Graphical abstract
Keywords: Silver nanoparticles, Plant extract, Green synthesis, Antimicrobial
Abstract
Metallic nanoparticles are being utilized in every phase of science along with engineering including medical fields and are still charming the scientists to explore new dimensions for their respective worth which is generally attributed to their corresponding small sizes. The up-and-coming researches have proven their antimicrobial significance. Among several noble metal nanoparticles, silver nanoparticles have attained a special focus. Conventionally silver nanoparticles are synthesized by chemical method using chemicals as reducing agents which later on become accountable for various biological risks due to their general toxicity; engendering the serious concern to develop environment friendly processes. Thus, to solve the objective; biological approaches are coming up to fill the void; for instance green syntheses using biological molecules derived from plant sources in the form of extracts exhibiting superiority over chemical and/or biological methods. These plant based biological molecules undergo highly controlled assembly for making them suitable for the metal nanoparticle syntheses. The present review explores the huge plant diversity to be utilized towards rapid and single step protocol preparatory method with green principles over the conventional ones and describes the antimicrobial activities of silver nanoparticles.
Introduction
Nanotechnology is an important field of modern research dealing with synthesis, strategy and manipulation of particle’s structure ranging from approximately 1 to 100 nm in size. Within this size range all the properties (chemical, physical and biological) changes in fundamental ways of both individual atoms/molecules and their corresponding bulk. Novel applications of nanoparticles and nanomaterials are growing rapidly on various fronts due to their completely new or enhanced properties based on size, their distribution and morphology. It is swiftly gaining renovation in a large number of fields such as health care, cosmetics, biomedical, food and feed, drug-gene delivery, environment, health, mechanics, optics, chemical industries, electronics, space industries, energy science, catalysis, light emitters, single electron transistors, nonlinear optical devices and photo-electrochemical applications. Tremendous growth in these expanding technologies had opened applied frontiers and novel fundamentals. This includes the production of nanoscale materials afterwards in investigation or utilization of their mysterious physicochemical and optoelectronic properties [1], [2], [3].
The nanoparticles used for all the aforesaid purposes, the metallic nanoparticles considered as the most promising as they contain remarkable antibacterial properties due to their large surface area to volume ratio, which is of interest for researchers due to the growing microbial resistance against metal ions, antibiotics and the development of resistant strains [2]. Among the all noble metal nanoparticles, silver nanoparticle are an arch product from the field of nanotechnology which has gained boundless interests because of their unique properties such as chemical stability, good conductivity, catalytic and most important antibacterial, anti-viral, antifungal in addition to anti-inflammatory activities which can be incorporated into composite fibres, cryogenic superconducting materials, cosmetic products, food industry and electronic components. [4], [5]. For biomedical applications; being added to wound dressings, topical creams, antiseptic sprays and fabrics, silver functions’ as an antiseptic and displays a broad biocidal effect against microorganisms through the disruption of their unicellular membrane thus disturbing their enzymatic activities.
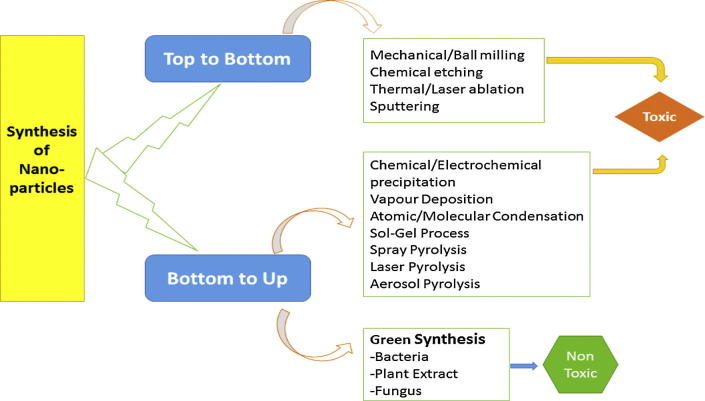
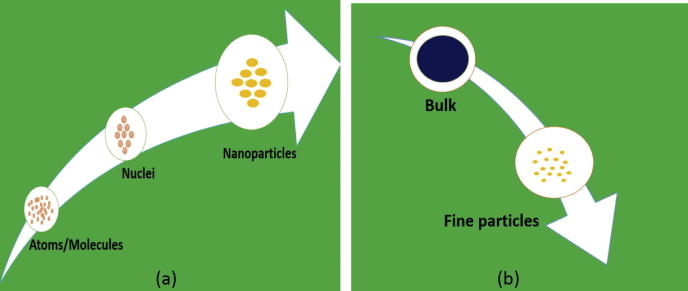
Synthesis of silver nanoparticles is of much interest to the scientific community because of their wide range of applications. These silver nanoparticles are being successfully used in the cancer diagnosis and treatment as well [6], [7]. Generally, nanoparticles are prepared by a variety of chemical and physical methods which are quite expensive and potentially hazardous to the environment which involve use of toxic and perilous chemicals that are responsible for various biological risks. The development of biologically-inspired experimental processes for the syntheses of nanoparticles is evolving into an important branch of nanotechnology. Generally there are two approaches which are involved in the syntheses of silver nanoparticles, either from “top to bottom” approach or a “bottom to up” approach (Fig. 1). In bottom to top approach, nanoparticles can be synthesized using chemical and biological methods by self-assemble of atoms to new nuclei which grow into a particle of nanoscale as shown in Fig. 2.a while in top to bottom approach, suitable bulk material break down into fine particles by size reduction with various lithographic techniques e.g. grinding, milling, sputtering and thermal/laser ablation. (Fig. 1, Fig. 2b).
Fig. 1.
Different approaches of synthesis of silver nanoparticles.
Fig. 2.
Protocols employed for synthesis of nanoparticles (a) bottom to top approach and (b) top to bottom approach.
In bottom to top approach, chemical reduction is the most common scheme for syntheses of silver nanoparticles [8], [9]. Different organic and inorganic reducing agents, such as sodium borohydride (NaBH4), sodium citrate, ascorbate, elemental hydrogen, Tollen’s reagent, N,N-dimethyl formamide (DMF) and poly (ethylene glycol) block copolymers are used for reduction of silver ions (Ag+) in aqueous or non-aqueous solutions [10], [11]. Capping agents are also used for size stabilization of the nanoparticles. One of the biggest advantages of this method is that a large quantity of nanoparticles can be synthesized in a short span of time. During this type of syntheses; chemicals used are toxic and led to non-eco-friendly by-products. This may be the reason which leads to the biosyntheses of nanoparticles via green route that does not employ toxic chemicals and hence proving to become a growing wanton want to develop environment friendly processes. Thus, the advancement of green syntheses of nanoparticles is progressing as a key branch of nanotechnology; where the use of biological entities like microorganisms, plant extract or plant biomass for the production of nanoparticles could be an alternative to chemical and physical methods in an eco-friendly manner [12].
In case of top to bottom approach; nanoparticles are generally synthesized by evaporation–condensation using a tube furnace at atmospheric pressure. In this method the foundation material; within a boat; place centred at the furnace is vaporized into a carrier gas. Ag, Au, PbS and fullerene nanoparticles have previously been produced using the evaporation/condensation technique. The generation of silver nanoparticles using a tube furnace has numerous drawbacks as it occupies a large space and munches a great deal of energy while raising the environmental temperature around the source material, and it also entails a lot of time to succeed thermal stability [13], [14], [15], [16], [17]. In addition; a typical tube furnace requires power using up of more than several kilowatts and a pre-heating time of several tens of minutes to attain a stable operating temperature. One of the biggest limitations in this method is the imperfections in the surface structure of the product and the other physical properties of nanoparticles are highly dependent on the surface structure in reference to surface chemistry.
In general, whatever the method is followed, it is generally concluded that the chemical methods have certain limitations with them either in the form of chemical contaminations during their syntheses procedures or in later applications. Yet; one cannot deny their ever growing applications in daily life. For instances; “The Noble Silver Nanoparticles” are striving towards the edge-level utilities in every aspect of science and technology including the medical fields; thus cannot be neglected just because of their source of generation. Due to their medicinal and antimicrobial properties, silver nanoparticles have been incorporated into more than 200 consumer products, including clothing, medicines and cosmetics. Their expanding applications are putting together chemists, physicist, material scientist, biologists and the doctors/pharmacologists to continue their latest establishments. Hence, it is becoming a responsibility of every researcher to emphasize on an alternate as the synthetic route which is not only cost effective but should be environment friendly in parallel. Keeping in view of the aesthetic sense, the green synthesis is rendering itself as a key procedure and proving its potential at the top.
The advancement of green syntheses over chemical and physical methods is: environment friendly, cost effective and easily scaled up for large scale syntheses of nanoparticles, furthermore there is no need to use high temperature, pressure, energy and toxic chemicals [18]. A lot of literature has been reported to till date on biological syntheses of silver nanoparticles using microorganisms including bacteria, fungi and plants; because of their antioxidant or reducing properties typically responsible for the reduction of metal compounds in their respective nanoparticles. Although; among the various biological methods of silver nanoparticle synthesis, microbe mediated synthesis is not of industrial feasibility due to the requirements of highly aseptic conditions and their maintenance. Therefore; the use of plant extracts for this purpose is potentially advantageous over microorganisms due to the ease of improvement, the less biohazard and elaborate process of maintaining cell cultures [19]. It is the best platform for syntheses of nanoparticles; being free from toxic chemicals as well as providing natural capping agents for the stabilization of silver nanoparticles. Moreover, use of plant extracts also reduces the cost of micro-organisms isolation and their culture media which enhance the cost competitive feasibility over nanoparticles synthesis by microorganisms. Hence, a review is compiled describing the bio-inspired syntheses of silver nanoparticles that provide advancement over physical and chemical methods which are eco-friendly, cost effective and more effective in a variety of applications especially in bactericidal activities.
Green syntheses of silver nanoparticles using plant extracts
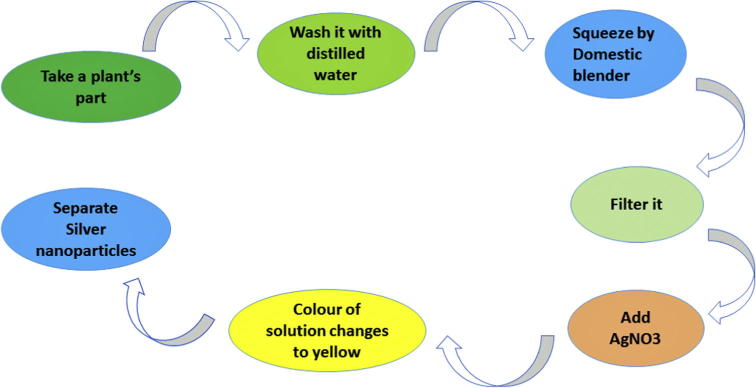
The use of plants as the production assembly of silver nanoparticles has drawn attention, because of its rapid, eco-friendly, non-pathogenic, economical protocol and providing a single step technique for the biosynthetic processes. The reduction and stabilization of silver ions by combination of biomolecules such as proteins, amino acids, enzymes, polysaccharides, alkaloids, tannins, phenolics, saponins, terpinoids and vitamins which are already established in the plant extracts having medicinal values and are environmental benign, yet chemically complex structures [20]. A large number of plants are reported to facilitate silver nanoparticles syntheses are mentioned (Table 1) and are discussed briefly in the presented review. The protocol for the nanoparticle syntheses involves: the collection of the part of plant of interest from the available sites was done and then it was washed thoroughly twice/thrice with tap water to remove both epiphytes and necrotic plants; followed with sterile distilled water to remove associated debris if any. These; clean and fresh sources are shade-dried for 10–15 days and then powdered using domestic blender. For the plant broth preparation, around 10 g of the dried powder is boiled with 100 mL of deionized distilled water (hot percolation method). The resulting infusion is then filtered thoroughly until no insoluble material appeared in the broth. To 10−3 M AgNO3 solution, on addition of few mL of plant extract follow the reduction of pure Ag(I) ions to Ag(0) which can be monitored by measuring the UV–visible spectra of the solution at regular intervals [21].
Table 1.
Green synthesis of silver nanoparticles by different researchers using plant extracts.
| Plants | Size (nm) | Plant’s part | Shape | References |
|---|---|---|---|---|
| Alternanthera dentate | 50–100 | Leaves | Spherical | [23] |
| Acorus calamus | 31.83 | Rhizome | Spherical | [24] |
| Boerhaavia diffusa | 25 | Whole plant | Spherical | [25] |
| Tea extract | 20–90 | Leaves | Spherical | [26] |
| Tribulus terrestris | 16–28 | Fruit | Spherical | [28] |
| Cocous nucifera | 22 | Inflorescence | Spherical | [37] |
| Abutilon indicum | 7–17 | Leaves | Spherical | [30] |
| Pistacia atlantica | 10–50 | Seeds | Spherical | [38] |
| Ziziphora tenuior | 8–40 | Leaves | Spherical | [31] |
| Ficus carica | 13 | Leaves | – | [32] |
| Cymbopogan citratus | 32 | Leaves | – | [39] |
| Acalypha indica | 0.5 | Leaves | – | [40] |
| Premna herbacea | 10–30 | Leaves | Spherical | [41] |
| Calotropis procera | 19–45 | Plant | Spherical | [42] |
| Centella asiatica | 30–50 | Leaves | Spherical | [43] |
| Argyreia nervosa | 20–50 | Seeds | – | [44] |
| Psoralea corylifolia | 100–110 | Seeds | – | [45] |
| Brassica rapa | 16.4 | Leaves | – | [46] |
| Coccinia indica | 10–20 | Leaves | – | [47] |
| Vitex negundo | 5 & 10–30 | Leaves | Spherical & fcc | [48] |
| Melia dubia | 35 | Leaves | Spherical | [49] |
| Portulaca oleracea | <60 | Leaves | – | [50] |
| Thevetia peruviana | 10–30 | Latex | Spherical | [51] |
| Pogostemon benghalensis | >80 | Leaves | – | [52] |
| Trachyspermum ammi | 87, 99.8 | Seeds | [53] | |
| Swietenia mahogani | 50 | Leaves | [54] | |
| Musa paradisiacal | 20 | Peel | [55] | |
| Moringa oleifera | 57 | Leaves | [56] | |
| Garcinia mangostana | 35 | Leaves | [57] | |
| Eclipta prostrate | 35–60 | Leaves | Triangles, pentagons, hexagons | [58] |
| Nelumbo nucifera | 25–80 | Leaves | Spherical, triangular | [59] |
| Acalypha indica | 20–30 | Leaves | Spherical | [60] |
| Allium sativum | 4–22 | Leaves | Spherical | [61] |
| Aloe vera | 50–350 | Leaves | Spherical, triangular | [62] |
| Citrus sinensis | 10–35 | Peel | Spherical | [63] |
| Eucalyptus hybrid | 50–150 | Peel | [64] | |
| Memecylon edule | 20–50 | Leaves | Triangular, circular, hexagonal | [65] |
| Nelumbo nucifera | 25–80 | Leaves | Spherical, triangular | [66] |
| Datura metel | 16–40 | Leaves | Quasilinear superstructures | [67] |
| Carica papaya | 25–50 | Leaves | [68] | |
| Vitis vinifera | 30–40 | Fruit | [69] |
A vast segment of flora had been utilized for the preparation of silver nanoparticles. Different plants and their respective portions have been exploited for the same as well. The green rapid syntheses of spherical shaped silver nanoparticles with dimensions of 50–100 nm were observed using Alternanthera dentate aqueous extract. The reduction of silver ions to silver nanoparticles by this extract was completed within 10 min. The extracellular silver nanoparticles syntheses by aqueous leaf extract validate quick, simple, economical process comparable to chemical and microbial methods. These silver nanoparticles exhibit antibacterial activity against Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumonia and Enterococcus faecal [22]. Acorus calamus was also used for the synthesis of silver nanoparticles to evaluate its antioxidant, antibacterial as well as anticancer effects [23]. Boerhaavia diffusa plant extract was used as a reducing agent for green synthesis of silver nanoparticles. XRD and TEM analysis revealed an average particle size of 25 nm of silver nanoparticles having face-centred cubic(fcc) structure with spherical shape. These nanoparticles were tested for antibacterial activity against three fish bacterial pathogens, viz. Pseudomonas fluorescens, Aeromonas hydrophila and Flavobacterium branchiophilum and demonstrated highest sensitivity towards F. Branchiophilumin in comparison with other two bacteria [24].
The relatively high levels of the steroids, sapogenins, carbohydrates and flavonoids act as reducing agents and phyto-constituents as the capping agents which provide stability to silver nanoparticles. The synthesized nanoparticles found to be of average size around 7–17 nm and are of spherical shaped. These nanoparticles were found to have a crystalline structure with face cantered cubic geometry as studied by XRD method. By using tea as a capping agent, 20–90 nm silver nanoparticles were synthesized with crystalline structure. Reaction temperature and the dosage of the tea extract showed an effect on the production efficiency and formation rate of nanoparticles [25]. The size of spherical shaped silver nanoparticles is ranging from 5 to 20 nm, as evident by TEM. With increasing intensity of extract during the period of incubation, silver nanoparticles showed gradual change in colour of the extracts to yellowish brown with callus extract of the salt marsh plant, Sesuvium portulacastrum L. [26]. The dried fruit body extract of the plant, Tribulus terrestris L. was mixed with silver nitrate in order to synthesize silver nanoparticles. The spherical shaped silver nanoparticles having size in range of 16–28 nm were achieved using this extract with antibacterial property observed by Kirby-Bauer method against multi-drug resistant bacteria such as Streptococcus pyogens, Pseudomonas aeruginosa, Bacillus subtilis, Escherichia coli and Staphylococcus aureus [27]. A silver nanoparticle of size 22 nm was synthesized using extracts of the tree Cocous nucifera in ethyl acetate and methanol (in ratio of EA:M40:60). It showed significant antimicrobial activity against human bacterial pathogens, viz. Salmonella paratyphi, Klebsiella pneumoniae, Bacillus subtilis and Pseudomonas aeruginosa [28].
A stable and spherical shaped silver nanoparticle was synthesized using extract of Abutilon indicum. These nanoparticles show high antimicrobial activities against S. typhi, E. coli, S. aureus and B. substilus microorganisms [29]. Ziziphoratenuior leaves were also used to prepare the silver nanoparticles and different techniques were employed to characterize these nanoparticles. Transmission electron microscopy (TEM) analysis showed that these nanoparticles were spherical and uniformly distributed having size from 8 to 40 nm, functionalized with biomolecules that have primary amine group, carbonyl group, hydroxyl groups and other stabilizing functional groups as shown by FTIR spectroscopic technique [30].
In a recent report, these nanoparticles have been synthesized on irradiation using an aqueous mixture of Ficuscarica leaf extract [31]. The silver nanoparticles were formed after three hour of incubation at 37 °C using aqueous solution of 5 mM silver nitrate. Cymbopogan citratus (DC) stapf (commonly known as lemon grass) a native aromatic herb from India and also cultivated in other tropical and subtropical countries showed strong antibacterial effect against P. aeruginosa, P. mirabilis, E. coli, Shigella flexaneri, S. Somenei and Klebsiella pneumonia [32].
Silver nanoparticles were rapidly synthesized by Krishnaraj et al. using leaf extract of Acalypha indica and the formation of nanoparticles was observed within 30 min [33]. Formation of stable silver nanoparticles at different concentration of AgNO3 gives mostly spherical particles with diameter ranging from 15 to 50 nm. In the pursuit of making the nanoscale-research greener, the utilization of the reductive potency of a common by-product of food processing industry i.e. orange peel (Citrussinensis) has been reported to prepare polymer bio-mimetic template “green” silver nanoparticles. TEM imaging showed well dispersed spherical articles of 3–12 nm size. It was also interesting to note that the highest fraction of particles had a diameter of 6 nm [34]. A facile and rapid biosynthesis of silver nanoparticles was reported by Dwivedi et al. from an obnoxious weed Chenopodium album. The leaf extract was prepared and successfully used for the synthesis of silver nanoparticles and gold nanoparticles having the size in range of 10–30 nm. The spherical nanoparticles were observed at higher leaf extract concentration, as infer from the TEM imaging [35].
Silver nanoparticles were synthesized on reduction of silver nitrate solution by aqueous extract of Azadirachta indica leaves by Prathna et al. and the growth kinetics of silver nanoparticles was investigated having size of 10–35 nm. Colloidal silver nanoparticles were synthesized by an easy green method using thermal treatment of aqueous solutions of silver nitrate and natural rubber latex extracted from Hevea brasilensis. The silver nanoparticles presented diameter ranging from 2 nm to 10 nm and had spherical shape with face centred cubic (fcc) crystalline structure [36].
Applications of silver nanoparticles
Due to their anti-bacterial properties, silver nanoparticles have been used most widely in the health industry, food storage, textile coatings and a number of environmental applications. In spite of decades of its use, it is important to note that the evidences of toxicity of silver are still not clear. Products prepared with silver nanoparticles have been approved by a range of accredited bodies including the US FDA, US EPA, Korea’s Testing, SIAA of Japan and Research Institute for Chemical Industry and FITI Testing and Research Institute [34]. The antimicrobial properties of silver nanoparticles have also been exploited both in the medicine and at home. Silver sulfadiazine creams use sometimes to prevent infection at the burn site and at least one appliance company has incorporated silver into their washing machines. Currently silver is used in the expanding field of nanotechnology and appears in many consumer products that include baby pacifiers, acne creams, and computer’s keyboard, clothing (e.g. socks and athletic wear) that protects from emitting body odour in addition to deodorizing sprays.
It is a well-known fact that silver nanoparticles and their composites show greater catalytic activities in the area of dye reduction and their removal. Kundu et al. studied the reduction of methylene blue by arsine in the presence of silver nanoparticle [70]. Mallick et al. studied the catalytic activity of these nanoparticles on the reduction of phenosafranine dye [71]. In this study, the application of silver nanoparticles as an antimicrobial agent was also investigated by growing E. coli on agar plates and in liquid LB medium, both supplemented with silver nanoparticles [72]. Single silver nanoparticles were applied to investigate membrane transport in living microbial cells (P. aeruginosa) in real times [73]. The triangular silver nanoparticles fabricated by nanosphere lithography indeed function as sensitive and selective nanoscale affinity biosensors. These nanosensors retain all of the other desirable features of Surface Plasmon Resonance (SPR) spectroscopy which is the fundamental principle behind many colour based biosensor applications and by changing nanoparticles size and shape, these nanosensors possess at least two unique characteristics: (i) modest refractive sensitivity and (ii) a short-range, sensing length scale determined by the characteristic decay length of the local electromagnetic field. These two factors combine to yield an area of mass sensitivity of ∼100–1000 pg/mm2, which is only a factor of 100 poorer than the best propagating SPR sensitivities [74].
Silver nanoparticles synthesized through green method have been reported to have biomedical applications as well as in controlling the pathogenic microbes. In a study, silver nanoparticles were synthesized using aqueous Piper longum fruit extract. The aqueous P. longum fruit extract and the green synthesized silver nanoparticles showed powerful antioxidant properties in vitro antioxidant assays [75]. The toxicity of starch-coated silver nanoparticles was studied using normal human lung fibroblast cells (IMR-90) and human glioblastoma cells (U251). The toxicity was evaluated using changes in cell morphology, cell viability, metabolic activity, and oxidative stress. These nanoparticles produced ATP content of the cell causing damage to mitochondria and increased production of reactive oxygen species (ROS) in a dose-dependent manner. DNA damage, as measured by single cell gel electrophoresis (SCGE) and cytokinesis blocked micronucleus assay (CBMN), was also dose-dependent and more prominent in the cancer cells [76]. The high frequency electrical behaviour of nanosilver based conductors is up to 220 GHz. [77]. Silver nanoparticles have proven to exert antiviral activity against HIV-1 at non-cyto-toxic concentrations, but the mechanism underlying their HIV-inhibitory activity has been not fully elucidated. These silver nanoparticles were evaluated to elucidate their mode of antiviral action against HIV-1 using a panel of different in vitro assays [78]. Special interest has been directed at providing enhanced bio-molecular diagnostics, including SNP detection gene expression profiles and biomarker characterization. These strategies have been focused on the development of nanoscale devices and platforms that can be used for single molecule characterization of nucleic acid, DNA or RNA, and protein at an increased rate when compared to traditional techniques [79].
Antimicrobial property of silver nanoparticles and its mechanism
Silver metal has been used widely across the civilizations for different purposes. Many societies use silver as jewellery, ornamentation and fine cutlery. Silver as jewellery, wares and cutlery was considered to impart health benefits to the users. Silver has a long history of anti-microbial use to discourage contamination of microbes dating back to the Phoenicians who used silver as a natural biocide to coat milk bottles. Silver is a well-known antimicrobial agent against a wide range of over 650 microorganisms from different classes such as gram-negative and gram-positive bacteria, fungi or viruses. More recently the metal is finding use in the form of silver nanoparticles. In ancient Indian medical system (called Ayurveda), silver has been described as therapeutic agent for many diseases. In 1884, during childbirth it became a common practice to administer drops of aqueous silver nitrate to new-born’s eyes to prevent the transmission of Neisseria gonorrhoea from infected mothers. Out of all the metals with antimicrobial properties, it was found that silver has the most effective antibacterial action and is least toxic to animal cells. Silver became commonly used in medical treatments, such as those of wounded soldiers in World War I, to deter microbial growth [80]. The medical properties of silver have been known for over 2000 years [81]. Silver is generally used in the nitrate form to induce antimicrobial effect but when silver nanoparticles are used, there is a huge increase in the surface area available for the microbes to be exposed to. Silver nanoparticles synthesized using plant extracts (from different sources) have been used for analysing their antimicrobial activities against different microbes (Table 2).
Table 2.
Antimicrobial activities of silver nanoparticles synthesized using plant extracts.
| Biological entity | Test microorganisms | Method | References |
|---|---|---|---|
| Alternanthera dentate | Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia and, Enterococcus faecalis | [23] | |
| Boerhaavia diffusa | Aeromonas hydrophila, Pseudomonas fluorescens and Flavobacterium branchiophilum | [15] | |
| Tea | E. coli | [26] | |
| Tribulus terrestris | Streptococcus pyogens, Pseudomonas aeruginosa, Escherichia coli, Bacillus subtilis and Staphylococcus aureus | Kirby-Bauer | [28] |
| Cocous nucifera | Klebsiella pneumoniae, Bacillus subtilis, Pseudomonas aeruginosa and Salmonella paratyphi | [38] | |
| Aloe vera | E. coli | Standard plate count | [91] |
| Solanus torvum | P. aeruginosa, S. aureus, A. flavus and Aspergillus niger | Disc diffusion | [92] |
| Trianthema decandra | E. coli and P. aeruginosa | Disc diffusion | [93] |
| Argimone mexicana | Escherichia coli; Pseudomonas aeruginosa; Aspergillus flavus | Disc diffusion for bacteria and food poisoning for fungi | [94] |
| Abutilon indicum | S. typhi, E. coli, S. aureus and B. substilus | [30] | |
| Cymbopogan citratus | P. aeruginosa, P. mirabilis, E. coli, Shigella flexaneri, S. somenei and Klebsiella pneumonia | Disc diffusion | [40] |
| Svensonia hyderabadensis | A. niger, Fusarium oxysporum, Curvularia lunata and Rhizopus arrhizus | Disc diffusion | [95] |
The antimicrobial properties of silver nanoparticles depend on:
-
1.
Size and environmental conditions (size, pH, ionic strength).
-
2.
Capping agent.
The exact mechanisms of antimicrobial or toxicity activities by silver nanoparticles are still in investigation and a well debated topic. The positive charge on the Ag ions is suggested vital for antimicrobial activities. In order for silver to have any antimicrobial properties, it must be in its ionized form. In its ionized form, silver is inert but on coming in contact with moisture it releases silver ions [83]. Ag+ ions are able to form complexes with nucleic acids and preferentially interact with the nucleosides rather than with the phosphate groups of nucleic acids. Thus, all forms of silver or silver containing compounds with observed antimicrobial properties are in one way or another sources of silver ions (Ag+); these silver ions may be incorporated into the substance and released slowly with time as with silver sulfadiazine, or the silver ions can come from ionizing the surface of a solid piece of silver as with silver nanoparticles [86], [87]. There is some literature showing the electrostatic attraction between positively charged nanoparticles and negatively charged bacterial cells [82] and they are suggested to be most suitable bactericidal agent [84], [85]. These nanoparticles have been shown to accumulate inside the membrane and can subsequently penetrate into the cells causing damage to cell wall or cell membranes. It is thought that silver atoms bind to thiol groups (—SH) of enzymes forming stable S—Ag bonds with thiol containing compounds and then it causes the deactivation of enzymes in the cell membrane that involve in trans membrane energy generation and ion transport. It was proposed that Ag(I) ion enters the cell and intercalates between the purine and pyrimidine base pairs disrupting the hydrogen bonding between the two anti-parallel strands and denaturing the DNA molecule. Bacterial cell lysis could be one of reason for its antibacterial property. Nanoparticles modulated phosphotyrosine profile of bacterial peptide that in turn affects signal transduction and inhibited growth of micro-organisms. Antibacterial effect is dose-dependent and is independent of acquisition of resistance by bacteria against antibiotics. E. coli cells treated with silver nanoparticles found to be accumulated in the bacterial membrane which results in the increase in permeability and death of cell.
Gram-positive bacteria are less susceptible to Ag+ than gram-negative bacteria. This is due to; the gram positive bacterial cell wall made up of peptidoglycan molecules and has more peptidoglycan than gram-negative bacteria. As cell wall of gram positive is thicker, as peptidoglycan is negatively charged and silver ions are positively charged; more silver may get stuck by peptidoglycan in gram-positive bacteria than in gram-negative bacteria. The decreased liability of gram-positive bacteria can also simply be explained by the fact that the cell wall of gram-positive bacteria is thicker than that of gram-negative bacteria [80]. Other mechanisms involving interaction of silver molecules with biological macromolecules such as enzymes and DNA through an electron-release mechanism [88] or free radical production [80] have been proposed. The inhibition of cell wall synthesis as well as protein synthesis shown to be induced by silver nanoparticles has been suggested by some literatures with the proteomic data having evidence of accumulation of envelope protein precursor or destabilization of outer membrane, which finally leads to ATP leaking [89]. Nanosilver is a much effective and a fast-acting fungicide against a broad spectrum of common fungi including genera such as Aspergillus, Candida and Saccharomyces [90].
The multi-resistant pathogens due to antigenic shifts and/or drifts are ineffectively managed with current medications. This resistance to medication by pathogens has become a stern problem in public health; therefore, there is a strong requirement to develop new bactericides and virucides. Silver is having a long history of use as an antiseptic and disinfectant and is able to interact with disulphide bonds of the glycoprotein/protein contents of microorganisms such as viruses, bacteria and fungi. Both silver nanoparticles and silver ions can change the three dimensional structure of proteins by interfering with disulphide bonds and block the functional operations of the microorganism [30], [96], [97]. Advancement of this route (green synthesis) over chemical and physical method is that it is cost effective, environment friendly, easily scaled up for large scale synthesis and there is no need to use high energy, pressure, temperature and toxic chemicals [15], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100]. The use of environmentally benign materials like bacteria, fungi, plant extracts and enzymes for the syntheses of silver nanoparticles offers numerous benefits of eco-friendly and compatibility for pharmaceutical and other biomedical applications as they do not use toxic chemicals for the synthesis protocol. These disadvantages insisted the use of novel and well refined methods that opened doors to explore benign and green routes for synthesizing nanoparticles (see Fig. 3).
Fig. 3.
Protocol for synthesis of silver nanoparticles using plant extract.
Conclusions
Nature has elegant and ingenious ways of creating the most efficient miniaturized functional materials. An increasing awareness towards green chemistry and use of green route for synthesis of metal nanoparticles lead a desire to develop environment-friendly techniques. Benefit of synthesis of silver nanoparticles using plant extracts is that it is an economical, energy efficient, cost effective; provide healthier work places and communities, protecting human health and environment leading to lesser waste and safer products. Green synthesized silver nanoparticles have significant aspects of nanotechnology through unmatched applications. For the syntheses of nanoparticles employing plants can be advantageous over other biological entities which can overcome the time consuming process of employing microbes and maintaining their culture which can lose their potential towards synthesis of nanoparticles. Hence in this regard; use of plant extract for synthesis can form an immense impact in coming decades.
Many reports have been published about the syntheses of silver nanoparticles using plant extracts like those as already discussed. There is still a need for commercially viable, economic and environment friendly route to find capacity of natural reducing constituent to form silver nanoparticles which has not yet been studied. There is a significant variation in chemical compositions of plant extract of same species when it collected from different parts of world and may lead to different results in different laboratories. This is the major drawback of syntheses of silver nanoparticles using plant extracts as reducing and stabilizing agents and there is need to resolve this problem. On identifying biomolecules present in the plant which are responsible for mediating the nanoparticles production for rapid single step protocol to overcome the above said problem can give a new facelift towards green syntheses of silver nanoparticles.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgement
The author, Shakeel Ahmed gratefully acknowledges financial support from the University Grants Commission (UGC), New Delhi in the form of Junior Research Fellowship. Corresponding author Saiqa Ikram is thankful to grant (AC-6(15)/RO-2014) sponsored by Jamia Millia Islamia, New Delhi.
Biographies

Shakeel Ahmed was born and raised in a small village Dhangri-Doba, in the Rajouri District of Jammu and Kashmir, India. He obtained his B.Sc. from Govt. P.G. College, Rajouri, University of Jammu, Jammu and later M.Sc. in Materials Chemistry from Jamia Millia Islamia, New Delhi, India. He was awarded with Junior Research Fellowship by UGC, New Delhi. Since September 2013, he is currently pursuing his Ph.D. at the Jamia Millia Islamia (a central university), New Delhi with group of Dr. Saiqa Ikram, where he is working on synthesis of biopolymer blended films for antimicrobial food packaging. His area of interest is polymer nanocomposites, green-technology, biocomposites, green synthesis of silver nanoparticles and biodegradable food packaging.

Mudasir Ahmad was born in 1988 in Dadasara-Tral Kashmir, India. Decade after matriculation from MPHS, he received his M.Sc chemistry and now pursuing his Doctorate in Chemistry from Jamia Millia Islamia (a central University), New Delhi. Nowadays, his current interest is focused in the development of new reactions and new methodologies for the synthesis of green adsorbent for the removal of heavy metals from waste water, metal complexes and nanoparticles.

Babu Lal Swami was born in Jaipur, India. He has completed his B.Sc. in 2002 and subsequently received his M.Sc. in 2008 from University of Rajasthan, India. He has been awarded as a Junior Research Fellow from University Grant Commission, India, and is presently working for his Ph.D. on Schiff base Ion selective electrodes at Jamia Millia Islamia (a central university), New Delhi, India. His area of interest is in green chemistry and ion selective electrodes.

Saiqa Ikram was born in 1973. She got her Master’s degree from one of the most prestigious college from one of the oldest university; Meerut University which has a glorious past in the freedom fighting movement of India. She completed her Ph.D. from University of Delhi, Delhi in 2000 in the area of polymer technology. During her Ph.D. she was awarded with Junior and Senior Research Fellowship/s from University Grants Commission and Council of Scientific & Industrial Research; the two most prestigious government funding organization in country. Later she joined as a Research Associate (again sponsored by CSIR, India) in Indian Institute of Technology, Delhi India. She is currently working as an Assistant Professor in Department of Chemistry, Faculty of Natural Sciences, Jamia Millia Islamia, (A Central University by an Act of Parliament) since February 2006. She has more than 20 peer reviewed research articles and a co-inventor in a patent. Her research area of interest is in biopolymers, green chemistry, biocomposites and green synthesis of nanoparticles.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Korbekandi H, Iravani S. Silver nanoparticles, the delivery of nanoparticles. In: Hashim Abbass A., editor, ISBN: 978-953-51-0615-9, InTech; 2012.
- 2.Khalil K.A., Fouad H., Elsarnagawy T., Almajhdi F.N. Preparation and characterization of electrospun PLGA/silver composite nanofibers for biomedical applications. Int J Electrochem Sci. 2013;8:3483–3493. [Google Scholar]
- 3.Kaviya S.S.J., Viswanathan B. Green synthesis of silver nanoparticles using Polyalthia longifolia leaf extract along with D-sorbitol. J Nanotech. 2011:1–5. [Google Scholar]
- 4.Ahmad A., Mukherjee P., Senapati S., Mandal D., Khan M.I., Kumar R., Sastry M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B: Biointerfaces. 2003;28:313–318. [Google Scholar]
- 5.Klaus-Joerger T., Joerger R., Olsson E., Granqvist C. Bacteria as workers in the living factory: metal accumulating bacteria and their potential for materials science. Trends Biotechnol. 2001;19:15–20. doi: 10.1016/s0167-7799(00)01514-6. [DOI] [PubMed] [Google Scholar]
- 6.Popescu M., Velea A., Lorinczi A. Biogenic production of nanoparticles. Dig J Nanomater Bios. 2010;5(4):1035–1040. [Google Scholar]
- 7.Baruwati B., Polshettiwar V., Varma R.S. Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem. 2009;11:926–930. [Google Scholar]
- 8.Elghanian R., Stohoff J.J., Mucic R.C., Letsinger R.L., Mirkin C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science. 1997;277:1078. doi: 10.1126/science.277.5329.1078. [DOI] [PubMed] [Google Scholar]
- 9.Hurst S.J., Lytton-Jean A.K.R., Mirkin C.A. Maximizing DNA loading on a range of gold nanoparticle sizes. Anal Chem. 2006;78(24):8313–8318. doi: 10.1021/ac0613582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran Q.H., Van Quy Nguyen V.Q., Le A.T. Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives. Adv Nat Sci: Nanosci Nanotechnol. 2013;4:1–21. [Google Scholar]
- 11.Iravani S., Korbekandi H., Mirmohammadi S.V., Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci. 2014;9(6):385–406. [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy G.A.K., Joy J.M., Mitra T., Shabnam S., Shilpa T. Nano silver – a review. Int J Adv Pharm. 2012;2(1):09–15. [Google Scholar]
- 13.Samberg M.E., Oldenburg S.J., Monteiro-Riviere N.A. Evaluation of silver nanoparticle toxicity in vivo skin and in vitro keratinocytes. Environ Health Persp. 2010;118(3):407–413. doi: 10.1289/ehp.0901398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sintubin L., De Gusseme B., Van der Meeren P., Pycke B.F., Verstraete W., Boon N. The antibacterial activity of biogenic silver and its mode of action. Appl Microbiol Biotechnol. 2011;91:153–162. doi: 10.1007/s00253-011-3225-3. [DOI] [PubMed] [Google Scholar]
- 15.Vijay Kumar P.P.N., Pammi S.V.N., Kollu P., Satyanarayana K.V.V., Shameem U. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti-bacterial activity. Ind Crops Prod. 2014;52:562–566. [Google Scholar]
- 16.Prathna T.C., Chandrasekaran N., Raichur A.M., Mukherje A. Kinetic evolution studies of silver nanoparticles in a bio-based green synthesis process. Colloids Surf A, Physicochem Eng Aspects. 2011;37:212–216. [Google Scholar]
- 17.Daniel M.-C., Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 18.Dhuper S., Panda D., Nayak P.L. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Mangifera indica. Nano Trends: J Nanotech App. 2012;13(2):16–22. [Google Scholar]
- 19.Kalishwaralal K., Deepak V., Pandian R.K., Kottaisamy Barath-mani S.M., Kartikeyan K.S., Gurunathan B.S. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf B: Biointerfaces. 2010;77:257–262. doi: 10.1016/j.colsurfb.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni N., Muddapur U. Biosynthesis of metal nanoparticles: a review. J Nanotechnol. 2014:1–8. [Google Scholar]
- 21.Sahayaraj K, Rajesh S. Bionanoparticles: synthesis and antimicrobial applications, science against microbial pathogens: communicating current research and technological advances. In: Méndez-Vilas A, editor, FORMATEX; 2011. p. 228–44.
- 22.Kumar D.A., Palanichamy V., Roopan S.M. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2014;127:168–171. doi: 10.1016/j.saa.2014.02.058. [DOI] [PubMed] [Google Scholar]
- 23.Nakkala J.R., Mata R., Kumar Gupta A., Rani Sadras S. Biological activities of green silver nanoparticles synthesized with Acorous calamus rhizome extract. Eur J Med Chem. 2014;85:784–794. doi: 10.1016/j.ejmech.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Nakkala J.R., Mata R., Gupta A.K., Sadras S.R. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their antibacterial activity. Indus Crop Prod. 2014;52:562–566. [Google Scholar]
- 25.Suna Q., Cai X., Li J., Zheng M., Chenb Z., Yu C.P. Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloid Surf A: Physicochem Eng Aspects. 2014;444:226–231. [Google Scholar]
- 26.Nabikhan A., Kandasamy K., Raj A., Alikunhi N.M. Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf B: Biointerfaces. 2010;79:488–493. doi: 10.1016/j.colsurfb.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Gopinatha V., Ali M.D., Priyadarshini S., MeeraPriyadharsshini N., Thajuddinb N., Velusamy P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach. Colloid Surf B: Biointerface. 2012;96:69–74. doi: 10.1016/j.colsurfb.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Mariselvam R., Ranjitsingh A.J.A., Usha Raja Nanthini A., Kalirajan K., Padmalatha C., Mosae Selvakumar P. Green synthesis of silver nanoparticles from the extract of the inflorescence of Cocos nucifera (Family: Arecaceae) for enhanced antibacterial activity. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2014;129:537–541. doi: 10.1016/j.saa.2014.03.066. [DOI] [PubMed] [Google Scholar]
- 29.Ashok kumar S., Ravi S., Kathiravan V., Velmurugan S. Synthesis of silver nanoparticles using A. indicum leaf extract and their antibacterial activity. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2015;134:34–39. doi: 10.1016/j.saa.2014.05.076. [DOI] [PubMed] [Google Scholar]
- 30.Sadeghi B., Gholamhoseinpoor F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2015;134:310–315. doi: 10.1016/j.saa.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 31.Ulug B., HalukTurkdemir M., Cicek A., Mete A. Role of irradiation in the green synthesis of silver nanoparticles mediated by fig (Ficus carica) leaf extract. Spectrochim Part A: Mol Biomol Spectrosc. 2015;135:153–161. doi: 10.1016/j.saa.2014.06.142. [DOI] [PubMed] [Google Scholar]
- 32.Geetha N., Geetha T.S., Manonmani P., Thiyagarajan M. Green synthesis of silver nanoparticles using Cymbopogan Citratus(Dc) Stapf. Extract and its antibacterial activity. Aus J Basic Appl Sci. 2014;8(3):324–331. [Google Scholar]
- 33.Krishnaraj C., Jagan E.G., Rajasekar S. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B. 2010;76:50–56. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Veeraputhiran V. Bio-catalytic synthesis of silver nanoparticles. Int J Chem Tech Res. 2013;5(5):255–2562. [Google Scholar]
- 35.Dwivedi A.D., Gopal K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Physicochem Eng Aspects. 2010;369:27–33. [Google Scholar]
- 36.Ramya1 M., Subapriya M.S. Green synthesis of silver nanoparticles. Int J Pharm Med Biol Sci. 2012;1(1):54–61. [Google Scholar]
- 37.Mariselvam R., Ranjitsingh A.J.A., Usha Raja Nanthini A., Kalirajan K., Padmalatha C., Selvakumar M.P. Green synthesis of silver nanoparticles from the extract of the inflorescence of Cocos nucifera (Family: Arecaceae) for enhanced antibacterial activity. Spectrochim Part A: Mol Biomol Spectrosc. 2014;129:537–541. doi: 10.1016/j.saa.2014.03.066. [DOI] [PubMed] [Google Scholar]
- 38.Sadeghi B., Rostami A., Momeni S.S. Facile green synthesis of silver nanoparticles using seed aqueous extract of Pistacia atlantica and its antibacterial activity. Spectrochim Part A: Mol Biomol Spectrosc. 2015;134:326–332. doi: 10.1016/j.saa.2014.05.078. [DOI] [PubMed] [Google Scholar]
- 39.Masurkar S.A., Chaudhari P.R., Shidore V.B., Kamble S.P. Rapid biosynthesis of silver nanoparticles using Cymbopogan Citratus (Lemongrass) and its antimicrobial activity. Nano-Micro Lett. 2011;3(3):189–194. [Google Scholar]
- 40.Kumarasamyraja D., jeganathan N.S. Green synthesis of silver nanoparticles using aqueous extract of acalypha indica and its antimicrobial activity. Int J Pharm Biol Sci. 2013;4(3):469–476. [Google Scholar]
- 41.Kumar S., Daimary R.M., Swargiary M., Brahma A., Kumar S., Singh M. Biosynthesis of silver nanoparticles using Premna herbacea leaf extract and evaluation of its antimicrobial activity against bacteria causing dysentery. Int J Pharm Biol Sci. 2013;4(4):378–384. [Google Scholar]
- 42.Gondwal M., Pant G.J.N. Biological evaluation and green synthesis of silver nanoparticles using aqueous extract of Calotropis procera. Int J Pharm Biol Sci. 2013;4(4):635–643. [Google Scholar]
- 43.Rout A., Jena P.K., Parida U.K., Bindhani B.K. Green synthesis of silver nanoparticles using leaves extract of Centella asiatica L. For studies against human pathogens. Int J Pharm Biol Sci. 2013;4(4):661–674. [Google Scholar]
- 44.Thombre R., Parekh F., Patil N. Green synthesis of silver nanoparticles using seed extract of Argyreia nervosa. Int J Pharm Biol Sci. 2014;5(1):114–119. [Google Scholar]
- 45.Sunita D., Tambhale D., Parag V., Adhyapak A. Facile green synthesis of silver nanoparticles using Psoralea corylifolia. Seed extract and their in-vitro antimicrobial activities. Int J Pharm Biol Sci. 2014;5(1):457–467. [Google Scholar]
- 46.Narayanan K.B., Park H.H. Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur J Plant Pathol. 2014;140:185–192. [Google Scholar]
- 47.Kumar A.S., Ravi S., Kathiravan V. Green synthesis of silver nanoparticles and their structural and optical properties. Int J Curr Res. 2013;5(10):3238–3240. [Google Scholar]
- 48.Zargar M., Hamid A.A., Bakar F.A., Shamsudin M.N., Shameli K., Jahanshiri F. Green synthesis and antibacterial effect of silver nanoparticles using Vitexnegundo L. Molecules. 2011;16:6667–6676. doi: 10.3390/molecules16086667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kathiravan V., Ravi S., kumar S.A. Synthesis of silver nanoparticles from Meliadubia leaf extract and their in vitro anticancer activity. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2014;130:116–121. doi: 10.1016/j.saa.2014.03.107. [DOI] [PubMed] [Google Scholar]
- 50.Firdhouse M.J., Lalitha P. Green synthesis of silver nanoparticles using the aqueous extract of Portulaca oleracea (L) Asian J Pharm Clin Res. 2012;6(1):92–94. [Google Scholar]
- 51.Rupiasih N.N., Aher A., Gosavi S., Vidyasagar P.B. Green synthesis of silver nanoparticles using latex extract of Thevetia peruviana: a novel approach towards poisonous plant utilization. J Phys Conf Ser. 2013;423:1–8. [Google Scholar]
- 52.Gogoi S.J. Green synthesis of silver nanoparticles from leaves extract of ethnomedicinal plants Pogostemon benghalensis (B) O. Ktz. Adv Appl Sci Res. 2013;4(4):274–278. [Google Scholar]
- 53.Vijayaraghavan K., Nalini S., Prakash N.U., Madhankumar D. One step green synthesis of silvernano/microparticles using extracts of Trachyspermum ammi and Papaver somniferum. Colloid Surf B Biointerfaces. 2012;94:114–117. doi: 10.1016/j.colsurfb.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 54.Mondal S., Roy N., Laskar R.A., Sk I., Basu S., Mandal D. Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahogani JACQ) leaves. Colloids Surf B Biointerfaces. 2011;82:497–504. doi: 10.1016/j.colsurfb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Bankar A., Joshi B., Kumar A.R., Zinjarde S. Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf A. 2010;368:58–63. doi: 10.1016/j.colsurfb.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 56.Prasad T.N.V.K.V., Elumalai E. Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac J Trop Biomed. 2011;1:439–442. doi: 10.1016/S2221-1691(11)60096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veerasamy R., Xin T.Z., Gunasagaran S., Xiang T.F.W., Yang E.F.C., Jeyakumar N. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc. 2010;15:113–120. [Google Scholar]
- 58.Rajakumar G., Abdul Rahuman A. Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrata leaf extract against filariasis and malaria vectors. Acta Trop. 2011;118:196–203. doi: 10.1016/j.actatropica.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Santhoshkumar T., Rahuman A.A., Rajakumar G., Marimuthu S., Bagavan A., Jayaseelan C. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res. 2011;108:693–702. doi: 10.1007/s00436-010-2115-4. [DOI] [PubMed] [Google Scholar]
- 60.Krishnaraj C., Jagan E., Rajasekar S., Selvakumar P., Kalaichelvan P., Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B Biointerfaces. 2010;76:50–56. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Ahamed M., Khan M., Siddiqui M., AlSalhi M.S., Alrokayan S.A. Green synthesis, characterization and evaluation of biocompatibility of silver nanoparticles. Phys E Low Dimens Syst Nanostruct. 2011;43:1266–1271. [Google Scholar]
- 62.Chandran S.P., Chaudhary M., Pasricha R., Ahmad A., Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006;22:577–583. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- 63.Kaviya S., Santhanalakshmi J., Viswanathan B., Muthumary J., Srinivasan K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochem Acta A Mol Biomol Spectrosc. 2011;79:594–598. doi: 10.1016/j.saa.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 64.Dubey M., Bhadauria S., Kushwah B. Green synthesis of nanosilver particles from extract of Eucalyptus hybrida (safeda) leaf. Dig J Nanomater Biostruct. 2009;4:537–543. [Google Scholar]
- 65.Elavazhagan T., Arunachalam K.D. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int J Nanomed. 2011;6:1265–1278. doi: 10.2147/IJN.S18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santhoshkumar T., Rahuman A.A., Rajakumar G., Marimuthu S., Bagavan A., Jayaseelan C. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res. 2011;108:693–702. doi: 10.1007/s00436-010-2115-4. [DOI] [PubMed] [Google Scholar]
- 67.Kesharwani J., Yoon K.Y., Hwang J., Rai M. Phytofabrication of silver nanoparticles by leaf extract of Daturametel: hypothetical mechanism involved in synthesis. J Bionanosci. 2009;3:39–44. [Google Scholar]
- 68.Jain D., Daima H.K., Kachhwaha S., Kothari S. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their antimicrobial activities. Dig J Nanomater Biostruct. 2009;4:557–563. [Google Scholar]
- 69.Gnanajobitha G., Paulkumar K., Vanaja M., Rajeshkumar S., Malarkodi C., Annadurai G., Kannan C. Fruit-mediated synthesis of silver nanoparticles using Vitis vinifera and evaluation of their antimicrobial efficacy. 2013;3(67):1–6. [Google Scholar]
- 70.Kundu S., Ghosh S.K., Mandal M., Pal Bull T. Silver and gold nanocluster catalyzed reduction of methylene blue by arsine in micellar medium. Mater Sci. 2002;25:577–579. [Google Scholar]
- 71.Mallick K., Witcomb M., Scurrell M. Silver nanoparticle catalysed redox reaction: an electron relay effect. Mater Chem Phys. 2006;97:283–287. [Google Scholar]
- 72.Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 73.Nancy Xu X.H., Brownlow W.J., Kyriacou S.V., Wan Q., Viola J.J. Real-time probing of membrane transport in living microbial cells using single nanoparticle optics and living cell imaging. Biochemistry. 2004;43:10400–10413. doi: 10.1021/bi036231a. [DOI] [PubMed] [Google Scholar]
- 74.Larguinho M., Baptista P.V. Gold and silver nanoparticles for clinical diagnostics – from genomics to proteomics. J Proteom. 2012;75(10):2811–2823. doi: 10.1016/j.jprot.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 75.Haes A.J., Van Duyne R.P. A nanoscale optical biosensor: sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. J Am Chem Soc. 2002;124:10596–10604. doi: 10.1021/ja020393x. [DOI] [PubMed] [Google Scholar]
- 76.Reddy N.J., NagoorVali D., Rani M., SudhaRani S. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater Sci Eng C. 2014;34:115–122. doi: 10.1016/j.msec.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 77.AshaRani P.V., KahMun G.L., Hande M.P., Valiyaveetti S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. Am Chem Soc. 2009;3(2):279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 78.Lara H.H., Ayala-Nuñez N.V., Ixtepan-Turrent L., Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goyal R.N., Oyama M., Bachheti N., Singh S.P. Fullerene C60 modified gold electrode and nanogold modified indium tin oxide electrode for prednisolone determination. Bioelectrochemistry. 2009;74(2):272–277. doi: 10.1016/j.bioelechem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Ankanna S., Prasad T.N.V.K.V., Elumalai E.K., Savithramma N. Production of biogenic silver nanoparticles using Boswelliao valifoliolata stem bark. Dig J Nanomater Biostruct. 2010;5:369–372. [Google Scholar]
- 81.Prabhu S., Poulose E.K. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2(32):1. [Google Scholar]
- 82.Cao Y.W., Jin R., Mirkin C.A. DNA-modified core–shell Ag/Au nanoparticles. J Am Chem Soc. 2001;123:7961–7962. doi: 10.1021/ja011342n. [DOI] [PubMed] [Google Scholar]
- 83.Klueh U., Wagner V., Kelly S., Johnson A., Bryers J.D. Efficacy of silver-coated fabric to prevent bacterial colonization and subsequent device-based biofilm formation. J Biomed Mater Res Part B: Appl Biomater. 2000;53:621–631. doi: 10.1002/1097-4636(2000)53:6<621::aid-jbm2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 84.Wright J.B., Lam K., Hanson D., Burrell R.E. Efficacy of topical silver against fungal burn wound pathogens. Am J Infect Control. 1999;27(4):344–350. doi: 10.1016/s0196-6553(99)70055-6. [DOI] [PubMed] [Google Scholar]
- 85.Matthew Eby D., Schaeublin Nicole M., Farrington Karen E., Hussain Saber M., Johnson G.R. Lysozyme catalyzes the formation of antimicrobial silver nanoparticles. ACS Nano. 2009;3(4):984–994. doi: 10.1021/nn900079e. [DOI] [PubMed] [Google Scholar]
- 86.Yakabe Y., Sano T., Ushio H., Yasunaga T. Kinetic studies of the interaction between silver ion and deoxyribonucleic acid. Chem Lett. 1980;4:373–376. [Google Scholar]
- 87.Sondi I., Sondi B.S. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for gram negative bacteria. J Colloid Interface Sci. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 88.Sharma V.K., Yngard R.A., Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 2009;145:83–96. doi: 10.1016/j.cis.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Park J., Lim D.H., Lim H.J., Kwon T., Choi J.S., Jeong S. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem Commun. 2011;47:4382–4384. doi: 10.1039/c1cc10357a. [DOI] [PubMed] [Google Scholar]
- 90.Yu H., Chen M., Rice P.M., Wang S.X., White R.L., Sun S. Dumbbell-like bifunctional Au-Fe3O4 nanoparticles. Nano Lett. 2005;5(2):379–382. doi: 10.1021/nl047955q. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y., Yang D., Kong Y., Wang X., Pandoli O., Gao G. Synergetic antibacterial effects of silver nanoparticles@Aloe Vera prepared via a green method. Nano Biomed Eng. 2010;2(4):252–257. [Google Scholar]
- 92.Govindaraju K., Tamilselvan S., Kiruthiga V., Singaravelu G. Biogenic silver nanoparticles by Solanumtorvum and their promising antimicrobial activity. J Biopest. 2010;3(1):394–399. [Google Scholar]
- 93.Geethalakshmi R., Sarada D.V.L. Synthesis of plant-mediated silver nanoparticles using Trianthema decandra extract and evaluation of their anti-microbial activities. Int J Eng Sci Technol. 2010;2(5):970–975. [Google Scholar]
- 94.Khandelwal N., Singh A., Jain D., Upadhyay M.K., Verma H.N. Green synthesis of silver nanoparticles using Argimone mexicana leaf extract and evaluation of their antimicrobial activities. Dig J Nanomater Biostruct. 2010;5(2):483–489. [Google Scholar]
- 95.Sun S., Zeng H., Robinson D.B., Raoux S., Rice P.M., Wang S.X., Li G.J. Monodisperse MFe2O4 (M = Fe Co, Mn) nanoparticles. Am Chem Soc. 2004;126:273. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- 96.Jia X., Ma X., Wei D., Dong J., Qian W. Direct formation of silver nanoparticles in cuttlebone derived organic matrix for catalytic applications. Colloids Surf A, Physicochem Eng Aspects. 2008;30:234–240. [Google Scholar]
- 97.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 98.Singh A., Jain D., Upadhyay M.K., Khandelwal, Verma H.N. Dig J Nanomater Biostruct. 2010;5:48. [Google Scholar]
- 99.Sahayaraj K, Rajesh S. Bionanoparticles: synthesis and antimicrobial applications, science against microbial pathogens: communicating current research and technological advances. In: Méndez-Vilas A, editor, FORMATEX; 2011. p. 228–44.
- 100.Panigrahi T. Synthesis and characterization of silver nanoparticles using leaf extract of Azadirachta indica. 2013:0–69. [Google Scholar]