (Suwanjang et al., 2013). However, the molecular mechanisms of GCs in pancreatic beta cell apoptosis are still poorly understood.
Glycogen synthase kinase-3β (GSK-3β) is a multifunctional serine/threonine kinase and is distributed in cytosol, mitochondria, and nuclei. It is constitutively active in resting cells and its inactivation is regulated by phosphorylation at Ser-9 (Force and Woodgett, 2009). GSK-3β plays an important role in energy metabolism and cell growth and also plays a role in apoptosis of various cell types (Beurel and Jope, 2006). GSK-3β has been considered to be a negative regulator of β-cells mass (Liu et al., 2010, Liu et al., 2008). Mice with conditional knockout of GSK-3β in β-cells leads to expansion of β-cells mass accompanied by enhanced proliferation and decreased apoptosis by promoting the insulin receptor/phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway(Liu et al., 2010). GSK-3β pathway has also been shown to mediate dexamethasone-induced osteoblast cell apoptosis (Yun et al., 2009). It is also involved in palmitate induced β cell apoptosis (Huang et al., 2014). However, whether GSK-3 β mediated dexamethasone- induced pancreatic β cell apoptosis is unknown.
In this study, we investigated the pro-apoptotic effects of GC, dexamethasone, on pancreatic β cells and found that GSK-3β is critical in apoptosis pathway. Our results are expected to provide a new mechanism of dexamethasone induced pancreatic β cell apoptosis and may serve as a new therapeutic option for treating GCs induced diabetes..
MATERIALS AND METHODS
Cell Culture
Rat insulinoma-derived insulin secreting cell line (INS-1) was generously provided by the Pathophysiological Laboratory in China-Japan Friendship Hospital. The cells were cultured in cell culture incubator containing 5% CO2 in RPMI-1640 media (Gibco) supplemented with 10 mM HEPES (Sigma), 10% fetal calf serum (Gibco), 2mM glutamine (Sigma), 1mM sodium pyruvate (Sigma), 50 μM 2-mercaptoethanol, 100 U/ml penicillin, and 100 mg/L streptomycin as described previously (de Leeuw van Weenen et al., 2010).
Cell proliferation and viability assay
INS-1 cells were cultured and seeded at 2×10 4 cells in 96 well cell culture plates with full culture media for 24 hours. Then, cell culture media were changed to RPMI-1640 media with 3% serum. Dexamethasone (0.1μM) was then added and treated cells for 48 hours. After treatment, cell proliferation rate was determined by using colorimetric MTT assay (Biomol) following the instruction manual. The absorbance was read with a microplate reader at 492 nm. In addition, Trypan Blue solution (Sigma) was used to stain cells to determine cell viability.
Apoptosis Determination
Cell apoptosis was evaluated by both TUNEL staining and flow cytometry after double staining with the Annexin V-FITC and propidium iodide (PI). For TUNEL staining: Apoptotic nuclei were detected using a transferase-mediated dUTP nick-end labeling (TUNEL) staining of cultured INS-1 cells. TUNEL staining was performed according to the manufacturer’s instructions (Roche) and analyzed under a microscope. Cells with brown granules in nuclei were TUNEL positive cells. The percent of TUNEL-positive cells was calculated.
For Annexin V and propidium iodide (PI) staining: After treatment, cells were harvested and washed in PBS and suspended in Annexin V binding buffer (10 mM HEPES, 140 mM NaCl, and 2.5 mM CaCl2, pH 7.4). Annexin V-FITC (Life technology) was added and cells were incubated in the dark for 15 minutes at room temperature. Then, 1: 10 diluted propidium iodide (Sigma) were added into cells and incubated in the dark for 15 minutes at room temperature. After washing, cells were collected and analyzed by flow cytometry (Bectondickinson). Annexin V positive and PI negative cells were considered apoptotic.
Western Blot Analysis
After treatment, INS-1 cells were harvested and cell lysates were made. After concentration being measured, protein was subjected to SDS-PAGE gel under reducing condition and transferred onto a nitrocellulose membrane. After blocking with 5% milk, the membrane was incubated with anti-β actin, anti-phospho-GSK-3β (Cell signaling), anti-GSK-3β (Cell signaling), anti-SOD (Abcam), and anti-NOX4 antibodies (Abcam) at 4°C overnight. After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (Jackson Labs). The reaction was visualized using an enhanced chemiluminescence system (Pierce). Immunoblots were analyzed by scanning densitometry and quantified by Quantity One gel Analysis software (Bio-Rad Laboratories).
Real-time PCR
Total RNA (2μg) extracted from treated INS-1 cells was converted to cDNA using MLV reverse transcriptase (Promega). The validity of primers and appropriate melting temperature (Tm) for real-time PCR were determined to ensure that only one band is amplified by PCR reaction. Primers were synthesized by Integrated DNA Technologies, with sequences as follows: 1). Beta-actin: 5′ GACATCCGTAAAGACCTCTATGCC-3′ and 5′-ATAGAGCCACCAATCCACACAGAG-3′; 2). GSK-3β: 5′-CACCTCTGGCTACCATCCTTAT-3′ and 5′-ATTATTGGTCTGTCCACGGTCT-3′; 3). NOX4: 5′-TAGCTGCCCACTTGGTGAACG-3′ and 5′-TGTAACCATGAGGAACAATACCACC-3′; 4). SOD: 5′-CGACCTTGCTCCTTATTG-3′ and 5′-TGACCTGCCTTACGACTA-3′. Real-time PCR analyses were performed using SYBR Green PCR Master Mix kit with a MyiQ real-time PCR thermal cycler (Bio-Rad). For each target gene, a standard curve was established by a series of 3 fold dilution of the gene of interest. All reactions were performed in triplicate in a final volume of 25 μl. Dissociation curves were run to detect nonspecific amplification, and we confirmed that single products were amplified in each reaction. The quantities of each test gene and internal control β-actin were then determined from the standard curve using the MyiQ system software, and mRNA expression levels of test genes were normalized to β-actin levels.
Immuno-fluorescence staining
After treatment, cells were washed with PBS and fixed in 4% PMSF for 20 minutes. After blocking, cells were incubated with primary antibody overnight at 4°C. Negative control was included omitting the primary antibody and substituting pre-immune IgG. After washing with PBS, FITC-labeled secondary antibody was applied for 40 min in the dark. After another 15 min washing, cells were stained with DAPI and observed under a fluorescence microscope.
Reactive Oxygen Species (ROS) Measurement
INS-1 cells were cultured in 96 well plate and treated with 0.1 μM Dex for 24 hours. Intracellular ROS levels were measured by fluorometric assay using an intracellular ROS probe, CM-H2DCFDA (Genmed) followed the instruction from the kit. RFU was measured by a fluorescence plate reader and expressed as intensity unit.
Statistical Analysis
Data are expressed as mean ± SEM. ANOVA followed by Turkey post-hoc test was used to analyze variation within groups. Student’s t-test was used to compare variation between groups. Statistical significance was accepted at a value of p<0.05.
RESULTS
Dexamethasone treatment induces INS-1 cells to undergo apoptosis
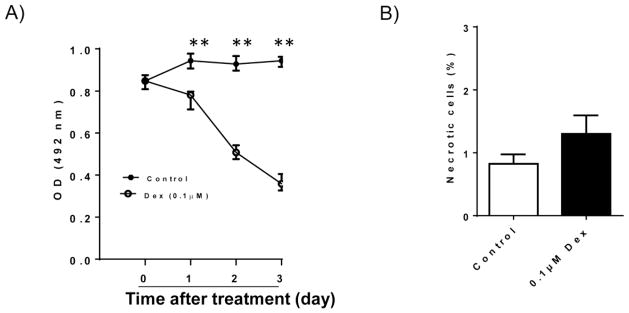
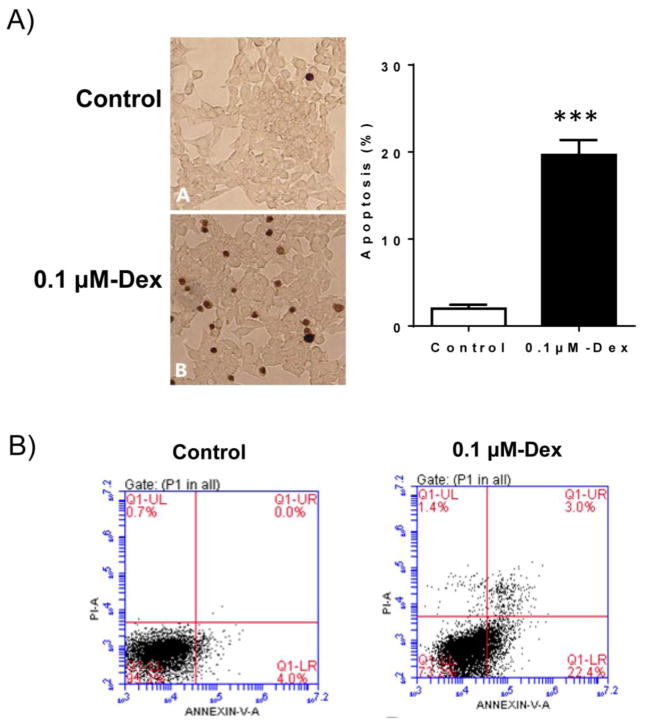
In this study, we determined the effect of Dex treatment on INS-1 cell viability. As shown in Figure 1A, Dex treatment significantly reduced INS-1 cell number after 1–3 day treatment determined by MTT assay. Trypan blue staining result reveled that Dex treatment did not induce INS-1 cell necrosis (Figure 1B). However, both TUNEL and FACS analysis provided strong evidence that Dex treatment induced INS-1 cells to undergo apoptosis (Figure 2), which is consistent with the previous studies (Avram et al., 2008, Ranta et al., 2006). This result suggests that apoptotic changes of INS-1 cells are major contributors of Dex mediated reduction in INS-1 cell viability.
Figure 1. Effect of Dex treatment on INS-1 cell proliferation and viability.
A). INS-1 cells were cultured and treated with 0.1 μM Dex for different days. After treatment, cell proliferation rate was determined by MTT assay. B). After 48 hours of 0.1 μM Dex treatment, cell necrosis was determined by Trypan Blue staining. The percentage of necrotic cells was calculated. Data are expressed as mean ± SEM (n= 3 separate experiments). **, p<0.01 vs. 0 time point.
Figure 2. Dex treatment induced INS-1 cell apoptosis.
A). INS-1 cells were cultured and treated with 0.1 μM Dex for 48 hours. After treatment, cell apoptosis was determined by TUNEL staining and the representative light micrographs of TUNEL staining (brown nuclear) were shown. Percent of apoptotic cells was calculated. Data are expressed as mean ± SEM (n= 3 separate experiments). ***, p<0.001 vs. control.
B). Dex-induced apoptosis was also determined by flow cytometry (FACS) after Annexin V-FITC/PI staining of INS-1 cells. The representative FACS images were shown.
Dexamethasone treatment activates GSK-3β pathway in INS-1 cells
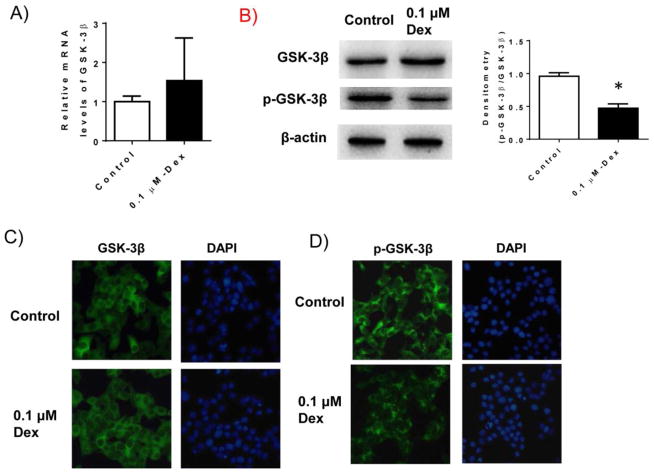
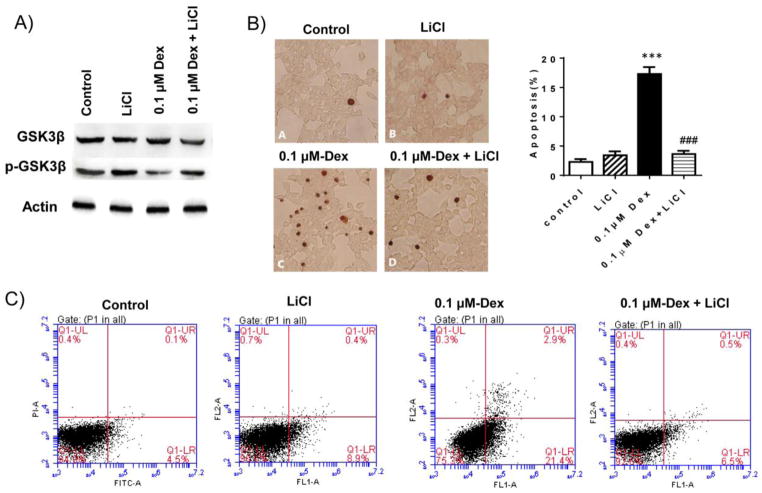
We determined whether GSK-3β pathway is activated in Dex treated INS-1 cells in our system. GSK-3β mRNA as well as protein levels were determined in Dex treated INS-1 cells. As shown in Figure 3, we found that Dex treatment did not stimulate total GSK-3β mRNA or protein levels. However, phosphorylation of GSK-3β at Ser-9 was significantly reduced in Dex treated INS-1 cells determined by either western blotting (B) or immunofluorescence staining (C–D), suggesting that GSK-3β pathway is activated in INS-1 cells by Dex treatment.
Figure 3. Dex treatment activated GSK-3β pathway.
INS-1 cells were cultured and treated with 0.1 μM Dex for 48 hours. After treatment, cells were harvested. A). mRNA levels of GSK-3β were determined by real-time PCR; B) The protein levels of total and phospho-GSK-3β in cell lysates were determined by western blotting. Beta-actin levels were used as loading control. Relative p-GSK-3β levels were determined by scanning densitometry of blots and normalized to total GSK-3β levles. Data are represented as mean ± SEM. * P<0.05 vs. control; C) Representative immunofluorescence staining of total GSK-3β in Dex treated INS-1 cells. DAPI staining showed the cell nuclei; and D) Representative immunofluorescence staining of phospho-GSK-3β in Dex treated INS-1 cells.
Dexamethasone treatment increases ROS production in INS-1 cells
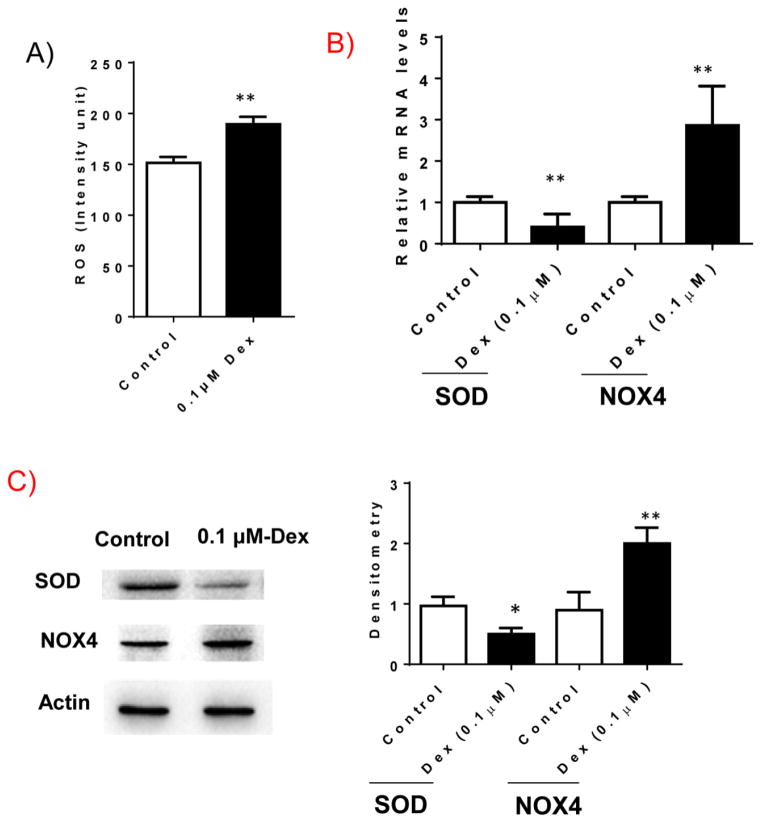
We determined whether Dex treatment increases ROS production in INS-1 cells. INS-1 cells were treated with Dex for 48 hours and cellular ROS levels were determined. As shown in Figure 4A, Dex treatment significantly increased ROS production in INS-1 cells. Furthermore, we found that Dex treatment regulated the expression of genes relating to ROS, including increased expression of NOX4 and reduced expression of SOD at both mRNA and protein levels (Figure 4B and C). These data indicate that Dex treated INS-1 cells had increased ROS production.
Figure 4. Dex treatment increases reactive oxygen species generation.
INS-1 cells were cultured and treated with 0.1 μM Dex for 48 hours. After treatment, cells were harvested. A) ROS production in Dex treated INS-1 cells were examined as described in methods section. B). mRNA levels of SOD and NOX4 were determined by real-time PCR; C) The protein levels of SOD and NOX4 in cell lysates were determined by western blotting. Beta-actin levels were used as loading control. The representative images were shown. Relative SOD or NOX4 levels were determined by scanning densitometry of blots. Results are expressed as mean ± SEM (n=3). * P<0.05 vs. control; ** P<0.01 vs. control.
Inhibition of GSK-3β pathway attenuates dexamethasone-induced INS-1 cell apoptosis through reduction of ROS generation
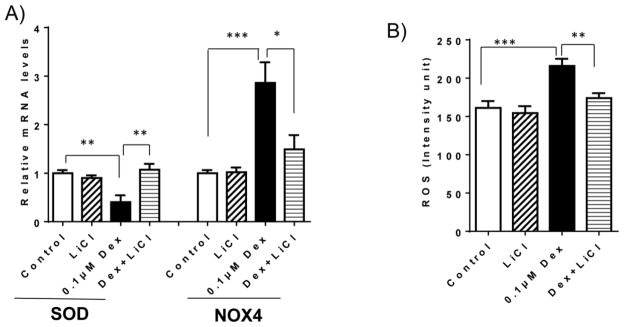
To determine whether GSK-3β activation mediates Dex induced INS-1 cell apoptosis, we used LiCl to inhibit GSK-3β activity and its effect on Dex-induced INS-1 apoptosis was determined. As expected, treatment of LiCl abolished Dex-mediated reduction in phosphorylated GSK-3β at Ser-9 in INS-1 cells (Figure 5A), indicating that Dex mediated activation of GSK-3β pathway was inhibited by LiCl treatment. Moreover, Dex induced INS-1 apoptosis was prevented by LiCl treatment (Figure 5B and C), suggesting that Dex induced INS-1 cell apoptosis is through the activation of GSK-3β. In addition, we found that LiCl treatment inhibited Dex stimulated expression of NOX4 and increased SOD expression, which as accompanied with reduced ROS production in Dex treated cells (Figure 6). Together, these data suggest that inhibition of GSK-3β pathway attenuates Dex-induced INS-1 cell apoptosis through reduction of ROS generation.
Figure 5. Inhibition of GSK-3β pathway by LiCl attenuated Dex-induced INS-1 cell apoptosis.
INS-1 cells were cultured and treated with 0.1 μM Dex in the absence or presence of 10 mMLiCl for 48 hours. A). After treatment, protein levels of total and phospho-GSK-3β in cell lysates were determined by western blotting. Beta-actin levels were used as loading control. The representative images were shown. B). Cell apoptosis was determined by TUNEL staining and the representative light micrographs of TUNEL staining (brown nuclear) were shown. Percent of apoptotic cells was calculated. Data are expressed as mean ± SEM (n= 3 separate experiments). ***, p<0.001 vs. control; ###, P<0.001 vs 0.1μM Dex. C). Cell apoptosis was also determined by flow cytometry (FACS) after Annexin V-FITC/PI staining of INS-1 cells. The representative FACS images were shown.
Figure 6. Inhibition of GSK-3β pathway by LiCl reduced Dex-induced ROS production in INS-1 cells.
INS-1 cells were cultured and treated with 0.1 μM Dex in the absence or presence of 10 mM LiCl for 48 hours. A). After treatment, cell were harvested. The mRNA levels of SOD and NOX4 were determined by real-time PCR; B) ROS production in INS-1 cells were examined as described in methods section. The results are expressed as mean ± SEM (n=3). * P<0.05, ** P<0.01 and ***P<0.001.
DISCUSSION
One major side effect of GCs therapy is the induction of diabetes. Although the effect of GCs on pancreatic β cell apoptosis and its contribution to diabetes development has been reported, the mechanism of GCs induced pancreatic β cell apoptosis is poorly understood. In this study, Dex-induced β cell apoptosis was confirmed in INS-1 cell line. Moreover, Dex treatment induced GSK-3β activation and accompanied with increased NOX 4 derived reactive oxygen species (ROS) production in INS-1 cells. LiCl treatment inhibited Dex-induced GSK-3β activation and abolished Dex-induced INS-1 cell apoptosis. Dex-induced increase in NOX4 and production of ROS were significantly reduced by LiCl treatment. Together, these results suggest that GSK-3β mediates dexamethasone-induced pancreatic β-cell apoptosis through upregulating NOX4-derived ROS production.
GSK-3β is a multifunctional serine/threonine kinase and is distributed in cytosol, mitochondria, and nuclei. It is constitutively active in resting cells and its inactivation is regulated by phosphorylation on Ser-9 (Force and Woodgett, 2009). However, the effect of Dex on expression and activity of GSK-3β is largely unknown. One report showed the reduced gene expression of GSK-3β in both male and female mice placentas after Dex administration (O’Connell et al., 2013). Another report showed a transient increase in the GSK-3β Ser9 phosphorylation in Dex treated thymocytes (Spokoini et al., 2010). In pancreatic β cell line INS-1 cells, we found that Dex treatment did not affect the expression of GSK-3β in these cells; while Dex treatment reduced phosphorylation of GSK-3β on Ser-9, indicating that Dex treatment induced GSK-3β activation in INS-1 cells. Several kinases have been implicated in Ser-9 phosphorylation of GSK-3β including PKCdelta (De Servi et al., 2005). Since Dex-induced Ras protein 1 (Dexras1) is a negative regulator of protein kinase C (PKC) delta (Nguyen and Watts, 2006), this suggests that Dex might indirectly phosphorylate GSK-3β (Ser-9) through a PKC dependent manner. The exact mechanism of Dex induced phosphorylation of GSK-3β is unknown currently and warrants further investigation.
In this study, we demonstrated that Dex treatment induced GSK-3β activation in pancreatic β cells. By using the GSK-3β inhibitor-LiCl, our studies further suggest that Dex induced INS apoptosis is through activation of GSK-3β pathway. LiCl treatment also inhibited Dex induced NOX4 expression (mRNA and protein levels) and ROS production. It is known that GSK-3β is an important regulator of protein synthesis. GSK-3β acts as a switch that regulates both transcription and mRNA translation by controlling the activity of transcription factors and translation initiation factor, respectively (Mariappan et al., 2008). Therefore, it is possible that Dex induced activation of GSK-3β directly regulates NOX4 gene transcription and/or translation, leading to increased NOX4 derived ROS and apoptotic signaling. In addition to the current study performed in pancreatic β cells, GSK-3β involves in apoptosis of other cell types (Beurel and Jope, 2006). In human neuroblastoma and lung carcinoma cells, studies found that apoptotic stimuli stimulate increases in both nuclear and mitochondrial levels of GSK-3β, which colocalize with p53 and contribute to p53-induced transcriptional and apoptosis signaling (Watcharasit et al., 2003, Watcharasit et al., 2002). This was supported by findings showing that LiC blocked p53-mediated increases in proapoptotic proteins p21 and Bax, as well as cytochrome c release and caspase-3 activation (Watcharasit et al., 2003). Studies also showed that the interaction of GSK-3β with p53 involves in glucose induced podocyte apoptosis (Peixoto et al., 2015). Together with our findings, these studies suggest that GSK-3β is a proapoptotic signaling for many stimuli.
CONCLUSIONS
Our studies demonstrated that Dex induces apoptosis of pancreatic β cells through activation of GSK-3β. Inhibition of GSK-3β by LiCl inhibited Dex induced NOX4-derived ROS production and protected INS-1 cells from Dex induced apoptosis. This study provides a new mechanism of Dex induced pancreatic β cell apoptosis and may serve as a new therapeutic option for treating GCs induced diabetes.
Acknowledgments
This study was supported by grants from National Nature Science Foundation of China (No. 81370918 to Men, No.81370873 to Zhang), and a NIH grant NIDDK DK098176 (S. Wang).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clinical science (London, England: 1979) 1999;96:513–23. doi: 10.1042/cs0960513. [DOI] [PubMed] [Google Scholar]
- Avram D, Ranta F, Hennige AM, Berchtold S, Hopp S, Haring HU, et al. IGF-1 protects against dexamethasone-induced cell death in insulin secreting INS-1 cells independent of AKT/PKB phosphorylation. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2008;21:455–62. doi: 10.1159/000129638. [DOI] [PubMed] [Google Scholar]
- Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Progress in neurobiology. 2006;79:173–89. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw van Weenen JE, Parlevliet ET, Maechler P, Havekes LM, Romijn JA, Ouwens DM, et al. The dopamine receptor D2 agonist bromocriptine inhibits glucose-stimulated insulin secretion by direct activation of the alpha2-adrenergic receptors in beta cells. Biochem Pharmacol. 2010;79:1827–36. doi: 10.1016/j.bcp.2010.01.029. [DOI] [PubMed] [Google Scholar]
- De Servi B, Hermani A, Medunjanin S, Mayer D. Impact of PKCdelta on estrogen receptor localization and activity in breast cancer cells. Oncogene. 2005;24:4946–55. doi: 10.1038/sj.onc.1208676. [DOI] [PubMed] [Google Scholar]
- Force T, Woodgett JR. Unique and overlapping functions of GSK-3 isoforms in cell differentiation and proliferation and cardiovascular development. J Biol Chem. 2009;284:9643–7. doi: 10.1074/jbc.R800077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Zhu M, Wu W, Rashid A, Liang Y, Hou L, et al. Valproate pretreatment protects pancreatic beta-cells from palmitate-induced ER stress and apoptosis by inhibiting glycogen synthase kinase-3beta. Journal of biomedical science. 2014;21:38. doi: 10.1186/1423-0127-21-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tanabe K, Baronnier D, Patel S, Woodgett J, Cras-Meneur C, et al. Conditional ablation of Gsk-3beta in islet beta cells results in expanded mass and resistance to fat feeding-induced diabetes in mice. Diabetologia. 2010;53:2600–10. doi: 10.1007/s00125-010-1882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Tanabe K, Bernal-Mizrachi E, Permutt MA. Mice with beta cell overexpression of glycogen synthase kinase-3beta have reduced beta cell mass and proliferation. Diabetologia. 2008;51:623–31. doi: 10.1007/s00125-007-0914-7. [DOI] [PubMed] [Google Scholar]
- Mariappan MM, Shetty M, Sataranatarajan K, Choudhury GG, Kasinath BS. Glycogen synthase kinase 3beta is a novel regulator of high glucose- and high insulin-induced extracellular matrix protein synthesis in renal proximal tubular epithelial cells. J Biol Chem. 2008;283:30566–75. doi: 10.1074/jbc.M801756200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CH, Watts VJ. Dexamethasone-induced Ras protein 1 negatively regulates protein kinase C delta: implications for adenylyl cyclase 2 signaling. Mol Pharmacol. 2006;69:1763–71. doi: 10.1124/mol.105.019133. [DOI] [PubMed] [Google Scholar]
- O’Connell BA, Moritz KM, Walker DW, Dickinson H. Treatment of pregnant spiny mice at mid gestation with a synthetic glucocorticoid has sex-dependent effects on placental glycogen stores. Placenta. 2013;34:932–40. doi: 10.1016/j.placenta.2013.06.310. [DOI] [PubMed] [Google Scholar]
- Peixoto EB, Papadimitriou A, Teixeira DA, Montemurro C, Duarte DA, Silva KC, et al. Reduced LRP6 expression and increase in the interaction of GSK3beta with p53 contribute to podocyte apoptosis in diabetes mellitus and are prevented by green tea. J Nutr Biochem. 2015;26:416–30. doi: 10.1016/j.jnutbio.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Ranta F, Avram D, Berchtold S, Dufer M, Drews G, Lang F, et al. Dexamethasone induces cell death in insulin-secreting cells, an effect reversed by exendin-4. Diabetes. 2006;55:1380–90. doi: 10.2337/db05-1220. [DOI] [PubMed] [Google Scholar]
- Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Spokoini R, Kfir-Erenfeld S, Yefenof E, Sionov RV. Glycogen synthase kinase-3 plays a central role in mediating glucocorticoid-induced apoptosis. Mol Endocrinol. 2010;24:1136–50. doi: 10.1210/me.2009-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanjang W, Abramov AY, Govitrapong P, Chetsawang B. Melatonin attenuates dexamethasone toxicity-induced oxidative stress, calpain and caspase activation in human neuroblastoma SH-SY5Y cells. The Journal of steroid biochemistry and molecular biology. 2013;138:116–22. doi: 10.1016/j.jsbmb.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Ullrich S, Zhang Y, Avram D, Ranta F, Kuhl D, Haring HU, et al. Dexamethasone increases Na+/K+ ATPase activity in insulin secreting cells through SGK1. Biochem Biophys Res Commun. 2007;352:662–7. doi: 10.1016/j.bbrc.2006.11.065. [DOI] [PubMed] [Google Scholar]
- Watcharasit P, Bijur GN, Song L, Zhu J, Chen X, Jope RS. Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J Biol Chem. 2003;278:48872–9. doi: 10.1074/jbc.M305870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watcharasit P, Bijur GN, Zmijewski JW, Song L, Zmijewska A, Chen X, et al. Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage. Proc Natl Acad Sci U S A. 2002;99:7951–5. doi: 10.1073/pnas.122062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SI, Yoon HY, Jeong SY, Chung YS. Glucocorticoid induces apoptosis of osteoblast cells through the activation of glycogen synthase kinase 3beta. Journal of bone and mineral metabolism. 2009;27:140–8. doi: 10.1007/s00774-008-0019-5. [DOI] [PubMed] [Google Scholar]