Abstract
Rationale
Alcohol use appears to decrease executive function acutely in a dose dependent manner, and lower baseline executive function appears to contribute to problematic alcohol use. However, no studies, to our knowledge, have examined the relationship between individual differences in working memory (a subcomponent of executive function) after alcohol consumption and drinking behaviors and consequences.
Objectives
The current study assessed the relationship between drinking behavior, alcohol-related consequences, and alcohol-induced changes in working memory (as assessed by Trails Making Test-B).
Method
Participants recruited from the community (n = 41), 57.3% male, mean age 39.2, took part in a three-session, within-subjects, repeated-measures design. Participants were administered a placebo, 0.4 g/kg, or 0.8 g/kg dose of alcohol. Working memory, past 30 day alcohol consumption, and consequences of alcohol use were measured at baseline; working memory was measured again after each beverage administration.
Results
Poorer working memory after alcohol administration (controlling for baseline working memory) was significantly associated with a greater number of drinks consumed per drinking day. Additionally, we observed a significant indirect relationship between the degree of alcohol-induced working memory decline and adverse consequences of alcohol use, which was mediated through greater average drinks per drinking day.
Conclusions
It is possible that greater individual susceptibility to alcohol-induced working memory decline may limit one’s ability to moderate alcohol consumption as evidenced by greater drinks per drinking day, and that this results in more adverse consequences of alcohol use.
Introduction
There are bidirectional associations between executive function, a higher-order cognitive construct involved in the self-regulation of goal-directed behavior (Lezak, Howieson, Bigler, & Tranel, 2012), and alcohol use. Specifically, alcohol use appears to acutely affect executive function (e.g., Fillmore & Vogel-Sprott, 2006; Duka et al., 2004) in a dose-dependent manner (Guillot, 2010), and lower baseline executive function appears to contribute to problematic alcohol use (e.g., Finn & Hall, 2004; Nigg et al., 2006).
Many studies examining executive function in relation to alcohol focus specifically on working memory (e,g. Peeters, et al., 2014; Houben, 2011), which has been conceptualized as a sub-component of executive functioning associated with updating information (Suchy, 2009). Working memory is particularly relevant for alcohol-related behaviors; an individual who initiates a drinking episode with the intention of stopping after a set number of drinks or foregoing risks associated with uncontrolled drinking, must rely, in part, on working memory to meet these behavioral goals. This potential for impaired control over drinking (e.g., Leeman et al., 2012) may in turn contribute to more alcohol related problems.
Despite evidence demonstrating that working memory is acutely affected by alcohol consumption, studies examining the relationship between these variables have almost exclusively assessed working memory at a point prior to alcohol consumption. This is problematic, as is it possible that working memory may be differentially affected following alcohol consumption, independent of an individual’s baseline working memory. Individual differences in alcohol-induced changes of other executive functions such as impulse inhibition (Weafer & Fillmore, 2008) have been linked to subsequent drinking behaviors. However, little to no information exists in the literature on individual differences in working memory following alcohol use and how this might affect subsequent behaviors. Indeed, it has been noted that there is a dearth of research examining the ways in which alcohol-induced executive function impairments might influence drinking as well as other risky behaviors, such as cigarette smoking, illicit drug use, driving after drinking, overeating, and risky sexual behavior (Day et al., 2015). Therefore, gaining a better understanding of how individuals may differ in terms of alcohol-induced working memory decline, and how this may relate to alcohol use behavior, is critical.
The current study examined the relationship between alcohol-induced declines in working memory performance and reported alcohol use behavior. Specifically, we examined changes in working memory performance as measured by Trail Making Test-B (TMT-B) following 3 doses of alcohol (placebo, low [0.4 g/kg], high [0.8 g/kg]) in relation to reported drinks consumed per drinking occasion. We hypothesized that poorer working memory after alcohol consumption (controlling for baseline working memory) would be associated with greater self-reported drinks per drinking day. Further, we hypothesized that alcohol-induced changes in processing and motor speed (Trail Making Test-A) would not be associated with self-reported drinking, indicating a specific effect of working memory change as opposed to other cognitive domains.
We built on this initial hypothesis by examining how the relationship between alcohol-induced working memory decline and reported drinking behaviors might relate to adverse consequences of alcohol use. We hypothesized a positive relationship between alcohol-induced working memory decline and adverse consequences of alcohol use through increased drinks per drinking day, but not through number of drinking days. This differential hypothesis was postulated based on the notion that acute alcohol-induced changes in working memory should be associated with number of drinks consumed, as this behavior occurs while alcohol is on board, but not closely associated with number of drinking days, as the decision to engage or not engage in a drinking session most often occurs while sober (except in rare cases of very heavy drinkers). Specifically, we posited that greater working memory impairment following alcohol consumption would be associated with diminished ability to moderate alcohol use as evidenced by greater drinks per drinking day, and that this increased alcohol consumption would be associated with more adverse consequences of alcohol use. It is possible that this hypothesized directionality is reversed; that is, chronic heavy drinking may impair one’s cognitive ability after alcohol administration. To control for the chronic effects of alcohol, we included past alcohol dependence as a covariate. Additionally, given the previous literature indicating that impulse inhibition may represent another key executive function relating to negative consequences of alcohol use (e.g. Rose et al, 2014; Albein-Urios et al., 2012; Weafer & Fillmore, 2008; Marczinski et al., 2005), we controlled for trait impulsivity.
Method
Participants
Full details of procedures used in the current study have been previously outlined (Kahler et al., 2014), and all procedures were approved by the Brown University Institutional Review Board. The current analyses were conducted with a subsample of individuals who completed executive function tasks. The parent study examined smoking related variables; therefore, all participants were daily smokers. Participants were recruited from the community and met the following inclusion criteria: 21 – 65 years old, use of 10–30 cigarettes daily, carbon monoxide level >10 ppm, current heavy drinking defined by ≥5 drinks per occasion for men or ≥4 drinks for women, at least twice a month, and endorsement of no history or intention to seek alcohol treatment. Exclusion criteria were: use of other tobacco or nicotine replacement therapy, plan to quit smoking within 30 days, inability to abstain from alcohol for 24 hours without significant withdrawal symptoms, current affective disorder or psychotic symptoms, current pregnancy or nursing, illicit drug use on more than four occasions in the past month, medical issues or medications contraindicated for alcohol consumption, and weight greater than 250lbs.
The current sample included (N= 41) participants with an average age of 39.2 (SD=10.84), and 57.3% were male. Participants drank on 53.1% of the 60 days prior to baseline, averaging 6.0 (SD=2.5) drinks per drinking day, and 67% had history of alcohol dependence (no participants met criteria for current alcohol dependence).
Procedure
A repeated-measures design included 3 counter-balanced sessions (mean days between = 9.23) (SD=2.13), in which participants received placebo (trace alcohol), 0.4g/kg, and 0.8 g/kg dose of alcohol. Dose was adjusted for weight and sex (women received 90% of the alcohol dose received by men). In line with methods used in prior research to control for effects of nicotine in alcohol challenge designs (King et al., 2014) participants smoked ad lib prior to the laboratory session and then smoked in the laboratory exactly 3 hours before beverage administration. Nicotine withdrawal symptoms did not differ across testing sessions. Participants refrained from alcohol use for 24 hours prior to testing, and .000 breath alcohol concentration (BrAC) was confirmed via Alco-Sensor IV (Intoximeters Inc., St Louis, MO, USA). Participants ate a standardized light meal in the laboratory 3 hours prior to beverage consumption. In all conditions, the beverage was divided into three glasses in equal-sized portions; each drink was consumed within 5 minutes for a total of 15 minutes. Working memory (TMT-B) and processing and motor speed (TMT-A) was assessed at baseline on the first testing day, and 20 minutes after initiation of beverage consumption in each condition. The mean BrAC reading assessed after administration (within 5 minutes of completion) of the Trail Making test was .035 (SD = .003) for the low alcohol condition and .079 (SD = .021) for the high alcohol condition, and TMT A/B took about 5 minutes to complete.
Measures
Working memory was assessed with the TMT-B (Sánchez-Cubillo et al., 2009) portion of the Trail-Making Test, processing and motor speed was assessed with the TMT-A portion of the Trail-Making Test (TMT, Reitan and Wolfson, 1995). Alcohol-induced working memory change was calculated by subtracting TMT-B after drink administration in each session from baseline TMT-B. Alternate versions of TMT were used at each session in order to decrease the likelihood of practice effects (Wagner et al., 2011). T-scores (Weaver et al., 2002; Reitan and Wolfson, 1995) adjusted for age, gender, and education, were used. The Timeline Followback Interview (28) was used to assess past 60-day alcohol use (Sobell & Sobell, 1992); which was used to calculate drinks per drinking day and number of drinking days. The Short Inventory of Problems (SIP; Miller, Tonigan, Longabaugh, 1995) was used to assess adverse consequences of alcohol use including problems related to health, mood, work, finance, and relationships. Current and past alcohol dependence was determined with the Structured Clinical Interview for DSM-IV Non-Patient Edition (First et al. 1995). Self-reported impulsivity was assessed via the UPPS Impulsive Behavior Scale which derives an overall impulsive behavior score from four factors: Urgency, Premeditation, Perseverance, and Sensation seeking (UPPS; Whiteside, et al., 2001).
Analytic Strategy
The first hypothesis, that working memory performance (TMT-B) after alcohol consumption would be associated with drinks per drinking day, was tested utilizing generalized estimating equations (GEE) to examine changes in working memory across beverage condition, with a normal distribution specified, along with an exchangeable working correlation matrix. The independent variables for the model were condition [within-subjects effect: placebo, low, or high alcohol, dummy coded with placebo as the reference group], and self-reported drinks per drinking day [between-subjects effect: (drinks coded as a linear effect)], as well as the two-way interaction (condition by drinks per drinking day). Baseline working memory was included as a covariate. In order to determine if observed effects were due to performance in working memory versus general impairment in cognitive performance after alcohol administration, we repeated the same model with a separate measure of cognitive performance (TMT-A) in place of our measure of working memory (TMT-B).
Following the GEE analyses, we tested whether change in working memory after alcohol consumption had significant direct and indirect effects on adverse consequences of alcohol use. Specifically, mediation analysis using bootstrapping with replacement (Preacher & Hayes, 2008) was utilized in order to estimate the indirect effects of alcohol induced working memory decline (as measured by TMT-B) on adverse consequences of alcohol (as measured by SIP total score) use via drinks per drinking day and number of drinking days (as measured by the TLFB), set as independent mediators. In this analysis, working memory decline score was calculated by subtracting the placebo condition working memory score from the working memory score measured after the high alcohol dose condition. Bivariate associations between variables identified as relevant in the extant literature and key outcome variables were examined; baseline working memory, past alcohol dependence, and impulsive behavior were included as covariates. Notably, Subjective Effects of Intoxication, was examined but omitted from subsequent analyses due to non-significant associations with variables of interest. Bias-corrected bootstrapping with 1000 bootstrap samples was chosen for its ability to maximize the power to detect mediation (Fritz & MacKinnon, 2007; Hayes & Scharkow, 2013; Preacher & Hayes, 2008). Mediation analyses were conducted using the SPSS PROCESS macro with bootstrapping which allows for non-normality (Preacher & Hayes, 2008). This modeling technique estimates simultaneous regression analyses and generates confidence intervals that correct for bias in estimating the indirect effects. An indirect effect is determined to be statistically significant if the confidence interval does not contain zero.
Results
Preliminary Analyses
Baseline working memory was not associated with drinks per drinking day, age, or past alcohol dependence. As detailed in Day et al. (2015), participants performed more poorly on TMT-B after the high dose of alcohol (B = 3.42, p = .01) compared to placebo, but not after the low dose versus placebo.
Experimental Analyses
Controlling for baseline working memory, the interaction between beverage condition and drinks per drinking day was significantly associated with change in working memory (as measured by TMT-B), B = −.691, SE = .3032, 95% CI = 1.285, .097, p = 0.023. Simple slope analysis indicated that lower working memory score in the high alcohol dose condition compared to placebo was associated with greater number of drinks per drinking day (1SD above mean); B = −4.807, SE = 1.645, 95% CI = −8.032, −1.581, p = 0.003 (See Table 1). This effect was not significant in the low dose alcohol condition. The interaction between beverage condition and drinks per drinking day was not associated with change in TMT-A performance, B = −.168, SE = .3173, 95% CI = −.790, .454, p = 0.597.
Table 1.
Effects of alcohol dose and drinks per drinking day on working memory performance.
| Parameter | B | Std. Error | Lower (95%Wald CI) | Upper (95% Wald CI) | p |
|---|---|---|---|---|---|
| Intercept | 17.950 | 7.1072 | 4.020 | 31.880 | .012 |
| Placebo vs. Low Dose Alcohol | −1.624 | 1.4513 | −4.468 | 1.221 | .263 |
| Placebo vs. High Dose Alcohol | −4.816 | 1.6477 | −8.046 | −1.587 | .003 |
| Baseline Working Memory | .748 | .1084 | .536 | .961 | .000 |
| Drinks per Drinking Day | .562 | .2077 | .155 | .969 | .053 |
| Low Dose x Drinks (1SD above) | −.256 | .2866 | −.818 | .306 | .372 |
| High Dose x Drinks (1SD above) | −.691 | .3032 | −1.285 | −.097 | .023 |
Dependent Variable = Working Memory Score (TMT-B standardized). Interaction terms are dummy coded with placebo as the reference group. Negative coefficients represent a decrease in working memory as compared to the reference group.
Mediation Analyses
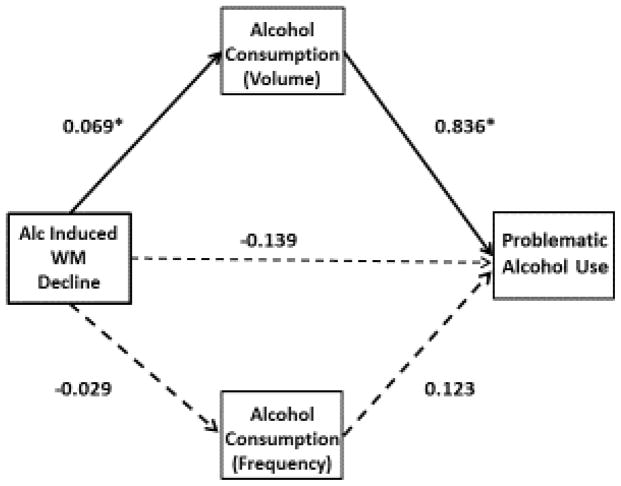
Next, we examined the indirect effects of alcohol-induced working memory decline on adverse consequences of alcohol use through reported drinking behaviors, controlling for the effects of baseline working memory, past alcohol dependence, and trait impulsivity. Consistent with our hypotheses, drinks per drinking day was a significant mediator of the relationship between alcohol induced working memory decline (baseline minus working memory after high alcohol dose), and adverse effects of alcohol (indirect effect = .025, SE = .02; 95% CI [0.0007, 0.1046]). Greater declines in working memory after alcohol consumption were associated with increased drinks per drinking day, which, in turn, was associated with increased adverse consequences of alcohol use. In order to examine the possibility that these effects were associated with alcohol consumption in general rather than acute effects of alcohol, we also included number of drinking days in the past month as a mediator in the model; drinking days was not a significant mediator of the relationship between alcohol induced working memory decline and adverse consequences of alcohol use (see Fig. 1).
Figure 1.
Dual mediator model of the relationship between alcohol-induced working memory decline and adverse consequences of alcohol use.
Indirect effects through Alcohol Consumption Volume = 0.025*, SE=0.020, 95%CI [0.0007, 0.1045] and Alcohol Consumption Frequency=−0.012, SE=0.047, 95%CI [−0.1026, 0.1028], controlling for baseline working memory, past alcohol dependence, and self-reported impulsivity.
*p < .05
Discussion
This study reports the first observed association, to our knowledge, between alcohol-induced changes in working memory performance, as assessed by TMT-B, and reported drinking behaviors. We followed this observation with an examination of the relationship between alcohol-induced working memory decline and adverse consequences of alcohol use through reported drinking behaviors. A significant indirect effect indicated that the relationship between greater alcohol-induced working memory decline and adverse consequences of alcohol use was mediated by drinks per drinking day, even when controlling for baseline working memory, alcohol dependence, and trait impulsivity. Additionally, we found that number of drinking days was not a significant mediator of the relationship, consistent with our expectations.
These results expand the literature by demonstrating a link between individual differences in changes in working memory after alcohol consumption and alcohol use behaviors and consequences; previous studies have examined only baseline working memory in relation to behaviors and consequences. These results are in line with previous reports indicating an association between individual differences in alcohol-induced changes in response inhibition and drinking behaviors (Weafer & Fillmore, 2008). It is important to note that the observed impairment in working memory only occurred in the high alcohol dose condition. It appears as though working memory in adult heavy drinkers is relatively unaffected at a lower dose (.4g/kg) of alcohol and that only changes in working memory after a higher dose relate to reported drinking behaviors and adverse consequences of drinking outside of the laboratory in this population. Taken together, these studies highlight the importance of examining individual differences in executive function after alcohol consumption rather than focusing on the effects of baseline executive functioning alone on subsequent alcohol use behaviors.
Furthermore, the current study separated the effects of drinks per drinking day and number of drinking days on adverse consequences of alcohol use. Examining these effects individually may provide useful information for selecting individualized interventions, as well as intervention development. Recent research has demonstrated that enhancing executive function through interventions such as working memory training results in decreased alcohol consumption (Houben, 2011). However, it is unclear if this decreased alcohol consumption occurs through decreased drinks per drinking occasion or decreased drinking occasions. This is an important distinction; working memory training might affect one’s ability to make decisions while sober, but may not protect individuals with a neuropharmacological predisposition toward alcohol-induced working memory decline. Moreover, it is possible that working memory performance after alcohol use may be a better indicator of the potential for problems related to the inability moderate alcohol consumption once initiated, whereas baseline working memory performance may be a better indicator of the potential for problems related to the decision to engage or not engage in a drinking session. Examining interventions such as working memory training as well as other intervention’s ability to curb alcohol induced working memory decline may be important in moderating drinks per drinking occasion – a variable intimately linked with the dangerous consequences of alcohol use.
Several important limitations apply to the current study. Due to the controlled laboratory environment, our findings on alcohol-induced changes in working memory may not generalize to natural drinking environments. It is also important to note that TMT-B may not be measuring working memory exclusively. TMT-B has been documented as measuring several specific constructs including attentional set shifting, cognitive control, and working memory (Langenecker et al., 2007; Rios et al., 2004). However, studies examining the constructs measured by TMT-B have found that performance on a separate task of working memory (when compared to other tasks of executive function) accounted for the greatest proportion of variance in TMT-B performance (Sanchez-Cubillo et al., 2009), and this is consistent with other reports (e.g., Crowe et al., 1998). Thus, while TMT-B has been used to measure several different cognitive constructs in the past, based on the evidence suggesting that the construct referred to as working memory accounts for the most variance in TMT-B, we used the term working memory for the purposes of the current study. Future studies might examine the extent to which other tasks of executive function (e.g., response inhibition, set-shifting) are impaired after alcohol use and how this might affect drinking behaviors. In addition, a third variable (e.g., genetics, family history) may contribute to both drinking more drinks per occasion and alcohol-induced working memory decline. The current study was conducted in a group of individuals who smoked between 10 and 30 cigarettes per day; it is possible that these results may not generalize to individuals who do not smoke regularly. Additionally, it is important to note that we cannot infer directionality in the current study. It is possible that individuals who drink more on drinking days may have incurred more accumulative neurodysregulating effects from these chronic repeated episodes of heavy alcohol consumption, which could make them more sensitive to acute alcohol-induced working memory decline. While the current study attempted to control for these effects by including past alcohol dependence as a covariate, the lack of prospective data precludes inference of direct causality. Lastly, the current sample consisted of heavy drinking adults with an average age of 39.2 years, most of whom (67%) have a history of alcohol dependence; it is possible that a younger sample with less history of alcohol use may produce different results. Thus, these findings set the stage for the next set of studies that could explore the mechanisms by which alcohol’s acute effects on executive functioning contribute to different types of alcohol-related consequences.
Future studies might examine these effects in vivo using Ecological Momentary Assessment to evaluate whether alcohol’s acute effects on executive function influence later choices to engage in risky or aggressive behavior. Future laboratory based studies may examine changes in working memory after alcohol consumption on subsequent drinks consumed in an alcohol self-administration paradigm (O’Malley, 2002), in order to more directly assess causality in the relationship.
Acknowledgments
This study was funded by the National Institute on Alcohol Abuse and Alcoholism, grant R01AA016978 to Dr. Kahler. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Footnotes
The authors have no financial relationship with the study sponsor, and no conflicts of interest to disclose.
References
- Albein-Urios N, Martinez-González JM, Lozano Ó, Clark L, Verdejo-García A. Comparison of impulsivity and working memory in cocaine addiction and pathological gambling: implications for cocaine-induced neurotoxicity. Drug Alcohol Depend. 2012;126:1–6. doi: 10.1016/j.drugalcdep.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Day AM, Kahler CW, Metrik J, Spillane NS, Tidey JW, Rohsenow DW. Working Memory Moderates the Association Between Smoking Urge and Smoking Lapse Behavior After Alcohol Administration in a Laboratory Analogue Task. 2014 doi: 10.1093/ntr/ntu259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AM, Kahler CW, Ahern DC, Clark US. Executive functioning in alcohol use studies: A brief review of findings and challenges in assessment. Current Drug Abuse Reviews. 2015;8:26–40. doi: 10.2174/1874473708666150416110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Townshend JM. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli. Psychopharmacology. 2004;176(3–4):353–361. doi: 10.1007/s00213-004-1906-7. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. Acute effects of alcohol and other drugs on automatic and intentional control. In: Wiers RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Thousand Oaks, CA: Sage; 2006. pp. 293–306. [Google Scholar]
- Finn PR, Hall E. Cognitive ability and risk for alcoholism: Short-term memory capacity and intelligence moderate personality risk for alcohol problems. Journal of Abnormal Psychology. 2004;113:569–581. doi: 10.1037/0021-843X.113.4.569. [DOI] [PubMed] [Google Scholar]
- Fritz MS, MacKinnon DP. Required sample size to detect the mediated effect. Psychological Science. 2007;18:233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot CR, Fanning JR, Bullock JS, McCloskey MS, Berman ME. Effects of alcohol on tests of executive functioning in men and women: a dose response examination. Exp Clin Psychopharmacol. 2010;18(5):409–417. doi: 10.1037/a0021053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Scharkow M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: Does method really matter? Psychological Science. 2013;24(10):1918–1927. doi: 10.1177/0956797613480187. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Metrik J, Spillane NS, et al. Acute effects of low and high dose alcohol on smoking lapse behavior in a laboratory analogue task. Psychopharmacology. 2014;231:4649–4657. doi: 10.1007/s00213-014-3613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, McNamara PA, Hasin DS, Cao D. Alcohol Challenge Responses Predict Future Alcohol Use Disorder Symptoms: A 6-Year Prospective Study. Biol Psychiatry. 2014;75:798–806. doi: 10.1016/j.biopsych.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Patock-Peckham JA, Potenza MN. Impaired control over alcohol use: An under-addressed risk factor for problem drinking in young adults? Experimental and Clinical Psychopharmacology. 2012;20(2):92–106. doi: 10.1037/a0026463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Abroms BD, Van Selst M, Fillmore MT. Alcohol-induced impairment of behavioral control: differential effects on engaging vs. disengaging responses. Psychopharmacology. 2005;182:452–459. doi: 10.1007/s00213-005-0116-2. [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS, Longabaugh R. Test Manual. Vol. 4. U.S. Government Printing Office; Washington, D.C: 2005. The drinker inventory of consequences: An instrument for assessing adverse consequences of alcohol abuse. NIAAA Project MATCH Monograph Series. 1995. Report No.: NIH Publication No. 395–3911. [Google Scholar]
- Montgomery C, Fisk JE, Murphy PN, Ryland I, Hilton J. The effects of heavy social drinking on executive function: A systematic review and meta-analytic study of existing literature and new empirical findings. Human Psychopharmacology: Clinical and Experimental. 2012;27(2):187–199. doi: 10.1002/hup.1268. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Puttler LI, Adams KM, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Peeters M, Monshouwer K, Janssen T, Wiers RW, Vollebergh WA. Working Memory and Alcohol Use in At-Risk Adolescents: A 2-Year Follow-Up. Alcoholism: Clinical and Experimental Research. 2014;38(4):1176–1183. doi: 10.1111/acer.12339. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology. 2002;160(1):19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. Category test and trail making test as measures of frontal lobe functions. Clinical Neuropsychologist. 1995;9(1):50–56. [Google Scholar]
- Rose AK, Jones A, Clarke N, Christiansen P. Alcohol-induced risk taking on the BART mediates alcohol priming. Psychopharmacology. 2014;231(11):2273–80. doi: 10.1007/s00213-013-3377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Cubillo I, Periáñez JA, Adrover-Roig D, Rodríguez-Sánchez JM, Ríos-Logo M, Tirapu J, Barceló F. Construct validity of the trail making test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc. 2009;15:438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A calendar method for assessing alcohol and drug use. Addiction Research Foundation; Toronto, Canada: 1996. [Google Scholar]
- Wagner S, Helmreich I, Dahmen N, Lieb K, Tadić A. Reliability of three alternate forms of the trail making tests a and B. Archives of Clinical Neuropsychology. 2011;26(4):314–321. doi: 10.1093/arclin/acr024. [DOI] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Individual differences in acute alcohol impairment of inhibitory control predict ad libitum alcohol consumption. Psychopharmacology. 2008;201(3):315–324. doi: 10.1007/s00213-008-1284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LK, Hopkins RO, Chan KJ, Churchill S, Elliott CG, Clemmer TP, Orme JF, Jr, Thomas FO, Morris AH. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347:1057–1067. doi: 10.1056/NEJMoa013121. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. doi: 10.1016/S0191-8869(00)00064-7. [DOI] [Google Scholar]