Abstract
Macrophages (Mϕ) and dendritic cells (DCs) are heterogeneous families of functionally and developmentally related immune cells that play crucial roles in tissue homeostasis and regulation of immune responses. In the past 5 years, immunologists have generated a considerable amount of data that challenged dogmas about the ontogeny and functions of these highly versatile cells. The male excurrent duct system plays a critical role in the establishment of fertility by allowing sperm maturation, transport and storage. In addition, it is challenged by pathogens and must establish a protective and tolerogenic environment for a continuous flow of autoantigenic spermatozoa. The post-testicular environment, and particularly the epididymis, contains an intricate network of DCs and Mϕ; however, the immunophysiology of this intriguing and highly specialized mucosal system is poorly understood. This review summarizes the current trends in mouse Mϕ and DC biology and speculates about their roles in the steady state epididymis. Unraveling immune cell functions in the male reproductive tract is an essential prerequisite for the design of innovative strategies aiming at controlling male fertility and treating infertility.
MeSH keywords: antigen-presenting cells, autoimmunity, dendritic cells, epididymis, macrophages, peripheral tolerance, sperm maturation, spermatozoa
Macrophages (Mϕ) and dendritic cells (DCs) are two families of mononuclear phagocytes (MPs) and professional antigen presenting cells (APCs) that have multiple immunoregulatory functions in physiological and pathological states, including tissue homeostasis, defense against pathogens, autoimmunity and cancer. The canonical function of Mϕ is the phagocytosis of pathogens, dying cells, and debris. Consequently, Mϕ are often described as the “scavengers” of the immune system, involved primarily in the maintenance of tissue hemostasis and innate immunity. In contrast, DCs are usually perceived as phagocytes specialized in antigen presentation and induction of adaptive immunity. As APCs, both Mϕ and DCs internalize foreign and self antigens, then process them and display the resulting peptides on their surfaces in association with major histocompatibility complex (MHC) molecules. MHC-antigen complexes are presented to “naïve” T cells (or T lymphocytes), which stimulates the proliferation and differentiation of cytotoxic or helper T cells. Antigen processing and presentation are critical for the initiation of immune responses, and MPs act as a bridge between the external environment and the body. Accordingly, Mϕ and DCs are found in every nonlymphoid mammalian tissue. The simplistic distinction between DCs and Mϕ, however, does not accurately reflect the complexity of the immune system. The generic terms “macrophage” and “dendritic cell” encompass numerous subsets of closely related, versatile cell types that are developmentally and functionally distinct. For the non-immunologist, understanding the nuance of cell nomenclature in the murine mononuclear phagocyte system (MPS) can be a challenge. Most markers used in flow cytometry-based phenotyping are not specific for a particular cellular subset. In addition, their ontogeny is still controversial, and their functions are overlapping: both DCs and Mϕ have phagocytic and antigen-presenting capabilities. Finally, the extreme plasticity of these cells allows them to adjust their morphology, location, phenotype and function rapidly in response to the variations of the inflammatory state of their environment. Whatever the outcome of semantic disputes might be, several types of mononuclear phagocytes are present in the epididymis and are therein likely to play a critical role. In order to better speculate on their importance in male reproductive function, the following section will briefly describe the current knowledge about murine MPs, on the basis of recent findings and proposed classifications.
Ontogeny and functions of murine dendritic cells and macrophages
Until recently, blood monocytes were thought to give rise to both Mϕ and DCs. This paradigm has shifted during the past 5 years (Ginhoux and Jung, 2014, Gomez Perdiguero, et al., 2015, Guilliams, et al., 2014, Hoeffel, et al., 2015); fate mapping and lineage tracing studies have demonstrated that, in the steady state, most tissue-resident macrophages are derived from erythro-myeloid progenitors that were seeded during embryological development and self-renew locally (Fig. 1). Following an inflammatory stimulus, circulating monocytes are recruited into tissues and differentiate into monocyte-derived Mϕ and DCs. The lamina propria of the small intestine represents a significant exception to this model: due to the continuous exposure to microbial products from the gut microbiota in the steady state, intestinal Mϕ are constantly replenished by circulating Ly6Chi monocytes (Bain, et al., 2014). This illustrates that each organ, depending on its content and the dynamics of interactions with the external environment, shapes highly specialized and unique subsets of mononuclear phagocytes. Thus, macrophages occasionally have organ-specific names, such as “microglia” in the central nervous system, “Kupffer cells” in the liver, or “Langerhans cells” in the skin. The “M1/M2 paradigm”, referring to macrophages with pro-inflammatory (M1) or anti-inflammatory (M2) properties, does not fully reflect the complexity and the plasticity of tissue Mϕ; the functional nomenclature will be progressively revised (Martinez and Gordon, 2014).
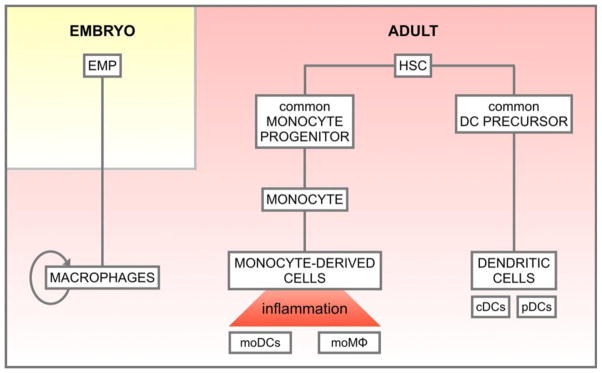
Figure 1. Simplified classification of mouse mononuclear phagocytes based on their ontogeny.
According to recent fate mapping studies, most tissue-resident macrophages (Mϕ) arise from erythro-myeloid progenitors (EMPs) in the embryo. They self-renew in adult tissues independently of hematopoietic stem cells (HSCs). In contrast, dendritic cell (DC) subsets originate from a common DC precursor (CDP) that derives from HSCs in the bone marrow. CDPs progressively differentiate to give rise to pre-DCs and, ultimately, classical DCs (cDCs) and plasmacytoid DCs (pDCs). In the steady state intestine, and following inflammation, circulating monocytes differentiate into monocyte-derived DCs (moDCs) and macrophages (mo Mϕ). Therefore, most peripheral organs are populated by various subsets of Mϕ and DCs with distinct origins and phenotypes. Ultimately, tissue mononuclear phagocytes are shaped by their microenvironment in order to exert organ-specific functions. Currently, the ontogeny of epididymal DCs and Mϕ is unknown.
The first distinction between DCs and Mϕ is based on their ontogeny: DCs are derived from the differentiation of hematopoietic stem cells (HSCs) in the adult bone marrow. HSCs give rise to a common DC precursor (CDP), which further differentiate into pre-DCs and 2 major families of terminally differentiated DCs: “classical” or “conventional” DCs (cDCs), and plasmocytoid DCs (pDCs) (Fig. 1). By producing large amounts of type I interferons upon activation, pDCs are mostly involved in antiviral immunity (Reizis, et al., 2011a, Reizis, et al., 2011b). cDCs, in contrast, exist in several subsets with functional specificities. Two major subsets of cDCs have been described in nonlymphoid tissues, based on the expression of CD103 (integrin E) and CD11b (integrin M). CD103+ DCs are specialized in cross-presentation: exogenous antigens are presented with MHC class I to CD8+ T cells (Haniffa, et al., 2013, Joffre, et al., 2012). Cross presentation is critical for the induction of immune defense against pathogens and tumors, as well as immune tolerance. CD11b+ (and CD103−) cDCs are less well characterized and do not cross present antigens. They express high levels of MHC class II and induce CD4+ T cell mediated immunity (Mildner and Jung, 2014).
Are macrophages and dendritic cells the sentinels of the epididymis?
Although the presence of Mϕ and DCs in the male reproductive tract is not surprising, their abundance and distribution in the murine steady state epididymis (in the absence of infection, inflammation or injury, Fig. 2) are intriguing (Da Silva, et al., 2011). The epididymis is a single, long and convoluted tube that connects the testis to the vas deferens. It is the primary site of sperm maturation and storage. The composition of luminal fluid is controlled by a pseudo-stratified epithelium, which contains three major cell types: principal cells, clear/narrow cells and basal cells (BCs). Altogether, epithelial cells establish a sequence of microenvironments within the lumen in which spermatozoa acquire motility, as well as the potential for capacitation (ability to undergo activation and fertilize an oocyte). The processes involved in sperm maturation and storage have been extensively documented; most studies focus on activities of the epithelium and their consequences on fluid composition and sperm characteristics (Arrighi, 2014, Belleannee, et al., 2012, Cornwall, 2009, Dacheux and Dacheux, 2014, Hermo and Robaire, 2002, Robaire and Hinton, 2002, Robaire, et al., 2006, Shum, et al., 2009, Shum, et al., 2011, Turner, 1995). In contrast, very few studies have been focused on interactions between the male excurrent ducts and the immune system.
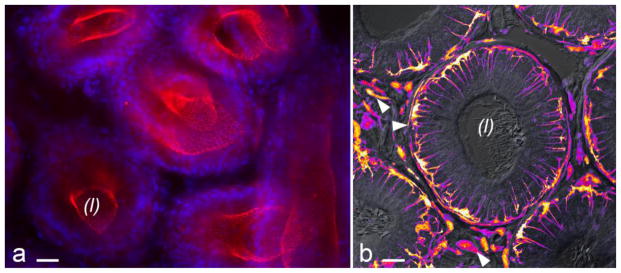
Figure 2. The blood-epididymis barrier is surveyed by mononuclear phagocytes.
The main component of the epididymis is a long tube lined by a pseudostratified epithelium that contains several cell types connected by apical tight junctions (TJs). ZO-1 immunolabeling (red, panel A) reveals the TJ network. Nuclei were labeled with DAPI (blue). This tight epithelium constitutes a physical barrier between the luminal compartment and the rest of the body, named the blood-epididymis barrier. The tubule is also populated by mononuclear phagocytes that closely interact with neighboring epithelial cells. Panel B shows a cross section of the mouse epididymal duct in the initial segment. Sperm are visible in the lumen. Cells that express green fluorescent protein under the control of CX3CR1 promoter are shown in pseudocolor to enhance contrast. CX3CR1 is expressed by subsets of monocytes, macrophages and dendritic cells. In the epididymis, CX3CR1+ cells are embedded in the epithelium, but are also present in the peritubular region and in the interstitium (arrowheads). Bars = 20 μm. (L): lumen.
During the entire reproductive life, sperm must be protected against ascending and blood-borne pathogens, as well as autoimmunity, while traveling through the epididymis. In accordance with the protective role of the excurrent ducts, immune cells have been identified in the epididymis of numerous species, including rodents and human (Nashan, et al., 1989, Pollanen and Cooper, 1994, Robaire and Hermo, 1988, Robaire, et al., 2006, Serre and Robaire, 2002). Previously, the predominant populations of cells were identified as intraepithelial lymphocytes (often referred to as “halo cells”) and macrophages. Due to their proximity and morphological similarities, BCs have occasionally been confused with Mϕ (Seiler, et al., 2000, Seiler, et al., 1999, Seiler, et al., 1998, Yeung, et al., 1994). Although a role of BCs in immune regulation cannot be excluded, others and we have shown that BCs and Mϕ constitute two clearly distinct cells types (Serre and Robaire, 1999, Shum, et al., 2014). In addition to halo cells and Mϕ, dendritic cells have been identified in the murine epididymis based on the expression of markers such as CD11c, CD103 and MHC class II (Da Silva, et al., 2011). The origin of epididymal DCs and Mϕ is unknown; fate mapping and lineage tracing studies will be necessary to determine whether Mϕ derive from a self-renewing embryological precursor or are replenished by circulating monocytes. Contrasting with the significant number of studies that describe the presence of Mϕ and DCs in the mammalian epididymis, functional data are sparse, and the precise role of immune cells in the post-testicular environment is poorly understood. Unraveling their functions is complicated by the aforementioned microenvironments, discrete segments in which numerous immune cells subsets likely coexist and exert niche-specific functions. Nevertheless, based on the canonical functions of DCs and Mϕ (phagocytosis and antigen presentation) as well as comparisons with others organ systems (Davies, et al., 2013), we can speculate about their roles in the homeostasis of the epididymis and in maintenance of male reproductive function.
Maintenance of the blood-epididymis barrier
The epididymal epithelium constitutes the main anatomical component of the blood epididymis barrier (BEB), the continuation of the blood-testis barrier that physically isolates the luminal compartment from the rest of the body (Dube and Cyr, 2012, Mital, et al., 2011). The importance of maintaining the integrity of the BEB is self-evident: any physical breach in the monolayer of epithelial cells connected by tight junctions (Fig. 2) would also constitute an opening in the physiological and immunological components of the barrier, thus altering the composition of the luminal microenvironment and creating an uncontrolled leak of sperm antigens. As the apparent rates of proliferation and apoptosis are relatively low in the adult rodent epididymis, the mucosa is likely maintained by very efficient mechanisms of phagocytic clearance of apoptotic cells (Yeung, et al., 2012). The clearance mechanisms that take place in the epididymis can be experimentally challenged using a simple surgical procedure: efferent duct ligation (EDL). Efferent ducts are short structures that connect the testis to the most proximal segment of the epididymis, the initial segment (IS); EDL therefore interrupts the luminal flow between the testis and the epididymis. This procedure has been used by several groups to study the influence of testicular luminal factors on the regulation of epididymal physiology (Kim, et al., 2015, Seiler, et al., 1999, Turner, et al., 2007). One of the most dramatic consequences of EDL is the rapid induction of extensive epithelial apoptosis in the proximal epididymis, which increases 50-fold shortly after EDL (Turner and Riley, 1999). The procedure deprives the epithelium of the high concentrations of testicular factors thought to be necessary for the survival of a large subset of principal cells, the most abundant epithelial cell type (Robaire, et al., 2006). Such a rapid and massive wave of cell death should cause breaches in the BEB. However, we have shown that a population of epithelial CX3CR1+ CD11c+ cells responds rapidly to the damage caused by EDL (Smith, et al., 2014). Intraepithelial macrophages alter their phenotype and morphology, engulf apoptotic cells and debris, and then return to their “steady state” appearance after the end of the apoptotic wave. The low apparent rate of epithelial apoptosis in physiological conditions could, therefore, result from the presence of a dense and dynamic population of macrophages that efficiently clear dead cells and debris, thus allowing the quick regeneration of the epididymal epithelium and preventing the release of debris (potentially harmful for spermatozoa) into the luminal milieu. The subsequent mechanisms of epithelial regeneration are still under investigation (Kim, et al., 2015).
Immune surveillance and initiation of inflammatory responses
Relative to other organs connected to the external environment, such as the intestine or the airways, the epididymis is a gentle and stable environment; the composition of the luminal milieu remains relatively constant throughout the entire reproductive life. However, the epididymis is constantly challenged by pathogens, particularly sexually-transmitted bacteria and viruses. Pathogens such as Chlamydia trachomatis, Neisseria gonorrhea, and Escherichia coli can ascend the urogenital tract and cause epididymitis (inflammation of the epididymis), ultimately, altering fertility either transiently or permanently (Hedger, 2011, Redgrove and McLaughlin, 2014). The epididymis is also a primary target for viruses such as HIV (Mullen, et al., 2003). Male reproductive function therefore relies on the presence of robust mechanisms of innate and adaptive immunity in all reproductive organs and ducts. Despite the immediate proximity of testis and epididymis, orchitis (inflammation of the testis) is rare compared to epididymitis, suggesting that they harbor distinct defense mechanisms; the epididymis could also contribute to the protection of the testis. The best-described effectors of innate immunity in the epididymis are antimicrobial proteins (particularly beta-defensins), which are abundantly secreted in the luminal fluid (Dorin and Barratt, 2014, Hall, et al., 2007).
With regard to their localization and antigen-presenting activity, Mϕ and DCs are very likely to be involved in the immunological surveillance of the epididymis. The adaptive immune response is initiated by the acquisition of antigen by APCs. Antigen uptake may be direct or indirect: in the initial segment, the highly ramified CX3CR1+ CD11c+ intraepithelial cells have the potential to penetrate the intraluminal compartment (Smith et al., 2015 American Society of Andrology meeting, Salt Lake City, USA). In addition, peritubular APCs could cooperate with surrounding bona fide epithelial cells in order to acquire luminal material. This division of labor has been observed in other mucosal systems such as the gut, in which luminal antigen acquisition and processing involve a functional cooperation between goblet cells, CX3CR1+ macrophages, and CD103+ migratory DCs (Mazzini, et al., 2014, McDole, et al., 2012). Although they reside in radically different environments, the mucosal systems of the small intestine and the epididymis share striking similarities with respect to the distribution of Mϕ and DCs.
In addition to ascending pathogens, the epididymis can be affected by blood-borne pathogens. The rodent epididymis is relatively poorly vascularized, excepting the initial segment. In the IS, the duct is surrounded by a very dense network of capillaries, including fenestrated capillaries (Abe, et al., 1984, Hirai, et al., 2010, Suzuki, 1982). One of the characteristics of the IS is its highly absorptive capacity: 90% of the testicular fluid is reabsorbed during transit in efferent ducts and the IS. This justifies the abundance of capillaries in charge of draining large amounts of fluid. However, the network is bidirectional, and we cannot exclude that the IS requires a quick supply of blood-borne material and/or cells. Although the functions of IS capillaries are not fully understood, the abundant fenestrated vessels in close proximity with the duct may constitute a fragile zone in the blood epididymis barrier. Interestingly, the IS is also a segment in which Mϕ and DCs are particularly abundant. Therefore, we hypothesize that mononuclear phagocytes in the IS are involved not only in the surveillance of the luminal compartment, but also in the monitoring of circulatory antigens. In the gut, lamina propria CX3CR1+ cells establish close interactions with fenestrated capillaries, acquire and process circulatory antigens, and induce the differentiation of a subset of CD8+ T cells (Chang, et al., 2013). Based on similarities between the epididymis and the small intestine mucosa, the IS is likely to represent a zone of very intense immunological activity, in which Mϕ and/or DCs could survey both the luminal compartment and the blood flow to regulate immune responses. In such a context, the immunological components of the BEB in the IS should be seen as a gate between the blood and the epididymis, rather than a barrier. This very immunologically active epididymal segment could also represent the ultimate opportunity to prevent the “immunologically privileged” (Forrester, et al., 2008) testis from being invaded by ascending pathogens.
Maintenance of immune tolerance to sperm antigens
The primary challenge of the immune system is to control the fragile equilibrium between immune defense against harmful antigens and tolerance to self antigen. Tolerance is induced by two mechanisms: central tolerance and peripheral tolerance. Central tolerance takes place in the thymus and in the bone marrow during fetal development and early life, and results in the deletion of autoreactive lymphocytes (lymphocytes that respond to self antigens). In contrast, peripheral tolerance takes place after maturation of the immune system and involves multiple mechanisms, including the induction of regulatory T cells (Tregs) and the inhibition of T cell growth by indoleamine 2,3-dioxygenase (Guiton, et al., 2013, Jrad-Lamine, et al., 2013). Impaired tolerance mechanisms contribute to numerous autoimmune and inflammatory conditions such as type 1 diabetes, rheumatoid arthritis and inflammatory bowel disease. With respect to immune tolerance, the epididymis is particularly challenging. Spermatozoa, which display antigens that are expressed nowhere else in the body and appear after the establishment of central tolerance, are abundant about 1,000 sperm cells enter the human epididymis every second (Hedger, 2011, Hedger and Hales, 2006). The epididymis and epididymal immune cells are ideally positioned to induce tolerance to sperm antigens newly exposed during the maturation process. We have shown that the epithelium of the initial segment is densely populated by CX3CR1+ CD11c+ mononuclear phagocytes with lumen-reaching properties (Da Silva, et al., 2011). The IS lumen is narrow and sperm concentration is relatively low, therefore the IS is the ideal site for a possible direct contact between sperm and mucosal macrophages. As the intestinal mucosa maintains tolerance to commensal bacterial by constantly surveying the luminal compartment via CX3CR1+ cells (Niess, et al., 2005, Niess and Reinecker, 2006), the IS epithelium could be the site of immunological monitoring of the luminal compartment. We have also shown that the murine epididymis contains at least one subset of CD103+ DCs. In the gut, migratory CD103+ DCs functionally cooperate with lamina propria CX3CR1+ macrophages to acquire luminal antigens and induce the differentiation of Foxp3+ T cells (Mazzini, et al., 2014). This division of labor between two distinct subsets of intestinal APCs is involved in the induction of tolerance to food antigens. Since very similar populations of DCs and Mϕ are positioned in the epididymal mucosa, it is reasonable to hypothesize that a similar functional cooperation could contribute to the induction of tolerance to sperm antigen as they transit in the IS.
Spermatophagy, sperm “quality control”, and other possible roles
One of the most controversial aspects of epididymal physiology is the possible presence of sperm “quality control” mechanisms, which would implicate the identification and removal of presumably defective spermatozoa (Cooper, et al., 2002, Jones, 2004, Sutovsky, 2003, Sutovsky, et al., 2001, Sutovsky, et al., 2002). Extreme situations such as the experimental ligation of the epididymal duct and vasectomy (ligation of the vas deferens) can cause a rupture of the epithelium, local inflammation, and, ultimately, formation of sperm granuloma (a mass of extravasated sperm and immune cells, including macrophages). However, although “spermatophagy” or “spermiophagy” have been reported (Arrighi, et al., 1994, Holstein, 1978, Jones, 2004), there is no convincing evidence to date of a biologically significant role of Mϕ in the detection or destruction of defective spermatozoa in the normally functioning mammalian epididymis. Another unexpected role of intestinal Mϕ has been described recently: a subset of muscularis Mϕ is involved in the regulation of peristaltic activity through an interaction with enteric neurons (Muller, et al., 2014). As the transit of spermatozoa in the epididymis also requires peristaltic contractions (Mietens, et al., 2014), some peritubular macrophages may be involved in a crosstalk with local neurons.
The epididymis, DCs and Mϕ in the 21st century
After half a century of research on the mammalian epididymis, relatively little is know about the sequence of mechanisms that control sperm maturation (Cooper, 2015). In addition, epididymal functions are influenced by other organ systems, and the presence of numerous populations of immune cells in the epididymis suggests a very dynamic crosstalk between the male excurrent duct and the immune system. During the past few decades, fundamental advances have been made in the understanding of immunoregulatory mechanisms in most organ systems. The most recent data point toward the presence of organ-specific subsets of macrophages and dendritic cells in peripheral tissues. These highly specialized cell populations are shaped by their microenvironment, by time, and by the inflammatory state of each organ. Niches drive the development of local macrophages by finely tuning cytokine secretion and modifying the enhancer landscape of each subset of cell (Gosselin, et al., 2014, Lavin, et al., 2014). Consequently, Mϕ and DCs (as well as other immune cells) must be studied in their native context: while epididymal MPs share phenotypical characteristics with MPs of the small intestine, kidney, and brain, the unique epididymal microenvironment is likely to have sculpted scarce yet highly specific populations of cells. Despite recent studies indicating that immune cells lose their context-specific features when isolated from their microenvironment, most immunological studies are based on the heavy usage of flow cytometry for cell analysis and sorting. While flow cytometry is the most convenient way to simultaneously analyze multiple cellular markers, it requires the disruption of tissues and the preparation of single-cell suspensions. This procedure can be misleading in the epididymis, particularly for the CX3CR1+ CD11c+ cells that are embedded in the initial segment epithelium (Da Silva, et al., 2011, Shum, et al., 2014, Smith, et al., 2014). These highly ramified cells are found mostly in the IS, where they establish extremely intricate interactions with surrounding epithelial cells and occasionally communicate with the luminal milieu. Furthermore, they are also strategically positioned to interact with a dense network of capillaries. Isolating these macrophages from their environment would disrupt their fundamental features and thereby prevent further understanding of their function. Powerful tools are available or in development to better analyze the ontogeny, distribution, and behavior of immune cells in situ and in vivo, including transgenic mouse models expressing fluorescent reporter proteins (such as CX3CR1-GFP and CD11c-EYFP mice) and modern imaging techniques (multiphoton intravital imaging, light sheet and super-resolution fluorescence microscopy). By allowing the quantitative analysis of multiple subsets of phenotypically distinct cells in situ, histo-cytometry (Gerner, et al., 2012) may aid in unraveling highly specialized niches like the epididymal segments. Characterizing the origin and phenotype of epididymal Mϕ and DC subsets will also be facilitated by fate mapping and cell lineage tracing studies (Yona, et al., 2013), as well as the extensive use of single cell RNA-Seq (RNA sequencing), enhancer and promoter landscape analyses, mass cytometry (Bjornson, et al., 2013), and other powerful “systems biology” technologies. Although epididymal research should not be led by technologies, new tools may help elucidate the complexity of the male excurrent duct system, as has recently been accomplished in other mucosal systems.
The epididymal mucosa should not be studied only for its role in reproductive physiology; this microenvironment has developed a very efficient way to maintain peripheral tolerance to a heavy and continuous load of autoantigens, while protecting its precious cargo from being attacked by the pathogens that constantly ascend the reproductive tract. Although Mϕ and DCs have critical immunoregulatory functions, mucosal homeostasis is regulated by a finely tuned crosstalk between myeloid, lymphoid (including halo cells) and non-immune cells; the potential role of all these cells in mucosal immunology should be evaluated. Additionally, the extremely low occurrence of cancer in the epididymis makes it unique compared to other organs of the male reproductive system, such as the testis and the prostate (Yeung, et al., 2012). Ultimately, deciphering the complex immunophysiology of the epididymis may help to characterize fundamental immunoregulatory mechanisms, better understand the control of male fertility, and identify potential targets for immuno-contraception.
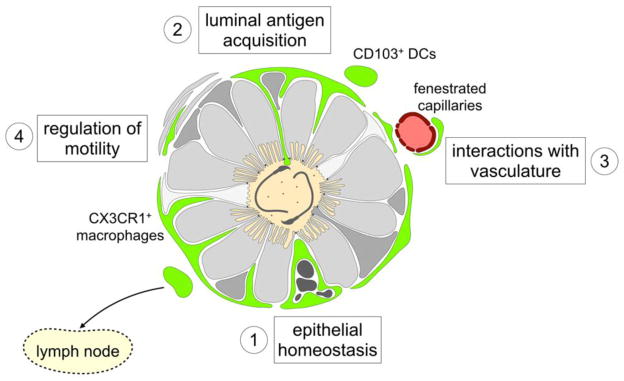
Figure 3. Possible roles of macrophages and dendritic cells in the epididymis.
Mononuclear phagocytes (MPs) are show in green in this schematic depiction of a cross section of the mouse epididymal duct. DCs and Mϕ express various levels of leukocyte surface markers and exhibit distinct morphological characteristics. In the proximal epididymis, a subset of CX3CR1+ CD11c+ cells establishes close interactions with neighboring epithelial cells. These intraepithelial Mϕ maintain the blood-epididymis barrier by rapidly removing apoptotic cells and debris (1). In addition, they project dendritic processes toward apical tight junctions and occasionally reach the luminal compartment. Such a mechanism could allow Mϕ and DCs to directly or indirectly acquire luminal antigens, including sperm antigens and pathogens, to initiate immune defense or tolerance (2). While intraepithelial macrophages are stationary, CD103+ DCs migrate into lymph nodes following antigen acquisition. Furthermore, the proximal epididymis interstitium contains a dense network of capillaries; fenestrated capillaries of the initial segments may be monitored by neighboring MPs (3). In the gut, a crosstalk between macrophages and neurons is involved in the regulation of intestinal motility; similarly, macrophages may regulate the contraction of peritubular smooth muscle cells in the epididymis (4).
Acknowledgments
Grant support: The authors are supported by U.S. Department of Health and Human Services - National Institutes of Health - National Institute of Child Health and Human Development grant R01HD069623.
References
- Abe K, Takano H, Ito T. Microvasculature of the mouse epididymis, with special reference to fenestrated capillaries localized in the initial segment. The Anatomical record. 1984;209:209–218. doi: 10.1002/ar.1092090208. [DOI] [PubMed] [Google Scholar]
- Arrighi S. Are the basal cells of the mammalian epididymis still an enigma? Reproduction, fertility, and development. 2014;26:1061–1071. doi: 10.1071/RD13301. [DOI] [PubMed] [Google Scholar]
- Arrighi S, Romanello MG, Domeneghini C. Ultrastructure of the epithelium that lines the ductuli efferentes in domestic equidae, with particular reference to spermatophagy. Acta anatomica. 1994;149:174–184. doi: 10.1159/000147574. [DOI] [PubMed] [Google Scholar]
- Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nature immunology. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleannee C, Thimon V, Sullivan R. Region-specific gene expression in the epididymis. Cell and tissue research. 2012;349:717–731. doi: 10.1007/s00441-012-1381-0. [DOI] [PubMed] [Google Scholar]
- Bjornson ZB, Nolan GP, Fantl WJ. Single-cell mass cytometry for analysis of immune system functional states. Current opinion in immunology. 2013;25:484–494. doi: 10.1016/j.coi.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SY, Song JH, Guleng B, Cotoner CA, Arihiro S, Zhao Y, Chiang HS, O’Keeffe M, Liao G, Karp CL, Kweon MN, Sharpe AH, Bhan A, Terhorst C, Reinecker HC. Circulatory antigen processing by mucosal dendritic cells controls CD8(+) T cell activation. Immunity. 2013;38:153–165. doi: 10.1016/j.immuni.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG. Epididymal research: more warp than weft? Asian journal of andrology. 2015 doi: 10.4103/1008-682X.146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG, Yeung CH, Jones R, Orgebin-Crist MC, Robaire B. Rebuttal of a role for the epididymis in sperm quality control by phagocytosis of defective sperm. Journal of cell science. 2002;115:5–7. doi: 10.1242/jcs.115.1.5. [DOI] [PubMed] [Google Scholar]
- Cornwall GA. New insights into epididymal biology and function. Human reproduction update. 2009;15:213–227. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva N, Cortez-Retamozo V, Reinecker HC, Wildgruber M, Hill E, Brown D, Swirski FK, Pittet MJ, Breton S. A dense network of dendritic cells populates the murine epididymis. Reproduction. 2011;141:653–663. doi: 10.1530/REP-10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux JL, Dacheux F. New insights into epididymal function in relation to sperm maturation. Reproduction. 2014;147:R27–42. doi: 10.1530/REP-13-0420. [DOI] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nature immunology. 2013;14:986–995. [Google Scholar]
- Dorin JR, Barratt CL. Importance of beta-defensins in sperm function. Molecular human reproduction. 2014;20:821–826. doi: 10.1093/molehr/gau050. [DOI] [PubMed] [Google Scholar]
- Dube E, Cyr DG. The blood-epididymis barrier and human male fertility. Advances in experimental medicine and biology. 2012;763:218–236. doi: 10.1007/978-1-4614-4711-5_11. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Xu H, Lambe T, Cornall R. Immune privilege or privileged immunity? Mucosal Immunol. 2008;1:372–381. doi: 10.1038/mi.2008.27. [DOI] [PubMed] [Google Scholar]
- Gerner MY, Kastenmuller W, Ifrim I, Kabat J, Germain RN. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity. 2012;37:364–376. doi: 10.1016/j.immuni.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nature reviews Immunology. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, Glass CK. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nature reviews Immunology. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiton R, Henry-Berger J, Drevet JR. The immunobiology of the mammalian epididymis: the black box is now open! Basic and clinical andrology. 2013;23 doi: 10.1186/2051-4190-23-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SH, Yenugu S, Radhakrishnan Y, Avellar MC, Petrusz P, French FS. Characterization and functions of beta defensins in the epididymis. Asian journal of andrology. 2007;9:453–462. doi: 10.1111/j.1745-7262.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- Haniffa M, Collin M, Ginhoux F. Identification of human tissue cross-presenting dendritic cells: A new target for cancer vaccines. Oncoimmunology. 2013;2:e23140. doi: 10.4161/onci.23140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger MP. Immunophysiology and pathology of inflammation in the testis and epididymis. Journal of andrology. 2011;32:625–640. doi: 10.2164/jandrol.111.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger MP, Hales DB. Immunophysiology of the male reproductive tract. In: Neill JD, editor. Knobil and Neill’s physiology of reproduction. Vol. 1. Elsevier Academic Press; 2006. pp. 1195–1286. [Google Scholar]
- Hermo L, Robaire B. Epididymal cell types and thier functions. In: Robaire B, Hinton BT, editors. The epididymis: from molecular to clinical practice. A comprehensive survey of the efferent ducts, the epididymis and the vas deferens. Kluwer Academic/Plenum; New York: 2002. pp. 81–102. [Google Scholar]
- Hirai S, Naito M, Terayama H, Ning Q, Miura M, Shirakami G, Itoh M. Difference in abundance of blood and lymphatic capillaries in the murine epididymis. Medical molecular morphology. 2010;43:37–42. doi: 10.1007/s00795-009-0473-8. [DOI] [PubMed] [Google Scholar]
- Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F. C-myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein AF. Spermatophagy in the seminiferous tubules and excurrent ducts of the testis in Rhesus monkey and in man. Andrologia. 1978;10:331–352. doi: 10.1111/j.1439-0272.1978.tb03044.x. [DOI] [PubMed] [Google Scholar]
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nature reviews Immunology. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- Jones R. Sperm survival versus degradation in the Mammalian epididymis: a hypothesis. Biol Reprod. 2004;71:1405–1411. doi: 10.1095/biolreprod.104.031252. [DOI] [PubMed] [Google Scholar]
- Jrad-Lamine A, Henry-Berger J, Damon-Soubeyrand C, Saez F, Kocer A, Janny L, Pons-Rejraji H, Munn DH, Mellor AL, Gharbi N, Cadet R, Guiton R, Aitken RJ, Drevet JR. Indoleamine 2,3-dioxygenase 1 (ido1) is involved in the control of mouse caput epididymis immune environment. PloS one. 2013;8:e66494. doi: 10.1371/journal.pone.0066494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Roy J, Shum WW, Da Silva N, Breton S. Role of testicular luminal factors on Basal cell elongation and proliferation in the mouse epididymis. Biol Reprod. 2015;92:9. doi: 10.1095/biolreprod.114.123943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity. 2014;40:248–261. doi: 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietens A, Tasch S, Stammler A, Konrad L, Feuerstacke C, Middendorff R. Time-lapse imaging as a tool to investigate contractility of the epididymal duct--effects of cGMP signaling. PloS one. 2014;9:e92603. doi: 10.1371/journal.pone.0092603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84:851–858. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen TE, Jr, Kiessling RL, Kiessling AA. Tissue-specific populations of leukocytes in semen-producing organs of the normal, hemicastrated, and vasectomized mouse. AIDS research and human retroviruses. 2003;19:235–243. doi: 10.1089/088922203763315740. [DOI] [PubMed] [Google Scholar]
- Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashan D, Malorny U, Sorg C, Cooper T, Nieschlag E. Immuno-competent cells in the murine epididymis. International journal of andrology. 1989;12:85–94. doi: 10.1111/j.1365-2605.1989.tb01289.x. [DOI] [PubMed] [Google Scholar]
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- Niess JH, Reinecker HC. Dendritic cells: the commanders-in-chief of mucosal immune defenses. Current opinion in gastroenterology. 2006;22:354–360. doi: 10.1097/01.mog.0000231807.03149.54. [DOI] [PubMed] [Google Scholar]
- Pollanen P, Cooper TG. Immunology of the testicular excurrent ducts. Journal of reproductive immunology. 1994;26:167–216. doi: 10.1016/0165-0378(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Redgrove KA, McLaughlin EA. The Role of the Immune Response in Chlamydia trachomatis Infection of the Male Genital Tract: A Double-Edged Sword. Frontiers in immunology. 2014;5:534. doi: 10.3389/fimmu.2014.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annual review of immunology. 2011a;29:163–183. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B, Colonna M, Trinchieri G, Barrat F, Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nature reviews Immunology. 2011b;11:558–565. doi: 10.1038/nri3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaire B, Hermo L. Efferent ducts, epididymis, and vas deferens: structure, functions, and their regulation. In: Knobil E, Neil J, et al., editors. The Physiology of Reproduction. Raven Press; New York: 1988. [Google Scholar]
- Robaire B, Hinton BT. The epididymis: from molecular to clinical practice. A comprehensive survey of the efferent ducts, the epididymis and the vas deferens. Kluwer Academic/Plenum; New York: 2002. [Google Scholar]
- Robaire B, Hinton BT, Orgebin-Crist MC. The epididymis. In: Neill JD, editor. Knobil and Neill’s physiology of reproduction. Vol. 1. Elsevier Academic Press; 2006. pp. 1072–1148. [Google Scholar]
- Seiler P, Cooper TG, Nieschlag E. Sperm number and condition affect the number of basal cells and their expression of macrophage antigen in the murine epididymis. International journal of andrology. 2000;23:65–76. doi: 10.1046/j.1365-2605.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- Seiler P, Cooper TG, Yeung CH, Nieschlag E. Regional variation in macrophage antigen expression by murine epididymal basal cells and their regulation by testicular factors. Journal of andrology. 1999;20:738–746. [PubMed] [Google Scholar]
- Seiler P, Wenzel I, Wagenfeld A, Yeung CH, Nieschlag E, Cooper TG. The appearance of basal cells in the developing murine epididymis and their temporal expression of macrophage antigens. International journal of andrology. 1998;21:217–226. doi: 10.1046/j.1365-2605.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- Serre V, Robaire B. Distribution of immune cells in the epididymis of the aging Brown Norway rat is segment-specific and related to the luminal content. Biol Reprod. 1999;61:705–714. doi: 10.1095/biolreprod61.3.705. [DOI] [PubMed] [Google Scholar]
- Serre V, Robaire B. Interactions of the immune system and the epididymis. In: Robaire B, Hinton BT, editors. The epididymis: from molecular to clinical practice. A comprehensive survey of the efferent ducts, the epididymis and the vas deferens. Kluwer Academic/Plenum; New York: 2002. pp. 219–231. [Google Scholar]
- Shum WW, Da Silva N, Brown D, Breton S. Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk. The Journal of experimental biology. 2009;212:1753–1761. doi: 10.1242/jeb.027284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum WW, Ruan YC, Da Silva N, Breton S. Establishment of cell-cell cross talk in the epididymis: control of luminal acidification. Journal of andrology. 2011;32:576–586. doi: 10.2164/jandrol.111.012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum WW, Smith TB, Cortez-Retamozo V, Grigoryeva LS, Roy JW, Hill E, Pittet MJ, Breton S, Da Silva N. Epithelial basal cells are distinct from dendritic cells and macrophages in the mouse epididymis. Biol Reprod. 2014;90:90. doi: 10.1095/biolreprod.113.116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TB, Cortez-Retamozo V, Grigoryeva LS, Hill E, Pittet MJ, Da Silva N. Mononuclear phagocytes rapidly clear apoptotic epithelial cells in the proximal epididymis. Andrology. 2014;2:755–762. doi: 10.1111/j.2047-2927.2014.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutovsky P. Ubiquitin-dependent proteolysis in mammalian spermatogenesis, fertilization, and sperm quality control: killing three birds with one stone. Microscopy research and technique. 2003;61:88–102. doi: 10.1002/jemt.10319. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Moreno R, Ramalho-Santos J, Dominko T, Thompson WE, Schatten G. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. Journal of cell science. 2001;114:1665–1675. doi: 10.1242/jcs.114.9.1665. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Neuber E, Schatten G. Ubiquitin-dependent sperm quality control mechanism recognizes spermatozoa with DNA defects as revealed by dual ubiquitin-TUNEL assay. Molecular reproduction and development. 2002;61:406–413. doi: 10.1002/mrd.10101. [DOI] [PubMed] [Google Scholar]
- Suzuki F. Microvasculature of the mouse testis and excurrent duct system. The American journal of anatomy. 1982;163:309–325. doi: 10.1002/aja.1001630404. [DOI] [PubMed] [Google Scholar]
- Turner TT. On the epididymis and its role in the development of the fertile ejaculate. Journal of andrology. 1995;16:292–298. [PubMed] [Google Scholar]
- Turner TT, Johnston DS, Finger JN, Jelinsky SA. Differential gene expression among the proximal segments of the rat epididymis is lost after efferent duct ligation. Biol Reprod. 2007;77:165–171. doi: 10.1095/biolreprod.106.059493. [DOI] [PubMed] [Google Scholar]
- Turner TT, Riley TA. p53 independent, region-specific epithelial apoptosis is induced in the rat epididymis by deprivation of luminal factors. Molecular reproduction and development. 1999;53:188–197. doi: 10.1002/(SICI)1098-2795(199906)53:2<188::AID-MRD8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Nashan D, Sorg C, Oberpenning F, Schulze H, Nieschlag E, Cooper TG. Basal cells of the human epididymis--antigenic and ultrastructural similarities to tissue-fixed macrophages. Biol Reprod. 1994;50:917–926. doi: 10.1095/biolreprod50.4.917. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Wang K, Cooper TG. Why are epididymal tumours so rare? Asian journal of andrology. 2012;14:465–475. doi: 10.1038/aja.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]