Abstract
Bacteriophage genomes contain an abundance of genes that code for hypothetical proteins with either a conserved domain or no predicted function. The Caulobacter phage CbK has an unusual shape, designated morphotype B3 that consists of an elongated cylindrical head and a long flexible tail. To identify CbK proteins associated with the phage particle, intact phage particles were subjected to SDS-PAGE, and the resulting protein bands were digested with trypsin, and analyzed using MALDI mass spectroscopy to provide peptide molecular weights. These peptide molecular weights were then compared with the peptides that would be generated from the predicted amino acid sequences that are coded by the CbK genome, and the comparison of the actual and predicted peptide masses resulted in the identification of single genes that could code for the set of peptides derived from each of the 20 phage proteins. We also found that CsCl density gradient centrifugation resulted in the separation of empty phage heads, phage heads containing material organized in a spiral, isolated phage tails, and other particulate material from the intact phage particles. This additional material proved to be a good source of additional phage proteins and preliminary results suggest that it may include a CbK DNA replication complex.
Introduction
Caulobacter crescentus is a Gram-negative, oligotrophic bacterium commonly found in freshwater and soils. It plays an important role in global carbon cycling by metabolizing dissolved organic materials. Since the genus Caulobacter is ubiquitous in the environment and bears the strongest known biological adhesive on its stalk-like polar appendage, stalked Caulobacter cells can attach themselves to a solid substrate, coating the substrate as a single layer biofilm, thereby performing remediation of natural water bodies and industrial wastewater [11].
In 1977, Johnson, Wood, and Ely [9] described a collection of bacteriophages that infect C. crescentus. Approximately three-fourths of these bacteriophages had an unusual shape similar to that of the previously characterized CbK bacteriophage. This unusual shape, designated morphotype B3, included an elongated cylindrical head and a long flexible tail [2]. Bacteriophages with the B3 morphotype are rare, comprising only 1.2% of the total number of characterized phages [1]. Since the B3 phages that infect Caulobacter comprise more than half of all the known B3 phages, bacteriophage CbK is an excellent starting point for studies aimed at understanding the reasons for the elongated head structure that is the hallmark of B3 phage morphology.
With genome sizes that are in excess of 200 kb, CbK and related C. crescentus bacteriophages are the largest of the B3 phages [6]. More recent studies have shown that the first interaction of CbK with its host is the attachment to the bacterial flagellum via a filament present at the top of the phage head [7]. The bacteriophage slides along the flagellum using this head filament until its tail can make contact near the base of the flagellum [7]. Contact with the flagellum facilitates the aggregation of viral particles around the receptor (pilus portals) on the C. crescentus bacterial cell surface. Once the phage tail makes contact with the cell surface, phage DNA is injected into the cytoplasm of the host cell. Inside the host cell, viral replication and virion assembly occurs before cell lysis releases the newly formed phage [2, 10]. The nucleotide sequence of the CbK genome has been determined [6, 12], but at the time this study was initiated, none of the phage structural proteins had been linked to the corresponding genes. Thus, the goal of this study was to identify proteins associated with the CbK phage particles and match them to the genes that code for them.
Materials and Methods
Growth Conditions
C. crescentus strain CB15, used a host for bacteriophage CbK, was cultured in a nutrient broth (PYE) containing 0.2% peptone, 0.1% yeast extract, 0.5 mM CaCl2, and 0.8 mM MgSO4 in distilled water [8]. For maximum growth of CbK, 2.5 ml of a fresh CB15 overnight culture was added to 250 ml of PYE broth in a 2000 ml Erlenmeyer flask and incubated at 4°C. After 24 hours, approximately 250 µl of bacteriophage CbK (109 plaque forming units) was added to the flask, and the culture was incubated at 30°C on a rotary shaker for 23 hours. The lysate was titered with C. crescentus CB15 in a PYE soft agar (0.3% agar) overlay, producing tiny plaques (~1 mm in diameter). Maximum titers obtained in this manner were in excess of 1011 phage/ml.
CbK Purification and Concentration
Bacteriophage CbK particles were concentrated and purified from 250 mL crude lysates previously grown with titers of >1011 phage/ml by combination of low-speed and high-speed centrifugation. Initially, the phage particles (250 mL) were purified by a series of centrifugations in 15 mL Falcon tubes at 5200 × g for 10 minutes at 4°C to remove bacteria and host debris. The final pooled supernatant (200 mL) was then spun in four tubes at 56,000 × g for 4.5 hours at 4°C to pellet the phage particles. The resulting concentrated CbK phage pellet was resuspended in a total of 500 µl TE (10 mM Tris-HCl, 1 mm EDTA). CsCl gradient purification was accomplished by layering 2 ml of a 1.45 g/ml CsCl solution on top of 3 ml of a 1.7 g/ml CsCl solution and subsequently layering 1 ml of the concentrated CbK phage preparation on top of the CsCl solutions. The centrifuge tube was then sealed and spun at 45,000 × g for 2 hr. The phage particles formed a band between the two CsCl layers, and the other phage components formed a second band just above the top CsCl layer. One ml volumes containing these bands were extracted from the gradient by carefully piercing the centrifuge tube just below the band using a syringe needle and removing the material above the syringe.
SDS PAGE
After concentration, 20 µl of the resuspended CbK phage pellet was combined with 10 µl of 3× SDS loading buffer (150 mM Tris-HCl (pH 7.5), 30% glycerol, 6% SDS, a trace of bromophenol blue) and heated at 100°C for 2 minutes. In each lane of either a 7.5% or 12% (8.6 × 6.7 × 0.1 cm) acrylamide gel, 25 µl of the heated phage sample was loaded and electrophoresed at 150 V in Tris-glycine-SDS buffer. Protein molecular weight standards were Prestained Protein Broad Range Ladder (10–230 kD) (New England BioLabs, Ipswich, MA) and BSA (bovine serum albumin). The gels were stained in 500 ml of 0.5 µg/ml Coomassie blue R-250 overnight at room temperature and placed in a destain solution (5% methanol, 7% glacial acetic acid) the following day for two hours at room temperature to visualize the protein bands.
Trypsin Digestion
Using a scalpel, bands of interest were excised from the Coomassie blue stained gels. Gel bands (one per tube for most bands, three per tube for the lightly stained bands marked with an asterisk) were placed in 50 µl of a second destaining solution (50% acetonitrile, 25 mM ammonium bicarbonate) for a total of one hour to totally remove the Coomassie blue stain. The protein gel bands were then incubated with 50 µl of 10 mM DTT for 30 minutes to eliminate disulfide bonds. After the DTT solution was removed with a pipette, the proteins were alkylated with 50 µl of 50 mM iodoacetamide in the dark for another 30 minutes to prevent cysteine residues from recombining. The gel bands were then washed with 50 µl of 100 mM ammonium bicarbonate for 10 minutes, dehydrated with 50 µl of 100% acetonitrile for 5 minutes, and rehydrated again with 50 µl of 100 mM ammonium bicarbonate for 10 minutes. This process of dehydration and rehydration was repeated two more times to remove the contaminants (salts) associated with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS), and then the gel bands were dried in a SpeedVac for 3 minutes. The dried gel bands were rehydrated with 30 µl of a trypsin solution (1 µl of 12.5 ng/µl trypsin [Trypsin Gold, Mass Spectrometry Grade, Promega, Madison, WI]), diluted with 40 µl of 40 mM ammonium bicarbonate containing 9% acetonitrile) and incubated at 35°C for 15 hours to cleave the protein at arginine and lysine residues. The reaction was stopped by adding 2 µl of 5% formic acid with 100 µl water. The resulting peptides in the gel bands were extracted by a series of three more centrifugations at 10,000 rpm for 5 minutes, transferring the supernatants to a tube containing 5 µl of 50% acetonitrile and 5% formic acid. The combined peptide solution was concentrated by evaporation under vacuum pressure and passed through a ZipTip C18 (Millipore, Billerica, MA) to remove metal ions and further concentrate the solution. Each sample was cycled in and out of a single Ziptip 15 times to load the maximum amount of peptide onto the column. The ZipTips loaded with protein were washed 5 times with the water/formic acid solution, followed by elution with a 700/290/10 acetonitrile/water/88% formic acid solution. The sample was then analyzed via MALDI MS.
MALDI-TOF MS
A 1 µl aliquot of the eluted the tryptic peptides was mixed with 1 µl of a CHCA matrix solution (0.02–0.03 M alpha-cyano-4-hydroxy-cinnamic acid in 0.1% TFA/acetonitrile 1:2) and deposited on a polished stainless steel target MALDI plate and allowed to crystallize. Mass spectra were acquired using a Bruker Ultraflex 1 (Bruker Daltonics, Billerica, MA) in the positive ion delayed extraction reflector for MS. The predicted amino acid sequences of each of the proteins encoded by the CbK genome were analyzed using Protein Prospector (http://prospector.ucsf.edu) to predict the peptide masses that would result from a trypsin digestion of each protein amino acid sequence in the genome. The predicted peptide masses from Protein Prospector were then compared to the mass spectrum for each excised protein to identify the CbK gene that codes for that protein. CbK gene designations correspond to those used by Gill et al. [6]. Generally the 4 to 6 peptide masses obtained from a single protein matched the predicted peptides from a single CbK gene. The predicted amino acid sequence of the matching gene was then compared to the NCBI database using the BLAST search program to determine if genes coding for homologous proteins were present in other genomes that had been submitted to the database.
Results
MALDI analysis of the most abundant phage protein resulted in five peptides (with molecular weights: 1428, 1542, 1685, 2078, 2842) that were identical to those predicted by Protein Prospector for only one of the CbK genes, gp068. A BLAST comparison to the GenBank database revealed that the gene matches other phage genes that code for major capsid proteins, the primary structural component of the phage head. Thus, this method matched the gp068 protein to a single gene.
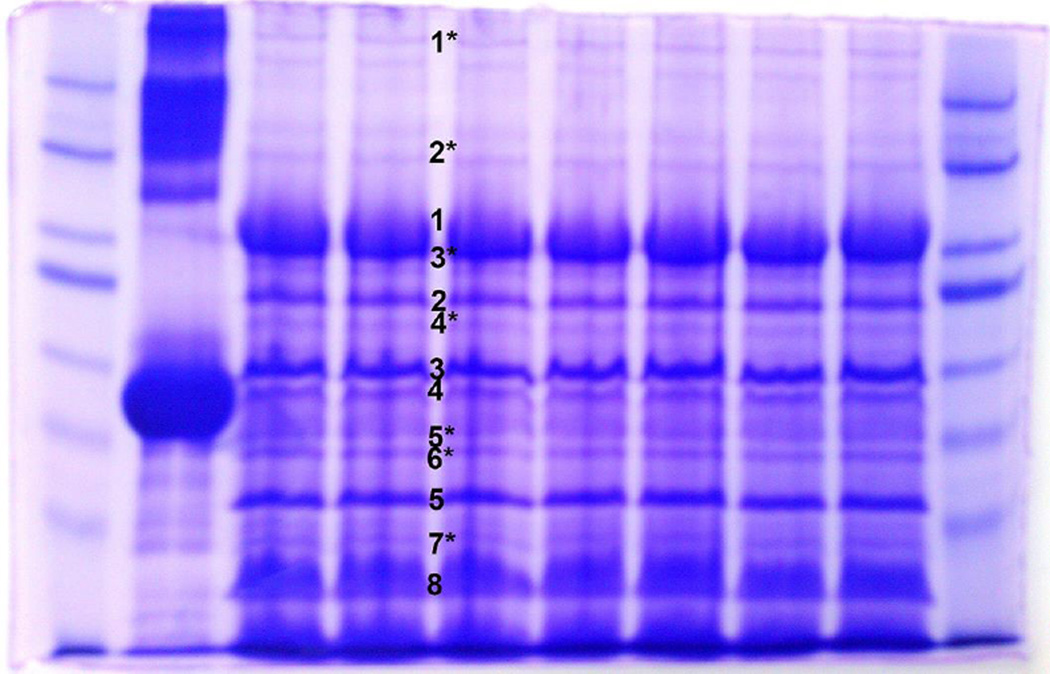
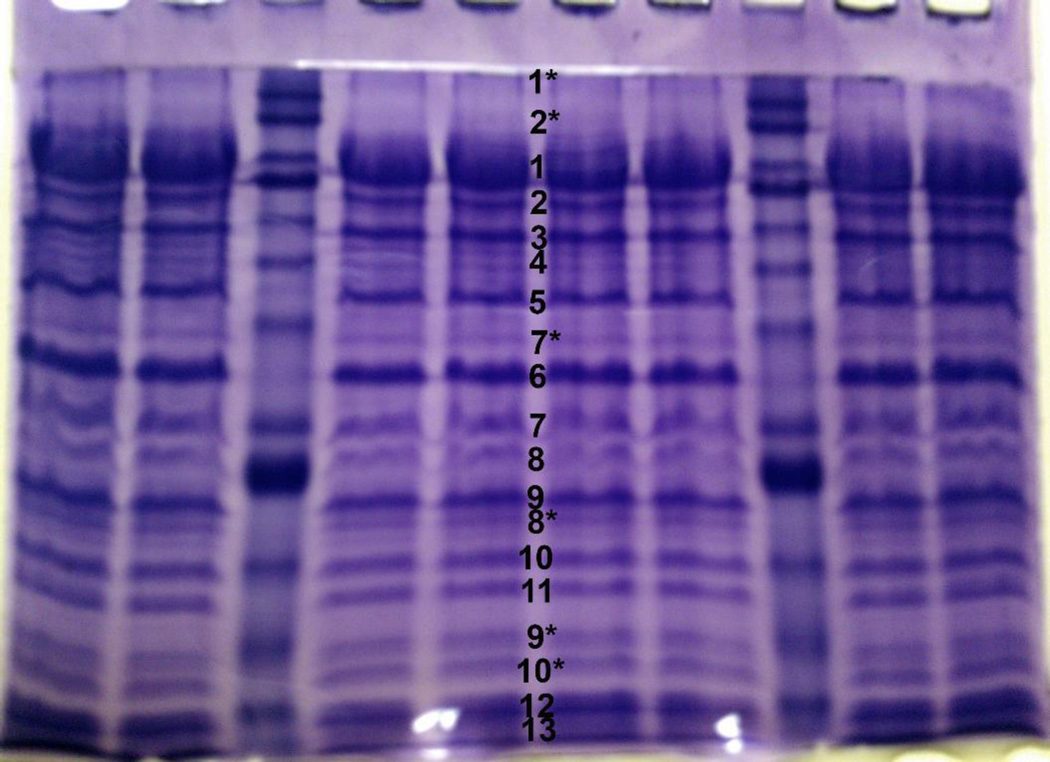
Applying this method to additional phage protein bands resolved by the 7.5% and 12% gels (Figures 1 and 2), the proteins in 20 different gel bands were uniquely matched to 20 different CbK genes (Table 1). Of those 20 proteins, three matched proteins with known structural functions, the major capsid protein (gp68), a portal protein (gp42), and a tail fiber protein (gp101) that have been reported previously by Gill et al. [6]. We also identified the large terminase subunit TerL (gp318) which interacts with the portal protein and gp198 which contains an HNH nuclease domain that could be involved in DNA packaging as well. In addition to the structural proteins, three enzymes involved in DNA replication or repair, a T7-like PolI DNA polymerase (gp123), a ribonucleotide-diphosphate reductase beta subunit (gp111), and a DHH phosphoesterase protein (gp60) were identified (Table 1). Other proteins included a nicotinate phosphoribosyl transferase (gp161), an rIIb-like protein (gp137), transcription termination factor Rho (gp119), and an HD-domain/PDEase-like protein (gp057). The remaining proteins we detected either did not match any other proteins in the GenBank database or only contained a conserved domain. However, every band we tested corresponded to a single predicted protein coded by a single Cbk gene.
Figure 1.
A Coomassie stained 7.5% SDS-PAGE gel showing proteins associated with the CbK phage particle. The (*) indicates bands that were too faint to analyze using only one band so bands from three gel lanes were used for the MALDI analysis.
Figure 2.
A Coomassie stained 12% SDS-PAGE gel showing proteins associated with the CbK phage particle. The (*) indicates bands that were too faint to analyze using only one band so bands from three gel lanes were used for the MALDI analysis.
Table 1.
Proteins associated with the CbK phage particles.
| Gel band | MW (kD) | CbK gene | Annotation |
|---|---|---|---|
| 1* | 270 | gp270 | Putative lectin-like domain protein |
| 2* | 149 | gp101 | Tail fiber protein |
| 1 | 102 | gp318 | Terminase large subunit |
| 3* | 86 | gp123 | T7-like Pol I DNA polymerase |
| 2 | 75 | gp273 | Conserved phage protein |
| 4* | 68 | gp42 | Portal protein |
| 3 | 59 | gp137 | Putative rIIb-like protein |
| 4 | 55 | gp111 | Ribonucleoside diphosphate reductase beta subunit |
| 5* | 52 | gp161 | Nicotinate phosphoribosyltransferase |
| 6* | 46 | gp164 | Hypothetical protein |
| 5 | 41.5 | gp119 | Transcription termination factor Rho |
| 7* | 39 | gp60 | DHH phosphoesterase protein |
| 6 | 36.5 | gp68 | Major capsid protein |
| 7 | 30 | gp180 | Core domain of the SPFH superfamily |
| 8 | 27 | gp198 | HNH nuclease domain |
| 9 | 25.5 | gp057 | HD-domain/PDEase-like protein |
| 10 | 21 | gp048 | Hypothetical protein |
| 11 | 19 | gp242 | Hypothetical protein |
| 12 | 13.5 | gp195 | Hypothetical protein |
| 13 | 10 | gp303 | Hypothetical protein |
Indicates that the band was a lightly staining band and that three bands were combined for the MALDI MS analysis.
While this work was in progress, Gill et al. published the identification of proteins associated with the CbK phage particle using a similar procedure [6]. They were able to identify peptides that were correlated to the predicted amino acid sequences of nine CbK genes. As described above, we found three of these proteins, the major capsid protein (gp068), a tail fiber protein (gp101), and a portal protein (gp042). However, we did not find peptides from the other six proteins. One difference between our procedures was that Gill et al. included a CsCl density gradient centrifugation as an additional purification step [6]. To determine the effect of the CsCl purification step, we prepared a new phage lysate and further purified half of the lysate with CsCl density gradient centrifugation. After the isopycnic equilibrium step two bands were observed, one at a density expected for DNA-containing phage particles and a second band with a density that was less than 1.45 g/ml. SDS-PAGE of the CsCl phage resulted in a pattern that was similar to that published by Gill et al. [6] indicating that many of the proteins that we had identified were not associated with the intact phage particles after CsCl purification. For example we found that a heavy band corresponding to approximately 60 kD was greatly reduced after the CsCl purification. When we analyzed the protein peptides derived from this band, we found peptides corresponding to a CbK rIIb-like protein (gp137). In contrast, Gill et al. were able to identify two phage tail protein bands in this region [6]. Thus, the abundant rIIb-like protein may have masked the presence of the tail proteins. We also observed that several of the proteins in the 15 to 25 kD size range were lost during the CsCl purification.
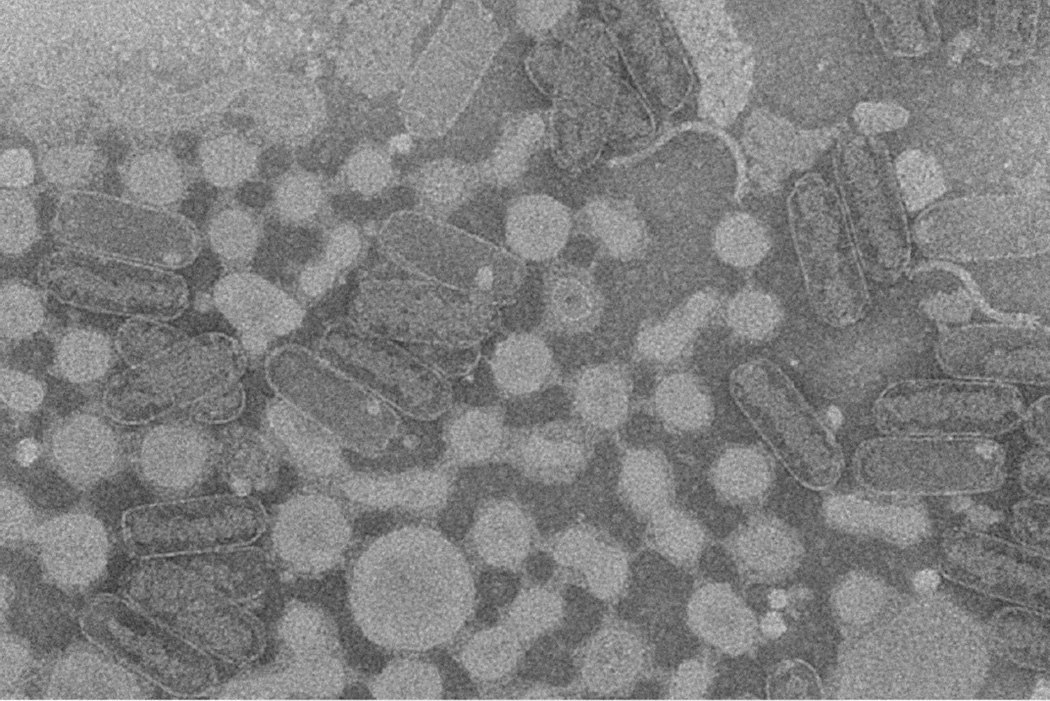
To determine what was in the low density band, we diluted it with two volumes of distilled water and spun the mixture at 85,000 × g for 2.5 h. An electron micrograph of the resulting pellet revealed an assemblage of empty phage heads, phage heads containing material organized in a spiral, isolated phage tails, and other particulate material (Figure 3). Therefore it is clear that some phage components that lack DNA co-sediment with the phage particles during high speed centrifugation, but they can be separated from the intact phage particles because of their different density.
Figure 3.
An electron micrograph showing empty phage heads, phage heads containing material organized in a spiral, isolated phage tails, and other particulate material separated from the intact phage by CsCl density gradient centrifugation.
Discussion
We have identified 20 genes in the ~200 kD bacteriophage CbK genome that code for proteins found in partially purified phage lysates. Five of these proteins, the major capsid protein (gp068), a tail fiber protein (gp101), a portal protein (gp042), the large terminase subunit and gp198, are likely to be phage capsid components. The remaining 15 proteins were associated with particulate material that could be separated from the intact phage by CsCl density gradient centrifugation and included an rIIB-like protein and several proteins involved in a DNA replication. Therefore, we propose that these phage proteins form complexes that can be isolated by high speed centrifugation of phage lysates. One of these complexes may be similar to a protein complex that was isolated by Chiu et al. from cell extracts of Escherichia coli during bacteriophage T4 infection [3]. The T4 protein complex containing T4 DNA polymerase, ribonucleotide reductase, the RIIA and B subunjts and several other proteins, was thought to convert ribonucleotides to deoxyribonucleotides so that they could be used for DNA replication. Since we also found DNA polymerase, ribonucleotide reductase, and an RIIB-like subunit in our particulate fraction, we propose that CbK infection also results in a protein complex that converts ribonucleotides to deoxyribonucleotides to facilitate DNA replication.
Half of the phage proteins that we identified in this study were considered hypothetical in the CbK genome annotations in the NCBI database since the corresponding genes did not code for proteins of known function. Thus, the analysis of proteins from partially purified phage that we performed is a way to identify non-structural proteins that are associated with partially-purified phage lysates. Some of these proteins may be part of a DNA replication protein complex. Others such as the HD-domain/PDEase-like protein (gp057) and the DHH phosphoesterase protein (gp066) may be associated with partially formed phage head structures since the genes that code for these proteins are located in a cluster containing other phage head proteins. Also, the spirals observed in some of the phage heads shown in Figure 3 may involve a CbK scaffolding protein. Scaffolding proteins are often required for the assembly of phage capsid proteins into the phage head structure, and electron micrographs have visualized the internal structure of the scaffolding proteins [13]. The scaffolding proteins appear to form a circular structure inside the developing phage head. However, these studies were done on phages that have icosahedral heads. Since CbK has an elongated head structure, it would be reasonable for the scaffolding proteins to form a spiral structure along the long axis of the head. Follow up experiments should allow us to purify the individual components of the particulate fraction, identify additional CbK proteins, and begin to elucidate the role of these proteins during phage infection. The scaffolding protein would be expected to be an abundant protein [4] so it should be readily identified.
Acknowledgements
This work was supported by National Science Foundation grant EF-0826792 and Public Health Service grants GM066526 and GM076277.
We thank Carlton Bequette and Kurt Ash for their advice and support.
References
- 1.Ackermann HW. Frequency of morphological phage descriptions in the year 2000. Arch Virol. 2001;146:843–857. doi: 10.1007/s007050170120. [DOI] [PubMed] [Google Scholar]
- 2.Agabian-Keshishian N, Shapiro L. Stalked bacteria: properties of deoxyribonucleic acid bacteriophage ɸCbK. J Virol. 1970;5:795–800. doi: 10.1128/jvi.5.6.795-800.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu CS, Cook KS, Greenberg GR. Characteristics of a bacteriophage T4-induced complex synthesizing deoxyribonucleotides. J Biol Chem. 1982;267:15087–15097. [PubMed] [Google Scholar]
- 4.Dai W, Fu C, Raytcheva D, Flanagan J, Khant HA, Liu X, Rochat RH, Hasse-Pettingell C, Piret J, Ludtke SJ, Nagayam K, Schmid MF, King JA, Chiu W. Visualizing virus assembly intermediates inside marine bacteria. Nature. 2003;502:707–710. doi: 10.1038/nature12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyer L, Pantůček R, Zdráhal Z, Konečná H, Kašpárek P, Růžičková V, Hernychová L, Preisler J, Doškař J. Structural protein analysis of the polyvalent staphylococcal bacteriophage 812. Proteomics. 2007;7:64–72. doi: 10.1002/pmic.200600280. [DOI] [PubMed] [Google Scholar]
- 6.Gill JJ, Berry JD, Russell WK, Lessor L, Escobar-Garcia DA, Hernandez D, Kane A, Keene J, Maddox M, Martin R, Mohan S, Thorn AM, Russell DH, Young R. The Caulobacter crescentus phage phiCbK: Genomics of a canonical phage. BMC Genomics. 2012;13:542. doi: 10.1186/1471-2164-13-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerrero-Ferreira RC, Viollier PH, Ely B, Poindexter JS, Georgieva M, Jensen GJ, Wright ER. Alternative mechanism for bacteriophage adsorption to the motile bacterium Caulobacter crescentus. Proc Nat Acad Sci USA. 2011;108:9963–9968. doi: 10.1073/pnas.1012388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson R, Ely B. Isolation of spontaneously derived mutants of Caulobacter crescentus. Genetics. 1977;86:25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson RC, Wood NB, Ely B. Isolation and characterization of bacteriophages for Caulobacter crescentus. J Gen Virol. 1977;37:323–335. [Google Scholar]
- 10.Lagenaur C, Farmer S, Agabian N. Adsorption properties of stage-specific Caulobacter phage ɸCbK. Virol. 1977;77:401–407. doi: 10.1016/0042-6822(77)90436-6. [DOI] [PubMed] [Google Scholar]
- 11.MacRae JD, Smit J. Characterization of caulobacters isolated from wastewater treatment systems. Appl Environ Microbiol. 1991;57:751–758. doi: 10.1128/aem.57.3.751-758.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panis G, Lambert C, Viollier PH. Complete genome sequence of Caulobacter crescentus bacteriophage ϕCbK. J Virol. 2012;86(18):10234–10235. doi: 10.1128/JVI.01579-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prevelige PE, Thomas D, King J. Scaffolding protein regulates the polymerization of P22 coat subunits into icosahedral shells in vitro. J Mol Biol. 1988;202:743–757. doi: 10.1016/0022-2836(88)90555-4. [DOI] [PubMed] [Google Scholar]
- 14.Thomas JA, Rolando MR, Carroll CA, Shen PS, Belnap DM, Weintraub ST, Serwer P, Hardies SC. Characterization of Psuedomonas chlororaphis myovirus 201ɸ2-1 via genomic sequencing, mass spectrometry, and electron microscopy. Virol. 2008;376:330–338. doi: 10.1016/j.virol.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]